Abstract
ECMO is a life-saving technology capable of restoring perfusion but is not without significant complications that limit its realizable therapeutic benefit. ECMO-induced hemodynamics increase cardiac afterload risking left ventricular distention and impaired cardiac recovery. To mitigate potentially harmful effects, multiple strategies to unload the left ventricle (LV) are used in clinical practice but data supporting the optimal approach is presently lacking. We reviewed outcomes of our ECMO population from September 2015 through January 2019 to determine if our LV unloading strategies were associated with patient outcomes. We compared reactive (Group 1, n=30) versus immediate (Group 2, n=33) LV unloading and then compared patients unloaded with an Impella CP (n=19) versus an intra-aortic balloon pump (IABP, n=16), analyzing survival and ECMO-related complications. Survival was similar between Groups 1 and 2 (33 vs 42%, p=0.426) with Group 2 experiencing more clinically-significant hemorrhage (40 vs. 67%, p=0.034). Survival and ECMO-related complications were similar between patients unloaded with an Impella versus an IABP. However, the Impella group exhibited a higher rate of survival (37%) than predicted by their median SAVE score (18%). Our findings correlate with recent large cohort studies and motivate further work to design clinical guidelines and future trial design.
Keywords: cardiogenic shock, extracorporeal membrane oxygenation, ventricular assist device, intraaortic balloon pumping
INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) is increasingly employed to provide mechanical circulatory support (MCS) despite incomplete understanding of its effects on the failing heart and lack of evidence-based approaches to mitigating harmful sequelae from its use.1–4 Deployed as an MCS device, ECMO shunts venous blood through a membrane oxygenator via a pump before returning oxygenated blood to the systemic arterial system.5,6 Cannulation strategies for returning oxygenated blood include: (1) central cannulation of the aortic arch to provide antegrade perfusion, most commonly performed for cardiac surgical patients unable to wean from cardiopulmonary bypass, and (2) peripheral cannulation, typically via percutaneous access or vascular cutdown of the femoral artery, in which the cannula terminates in the iliac artery or distal aorta to provide retrograde perfusion.7,8
ECMO-generated systemic perfusion increases cardiac afterload which may lead to distention of the left ventricle (LV) and, in cases of profound heart failure, loss of aortic valve opening.9,10 Possible sequelae of ECMO support on the LV include elevated wall tension, increased myocardial oxygen demand, subendocardial ischemia, dysrhythmias, pulmonary edema, hemostasis, and intracardiac thrombus formation.11 These effects may impair cardiac recovery or induce catastrophic complications.12,13
Medical therapy to maintain forward flow through the LV consists of optimizing preload and afterload and use of inotropes to augment contractility.3 In cases in which medical therapy is insufficient or not tolerated, mechanical means of unloading the LV are considered. Multiple approaches to LV unloading have been described and include: (1) atrial septostomy; (2) a surgically inserted transapical catheter; (3) intra-aortic balloon pump (IABP); (4) percutaneous pulmonary artery venting via the jugular vein; and, (5) transvalvular percutaneous ventricular assist device (pVAD) such as the Impella CP (Abiomed, Danvers, MA).14–17 Atrial septostomy and pulmonary artery venting act via reducing LV preload while pVADs and surgically placed transapical catheters directly unload the LV.18 IABPs appear to promote LV unloading, even in cases of peripheral VA ECMO with retrograde perfusion, via decrease in afterload during cardiac contraction.19
At present, the optimal venting strategy, including considerations of both when and how to achieve venting, is unknown. In an effort to further explore the association of venting approaches with patient outcomes, we performed a retrospective review of our institution’s experience with additional mechanical support via either an IABP or pVAD to achieve LV unloading during ECMO. We evaluated outcomes of patients treated with a strategy of immediate unloading versus those in whom unloading was performed based on specific criteria and then compared patients who underwent LV unloading via Impella CP versus IABP.
MATERIALS AND METHODS
This work was performed under the auspices of a protocol approved by the Brigham and Women’s Hospital Institutional Review Board. Informed consent was waived by the IRB for data collection in this protocol.
Study Design
We performed two retrospective analyses of patients supported by ECMO at Brigham and Women’s Hospital (Boston, MA) from September 2015 through January 2019. We included all patients cannulated either centrally (n=17) or via femoral cannulation (n=46) with the exception of a small cohort of patients supported with atypical cannulation strategies (n=7). We first compared patients supported by ECMO with an expectant unloading strategy as determined by clinical criteria (n=30) versus those on ECMO with immediate LV unloading (n=33), including those with an LV support device placed prior to escalation to ECMO. For simplicity, the group consisting of patients supported by ECMO with an expectant unloading strategy will be termed Group 1 while the patients in the immediate LV unloading group will be referred to as Group 2. Findings of worsening pulmonary edema on chest radiography, progressive LV dilation on serial echocardiography, or clinical assessment of inadequate systemic pulsatility were used to determine need for LV unloading in Group 1 patients. We then analyzed patient outcomes based on unloading strategy comparing those supported with an Impella CP (n=19) versus an IABP (n=16).
Institutional Approach to LV Unloading and ECMO Management
Patients meeting our institutional criteria for initiation of ECMO to provide circulatory support are monitored for evidence of ongoing aortic valve opening following cannulation. Monitoring consists of intermittent bedside ultrasonagraphy to assess cardiac contractility and continuous invasive measurement of the systemic arterial pressure via the right radial artery. Mean arterial pressure is maintained at 65–80 mm Hg while pulse pressure, defined as the peak difference between systolic and diastolic blood pressure measurements, is used as a surrogate for aortic valve opening. Continuous inotropic support is initiated if arterial pulse pressure is < 10 mm Hg or if there is ultrasonographic evidence of LV dilation or lack of aortic valve opening. Mechanical unloading with either an IABP or Impella CP was initiated if patients failed or were unable tolerate inotropic support (e.g. due to tachyarrhythmias). Following initiation of mechanical unloading, inotropic support was employed if there was persistent evidence of LV distention or inadequate end-organ perfusion.
All patients were maintained on anticoagulation with continuous infusions of unfractionated heparin administered directly through the ECMO circuit titrated to a goal PTT of 60–80 seconds for peripheral cannulation or 40–60 seconds for either central cannulation or high risk of bleeding complications. Patients maintained on Impella CP support received unfractionated heparin via the device as per the manufacturer’s recommendation.
Outcomes of Interest
Overall survival was the primary outcome of interest. Actual survival was compared to predicted survival as calculated from the Survival After Veno-Arterial ECMO (SAVE) score predictive index (www.save-score.com).20 Secondary outcomes were ECMO-related complications and time to decannulation. ECMO-related complications consisted of stroke (defined by presence of neurologic symptoms and positive findings on computed tomography), intracranial hemorrhage, clinically significant hemorrhage (defined as need for surgical or endoscopic intervention or transfusion requirement greater than three units of packed red blood cells in twenty-four hours), vascular complication requiring surgical or endovascular intervention, hemolysis (defined by a serum lactate dehydrogenase level greater than 1,000 units/L), sepsis (based upon findings of systemic inflammatory response syndrome in the context of a documented infection), need for renal replacement therapy, mesenteric ischemia confirmed endoscopically or requiring laparotomy, abdominal compartment syndrome requiring laparotomy, and intracardiac thrombus.
Statistical Analysis
Continuous variables are reported as median (1st, 3rd quartile) or mean ± standard deviation. Categorial variables are presented as absolute numbers (percentage). Student’s t-tests (for normally distributed continuous variables), Kruskal Wallis (for non-parametric continuous distributions), and Fisher exact tests (for categorical variables) were used to compare groups. All-cause mortality was compared via log-rank analysis with the date of ECMO cannulation used as initial time point of reference. Patients were censored at the time of death regardless of weaning from ECMO or at the time of last known follow up. Kaplan-Meier curves are depicted over thirty days. In total, four patients from the cohort were discharged and subsequently lost to follow up within 30 days (2 from Group 1, 2 from Group 2). These patients remained within the analyses. Computations were performed using Stata SE version 15.1 (StataCorp, College Station, Texas, USA) and GraphPad Prism 8.0.2 (GraphPad Software, San Diego, CA, USA).
RESULTS
Patient Characteristics
The study patient characteristics are shown in Table 1. Patients in both Group 1 (reactive unloading) and Group 2 (immediate unloading) were predominantly male, 77% versus 66%, respectively. Patients in Group 2 were significantly older (60 ± 11.0 years versus 52 ± 16.5 years; p = 0.0255). Both groups otherwise were similar in pre-existing cardiac disease and co-morbid conditions. No patient in Group 2 had prior valve repair or replacement versus four patients (13%, p = 0.030) in Group 1. Distribution of other prior cardiothoracic interventions – including coronary artery bypass grafting, percutaneous coronary intervention, and heart transplant – were similar between groups. Clinical metrics of acid-base status, serum lactate levels, and serum creatinine prior to initiation of ECMO support were similar between the two groups. Four patients in Group 1 (13%) required reactive unloading with one each receiving an Impella CP, IABP, atrial septostomy, or trans-septal catheter.
Table 1.
Baseline patient characteristics.
| Characteristic | Group 1 [Reactive venting] (n=30) | Group 2 [Immediate venting] (n=33) | p-value |
|---|---|---|---|
| Age, years | 52 ± 16.5 | 60 ± 11.0 | 0.0255 |
| Male sex | 23 (77) | 22 (67) | 0.38 |
| Preexisting cardiac disease | |||
| Coronary artery disease | 12 (40) | 17 (52) | 0.36 |
| Non-ischemic cardiomyopathy | 6 (20) | 7 (21) | 0.905 |
| Prior myocardial infarction | 1 (3) | 3 (9) | 0.349 |
| Pulmonary arterial hypertension | 6 (20) | 3 (9) | 0.217 |
| Systemic hypertension | 17 (57) | 17 (52) | 0.682 |
| Other comorbidities | |||
| Chronic kidney disease | 6 (20) | 4 (12) | 0.393 |
| Diabetes mellitus | 7 (23) | 8 (24) | 0.933 |
| Hyperlipidemia | 13 (43) | 13 (39) | 0.751 |
| Peripheral arterial disease | 1 (3) | 1 (3) | 0.945 |
| Prior CT interventions | |||
| Coronary artery bypass grafting | 1 (3) | 3 (9) | 0.349 |
| Percutaneous coronary interventions | 4 (13) | 4 (12) | 0.885 |
| Valve repair/replacement | 4 (13) | 0 | 0.030 |
| Heart transplant | 2 (7) | 1 (3) | 0.498 |
| Pre-ECMO pH | 7.19 ± 0.14 | 7.24 ± 0.13 | 0.196 |
| Pre-ECMO Lactate | 8.33 ± 6.20 | 6.24 ± 3.91 | 0.133 |
| Pre-ECMO serum creatinine | 1.93 ± 1.12 | 1.84 ± 1.24 | 0.763 |
| SAVE score | −10.5 (−14 to −5) | −9 (−12 to −5) | 0.366 |
Values presented as number (percentage) or mean ± standard deviation
Indications for MCS Support
We categorized primary patient indication for MCS as either acute myocardial infarction, ischemic cardiomyopathy, non-ischemic cardiomyopathy, myocarditis, post-cardiotomy shock or post-transplant graft dysfunction. Distribution of indications is shown in Table 2 and is similar between both groups. Numerically more patients underwent active cardiopulmonary resuscitation efforts at the time of ECMO cannulation (E-CPR) in Group 1 compared with Group 2 (33% versus 15%, respectively, p=0.091). The majority of patients in both groups were peripherally cannulated (77 vs 70%, p = 0.53) while time to decannulation and total MCS duration (time of ECMO plus any time of LV unloading device alone) were similar between patient groups.
Table 2.
Details of MCS therapy.
| Parameter | Group 1 (n=30) | Group 2 (n=33) | p-value |
|---|---|---|---|
| Indication for ECLS | |||
| Acute myocardial infarction | 12 (40) | 12 (36) | 0.767 |
| Ischemic cardiomyopathy | 1 (3) | 3 (9) | 0.349 |
| Non-ischemic cardiomyopathy | 7 (23) | 5 (15) | 0.409 |
| Myocarditis | 1 (3) | 4 (12) | 0.197 |
| Post-cardiotomy shock | 5 (17) | 6 (18) | 0.874 |
| Post-transplant graft dysfunction | 4 (13) | 3 (9) | 0.593 |
| ECPR | 10 (33) | 5 (15) | 0.091 |
| Bridge to ventricular assist device | 1 (3) | 3 (9) | 0.349 |
| Bridge to heart transplant | 0 | 2 (6) | 0.171 |
| Duration of VA-ECMO support, days | 4.97 ± 5.10 | 6.55 ± 4.89 | 0.215 |
| Duration of MCS support, days | 5.45 ± 5.37 | 8.45 ± 7.82 | 0.087 |
| Peripheral cannulation strategy | 23 (77) | 23 (70) | 0.534 |
Values presented as number (percentage) or mean ± standard deviation.
Survival and Complications
Patients in Group 1 experienced similar 30-day survival to those in Group 2 (33% vs. 42%, p=0.426) as shown in Figure 1 and detailed in Table 3. Causes of death – classified as hemorrhage, cardiac death, infection, multiple system organ failure, cerebrovascular accident, and breath death – were similarly distributed between patient groups. Regarding ECMO-related complications, Group 1 patients experienced less hemorrhage than patients in Group 2 (40 vs 67%, p = 0.034). Rates of remaining complications of interest, including ischemic stroke, intracranial hemorrhage, vascular complication, hemolysis, sepsis, need for renal replacement therapy, mesenteric ischemic, abdominal compartment syndrome, and intracardiac thrombus, were otherwise statistically similar between groups.
Figure 1. 30-day all-cause survival grouped by unloading strategy.
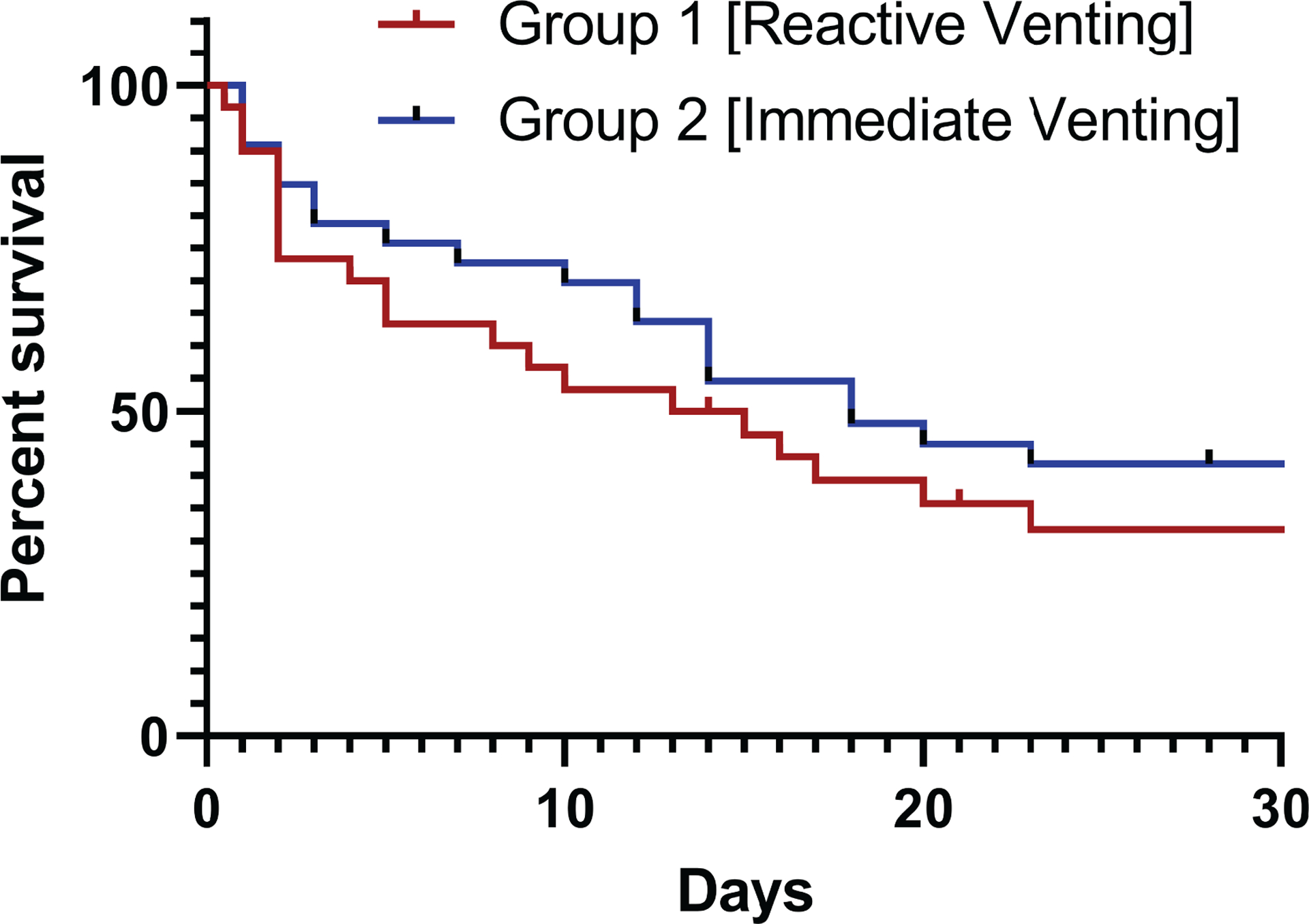
Group 1 – ECMO with reactive venting. Group 2 – ECMO with immediate venting. Kaplan-Meier curve demonstrating statistically equivalent survival between unloading strategies (p=0.426).
Table 3.
Survival rates and complications.
| Parameter | Group 1 (n=30) | Group 2 (n=33) | p-value |
|---|---|---|---|
| 30-day survival | 10 (33) | 14 (42) | 0.458 |
| Survival to decannulation | 15 (50) | 21 (64) | 0.275 |
| Survival to discharge | 9 (30) | 11 (33) | 0.777 |
| Cause of death | |||
| Hemorrhage | 0 | 1 (3) | 0.336 |
| Cardiac death | 8 (27) | 9 (27) | 0.957 |
| Infection | 2 (7) | 0 | 0.132 |
| Multiple system organ failure | 11 (37) | 12 (36) | 0.980 |
| Cerebrovascular accident | 1 (3) | 0 | 0.290 |
| Brain death | 1 (3) | 0 | 0.290 |
| Complications | |||
| Ischemic stroke | 2 (7) | 6 (18) | 0.170 |
| Intracranial hemorrhage | 1 (3) | 1 (3) | 0.945 |
| Hemorrhage | 12 (40) | 22 (67) | 0.034 |
| Vascular complication | 8 (27) | 6 (18) | 0.418 |
| Hemolysis | 3 (10) | 7 (21) | 0.224 |
| Sepsis | 3 (10) | 4 (12) | 0.789 |
| Need for renal replacement therapy | 13 (43) | 15 (45) | 0.866 |
| Mesenteric ischemia | 4 (13) | 2 (6) | 0.326 |
| Abdominal compartment syndrome | 2 (7) | 0 | 0.132 |
| Intracardiac thrombus | 5 (17) | 9 (27) | 0.312 |
Values presented as number (percentage) or mean ± standard deviation.
Patients undergoing LV unloading with Impella CP and IABP
In our second analysis, we compared patients with an Impella unloading strategy against those with an IABP unloading strategy with the characteristics of each group detailed in Table 4. Patients with IABP unloading were significantly older compared to the Impella group (66 ± 10.7 years versus 55 ± 7.1 years, p = 0.0013). The distribution of pre-existing cardiac disease, comorbid conditions, and prior cardiothoracic interventions were similar between these patient groups. Additionally, pre-ECMO clinical metrics including acid-base status, serum lactate, and serum creatinine were again similar. Median SAVE scores were also statistically equivalent (p = 0.97).
Table 4.
Baseline characteristics of patients undergoing ECMO with unloading via Impella versus IABP.
| Characteristic | ECMO + IMPELLA (n=19) | ECMO + IABP (n=16) | p-value |
|---|---|---|---|
| Age, years | 55 ± 7.1 | 66 ± 10.7 | 0.0013 |
| Male sex | 13 (81) | 10 (63) | 0.71 |
| Preexisting cardiac disease | |||
| Coronary artery disease | 10 (53) | 8 (50) | 0.88 |
| Non-ischemic cardiomyopathy | 4 (21) | 3 (19) | 0.87 |
| Prior myocardial infarction | 3 (16) | 0 | 0.096 |
| Pulmonary arterial hypertension | 2 (11) | 1 (6) | 0.65 |
| Systemic hypertension | 7 (37) | 11 (69) | 0.060 |
| Other comorbidities | |||
| Chronic kidney disease | 3 (16) | 1 (6) | 0.72 |
| Diabetes mellitus | 6 (32) | 4 (25) | 0.72 |
| Hyperlipidemia | 7 (37) | 8 (50) | 0.43 |
| Peripheral arterial disease | 0 | 1 (6) | 0.46 |
| Prior CT interventions | |||
| Coronary artery bypass grafting | 1 (5) | 2 (13) | 0.58 |
| Percutaneous coronary interventions | 4 (21) | 0 | 0.11 |
| Valve repair/replacement | 0 | 0 | - |
| Heart transplant | 1 (5) | 0 | 1.00 |
| Pre-ECMO pH | 7.26 ± 0.10 | 7.23 ± 0.15 | 0.56 |
| Pre-ECMO Lactate | 7.32 ± 5.03 | 6.55 ± 4.46 | 0.66 |
| Pre-ECMO serum creatinine | 1.88 ± 1.33 | 1.86 ± 1.07 | 0.96 |
| SAVE score | −10 (−13 to −5.5) | −8.5 (−13 to −5) | 0.97 |
Values presented as number (percentage) or mean ± standard deviation.
Clinical Setting of ECMO Unloading Strategies
We found the clinical location/service at the time of initial shock presentation contributed to the choice of LV venting strategy as detailed in Table 5. No patient with post-cardiotomy shock received LV venting with an Impella CP consistent with institutional use of IABPs for initial choice of mechanical support post-cardiotomy. This observation is in contrast to patients with ischemic cardiomyopathy presenting to our medical cardiac intensive care unit where the Impella CP is often used as the initial MCS device. Remaining indications including acute myocardial infarction, non-ischemic cardiopathy, myocarditis, and post-transplant graft dysfunction, were distributed similarly among patient groups. Notably, 26% of patients in the Impella group were cannulated in the context of CPR compared to none of the IABP patients (p = 0.027). Time to ECMO decannulation was similar between these patient groups (p = 0.76). The majority of patients in each group were peripherally cannulated although more common in the Impella group (84 vs 50%, p = 0.065). Notably, we found that 25% of patients who underwent unloading with an IABP required an additional device for LV venting during their course while none of the Impella patients required further unloading (p = 0.035). This finding highlights the direct nature of LV decompression provided by a transvalvular mechanism such as that employed by the Impella versus the indirect nature of LV unloading afforded by the IABP.
Table 5.
Indications for MCS therapy for patients undergoing VA ECMO with unloading via Impella versus IABP.
| Parameter | ECMO + IMPELLA (n=19) | ECMO + IABP (n=16) | p-value |
|---|---|---|---|
| Indication for ECLS | |||
| Acute myocardial infarction | 7 (37) | 5 (31) | 0.73 |
| Ischemic cardiomyopathy | 4 (21) | 0 | 0.11 |
| Non-ischemic cardiomyopathy | 3 (16) | 2 (13) | 1.00 |
| Myocarditis | 3 (16) | 1 (6) | 0.61 |
| Post-cardiotomy shock | 0 | 7 (44) | 0.002 |
| Post-transplant graft dysfunction | 2 (11) | 1 (6) | 1.00 |
| ECPR | 5 (26) | 0 | 0.027 |
| Bridge to ventricular assist device | 1 (5) | 2 (13) | 0.58 |
| Bridge to heart transplant | 1 (5) | 1 (6) | 1.00 |
| Duration of VA-ECMO support, days | 6.68 ± 4.52 | 6.19 ± 5.17 | 0.76 |
| Peripheral cannulation strategy | 16 (84) | 8 (50) | 0.065 |
| Need for additional LV vent | 0 | 4 (25) | 0.035 |
Values presented as number (percentage) or mean ± standard deviation.
We found that ECMO and the unloading device were most often initiated concomitantly in both the ECMO+Impella Group and the ECMO+IABP Group as detailed in Table 6 (74 vs 63%, p = 0.48). The order in which the devices were discontinued was more variable without a clear predominance and there were no statistical differences between the groups in this regard. Duration of the LV-unloading device and of total MCS support were not different between groups.
Table 6.
Details of MCS implementation for patients unloaded with an Impella versus IABP.
| Parameter | ECMO + IMPELLA (n=19) | ECMO + IABP (n=16) | p-value |
|---|---|---|---|
| Timing of ECMO cannulation and LV-unloading device initiation | |||
| Concomitant | 14 (74) | 10 (63) | 0.48 |
| LV-unloading device first | 4 (21) | 5 (31) | 0.70 |
| ECMO first | 1 (5) | 1 (6) | 1.00 |
| Duration of LV-unloading device | 8.84 ± 9.75 | 4.44 ± 2.83 | 0.091 |
| Timing of ECMO decannulation and LV-unloading device discontinuation | |||
| Concomitant | 8 (42) | 5 (31) | 0.73 |
| LV-unloading device removed first | 3 (16) | 6 (38) | 0.25 |
| ECMO discontinued first | 8 (42) | 5 (31) | 0.73 |
| Total duration of MCS support | 9.89 ± 9.22 | 6.81 ± 5.01 | 0.24 |
Values presented as number (percentage) or mean ± standard deviation.
Survival and Predicted Survival Based on Unloading Strategies
Thirty-day survival was similar between the ECMO+Impella Group and the ECMO+IABP Group patients as detailed in Table 7 and shown in Figure 2 (47% vs 31%, p=0.49). Survival to decannulation and survival to discharge were also similar between groups. No patients in these groups died due to infection or brain death. Death due to hemorrhage, cardiac death, multiple system organ failure, and cerebrovascular accident were similar in both groups and there were no statistical differences between groups in ECMO-related complications. Notably, hemorrhage was more common in patients unloaded with an Impella compared to those unloaded by an IABP but this finding did not reach clinical significance (79 vs 56%, p=0.15).
Table 7.
Survival rates and complications by unloading device.
| Parameter | ECMO + IMPELLA (n=19) | ECMO + IABP (n=16) | p-value |
|---|---|---|---|
| 30-day survival | 9 (47) | 5 (31) | 0.49 |
| Survival to decannulation | 14 (74) | 8 (50) | 0.15 |
| Survival to discharge | 7 (37) | 4 (25) | 0.49 |
| Cause of death | |||
| Hemorrhage | 0 | 1 (6) | 0.46 |
| Cardiac death | 4 (21) | 5 (31) | 0.70 |
| Infection | 0 | 0 | - |
| Multiple system organ failure | 6 (32) | 7 (44) | 0.46 |
| Cerebrovascular accident | 1 (5) | 0 | 1.00 |
| Brain death | 0 | 0 | - |
| Complications | |||
| Ischemic stroke | 5 (26) | 2 (13) | 0.415 |
| Intracranial hemorrhage | 2 (11) | 0 | 0.49 |
| Hemorrhage | 15 (79) | 9 (56) | 0.15 |
| Vascular complication | 4 (21) | 3 (19) | 1.00 |
| Hemolysis | 5 (26) | 3 (19) | 0.70 |
| Sepsis | 3 (16) | 2 (13) | 1.00 |
| Need for renal replacement therapy | 9 (47) | 7 (44) | 0.83 |
| Mesenteric ischemia | 1 (5) | 1 (6) | 1.00 |
| Abdominal compartment syndrome | 0 | 1 (6) | 0.46 |
| Intracardiac thrombus | 6 (32) | 3 (19) | 0.46 |
Values presented as number (percentage) or mean ± standard deviation.
Figure 2. 30-day all-cause survival grouped by unloading device.
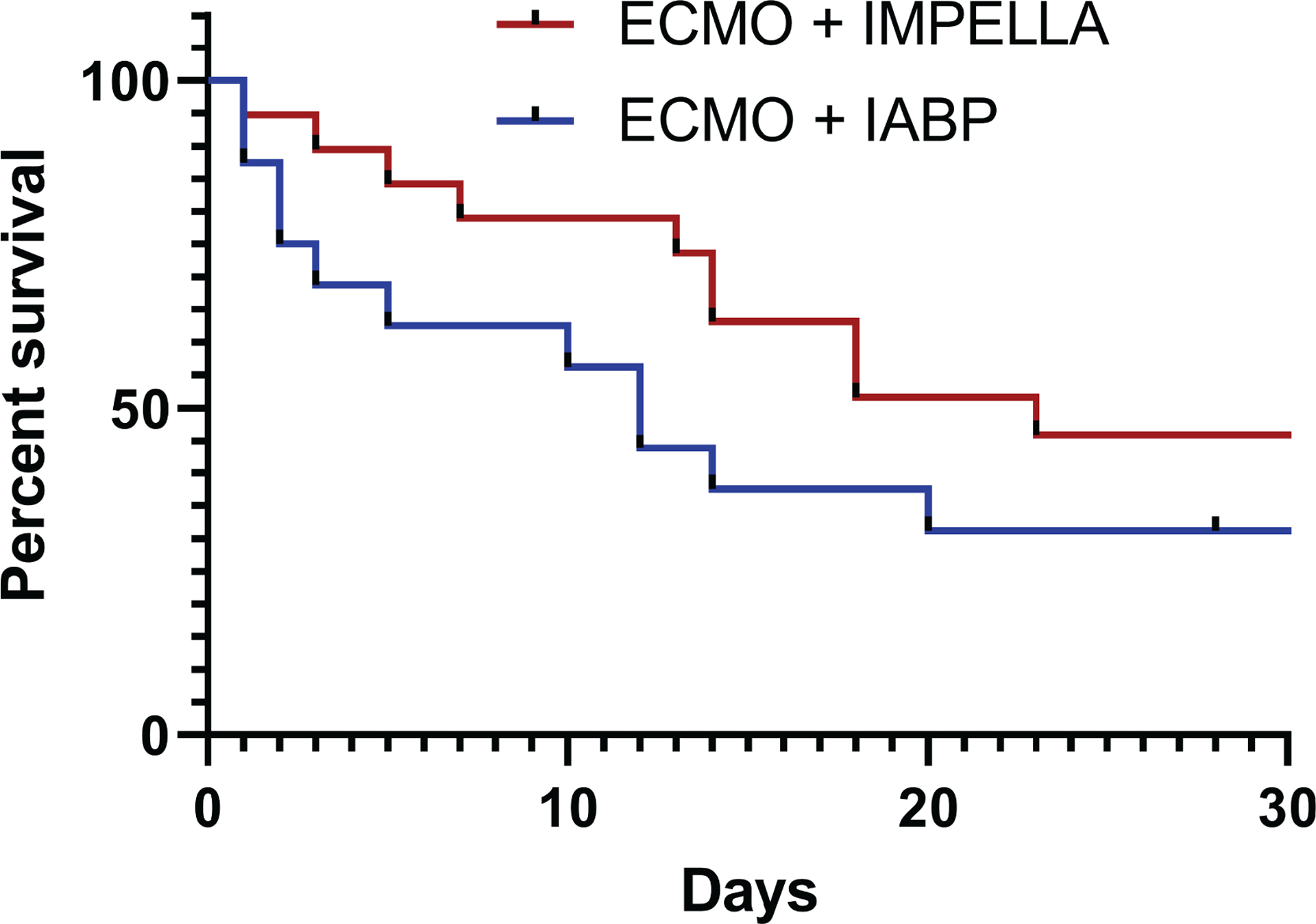
Kaplan-Meier curve demonstrating statistically equivalent survival between unloading devices (p=0.114).
Observed survival rates were compared to those predicted by SAVE scores. The median SAVE score of the Impella group (−10) corresponds to a predicted survival to discharge of 18%; the actual rate for this group was appreciably greater at 37% as shown in Table 8. In contrast, the median SAVE score for the IABP group (−8.5) corresponds to a predicted survival to discharge of 30% and we observed a slightly lower rate of 25%.
Table 8.
Actual versus predicted survival to discharge by unloading device.
| ECMO + IMPELLA (n=19) | ECMO + IABP (n=16) | |
|---|---|---|
| SAVE Score | −10 (−13 to −5.5) | −8.5 (−13 to −5) |
| Predicted survival to discharge | 18 % | 30 % |
| Actual survival to discharge | 37 % | 25 % |
Values are presented as median (interquartile range) or survival percentage. Predicted survival to discharge corresponds to that predicted by the median SAVE score.
DISCUSSION
Cardiogenic shock remains a highly morbid condition despite advances in critical care and clinical adoption of ECMO to provide mechanical circulatory support.21 While ECMO is capable of rapidly restoring systemic perfusion, clinical management is particularly challenging as it profoundly alters physiological circulation with poorly understood effects on the failing heart.3 In our study, we sought to investigate both the role of LV unloading and the effect of specific unloading strategy on survival of CS patients supported by ECMO.
Expectant management of LV distention versus Immediate LV Unloading
By shunting blood from the venous to the arterial system, ECMO both decreases cardiac preload and increases afterload.22 While improving cardiac function through augmented coronary perfusion, increased afterload may induce deleterious consequences for the failing heart. In profound failure, the left ventricle is unable to eject leading to distention, blood stasis, and risk of thrombus formation while rising intraventricular pressure acts to decrease coronary perfusion pressure further impairing cardiac function.23 Motivation for immediate LV unloading is driven by the theoretical benefit of reducing deleterious effects, minimizing further cardiac injury, and improving clinical outcomes.24 Immediate unloading conceivably may reduce need for arrhythmogenic inotropic agents otherwise necessary to overcome diminished cardiac contractility.
Developing an LV-unloading strategy has been advocated for as an important component of ECMO support with an estimated 70% of ECMO patients experiencing LV overload.12,25 A variety of diagnostic modalities to monitor for LV distention are employed in clinical practice including assessment of pulmonary edema on radiography, elevated pulmonary capillary wedge pressure, and increased LV diameter on echocardiography.26 Each of these modalities is intermittently performed with the risk that patients may develop potentially harmful LV distention between observation intervals. Additionally, the significance of subclinical LV distention on clinical outcomes is unknown. Both the possibility of missed LV distention and potential benefit derived from reducing subclinical LV distention argue for an immediate LV-unloading approach.
Despite the theoretical benefits of unloading, we observed no statistically significant differences in mortality in the reactive versus immediate LV unloading patient groups in our study. Examination of the Kaplan-Meier curves demonstrates that survival with immediate venting tended to be higher than survival with delayed venting. However, this difference was not statistically significant. Our study may have been underpowered to detect such a difference if one exists. CS patients are heterogeneous, differing significantly by their etiology of shock, co-morbid conditions, and likelihood for recovery. Although there were no differences between the delayed and immediate unloading groups in direct comparison of baseline characteristics and indication for MCS, it is difficult to account for these factors in aggregate. Our small sample size prohibits subgroup analyses which might otherwise provide insight into specific patient groups. We lacked sufficiently granular data to assess duration of shock prior to initiation of MCS which has been shown to affect outcomes.27 Further variability in care is introduced by choice of MCS platform, timing of its initiation, and its titration. While the theoretical basis for immediate LV unloading remains persuasive, realizing the significance of the clinical impact will likely require prospective randomized studies.
Given lack of evidence-based guidelines, the decision for immediate versus reactive LV unloading and the choice of device was substantially impacted by logistical considerations including device availability, staffing, cost, and experience. In our study, no patients with post-cardiotomy shock underwent Impella CP placement consistent with existing cardiac surgical preference for IABP support at our institution. Similarly, all patients experiencing E-CPR were unloaded with an Impella and were in the cardiac catheterization laboratory, our institution’s primary users of the Impella, at the time of arrest. As guidelines are lacking, we posit that such logistical and non-medical factors contribute to the variability of findings in the literature to date.
IABP versus Impella LV Unloading Device
The Impella CP and IABP are commonly used mechanical support devices with each having a unique operational paradigm. The Impella is an axial flow percutaneous ventricular assist device positioned across the aortic valve that propels blood antegrade into the aorta to directly offload the LV throughout the cardiac cycle.28–30 In contrast, IABP counterpulsation unloads the LV indirectly through decreasing afterload during systole to promote LV ejection.18,31,32 However, in the setting of ECMO support, and particularly peripheral cannulation with retrograde perfusion of the aorta, the mechanism by which an IABP may achieve LV unloading is unclear. Unlike the IABP, which has limited range of titration, the Impella provides multiple clinician-controlled flow settings enabling a greater degree of titration to a specific physiological state. Limiting adoption of the Impella as an LV venting device is its substantially higher cost in comparison with an IABP and concerns about increasing the risk of hemolysis by introducing a second mechanical pump in a patient maintained on ECMO. Each mechanical unloading device also presents the risk of vascular complications.33 The Impella CP is introduced through the femoral artery via a 14 Fr sheath in comparison to IABPs which typically require a 7–8 Fr sheath. While we observed no leg ischemia complications attributed to either mechanical unloading device, the larger vascular sheath required for the Impella CP is an important consideration.
The Impella pVAD platform consists of several different devices with varying capabilities and specifications. In general, the larger the device, the higher the amount of blood flow and the degree of support the device is capable of providing. The smaller Impella 2.5 has a 12 Fr diameter and is capable of providing up to 2.5 L/min of flow while the newer Impella 5.5 has a 19 Fr diameter and can provide up to 6.2 L/min of flow. The Impella CP provides a peak flow of 4.3 L/min with a size profile only slightly larger than the Impella 2.5. The Impella CP provides for a balance between size and capability leading to our institutional preference to use this device for both mechanical circulatory support and to provide for mechanical LV venting. Ease of placement is another factor as both the Impella 2.5 and Impella CP can be placed by interventional cardiologists with imaging assistance whereas the larger Impella devices are placed via axillary graft requiring surgical expertise.
Despite the apparent physiological advantages of the Impella CP as an offloading device, we found no difference in outcomes between the Impella and IABP patient groups. Although we noted no mortality benefit for unloading strategies, we found that our Impella patients had greater inpatient survival than predicted by their SAVE score (37 versus 18%). Our findings are consistent with those of Schrage et al. who recently reported results from their cohort of 106 Impella-unloaded patients.34 In their cohort, thirty-day all-cause mortality was 35.8% which was greater than the 20% survival predicted by the SAVE score or the 6.9% survival estimated by SAPS-II score. This group previously reported 21-patient cohort unloaded with Impella propensity matched with controls with a lower in-hospital mortality in the Impella group (47% vs 80%, p < 0.001).35 These retrospective studies support the hypothesis of a benefit of dual ECMO-Impella mechanical support in patient populations with a high mortality. Definitive testing of this hypothesis likely requires an adequately powered prospective trial.
ECMO-related complications
Rates of complications in both Group 1 and Group 2 patients were not detectably different in our study with the exception of significant hemorrhage in which patients who underwent immediate unloading experienced more hemorrhage than the reactive-unloading group (67% versus 40%, p=0.03). The etiology of this difference is unclear although may be related to dosing of anticoagulants at the initiation of support or challenges in obtaining vascular access at multiple sites simultaneously in patients experiencing active shock. We observed no difference in hemorrhage comparing unloading with an Impella versus an IABP. Similarly, Pappalardo et al. saw no significant difference in major bleeding between patients unloaded with an Impella vs. without and their propensity-matched VA ECMO cohort.35 Despite the general concern that multiple mechanical support devices with multiple vascular access site may increase risk of hemorrhage and hemolysis, this does not appear to be a consistent finding across observational studies reported to date.35,36
Study Limitations and Future Directions
Our work is limited by small sample size reducing its statistical power. Small patient numbers prohibited further subgroup or matched analyses that may identify significant differences for specific patient subtypes. The retrospective nature of our study prevents definitive conclusion regarding the causation of findings. While LV distention in the setting of central ECMO is poorly characterized, prior study has demonstrated benefit to LV decompression for patients maintained on central ECMO circulatory support.37 The inclusion of centrally-cannulated patients in our study is a potential confounder but small number of study patients limits the efficacy of further subgroup analysis. Of interest is that prior study has shown no significant differences in outcomes for post-cardiotomy patients with cardiogenic shock maintained on either central or peripheral ECMO.38 Although prospective randomized clinical trials remain the premium for defining patient care standards, observational studies such as our current report may inform the development of formalized protocols in advance of randomized controlled trials that may facilitate the conduct of such studies.
In absence of evidence-based guidelines, there is significant inter- and intra-institutional variability in the care of patients requiring mechanical circulatory support. As mechanical circulatory support platforms are not a therapy akin to a drug prescribed at a set dose and frequency or even a device with an “on-off” switch, their use requires near-constant provider decision making integrating hemodynamics, underlying pathology, titration of vasoactive agents, ventilation strategy, and management of associated devices, among other factors. As these patients are critically ill, their care is tenuous, and providing additional information for MCS providers to standardize and improve their overall strategy is vital for achieving optimal outcomes for these patients. Further studies are necessary to drive management consensus for CS patients requiring VA ECMO. Given the heterogeneity of this patient population, we propose that large-scale studies of patients stratified by specific shock etiology will be necessary to provide definitive evidence regarding LV unloading strategies. Physiologic principles suggest patients with ischemic etiologies of shock may benefit most from immediate LV unloading and targeting this subgroup is feasible for a prospective, controlled trial. Additionally, robust and direct comparison of LV unloading devices is needed to inform best approaches.
Source of funding statement:
S.P.K. was funded by NIH 5K08HL143342-02
Abbreviations:
- ECMO
extracorporeal membrane oxygenation
- LV
left ventricle
- IABP
intraaortic balloon pump
- MCS
mechanical circulatory support
- CS
cardiogenic shock
- L
liters
- E-CPR
ECMO cardiopulmonary resuscitation
Footnotes
Conflict of interest:
S.P.K. serves on the Critical Care Advisory Board for Abiomed, Inc.
REFERENCES
- 1.Stretch R, Sauer CM, Yuh DD, Bonde P: National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 64: 1407–15, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Sanaiha Y, Bailey K, Downey P, et al. : Trends in mortality and resource utilization for extracorporeal membrane oxygenation in the United States: 2008–2014 Surgery 165: 381–388, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Keller SP: Management of Peripheral Venoarterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock Crit Care Med 47: 1235–1242, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghodsizad A, Koerner MM, Brehm CE, El-Banayosy A: The role of extracorporeal membrane oxygenation circulatory support in the “crash and burn” patient: From implantation to weaning Curr Opin Cardiol 29: 275–280, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Guglin M, Zucker MJ, Bazan VM, et al. : Venoarterial ECMO for Adults J Am Coll Cardiol 73: 698–716, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Le Gall A, Follin A, Cholley B, Mantz J, Aissaoui N, Pirracchio R: Veno-arterial-ECMO in the intensive care unit: From technical aspects to clinical practice Anaesth Crit Care Pain Med 37: 259–268, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Jayaraman A, Cormican D, Shah P, Ramakrishna H: Cannulation strategies in adult veno-arterial and veno-venous extracorporeal membrane oxygenation: Techniques, limitations, and special considerations Ann Card Anaesth 20: 11, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD: Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest Circ Heart Fail 11: e004905, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Bavaria JE, Ratcliffe MB, Gupta KB, Wenger RK, Bogen DK, Edmunds LH: Changes in left ventricular systolic wall stress during biventricular circulatory assistance. Ann Thorac Surg 45: 526–32, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Ostadal P, Mlcek M, Kruger A, et al. : Increasing venoarterial extracorporeal membrane oxygenation flow negatively affects left ventricular performance in a porcine model of cardiogenic shock. J Transl Med 13: 266, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amarelli C, Musumeci F, Loforte A, Montalto A, Di Franco S, Hernandez-Montfort J: Flow Optimization, Management, and Prevention of LV Distention during VA-ECMO, in: Advances in Extra-corporeal Perfusion Therapies. IntechOpen, 2019. [Google Scholar]
- 12.Soleimani B, Pae W: Management of left ventricular distension during peripheral extracorporeal membrane oxygenation for cardiogenic shock Perfusion 27: 326–331, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Truby LK, Takeda K, Mauro C, et al. : Incidence and Implications of Left Ventricular Distention During Venoarterial Extracorporeal Membrane Oxygenation Support ASAIO J 63: 257–265, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Xie A, Forrest P, Loforte A: Left ventricular decompression in veno-arterial extracorporeal membrane oxygenation Ann Cardiothorac Surg 8: 9–18, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwo PY, Mantry PS, Coakley E, et al. : An Interferon-free Antiviral Regimen for HCV after Liver Transplantation N Engl J Med 371: 2375–2382, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Loforte A, Baiocchi M, Dal Checco E, et al. : Percutaneous Pulmonary Artery Venting via Jugular Vein While on Peripheral Extracorporeal Life Support ASAIO J: 1, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Prasad A, Ghodsizad A, Brehm C, et al. : Refractory Pulmonary Edema and Upper Body Hypoxemia During Veno-Arterial Extracorporeal Membrane Oxygenation—A Case for Atrial Septostomy Artif Organs 42: 664–669, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Meani P, Gelsomino S, Natour E, et al. : Modalities and Effects of Left Ventricle Unloading on Extracorporeal Life support: a Review of the Current Literature Eur J Heart Fail 19: 84–91, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Griffin JM, Restaino S, Takeda K, Garan AR: Left ventricular decompression on Veno-arterial extracorporeal membrane oxygenation with intra-aortic balloon Counterpulsation J Cardiothorac Surg 14: 153, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt M, Burrell A, Roberts L, et al. : Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score Eur Heart J 36: 2246–2256, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Csepe TA, Kilic A: Advancements in mechanical circulatory support for patients in acute and chronic heart failure J Thorac Dis 9: 4070–4083, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baran DA: Extracorporeal Membrane Oxygenation (ECMO) and the Critical Cardiac Patient Curr Transplant Reports 4: 218–225, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delewi R, Zijlstra F, Piek JJ: Left ventricular thrombus formation after acute myocardial infarction Heart 98: 1743–1749, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curran J, Burkhoff D, Kloner RA: Beyond Reperfusion: Acute Ventricular Unloading and Cardioprotection During Myocardial Infarction J Cardiovasc Transl Res 12: 95–106, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donker DW, Brodie D, Henriques JPS, Broomé M: Left ventricular unloading during veno-arterial ECMO: a review of percutaneous and surgical unloading interventions Perfusion 34: 98–105, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camboni D, Schmid C: To vent or not on veno-arterial extracorporeal membrane oxygenation, does it improve myocardial recovery and outcome? J Thorac Dis 9: 4915–4918, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basir MB, Schreiber TL, Grines CL, et al. : Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock Am J Cardiol 119: 845–851, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Rihal CS, Naidu SS, Givertz MM, et al. : 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care J Am Coll Cardiol 65: e7–e26, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Ergle K, Parto P, Krim SR: Percutaneous Ventricular Assist Devices: A Novel Approach in the Management of Patients With Acute Cardiogenic Shock. Ochsner J 16: 243–9, 2016 [PMC free article] [PubMed] [Google Scholar]
- 30.Chera HH, Nagar M, Chang NL, et al. : Overview of Impella and mechanical devices in cardiogenic shock Expert Rev Med Devices 15: 293–299, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Madershahian N, Wippermann J, Liakopoulos O, et al. : The acute effect of IABP-induced pulsatility on coronary vascular resistance and graft flow in critical ill patients during ECMO. J Cardiovasc Surg (Torino) 52: 411–8, 2011 [PubMed] [Google Scholar]
- 32.Brasseur A, Scolletta S, Lorusso R, Taccone FS: Hybrid extracorporeal membrane oxygenation J Thorac Dis 10: S707–S715, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boudoulas KD, Pederzolli A, Saini U, et al. : Comparison of Impella and intra-aortic balloon pump in high-risk percutaneous coronary intervention: Vascular complications and incidence of bleeding Acute Card Care 14: 120–124, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Schrage B, Burkhoff D, Rübsamen N, et al. : Unloading of the Left Ventricle During Venoarterial Extracorporeal Membrane Oxygenation Therapy in Cardiogenic Shock JACC Hear Fail 6: 1035–1043, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Pappalardo F, Schulte C, Pieri M, et al. : Concomitant implantation of Impella ® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock Eur J Heart Fail 19: 404–412, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Badiye AP, Hernandez GA, Novoa I, Chaparro SV.: Incidence of Hemolysis in Patients with Cardiogenic Shock Treated with Impella Percutaneous Left Ventricular Assist Device ASAIO J 62: 11–14, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Schmack B, Seppelt P, Weymann A, et al. : Extracorporeal life support with left ventricular decompression—improved survival in severe cardiogenic shock: results from a retrospective study PeerJ 5: e3813, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Djordjevic I, Eghbalzadeh K, Sabashnikov A, et al. : Central vs peripheral venoarterial ECMO in postcardiotomy cardiogenic shock J Card Surg, 2020 [DOI] [PubMed] [Google Scholar]


