Abstract
IL-17 producing CD4 T cells (Th17) cells increase significantly with disease severity in myasthenia gravis (MG) patients. To suppress the generation of Th17 cells, we examined the effect of inhibiting retinoic acid receptor-related-orphan-receptor-C (RORγ), a Th17-specific transcription factor critical for differentiation. RORγ inhibition profoundly reduced Th17 cell frequencies, including IFN-γ and IL-17 co-producing pathogenic Th17 cells. Other T helper subsets were not affected. In parallel, CD8 T cell subsets producing IL-17 and IL-17/IFN-γ were increased in MG patients and inhibited by the RORγ inhibitor. These findings provide rationale for exploration of targeted Th17 therapies, including ROR-γ inhibitors, to treat MG patients.
Keywords: myasthenia gravis, Th17 cell, AChR, IL-17, receptor-related orphan receptor gamma, interferon-gamma
1. Introduction
Myasthenia gravis (MG) is a B cell mediated, T cell dependent autoimmune disease (Conti-Fine et al., 2006). In MG, autoantibodies directed against neuromuscular junction proteins, most commonly the acetylcholine receptor (AChR), cause impaired neuromuscular transmission and weakness of voluntary muscles that can be life threatening. Most patients with MG require treatment with immunosuppressive drugs such as prednisone, azathioprine, and mycophenolate mofetil (Sanders and Guptill, 2014). These drugs broadly suppress the immune system and often cause unwanted side effects in patients. Therefore, there is a unmet need for targeted treatments in MG that may reduce off target side effects and yield better efficacy.
Th17 cells are a distinct subset of IL-17 producing CD4 T cells that play an important role in clearing extracellular pathogens including gram negative bacteria and systemic fungal infections (Chen and Kolls, 2013; Hernandez-Santos et al., 2013; Oukka, 2008). Differentiation into Th17 cells require the activation of the transcription factor retinoic acid receptor-related-orphan-receptor-C (RORγ) along with TGF-β and IL-6 cytokines (Korn et al., 2008; Lee et al., 2012; Yang et al., 2008; Zielinski et al., 2012). Studies have demonstrated that IL-17 mediates inflammation in several autoimmune diseases, including rheumatoid arthritis, psoriasis, inflammatory bowel disease, and multiple sclerosis (Noack and Miossec, 2014; Uyttenhove and Van Snick, 2006; Zhu and Qian, 2012). In rheumatoid arthritis and psoriasis, clinical trials targeting Th17 cell activity with anti-IL-17 monoclonal antibodies demonstrated positive results and are currently available to treat patients (2015; Campa et al., 2016; Leonardi et al., 2012; Papp et al., 2012). Therapeutics directly targeting RORγ are also in development for the treatment of autoimmune disease. In preclinical studies, a small molecule RORγ inhibitor was demonstrated to bind the ligand binding domain of RORγ resulting in displacement of a co-activator peptide, down-regulation of permissive histone H3 acetylation, and methylation of the IL17A and IL23R promoter regions (Guendisch et al., 2017). In an antigen-induced arthritis model with this RORγ inhibitor, selective blockade of the proinflammatory Th17/IL-17A pathway was demonstrated with a dose dependent reduction in knee swelling in treated rats (Guendisch et al., 2017).
Several lines of evidence in animal models and patient studies suggest that IL-17 and Th17 cells are important to MG pathogenesis, and IL-17 directed therapies may be a promising, targeted treatment for autoimmune MG (Aguilo-Seara et al., 2017; Cao et al., 2016; Gradolatto et al., 2012; Roche et al., 2011; Schaffert et al., 2015; Xie et al., 2016; Yi et al., 2014). IL-17 knockout mice with induced experimental autoimmune myasthenia gravis (EAMG) demonstrated a less severe phenotype consisting of no muscle weakness, low AChR antibody levels, and evidence of reduced pro-inflammatory signaling compared with wildtype BL/6 EAMG mice (Aguilo-Seara et al., 2017). In human studies increased circulating IL-17 levels in MG patients positively correlated with disease severity, and was highly associated with early onset disease in women with thymic hyperplasia (Wang et al., 2019; Xie et al., 2016). These and other studies lead us to believe IL-17 targeted treatments may benefit MG patients.
To determine the efficacy and feasibility of targeting Th17 cells in MG, we performed cellular analysis of IL-17 producing Th17 cells in AChR-MG patients and healthy controls, and then examined the effect of ROR-γ inhibition on suppressing Th17 cell differentiation. We demonstrate that AChR-MG patients exhibit higher frequencies of overall Th17 cells, as well as pathogenic Th17 cells, that coproduce IL-17+ and IFN-γ, compared with healthy controls. Furthermore, the frequency of IL-17+ CD4 T cells is associated with disease severity in AChR-MG. Inhibition of ROR-γ with a small molecule suppressed the generation of Th17 cells, including the pathogenic phenotype, without affecting the frequencies of other Th subsets. Collectively, these studies demonstrate a targeted strategy to inhibit Th17 development and CD4-mediated immunopathology in AChR-MG.
2. Materials and Methods
2.1. Study Population and controls
Fourty-Nine MG patients were enrolled from the Duke MG clinic. All patients had detectable anti-AChR binding antibodies according to commercially available testing (Mayo Medical Laboratory, Rochester, MN) as well as clinical and electrodiagnostic features consistent with the disease. Severity of MG disease was defined according to the MGFA Severity Class and MG-Manual Muscle Test (MG-MMT) (Jaretzki et al., 2000; Sanders et al., 2003). MG patients were grouped by MGFA severity classification 0-II (ocular/mild disease) or III-V (moderate/severe). Control blood samples were obtained from 23 healthy individuals, who were age- and gender-matched to MG patients as closely as possible. Controls were not receiving therapy for any chronic disease. This study was approved by the Duke University Institutional Review Board and written and informed consent was obtained from subjects.
2.2. Isolation and storage of peripheral blood mononuclear cells (PBMC)
Following venipuncture, peripheral blood was collected in acid-citrate-dextrose tubes (BD Vacutainer). Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll density gradient centrifugation and were resuspended in a 90% FBS (Gemini, Sacramento, CA) and 10% DMSO (Sigma-Aldrich, St. Louis, MO) freezing solution. PBMCs were viably cryopreserved and stored in vapor phase liquid nitrogen. Plasma was isolated after Ficoll density gradient centrifugation and stored at −80°C.
2.3. Polarization of IL-17 producing T cells
To polarize T cells into Th17 cells, we adapted “BD Bioscience’s (BDB) protocol for Generation of Human IL-17-Producing Cells in Vitro (2013).” 3×106 PBMCs were plated in 24-well flat bottom plates in R10 (RPMI 1640 media [Gibco] + 10% FBS + 1% Penicillin-Streptomycin-L-Glutamine [Sigma-Aldrich]) and stimulated with αCD3 (1μg/mL, BDB) and αCD28 (1μg/mL, BDB) in the presence of IL-6 (10ng/mL, BDB), IL-1β (10ng/mL, BDB), IL-23 (10ng/mL, R&D Systems), TGF-β1 (10ng/mL, BDB), along with anti-IL-4 (10μg/mL, BDB) and anti-IFN-γ (10μg/mL, BDB) for 7 days at 37°C in a 5% CO2 incubator. For the last 5 hours, cells were restimulated with PMA (1μg/mL, Sigma-Aldrich), ionomycin (0.25μg/mL, Sigma-Aldrich) and brefeldin A (BFA;1μg/mL, BDB).
2.4. Intracellular cytokine staining
For intracellular cytokine staining of cells at baseline and following 7 days of Th17 polarization, cells were stained with Zombie Violet (BL) at room temperature for 15 minutes. Next, cells were surface stained with anti-CXCR5 Alexa Fluor 647 (RF8B2, BDB), anti-CD14 Pacific Blue (M5E2, BL), anti-CD45RA Brilliant Violet 510 (HI100, BL), and anti-CCR7 PE-Dazzle 594 (G043H7, BL) for 25 minutes at 4°C. Following cell surface staining, cells were treated with Cytofix/Cytoperm (BDB) in accordance with the manufacturer’s recommendations. To detect intracellular markers, cells were stained with fluorescent antibodies for anti-CD3 APC-Cy7 (SK3), anti-CD4 PE-Cy7 (SK3), anti-CD8 Alexa Fluor 700 (SK1), anti-IFN-γ FITC (B27), anti-IL-17A PerCP-Cy5.5 (BL168), and anti-IL-4 PE (MP4–25D2) for 30 minutes at 4°C. The fluorescent antibodies used for intracellular staining were obtained from Biolegend. Lastly, cells were fixed with 1% paraformaldehyde (PFA) and acquired on a LSRII flow cytometer (BDB).
2.5. ROR-γ Inhibition Assay
Three million PBMCs were plated in a 24-well flat bottom plate in R10 and stimulated with the described Th17 cocktail or the Th17 cocktail plus a high affinity small molecule inhibitor of ROR-γ transcription factor, ROR-c (gift from Novartis) (Guendisch et al., 2017; Guntermann et al., 2017). Cells were placed in a 37°C 5% CO2 incubator for 7 days. Five hours prior to the end of in the incubation period, cells from each condition were re-stimulated with PMA and ionomycin in the presence of BFA. 50μM of ROR-c was selected for subsequent experiments because it maintained viability and provided maximum inihibition of IL-17 production.
2.6. Data analysis
The studied population of MG patients was compared with healthy controls. To determine whether Th17 cell frequencies changed with MG disease severity we compared ocular/mild generalized MG with moderate/severe MGFA Severity Classes. T cell subset frequencies were compared with and without ROR-γ inhibition. Data analysis was performed using Flowjo software (Tree Star, Ashland, OR). Student T-tests determined statistical significance (α<0.05) between groups. The p values were calculated using Prism software (Graph Pad, LaJolla, CA). Samples in which stimulation with αCD3 and αCD28 failed to generate increased IL-17 over background were not analyzed.
3. Results
3.1. Study population
The demographics of the study population are shown in Table 1. The average age of the MG population was 63 years, 47% were female, 76% were Caucasian, and 53% of patients had ocular or mild disease. 39% of patients were not receiving any immunotherapy at the time of blood collection, while the others were receiving immunomodulatory therapy predominantly with corticosteroids, azathioprine, and mycophenolate mofetil. There were no patients in MG crisis, 8 had undergone thymectomy in the remote past, and three had a thymoma. A total of 49 MG patients were enrolled in the study, and the studies associated with individual PBMC sample is marked with a Y (yes) and N (no) in Table 1.
Table 1.
MG patient demographics
| Subj # | Age (Yr) | Gender | Race | MGFA Severity Class | MG-MMT | Thymectomy | MG Immunosuppressives | F1 | F3 | F4 | F5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | Male | White | 1 | 6 | No | Pred 3.75 mg/d MMF 3000 mg/d | Y | Y | Y | Y |
| 2 | 75 | Female | White | 2A | 12 | No | Pred 10 mg/d MMF 1000 mg/d | Y | Y | Y | Y |
| 3 | 74 | Male | White | 2B | 15 | No | Pred 80 mg/d | Y | N | N | Y |
| 4 | 51 | Female | Black | 4A | 48 | No | none | Y | N | N | Y |
| 5 | 71 | Male | White | 2B | 2 | No | Pred 2.5 mg/d MMF 2500 mg/d | Y | N | N | Y |
| 6 | 67 | Male | White | 3B | 29 | No | none | Y | N | N | Y |
| 7 | 76 | Male | White | 3B | 18 | No | none | Y | Y | N | Y |
| 8 | 29 | Male | Black | 1 | 2 | No | Pred 10 mg/d AZA 200 mg/d | Y | N | N | Y |
| 9 | 48 | Female | White | 2B | 3 | No | AZA 350 mg/d | Y | N | N | Y |
| 10 | 61 | Female | White | 2A | 17 | No | Pred 15mg/d | N | N | N | Y |
| 11 | 81 | Male | White | 2A | 5 | No | MMF 2000 mg/d | Y | Y | N | Y |
| 12 | 77 | Male | White | 1 | 2 | No | Pred 2.5 mg/d | Y | N | N | Y |
| 13 | 76 | Female | White | 3B | 31 | No | none | Y | Y | N | Y |
| 14 | 36 | Female | Black | 2A | 8 | No | Pred 15 mg/d | Y | N | N | Y |
| 15 | 68 | Male | Black | 3A | 36 | Yes | TPEa | Y | Y | N | Y |
| 16 | 31 | Female | Asian | 3A | 18 | No | none | Y | Y | N | Y |
| 17 | 68 | Male | White | 2B | 5 | No | Pred 3 mg/d MMF 3000 mg/d | Y | Y | Y | Y |
| 18 | 47 | Male | Black | 4B | 13 | No | none | Y | Y | Y | Y |
| 19 | 80 | Male | White | 1 | 8 | No | MMF 1000 mg/d | Y | N | N | Y |
| 20 | 63 | Male | White | 2B | 14 | No | none | Y | Y | N | Y |
| 21 | 58 | Female | Black | 1 | 4 | Yes | AZA 200 mg/d | Y | Y | Y | Y |
| 22 | 73 | Female | White | 0 | 0 | No | MMF 2000 mg/d | Y | N | N | Y |
| 23 | 54 | Male | Black | 0 | 0 | Yes | MMF 1000 mg/d | Y | N | N | Y |
| 24 | 70 | Male | Black | 2B | 4 | No | none | Y | Y | N | Y |
| 25 | 72 | Female | White | 1 | 1 | No | MMF 2000 mg/d | Y | Y | Y | Y |
| 26 | 53 | Female | White | 2A | 12 | No | TPEa | N | N | N | Y |
| 27 | 48 | Male | White | 2B | 7 | No | none | Y | Y | N | Y |
| 28 | 79 | Female | White | 0 | 0 | No | MMF 2000 mg/d | Y | N | N | Y |
| 29 | 47 | Female | White | 2A | 12 | No | AZA 200 mg/d IVIga | Y | Y | Y | Y |
| 30 | 59 | Female | Black | 1 | 5 | Yes | AZA 200 mg/d | Y | Y | Y | Y |
| 31 | 66 | Female | White | 3A | 16 | No | RTXb MMF 2500 mg/d | Y | Y | Y | N |
| 32 | 66 | Male | White | 2B | 4 | Yes | None | Y | Y | N | N |
| 33 | 87 | Male | White | 1 | 3 | No | Pred 15 mg/d AZA 150 mg/d | Y | Y | N | N |
| 34 | 72 | Female | White | 0 | 2 | No | RTXa Pred 7.5 mg/d AZA 150 mg/d | Y | Y | N | N |
| 35 | 45 | Male | White | 3B | 14 | No | None | Y | Y | Y | Y |
| 36 | 71 | Male | White | 3B | 14 | No | none | Y | Y | Y | Y |
| 37 | 48 | Female | Black | 3A | 12 | Yes | AZA 200mg/d | Y | Y | Y | Y |
| 38 | 67 | Female | White | 3B | 21 | No | None | Y | Y | Y | Y |
| 39 | 65 | Male | White | 3B | 13 | No | None | Y | Y | N | Y |
| 40 | 61 | Male | White | 3B | 21 | No | None | Y | Y | Y | Y |
| 41 | 75 | Male | White | 3B | 14 | No | Pred 40 mg/d MMF 1000 mg/d | Y | Y | Y | Y |
| 42 | 67 | Female | White | 4A | 37 | No | Pred 20 mg/d | Y | Y | Y | Y |
| 43 | 61 | Male | White | 3A | 19 | No | None | Y | Y | Y | Y |
| 44 | 31 | Female | Black | 3A | 24 | Yes | None | Y | Y | Y | Y |
| 45 | 71 | Female | White | 3B | 13 | Yes | Pred 30 mg/d IVIga | Y | Y | Y | Y |
| 46 | 61 | Male | White | 3A | n/a | No | None | Y | Y | Y | Y |
| 47 | 86 | Female | White | 3B | 13 | No | Pred 60 mg/d TPEa | Y | Y | Y | Y |
| 48 | 77 | Male | White | 3B | 15 | No | MMF 500mg/d | Y | Y | Y | Y |
| 49 | 62 | Female | White | 3A | 24 | No | None | Y | Y | Y | Y |
Abbreviations: AZA=azathioprine; d=day; g=grams; MG=Myasthenia Gravis; mg=milligrams; MGFA=Myasthenia Gravis Foundation of America; MMF=mycophenolate mofetil; MG-MMT=myasthenia gravis manual muscle testing score at time of blood draw; n/a= not available; Pred=prednisone; RTX=rituximab, TPE=therapeutic plasma exchange.
Within 90 days of blood draw
Within 12 months of blood draw
3.2. Frequency of IL-17 producing CD4 T cells is associated with disease severity
To determine the capacity of CD4 T cells, from AChR-MG patients and healthy controls, to produce IL-17, we polarized PBMCs with αCD3/αCD28, IL-6, IL-1β, IL-23, TGF-β1, αIL-4, and αIFN-γ for seven days. As expected, following PMA and ionomycin restimulation, we observed an increase in IL-17 expressing CD4 (data not shown). Th17 cell frequencies in the overall MG sample population were not significantly increased compared with controls (3.19 ± 3.63 vs 1.72 ± 1.91, p<0.095 When AChR-MG patients were categorized according to disease severity, the frequency of IL-17+ CD4 T cells was significantly associated with disease severity, with moderate to severe disease AChR-MG patients expressing the highest levels of IL-17 (Figure 1). Additionally, immunosuppression status did not affect the frequency of IL-17+ CD4 T cells (data not shown). These findings support that AChR-MG patients have an enhanced capacity to produce IL-17 and that Th17 cell frequencies may serve as a biomarker of MG disease severity.
Figure 1. Th17 cells frequencies in MG are associated with disease severity.
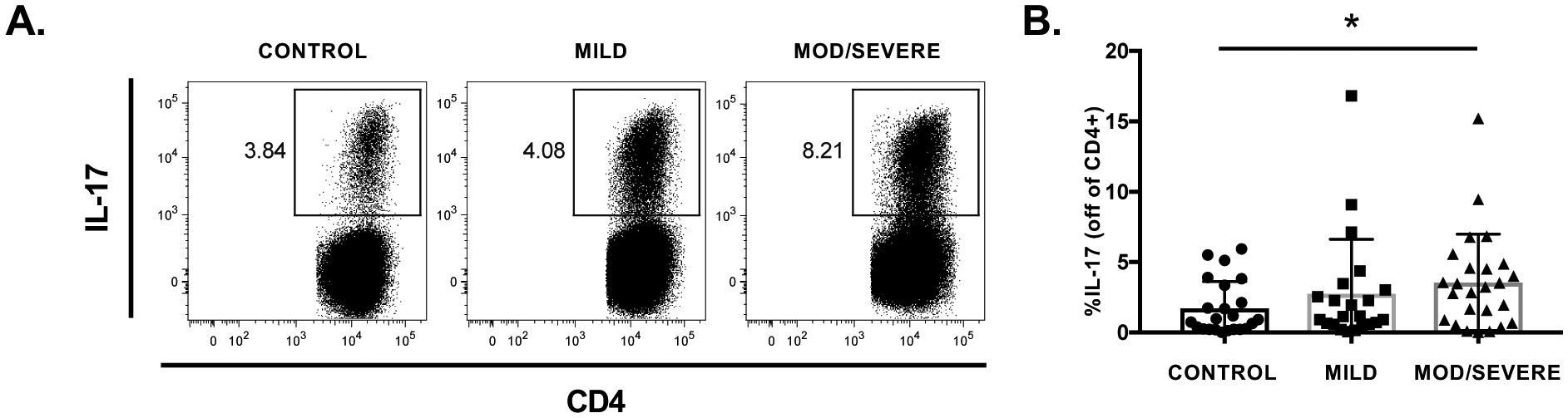
A: Representative flow plots of Th17 cell frequencies following Th17 cell polarization in a healthy control and a mild and severely affected MG patient. Th17 frequencies, indicated by are highest in the MG patient with severe disease. B) Th17 cell frequencies are increased in moderate/severe MG (n=25) compared with healthy controls (n=23), and mild MG (n=22). Statistical significance is represented as follows: * p≤0.05.
3.3. ROR-γ inhibitor suppresses the generation of Th17 cells.
Based on the increase in IL-17 frequency in AChR-MG and its association with disease severity, we investigated the efficacy of a small molecule inhibitor of ROR-γ transcription factor for suppression of Th17 cells. Initially, ROR-c was titrated in Th17 polarization experiments to identify the concentration at which ROR-c maximally inhibited IL-17 production without affecting cell viability. We observed a concentration dependent decrease in IL-17 production with the addition from 1μM to 100μM of ROR-γ inhibitor (Figure 2A, B), and cell viability diminished with concentrations greater than 100μM (Figure 2C). To maximize inhibition of IL-17 production and maintain cell viability 50μM ROR-γ inhibitor was chosen as the working concentration for experiments moving forward.
Figure 2. Inhibition of ROR-γ reduces IL-17 production in vitro.
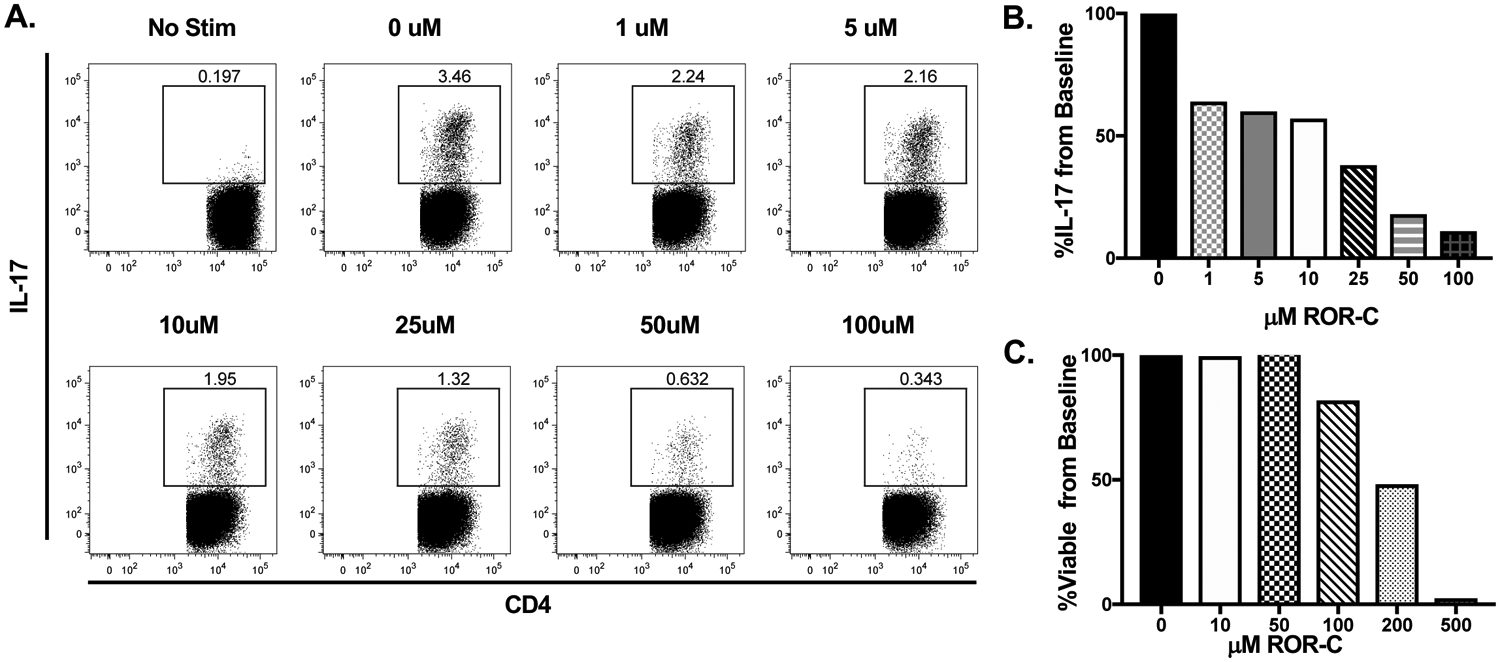
A, B: Representative flow plots and mean responses during ROR-c titration experiments demonstrate dose dependent inhibition of Th17 cells with maximal inhibition at 50μM and 100μM concentrations. C: Cell viability is maintained at 10μM and 50μM concentrations of ROR-c but reduced at concentrations ≥100μM. In order to maximize Th17 cell inhibition and cell viability, the 50μM concentration was selected for additional experiments.
After optimizing the ROR-c concentration, we determined the in-vitro effect of ROR-γ inhibition on Th subsets from MG patient samples. ROR-c exposure reduced Th17 cell frequencies in MG patients (Figure 3A). Parallel studies were performed to determine whether inhibition of ROR-γ had any effects on the frequencies of other Th subsets. Th1 (IFN-γ), Th2 (IL-4), Tfh (CXCR5), and Treg (FOXP3) cells were identified by their hallmark cytokines, transcription factor, or surface marker following a 7-day Th17 polarization with 0μM or 50μM ROR-γ inhibitor. The addition of ROR-γ inhibitor had no effect on Th1, Th2, Tfh, and Treg subsets (Figure 3B–F).
Figure 3. ROR-γ inhibition in patients with MG reduces Th17 cell frequencies but does not affect other Th cell subsets.
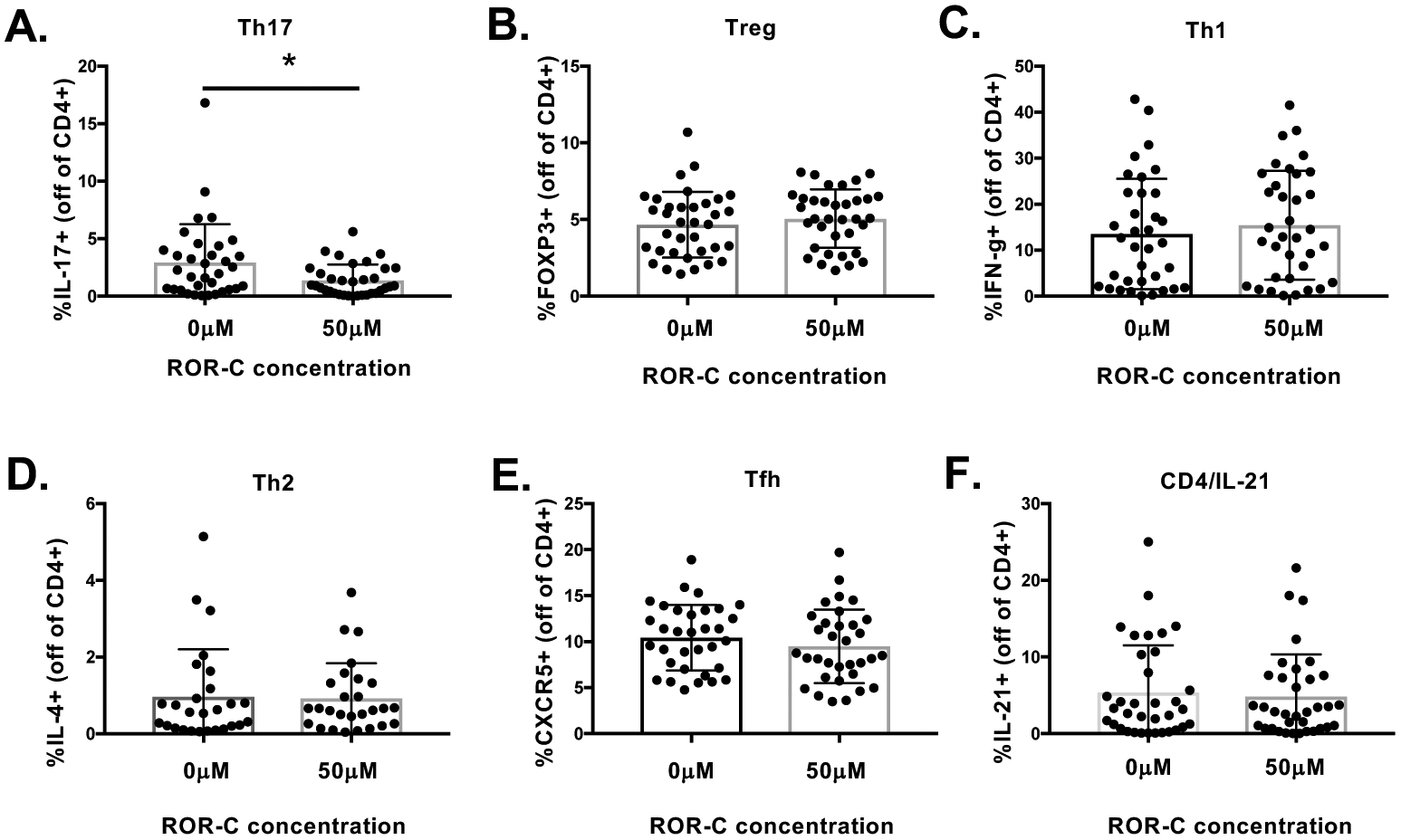
A) ROR-γ inhibition in MG patients produced a strong trend towards reduced frequencies of Th17 cells (CD4+IL-17+; n=34), but not B) Tregs (CD4+ FOXP3+, n=35), C) Th1 (CD4+ IFN-γ+, n=35), D) Th2 (CD4+IL-4+, n=27), E) Tfh (CD4+CXCR5, n=33), or F) CD4+IL-21+ (n=35) T cells subsets. Statistical significance is represented as follows: * p≤0.05.
Collectively, these data demonstrate the specificity of the ROR-γ inhibitor to suppress the differentiation of Th17 cells without affecting other Th subsets.
3.4. Inhibition of ROR-γ reduces pathogenic Th17 cell frequencies in MG patients
We also investigated the effect of ROR-γ inhibition on the generation of pathogenic Th17 cells, a subset of Th17 cells that coproduce IL-17+ and IFN-γ (Zielinski et al., 2012). Under conditions without ROR-c, frequencies of pathogenic Th17 cells were slightly higher in MG patients. Pathogenic Th17 cells were strongly inhibited by ROR-c, and the effect was more striking in MG patients than healthy controls (Figure 4).
Figure 4. Pathogenic Th17 cells in MG patients are reduced by ROR-γ inhibition.
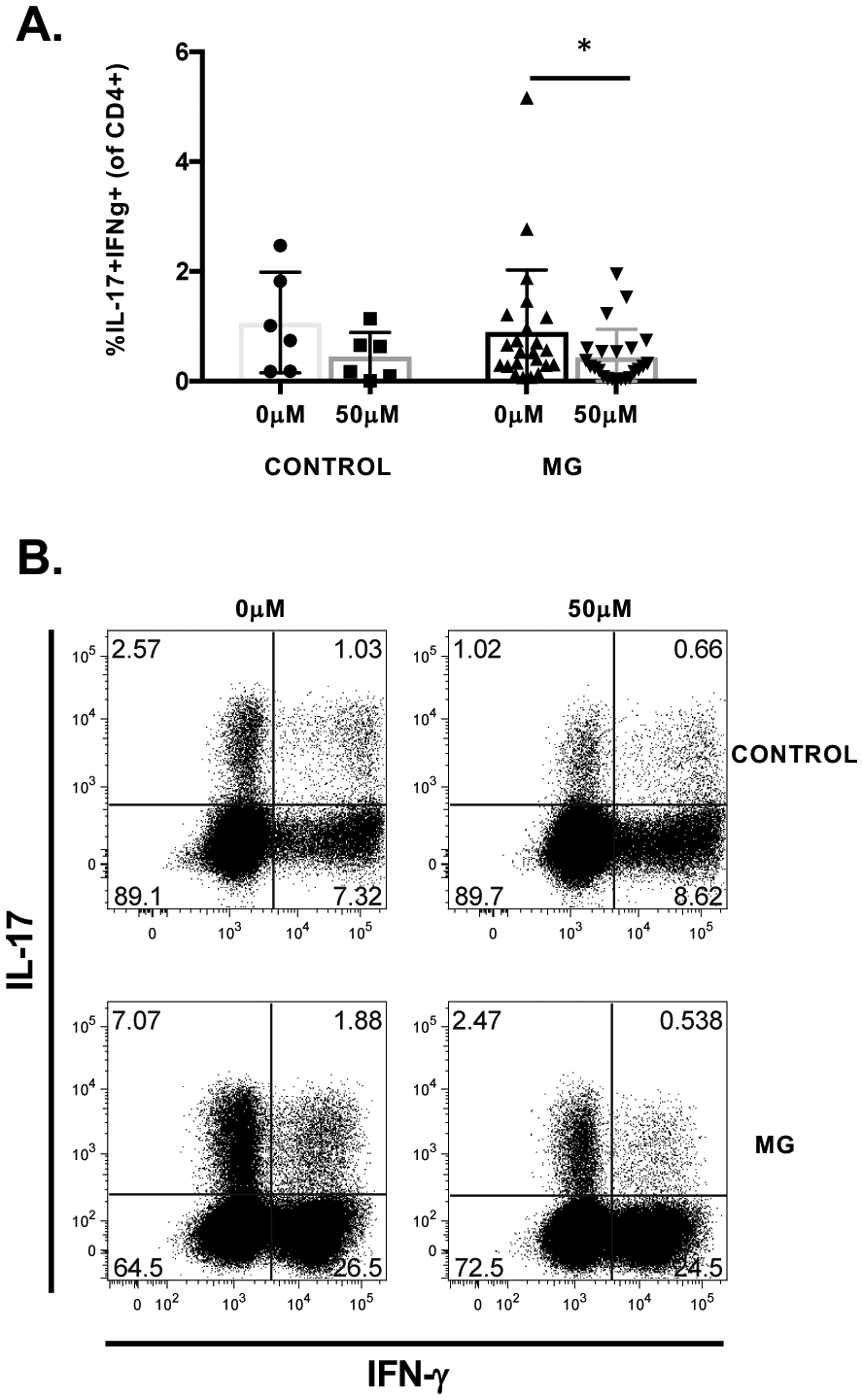
A) Pathogenic Th17 cell frequencies following polarization with and without 50μM ROR-c in healthy controls (n=6) and MG patients (n=22). Exposure to ROR-γ inhibitor in MG patients resulted in significantly reduced frequencies of pathogenic Th17 cells. B) Representative flow plots and frequencies of pathogenic Th17 cells in a MG and control patient on exposure to 0μM or 50μM of ROR-c. Pathogenic Th17 cells co-producing IL-17 and IFN-γ are in the upper right hand boxes. Statistical significance is represented as follows: * p≤0.05.
3.5. CD8 IL-17 producing T cells
We investigated a rare subset of CD8 T cells that produce IL-17, so-called Tc17 cells, that have recently been implicated in proinflammatory responses and autoimmunity (Srenathan et al., 2016). Tc17 cells were increased in MG patients compared with controls following polarization, though the difference was not statistically significant (Figure 5A). Investigation into IFN-γ and IL-17 double positive CD8 T cells revealed a significant difference between MG patients and controls (Figure 5B). Interestingly, ROR-c exposure resulted in a significant reduction in Tc17 cell frequencies in MG patients (Figure 5C). Overall, we demonstrate the capacity of CD8 T cells to produce IL-17 and the ability to inhibit both CD4 and CD8 from producing IL-17.
Figure 5. Tc17 cells are increased in MG patients and significantly reduced with exposure to ROR-γ inhibitor.
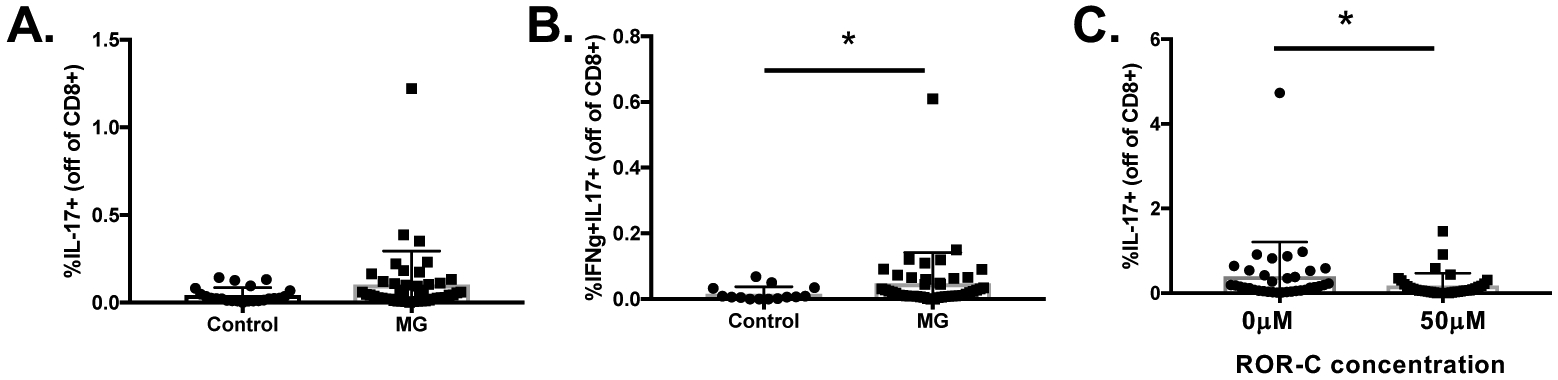
A) Overall Tc17 cell frequencies are increased in MG patients (n=45) compared with controls (n=23). B) IFN-γ and IL-17 co-producing Tc17 cell frequencies were increased in MG patients (n=45) compared with controls (n=23). C) Exposure to ROR-γ inhibitor resulted in a significant reduction of Tc17 cells in MG patients (n=35). Statistical significance is represented as follows: * p≤0.05.
4. Discussion
Beginning with the first report of elevated peripheral IL-17A levels in patients with MG (Roche et al., 2011), studies in animal models of MG and in patients with AChR-MG have consistently shown Th17 associated pathology (Aguilo-Seara et al., 2017; Cao et al., 2016; Gradolatto et al., 2012; Roche et al., 2011; Schaffert et al., 2015; Xie et al., 2016; Yi et al., 2014). Following induction of EAMG, increased serum IL-17 levels are accompanied by increases in Th17 cells as the disease progresses (Mu et al., 2009). Furthermore, IL-17 stimulation in established EAMG results in T cell proliferation and increases in anti-AChR secreting B cells. Knockout of the IL-17 gene in animal studies have demonstrated minimal weakness following inoculation with AChR, reduced muscle endplate complement deposition, and reduced complement fixing antibodies, confirming a significantly milder disease phenotype (Aguilo-Seara et al., 2017; Schaffert et al., 2015). In human AChR-MG the thymus shows upregulation of Th1, Tfh, and Th17 genes and increased IL-17 expression (Gradolatto et al., 2014), and there is increased IL-17 production in response to AChR stimulation (Cao et al., 2016). In addition, prior observations showed that peripheral IL-17 levels are higher in patients with generalized disease (Xie et al., 2016). The results of our study from patients with MG support these findings by demonstrating that peripheral Th17 cell frequencies are higher in MG patients with moderate to severe generalized disease compared with controls. Patients with ocular and mild MG did not have increased frequencies of Th17 cells following polarization, and it is likely that we did not observe a statistically significant increase in overall Th17 cell frequencies because our study population was weighted towards patients with mild disease. Overall, there is a large body of evidence implicating Th17 pathology, though therapeutics directed against this immunopathology are lacking in MG.
With the strong support for Th17 pathology in EAMG and humans, targeting Th17 cell pathology in MG is a rational strategy to explore for treatment of the disease (Cosmi et al., 2014). Current immunotherapies to treat MG may impact Th17 cell populations, but also cause more off target immunological effects leading to more widespread immune system suppression (Li et al., 2019). ROR-c is a small molecule that inhibits the key transcription factor in Th17 cell differentiation, ROR-γ (Guntermann et al., 2017; Ivanov et al., 2006). Inhibiting ROR-γ affects thymopoiesis, resulting in reduced thymic T cell output with a condensed T cell receptor repertoire, and resistance to development of experimental autoimmune encephalomyelitis (EAE) in mice (Guo et al., 2016). This decrease in the quantity and quality of thymic T cells affect the number of self reactive T cells, as well as T cells responding to foreign antigens. In a series of experiments we show that ROR-γ inhibition effectively reduces Th17 cell frequencies in human autoimmunity.
Furthermore, a subset of Th17 cells that has garnered significant interest as a mediator of autoimmune disease are pathogenic Th17 cells. This proinflammatory Th subset is defined by the co-production of the IL-17 and IFN-γ and has recently been shown to hold significance in multiple sclerosis (Hu et al., 2017; Zielinski et al., 2012). Pathogenic Th17 cells, which have been shown to be elevated in autoreactive T cells from patients with MG (Cao et al., 2016), were strongly inhibited by ROR-c. Importantly, we did not observe effects of ROR-γ inhibition on other Th cell subsets including Th1, Th2, Tfh, and Tregs. These data suggest that selective ROR-γ inhibition may be an effective and targeted therapeutic approach for addressing Th17 immunopathology in MG without the more widespread immune system effects observed with currently available drugs, such as corticosteroids. Targeted ROR-γ inhibition could also help clarify the relative contribution of Th17 cells as mediators of the disease, compared with other implicated immune subsets not affected by ROR-γ inhibitors, such as Th1 cells.
Given the importance of the CD4 T cells in MG pathogenesis, CD8 T cells have received less scrutiny. A novel finding in this study is the demonstration that CD8 T cell subsets producing IL-17 can be inhibited pharmacologically. Most knowledge about this T cell subset, also called Tc17 cells, comes from animal models (Srenathan et al., 2016). It has been shown that Tc17 cells share phenotypic properties with Th17 cells and promote inflammation through production of proinflammatory cytokines, and, in contrast to other CD8 T cell populations, have limited cytotoxic capacity (Huber et al., 2009). Tc17 cells express ROR-γ and are promoted under the polarizing conditions used to differentiate Th17 cells (Gras et al., 2012; Kondo et al., 2009; Liu et al., 2007). In humans, Tc17 cells co-expressing IFN-γ have been identified in skin plaques from patients with psoriasis and appear to have higher proinflammatory potential than Tc17 cells that do not produce IFN-γ (Billerbeck et al., 2010; Ortega et al., 2009).
To this point, descriptions of cultured Tc17 cells in MG patients are limited to a single study (Hosseini et al., 2017). In that study, Tc17 cell frequencies were measured in 12 early-onset MG patients, including 3 with thymoma, before and after thymectomy. The authors found that Tc17 cell frequencies were increased compared with controls prior to thymectomy and that Tc17 cell frequencies were decreased 6 months post-operatively. In our study conducted in predominantly adult-onset MG patients, Tc17 cells frequencies were increased compared with controls and frequencies were associated with MG disease severity. In addition, the more pathogenic subset of Tc17 cells co-producing IFN-γ were also increased in MG patients. ROR-γ inhibition markedly reduced all Tc17 subset frequencies. This suggests that some of the effect of ROR-γ inhibitors in ameliorating inflammatory diseases may be related to an effect on Tc17 cells and not exclusively Th17 cells. Collectively, these data implicate Tc17 immunopathology in all subtypes of AChR-MG and that show Tc17 pathology can be addressed by therapeutics targeting ROR-γ.
In summary, this study demonstrates that Th17 and Tc17 cell frequencies are increased in MG patients with more severe disease and that differentiation of these proinflammatory cell subsets is greatly reduced with inhibition of the transcription factor ROR-γ. Furthermore, cell populations with a pathogenic phenotype expressing IFN-γ were also strongly inhibited. These results strongly support further exploration of Th17 directed therapies as a rationale target for patients with MG and that inhibitors of the transcription factor ROR-γ may be a particularly good approach. In addition to the ROR-γ inhibitor used in our experiments, other topical and systemic ROR-γ modulators are in development for use in patients with psoriasis and autoimmune diseases (Imura et al., 2019; Smith et al., 2016; Xue et al., 2016), and there is intense interest to develop even more selective Th17 cell inhibitors to limit potential off target effects (Rutz et al., 2016). If studies of Th17 targeted therapeutics advance to MG patients, those with more severe disease and higher levels of Th17 inflammation may be most appropriate to establish proof-of-concept and provide an enriched population for clinical trials.
Highlights:
Th17 cell frequencies are associated with disease severity in MG.
RORγ inhibitor suppresses Th17 differentiation in MG.
IL-17 producing CD8 T cells are enhanced in MG, and inhibited by RORγ inhibitor.
Acknowledgement statement:
This study was funded by a Transformative Research Grant from the Myasthenia Gravis Foundation of America. JTG is supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number K23NS085049.
Footnotes
Conflicts of interest:
The authors declare that they have no conflicts of interest.
References
- 2013. BD Biosciences. Generation of Human IL-17-Producing Cells (Th17-like cells) in Vitro.
- 2015. Novartis Pharmaceuticals Corporation. Cosentyx (secukinumab) Package Insert.
- Aguilo-Seara G, Xie Y, Sheehan J, Kusner LL, Kaminski HJ, 2017. Ablation of IL-17 expression moderates experimental autoimmune myasthenia gravis disease severity. Cytokine 96, 279–285. [DOI] [PubMed] [Google Scholar]
- Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V, Flint J, Willberg CB, Bengsch B, Seigel B, Ramamurthy N, Zitzmann N, Barnes EJ, Thevanayagam J, Bhagwanani A, Leslie A, Oo YH, Kollnberger S, Bowness P, Drognitz O, Adams DH, Blum HE, Thimme R, Klenerman P, 2010. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci U S A 107, 3006–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campa M, Mansouri B, Warren R, Menter A, 2016. A Review of Biologic Therapies Targeting IL-23 and IL-17 for Use in Moderate-to-Severe Plaque Psoriasis. Dermatol Ther (Heidelb) 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Amezquita RA, Kleinstein SH, Stathopoulos P, Nowak RJ, O’Connor KC, 2016. Autoreactive T Cells from Patients with Myasthenia Gravis Are Characterized by Elevated IL-17, IFN-gamma, and GM-CSF and Diminished IL-10 Production. J Immunol 196, 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kolls JK, 2013. T cell-mediated host immune defenses in the lung. Annu Rev Immunol 31, 605–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Fine BM, Milani M, Kaminski HJ, 2006. Myasthenia gravis: past, present, and future. J Clin Invest 116, 2843–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmi L, Santarlasci V, Maggi L, Liotta F, Annunziato F, 2014. Th17 plasticity: pathophysiology and treatment of chronic inflammatory disorders. Curr Opin Pharmacol 17, 12–16. [DOI] [PubMed] [Google Scholar]
- Gradolatto A, Nazzal D, Foti M, Bismuth J, Truffault F, Le Panse R, Berrih-Aknin S, 2012. Defects of immunoregulatory mechanisms in myasthenia gravis: role of IL-17. Ann N Y Acad Sci 1274, 40–47. [DOI] [PubMed] [Google Scholar]
- Gradolatto A, Nazzal D, Truffault F, Bismuth J, Fadel E, Foti M, Berrih-Aknin S, 2014. Both Treg cells and Tconv cells are defective in the Myasthenia gravis thymus: roles of IL-17 and TNF-alpha. J Autoimmun 52, 53–63. [DOI] [PubMed] [Google Scholar]
- Gras R, Garcia MI, Gomez R, de la Mata FJ, Munoz-Fernandez MA, Lopez-Fernandez LA, 2012. Carbosilane dendrimer 2G-NN16 represses Tc17 differentiation in primary T CD8+ lymphocytes. Mol Pharm 9, 102–110. [DOI] [PubMed] [Google Scholar]
- Guendisch U, Weiss J, Ecoeur F, Riker JC, Kaupmann K, Kallen J, Hintermann S, Orain D, Dawson J, Billich A, Guntermann C, 2017. Pharmacological inhibition of RORgammat suppresses the Th17 pathway and alleviates arthritis in vivo. PLoS One 12, e0188391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntermann C, Piaia A, Hamel ML, Theil D, Rubic-Schneider T, Del Rio-Espinola A, Dong L, Billich A, Kaupmann K, Dawson J, Hoegenauer K, Orain D, Hintermann S, Stringer R, Patel DD, Doelemeyer A, Deurinck M, Schumann J, 2017. Retinoic-acid-orphan-receptor-C inhibition suppresses Th17 cells and induces thymic aberrations. JCI Insight 2, e91127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, MacIsaac KD, Chen Y, Miller RJ, Jain R, Joyce-Shaikh B, Ferguson H, Wang IM, Cristescu R, Mudgett J, Engstrom L, Piers KJ, Baltus GA, Barr K, Zhang H, Mehmet H, Hegde LG, Hu X, Carter LL, Aicher TD, Glick G, Zaller D, Hawwari A, Correll CC, Jones DC, Cua DJ, 2016. Inhibition of RORgammaT Skews TCRalpha Gene Rearrangement and Limits T Cell Repertoire Diversity. Cell Rep 17, 3206–3218. [DOI] [PubMed] [Google Scholar]
- Hernandez-Santos N, Huppler AR, Peterson AC, Khader SA, McKenna KC, Gaffen SL, 2013. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol 6, 900–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini M, Robat-Jazi B, Shaygannejad V, Naffisi S, Mirmossayeb O, Rezaei A, Mansourian M, Esmaeil N, 2017. Increased Proportion of Tc17 and Th17 Cells and Their Significant Reduction after Thymectomy May Be Related to Disease Progression in Myasthenia Gravis. Neuroimmunomodulation 24, 264–270. [DOI] [PubMed] [Google Scholar]
- Hu D, Notarbartolo S, Croonenborghs T, Patel B, Cialic R, Yang TH, Aschenbrenner D, Andersson KM, Gattorno M, Pham M, Kivisakk P, Pierre IV, Lee Y, Kiani K, Bokarewa M, Tjon E, Pochet N, Sallusto F, Kuchroo VK, Weiner HL, 2017. Transcriptional signature of human pro-inflammatory TH17 cells identifies reduced IL10 gene expression in multiple sclerosis. Nat Commun 8, 1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, Elflein K, Hunig T, Mittrucker HW, Brustle A, Kamradt T, Lohoff M, 2009. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol 39, 1716–1725. [DOI] [PubMed] [Google Scholar]
- Imura C, Ueyama A, Sasaki Y, Shimizu M, Furue Y, Tai N, Tsujii K, Katayama K, Okuno T, Shichijo M, Yasui K, Yamamoto M, 2019. A novel RORgammat inhibitor is a potential therapeutic agent for the topical treatment of psoriasis with low risk of thymic aberrations. J Dermatol Sci 93, 176–185. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR, 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133. [DOI] [PubMed] [Google Scholar]
- Jaretzki A 3rd, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, Sanders DB, 2000. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 55, 16–23. [DOI] [PubMed] [Google Scholar]
- Kondo T, Takata H, Matsuki F, Takiguchi M, 2009. Cutting edge: Phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J Immunol 182, 1794–1798. [DOI] [PubMed] [Google Scholar]
- Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M, 2008. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 105, 18460–18465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, Sobel RA, Regev A, Kuchroo VK, 2012. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol 13, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S, 2012. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 366, 1190–1199. [DOI] [PubMed] [Google Scholar]
- Li Y, Guptill JT, Russo MA, Massey JM, Juel VC, Hobson-Webb LD, Howard JF, Chopra M, Liu W, Yi JS, 2019. Tacrolimus inhibits Th1 and Th17 responses in MuSK-antibody positive myasthenia gravis patients. Exp Neurol 312, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh CL, Yeh YC, Chou AH, Chang SR, Hsiao KN, Yu FW, Chen HW, 2007. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. J Leukoc Biol 82, 354–360. [DOI] [PubMed] [Google Scholar]
- Mu LL, Sun B, Kong QF, Wang JH, Wang GY, Zhang SJ, Wang DD, Liu YM, Liu YX, An HX, Li HL, 2009. Disequilibrium of T helper type 1, 2 and 17 cells and regulatory T cells during the development of experimental autoimmune myasthenia gravis. Immunology 128, e826–e836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack M, Miossec P, 2014. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev 13, 668–677. [DOI] [PubMed] [Google Scholar]
- Ortega C, Fernandez AS, Carrillo JM, Romero P, Molina IJ, Moreno JC, Santamaria M, 2009. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol 86, 435–443. [DOI] [PubMed] [Google Scholar]
- Oukka M, 2008. Th17 cells in immunity and autoimmunity. Ann Rheum Dis 67 Suppl 3, iii26–29. [DOI] [PubMed] [Google Scholar]
- Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, Baumgartner S, 2012. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 366, 1181–1189. [DOI] [PubMed] [Google Scholar]
- Roche JC, Capablo JL, Larrad L, Gervas-Arruga J, Ara JR, Sanchez A, Alarcia R, 2011. Increased serum interleukin-17 levels in patients with myasthenia gravis. Muscle Nerve 44, 278–280. [DOI] [PubMed] [Google Scholar]
- Rutz S, Eidenschenk C, Kiefer JR, Ouyang W, 2016. Post-translational regulation of RORgammat-A therapeutic target for the modulation of interleukin-17-mediated responses in autoimmune diseases. Cytokine Growth Factor Rev 30, 1–17. [DOI] [PubMed] [Google Scholar]
- Sanders DB, Guptill JT, 2014. Myasthenia gravis and Lambert-Eaton myasthenic syndrome. Continuum (Minneap Minn) 20, 1413–1425. [DOI] [PubMed] [Google Scholar]
- Sanders DB, Tucker-Lipscomb B, Massey JM, 2003. A simple manual muscle test for myasthenia gravis: validation and comparison with the QMG score. Ann N Y Acad Sci 998, 440–444. [DOI] [PubMed] [Google Scholar]
- Schaffert H, Pelz A, Saxena A, Losen M, Meisel A, Thiel A, Kohler S, 2015. IL-17-producing CD4(+) T cells contribute to the loss of B-cell tolerance in experimental autoimmune myasthenia gravis. Eur J Immunol 45, 1339–1347. [DOI] [PubMed] [Google Scholar]
- Smith SH, Peredo CE, Takeda Y, Bui T, Neil J, Rickard D, Millerman E, Therrien JP, Nicodeme E, Brusq JM, Birault V, Viviani F, Hofland H, Jetten AM, Cote-Sierra J, 2016. Development of a Topical Treatment for Psoriasis Targeting RORgamma: From Bench to Skin. PLoS One 11, e0147979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srenathan U, Steel K, Taams LS, 2016. IL-17+ CD8+ T cells: Differentiation, phenotype and role in inflammatory disease. Immunol Lett 178, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C, Van Snick J, 2006. Development of an anti-IL-17A auto-vaccine that prevents experimental auto-immune encephalomyelitis. Eur J Immunol 36, 2868–2874. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiang H, Qiu Y, Wang Y, Sun G, Zhao J, 2019. Effector memory regulatory T cells were most effective at suppressing RANKL but their frequency was downregulated in tibial fracture patients with delayed union. Immunol Lett 209, 21–27. [DOI] [PubMed] [Google Scholar]
- Xie Y, Li HF, Jiang B, Li Y, Kaminski HJ, Kusner LL, 2016. Elevated plasma interleukin-17A in a subgroup of Myasthenia Gravis patients. Cytokine 78, 44–46. [DOI] [PubMed] [Google Scholar]
- Xue X, Soroosh P, De Leon-Tabaldo A, Luna-Roman R, Sablad M, Rozenkrants N, Yu J, Castro G, Banie H, Fung-Leung WP, Santamaria-Babi L, Schlueter T, Albers M, Leonard K, Budelsky AL, Fourie AM, 2016. Pharmacologic modulation of RORgammat translates to efficacy in preclinical and translational models of psoriasis and inflammatory arthritis. Sci Rep 6, 37977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA, 2008. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 454, 350–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Guidon A, Sparks S, Osborne R, Juel VC, Massey JM, Sanders DB, Weinhold KJ, Guptill JT, 2014. Characterization of CD4 and CD8 T cell responses in MuSK myasthenia gravis. J Autoimmun 52, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Qian Y, 2012. IL-17/IL-17 receptor system in autoimmune disease: mechanisms and therapeutic potential. Clin Sci (Lond) 122, 487–511. [DOI] [PubMed] [Google Scholar]
- Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F, 2012. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature 484, 514–518. [DOI] [PubMed] [Google Scholar]


