Abstract
Purpose of review
This review is focused on the existing evidence for circadian control of innate and adaptive immune responses to provide a framework for evaluating the contributions of diurnal rhythms to control of infections and pathogenesis of disease.
Recent findings
Circadian rhythms driven by cell-autonomous biological clocks are central to innate and adaptive immune responses against microbial pathogens. Research during the past few years has uncovered circadian circuits governing leukocyte migration between tissues, the magnitude of mucosal inflammation, the types of cytokines produced, and the severity of immune diseases. Other studies revealed how disruption of the circadian clock impairs immune function or how microbial products alter clock machinery.
Summary
Revelations concerning the widespread impact of the circadian clock on immunity and homeostasis highlight how the timing of inflammatory challenges can dictate pathological outcomes and how the timing of therapeutic interventions likely determines clinical efficacy. An improved understanding of circadian circuits controlling immune function will facilitate advances in circadian immunotherapy
Keywords: clock, migration, allergy, asthma, microbiota, infection
Introduction
In order to anticipate and respond to daily environmental changes, virtually every living organism has evolved circadian clocks that drive daily oscillations in behavior and physiology. The 24-hour light-dark cycle along with other stimuli (e.g. feeding/fasting, oxygen, etc.) entrain cell-autonomous circadian clocks that regulate sleep, body temperature, digestion, locomotion, and metabolism [1]. Importantly, the responsiveness, localization, and activity of immune cells exhibit robust circadian rhythmicity. Mechanistically, this likely evolved to maximize immune defense during the greatest time of pathogen exposure and to facilitate tissue repair during rest [2, ●3].
This review summarizes recent advances in our understanding of diurnal rhythms and circadian clock circuitry that impact the magnitude and character of immune responses. Intricate changes in expression of migration factors and localization of immune cells are discussed in the context of immune surveillance, tissue repair, and disease pathogenesis. The impact of timing of vaccination or inflammatory challenge is described as a potent modulator of immune cell function and pathogen control. In addition, this review reveals the mechanism of microbe-mediated or disease-associated disruption of the circadian clock in immune cells as a driver of disease. In light of these factors, attention is devoted to the growing field of circadian medicine as a method for maximizing therapeutic efficacy via optimization of treatment timing or manipulation of the circadian clock.
Organism-level synchronization of the clock
The coordination of clocks within individual cells of the body is regulated by a master oscillator (Figure 1) in the hypothalamus called the suprachiasmatic nucleus (SCN) [4]. The SCN is entrained by light-dark cycles via innervation from the retina. In turn, the SCN clock transmits behavioral and rhythmic cues to peripheral oscillators via both neuropeptides and autonomic innervation [5]. Through SCN networking, the central clock also encodes day length and thereby drive seasonal responses [4]. Recent data revealing the astrocyte clocks can drive circadian rhythms of SCN [6], reveal evolutionary redundancy and importance of maintaining a master timekeeper.
Figure 1. Organization of central and cell-intrinsic peripheral oscillators.
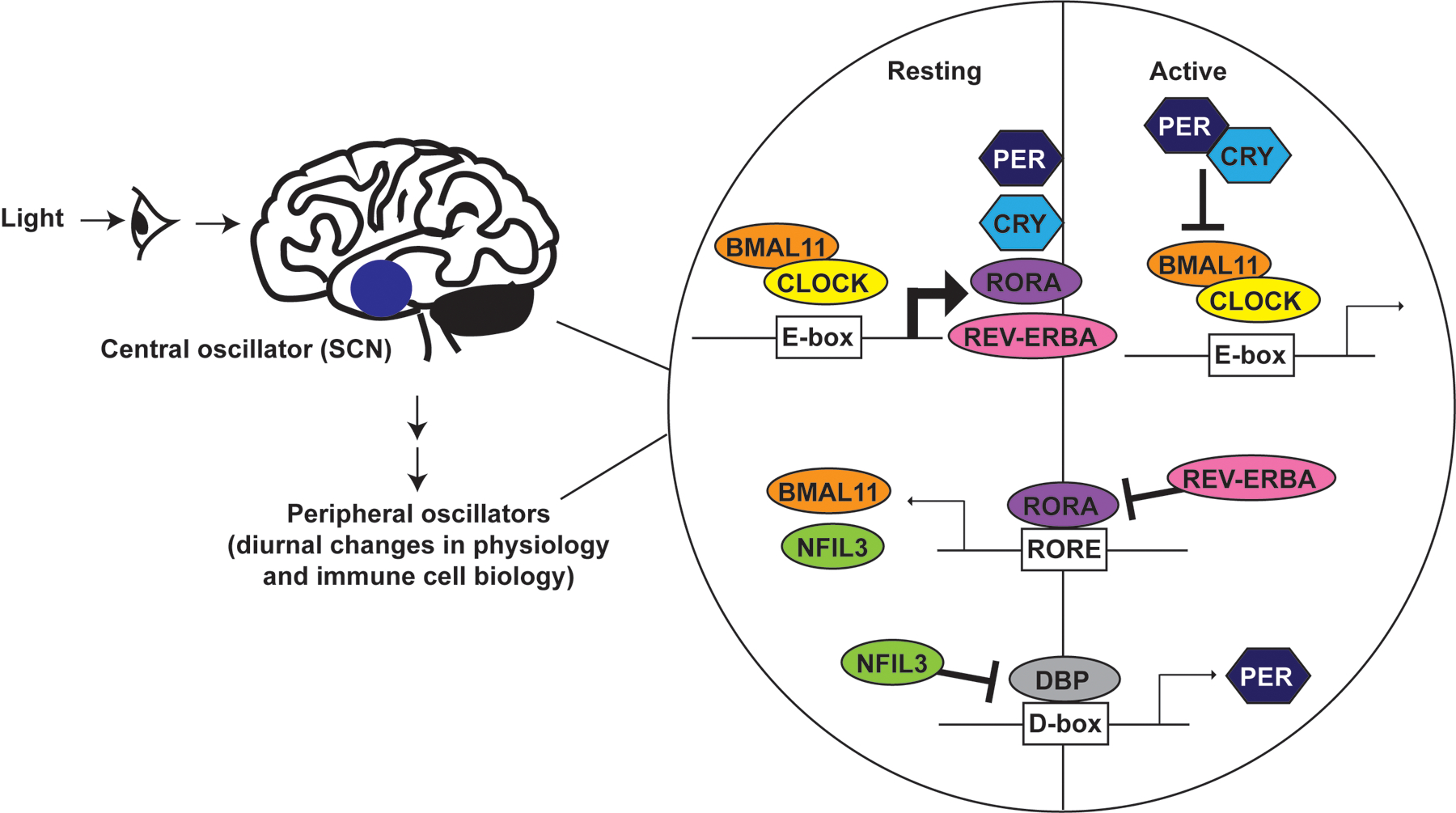
The central oscillator is comprised of the SCN in the hypothalamus, which receives light signals and transmits rhythmic cues to the peripheral oscillators via hormone and neural pathways that are further modulated by timing of food intake and other environmental cues. The peripheral clock machinery is comprised of three interlocking feedback loops. BMAL1 and CLOCK binding to E-box elements drives transcription of multiple clock-controlled genes. As PER and CRY accumulate, they translocate back into the nucleus to repress BMAL1:CLOCK driven gene transcription, including that of PER and CRY. REV-ERBα and RORα alternatively regulate expression of RORE element associated genes, including BMAL1 and NFIL3. NFIL3 and DBP in turn alternatively regulate expression of genes like PER via D-box elements. Each element modulates expression of clock-controlled genes that may either contribute to circadian expression of other genes or have a circadian expression profile without feeding back into the molecular clock machinery.
Anatomy of the molecular clock
The 2017 Nobel Prize in Physiology or Medicine was awarded for the discovery of evolutionarily conserved molecular mechanisms governing the circadian clock [7]. Mammalian circadian rhythms are a consequence of a transcriptional- translational feedback loop controlled by self-sustained cell-autonomous molecular clocks (Figure 1). These oscillating transcription-translation feedback loops are driven by a set of dedicated clock proteins [8]. The transcription factors brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK) heterodimerize and bind E-box regulatory sequence motifs throughout the genome to drive gene expression [9, 10]. Importantly, CLOCK-BMAL1 regulate expression of their own repressors, period (PER) proteins and cryptochromes (CRY), which increase in concentration over time, oligomerize and enter the nucleus to suppress CLOCK-BMAL1 activity [8]. This feedback loop operates in conjunction with two additional circuits to establish a 24-h rhythm. First, the timing and amplitude of BMAL1 expression is controlled by competitive binding of REV-ERBs (NR1D1 and NR1D2) and retinoic acid-related orphan receptor alpha (RORα) to the BMAL1 promoter, respectively, resulting in either repression or activation of BMAL1 transcription [11, 12]. Second, albumin D-box binding protein (DBP) transcriptional activator and its repressor, nuclear factor interleukin 3 regulated (NFIL3; also known as E4BP4), synergistically regulate expression of PER and other D-box genes [13]. The translation, trafficking, and degradation of clock proteins generates oscillations in gene expression that collectively establish the 24-h period of circadian timing (Figure 1).
Role of the clock in immune cell development
The majority of immune cell lineages possess intrinsic clocks that provide temporal control of development, differentiation, migration, and function of these cells [2, ●3]. In some cases, the circadian clock machinery contributes to development and differentiation of immune cell lineages. A primary example is NFIL3, which is required for the development of innate lymphoid cells (ILCs), including natural killer (NK) cells [14–18]. In the case of interferon-gamma (IFN-γ) producing ILC1 and NK cells, NFIL3 is essential both for development of these cells from a common ILC progenitor [15, 16, 19, 20], as well as, for the homeostatic maintenance of these lineages within intestines and lymphoid tissues [17, 18]. In contrast, group 3 ILC only require NFIL3 at the level of the common ILC progenitor [15].
Similar to the role of NFIL3 in ILC, the development of B cells depends on BMAL1. Bmal1−/− mice exhibit marked deficits in peripheral B cell numbers and immunoglobulin (IgG) titers [21]. However, cell transfer and conditional gene deletion experiments revealed a B cell-extrinsic role of BMAL1 in modulating the bone marrow microenvironment in which B cells develop [21, 22].
The circadian clock machinery is also implicated in the development of specific subsets of CD4⁺ T cells. Neither global nor T-cell-specific ablation of Bmal1 appreciably affected overall or subset-specific frequencies of T cells [21, 22]. However, loss of NFIL3 in T cells resulted in increased frequencies of T helper 17 (Th17) cells [23], whereas mice expressing a dominant-negative CLOCK protein or lacking either REV-ERBα or RORα exhibited reduced Th17 frequencies [23, 24]. Given the vital role of Th17 cells in intestinal health and immunity, the role of clock genes in Th17 homeostasis likely serves to balance the activity of the cells for optimal protection against pathogens with minimal tissue damage.
Clocks control leukocyte trafficking
During homeostasis, inflammatory cells undergo daily flux between the bone marrow, blood, and tissues. As a result, the number of circulating leukocytes in both mice and humans changes dynamically over the day [25, 26]. The rhythmic trafficking of immune cells during homeostasis is largely controlled via cell-extrinsic mechanisms [27]. Central clock cues from adrenergic nerves regulate expression of C-X-C motif chemokine ligand 12 (CXCL12) by bone marrow stromal cells. CXCL12 is a vital bone marrow retention signal and this mechanism controls the rhythmic egress of cells into the periphery.
Similar cell-extrinsic mechanisms control temporal variation in leukocyte numbers in tissues [28]. During acute inflammation, the rhythmic release of CXCL5 from bronchiolar epithelial Club cells in the lung promotes increased recruitment of neutrophils during the resting phase (daytime in mice) [29]. Elevated endogenous glucocorticoids dampen CXCL5 and neutrophil recruitment during the active phase (nighttime in mice), and BMAL1 deletion in Club cells abrogates this circadian circuit, resulting in a non-rhythmic pro-inflammatory influx of neutrophils into the lung.
Of note, diurnal expression of glucocorticoids also regulates IL-7 receptor (IL-7R) and CXCR4 in T cells [●30]. Circulating T cell frequencies in mouse blood peak during the resting phase when IL-7R expression is low, whereas elevations in IL-7R expression during the active phase enhanced T cell survival and accumulation in spleen and lymph nodes. As a result of active phase accumulation of T cells in secondary lymphoid tissues, antigen-specific T cell responses and humoral immunity induced by systemic bacterial infections and soluble antigen during this active phase were enhanced [●30].
A recent organism-wide analysis revealed that both the microenvironment and leukocyte-autonomous oscillations in migratory factors control the time-of-day-dependent homing of particular cell subsets to specific organs [●●31]. In multiple lymphoid and nonlymphoid tissues, pro-migratory molecules like intercellular adhesion molecule 1 (ICAM1), ICAM2, and vascular cell adhesion molecule 1 (VCAM1) undergo rhythmic oscillations. In contrast, addressins and selectins display tissue-restricted oscillations in the liver (E-selectin), lymph node (mucosal vascular addressin cell adhesion molecule 1, MAdCAM-1), gut (CD44 and peripheral lymph node addressin, PNAd), and skin (CD44). Correspondingly, some chemokine receptors (CXCR4) and adhesion molecules (P-selectin glycoprotein ligand-1, PSGL-1) exhibit circadian oscillations in nearly every leukocyte subset analyzed, including lymphocytes, myeloid cells, and granulocytes [●●31]. Genetic ablation of the clock in either the endothelium or in leukocyte ablates these time-of-day differences. Similarly, paired phasing of CC-chemokine receptor 7 (CCR7) on T cells and high endothelial venules CCL21 favored accumulation of mouse T cells in lymph nodes during the active phase [●32]. The authors conclude that an extensive circadian trafficking zip code system guides homeostatic leukocyte migration between circulation and organs.
Cell-intrinsic clocks also coordinate immune defense during inflammatory insult. Trafficking of inflammatory monocytes from blood to infected tissues is constrained by BMAL1 regulation of CXCR4 and CCL2 expression [25]. Likewise, BMAL1 regulation of CXCL2 expression modulates the migratory properties of circulating neutrophils, which is antagonized by CXCR4 [●33]. This process favors the egress of neutrophils from blood vessels of mice during the active phase, thereby enhancing anti-microbial activity within tissues. Disruption of this internal timer resulted in intravascular accumulation of neutrophils that predisposed mice to fatal vascular injury [●33]. Collectively, these studies reveal that leukocyte localization within the body is under strict environmental and cell-intrinsic circadian control that is vital to limit harmful pathology and promote optimal immune defense. In addition, these adaptations presumably optimize energy expenditure while pairing appropriate immune responses with the likely timing of pathogen encounter.
Rhythms of immune responses and function
In addition to controlling the location of leukocytes, circadian clocks impact the nature and amplitude of inflammatory responses induced by both pathogens and vaccines [●3]. The expression of pattern recognition receptors, secretion of complement or coagulation factors, the release of histamine, production of cytokines, and phagocytic activity of macrophages all exhibit circadian oscillations [2, 25, 26, 34–36]. For example, oscillations in NK cell capacity to make IFN-γ or kill target cells are dampened in the absence of PER1 [37]. Likewise, circadian control of IL-5 and IL-13 production by ILC2 determines blood eosinophilia and recruitment of eosinophils to tissue [38]. In the case of macrophages, REV-ERBs suppress CX3CR1, CCL2, CCL5, IL-12, and IL-6 expression to limit inflammation in mice during the resting phase [39, 40]. Desynchronization of the molecular clock among a population of macrophages promotes heterogeneity in inflammatory responses, with NFIL3 and DBP determining IL-12 production in response to lipopolysaccharide [●41].
Recent studies expanded our knowledge of circadian circuits on antimicrobial immunity to numerous bacteria, viruses, and parasites. Macrophage-associated BMAL1 is critical for control of Leishmania infection [42]. Dendritic cell (DC) BMAL1 dictates the Th1/Th2 balance via regulated production of IL-12, thereby contributing to strong Th2 bias and robust worm expulsion in morning-infected mice [43]. BMAL1 also controls IL-1 expression in myeloid cells [44].
In a similar fashion, BMAL1 determines innate immune control of infections with Sendai, respiratory syncytial, parainfluenza, hepatitis C, dengue, Zika, and influenza A virus at the level of both lung fibroblasts in vitro and intact mice [45–●47]. This likely drives changes in time-of-day susceptibility to influenza A and herpesvirus infections [48], while the absence of BMAL1-driven rhythms contributes to marked asthma-like airway changes [●47]. Importantly, oscillating leukocyte frequencies in tissues and circadian control of inflammatory gene expression critically affect harmful lung inflammation during influenza A virus infection [●●49]. As a result, infection just before the onset of the active phase resulted in more weight loss, increased clinical scores, and greater mortality than observed in mice infected just prior to the resting phase. Survival was independent of viral control but associated with the circadian influx of NK and NKT cells into the lung [●●49].
Microbial control of circadian immunity
Critical interactions between the microbiota and intestinal epithelium are orchestrated by the circadian clock. REV-ERBα and RORα control rhythmic gene expression of toll-like receptors (TLR) in intestinal epithelial cells (IEC) [50]. Moreover, intestinal bacteria trigger DC release of IL-23 during active phase in mice, thereby activating ILC3 to make IL-22 that in turn inhibits REV-ERBα in IEC [●51]. The resulting rhythmic expression of NFIL3 in IEC crucially regulates both Th17 differentiation and lipid metabolism. Intriguing new evidence points to synergistic contributions of light-dark cycles, food intake, and microbial cues via both the central clock and ILC3-intrinsic BMAL1 that shape intestinal health and lipid metabolism [●●52].
In fact, metabolism is synchronized withsleep-wake cycles and food intake. Recent work reveals an epigenetic mechanism by which gut microbiota contribute to daily metabolic cycles [●●53]. In small intestine but not colon IEC, microbiota drive oscillation in expression of metabolic genes involved in nutrient transport and lipid metabolism via rhythmic changes in histone deacetylase 3 (HDAC3) activity. Thus, mice lacking gut microbiota exhibit disrupted oscillations in metabolism and become obese on high-fat chow, providing a potential explanation for human obesity-associated with antibiotic damage to the microbiota.
Circadian disruption and clock contributions to disease
Circadian control of T and B cell responses affects the amplitude of pathogen-specific, allergen reactive and potentially harmful self-reactive lymphocytes. CLOCK promotes rhythmic proliferation responses of these cells by modulating timing of T-cell interaction with antigen-presenting cells [54]. Induction of sterile inflammation coincident with circadian timing of high cell counts in lymphoid tissues results in strong T [●●55, ●56] and B cell responses [●32, ●57]. This observation potentially explains stronger vaccine responses in humans immunized in the morning (early active phase) relative to the afternoon (late active phase) [58]. BMAL1 in DCs or myeloid cells was essential for oscillations in CD8 T cell responses [●●55, ●56]. Importantly, loss of myeloid BMAL1 increases inflammation in CNS with pathogenic expansions of Th1 and Th17 cells that exacerbate experimental autoimmune encephalomyelitis (EAE) [●●55]. Likewise, Cry1−/−/Cry2−/− mice exhibit marked elevations in IgG and autoantibody titers [●59], suggesting multiple circadian mechanisms exist to limit autoimmune disease.
The circadian clock has also been implicated in tumorigenesis [60]. In addition to the formation of tumors, some cancers (adenocarcinoma) mediate circadian remodeling in the liver via inflammatory mediators, where this process promotes a metabolic profile favorable to tumor growth [61]. In addition, therapies that disrupt circadian clock processes (REV-ERB agonists) selectively undermine the survival of tumor cells [●62], highlighting the importance of circadian rhythms in cancer. Intriguingly, BMAL1 was also implicated in suppression of programmed death-ligand 1 (PD-L1) expression on myeloid cells [63], which likely has implications for checkpoint blockade therapy targeting programmed cell death protein 1 (PD-1) on tumor-specific T cells.
Of note, asthma and allergic disease present robust circadian rhythms [●64]. There are marked day-night differences in clinical presentation of the disease, with symptoms usually worsening overnight (resting phase). Circadian variations in IgE and mast cells are likely important drivers of these disease oscillations [34]. As was the case with T cells [●●55], BMAL1 in myeloid cells constrains allergic asthma [65]. Likewise, CLOCK modulates the IL-33-mast cell axis through both temporal variation in IL-33-induced cytokine expression and oscillations in mast cell expression of the IL-33 receptor, ST2 [●66].
Therapeutic potential of clock modifiers
A large number of drugs used in the treatment of inflammatory and metabolic diseases directly target genes that exhibit circadian oscillations [●3, 67]. For example, targeting REV-ERB is beneficial in both atherosclerosis [68] and cancer [●62], whereas CRY-stabilizing drugs are anti-inflammatory [69]. The short in vivo half-life of many drugs and clock-dependent changes in drug metabolism genes provide further rationale for considerations of revisiting the time-of-day administration of these therapies. Some examples include the timing of vaccination [58], bone marrow transplantation [70], chemotherapy [71], and anti-inflammatory medication administration in arthritis, asthma, and multiple sclerosis [●3]. These promising results portend a future with carefully rationalized application of medical interventions and multiple new tools for modulation of the molecular clock to curtail disease.
Conclusions
Homeostasis and inflammatory responses of the immune system exhibit marked time-of-day variations. These oscillations are a product of cell-intrinsic molecular clocks and environmental cues- light, food, or microbiota. As a result, the number and function of leukocytes in specific tissues are highly dependent on circadian rhythms that determine the amplitude and nature of immune responses to immunological challenges. Circadian disruption of the rhythms is often observed in inflammatory diseases, including autoimmunity, atherosclerosis, and asthma. An improved understanding of the complex interplay between cell-intrinsic clocks in different immune cells and the central clock will be necessary to advance new circadian medicine opportunities.
Acknowledgements
The author is supported by National Institutes of Health (NIH) grants DA038017, AI148080, and AR073228, as well as by Cincinnati Children’s Research Foundation. I wish to thank Lauren Francey and John Hogenesch for reading the manuscript and providing helpful feedback.
References
- 1.Turek FW, Circadian clocks: Not your grandfather’s clock. Science, 2016. 354(6315): p. 992–993. [DOI] [PubMed] [Google Scholar]
- 2.Man K, Loudon A, and Chawla A, Immunity around the clock. Science, 2016. 354(6315): p. 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ●.Scheiermann C, et al. , Clocking in to immunity. Nat Rev Immunol, 2018. 18(7): p. 423–437. [DOI] [PubMed] [Google Scholar]; Outstanding comprehensive review of circadian aspects of immunity.
- 4.Hastings MH, Maywood ES, and Brancaccio M, Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci, 2018. 19(8): p. 453–469. [DOI] [PubMed] [Google Scholar]
- 5.Cheng MY, et al. , Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature, 2002. 417(6887): p. 405–10. [DOI] [PubMed] [Google Scholar]
- 6.Brancaccio M, et al. , Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science, 2019. 363(6423): p. 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burki T, Nobel Prize awarded for discoveries in circadian rhythm. Lancet, 2017. 390(10104): p. e25. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi JS, Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet, 2017. 18(3): p. 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young MW and Kay SA, Time zones: a comparative genetics of circadian clocks. Nat Rev Genet, 2001. 2(9): p. 702–15. [DOI] [PubMed] [Google Scholar]
- 10.Allada R, et al. , Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci, 2001. 24: p. 1091–119. [DOI] [PubMed] [Google Scholar]
- 11.Preitner N, et al. , The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell, 2002. 110(2): p. 251–60. [DOI] [PubMed] [Google Scholar]
- 12.Sato TK, et al. , A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron, 2004. 43(4): p. 527–37. [DOI] [PubMed] [Google Scholar]
- 13.Mitsui S, et al. , Antagonistic role of E4BP4 and PAR proteins in the circadian oscillatory mechanism. Genes Dev, 2001. 15(8): p. 995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kostrzewski T, et al. , Multiple Levels of Control Determine How E4bp4/Nfil3 Regulates NK Cell Development. J Immunol, 2018. 200(4): p. 1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiger TL, et al. , Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med, 2014. 211(9): p. 1723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seillet C, et al. , Nfil3 is required for the development of all innate lymphoid cell subsets. J Exp Med, 2014. 211(9): p. 1733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Male V, et al. , The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. J Exp Med, 2014. 211(4): p. 635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamizono S, et al. , Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J Exp Med, 2009. 206(13): p. 2977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu W, et al. , NFIL3 orchestrates the emergence of common helper innate lymphoid cell precursors. Cell Rep, 2015. 10(12): p. 2043–54. [DOI] [PubMed] [Google Scholar]
- 20.Yu X, et al. , The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. Elife, 2014. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, et al. , MOP3, a component of the molecular clock, regulates the development of B cells. Immunology, 2006. 119(4): p. 451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemmers S and Rudensky AY, The Cell-Intrinsic Circadian Clock Is Dispensable for Lymphocyte Differentiation and Function. Cell Rep, 2015. 11(9): p. 1339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu X, et al. , TH17 cell differentiation is regulated by the circadian clock. Science, 2013. 342(6159): p. 727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang XO, et al. , T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity, 2008. 28(1): p. 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen KD, et al. , Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science, 2013. 341(6153): p. 1483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casanova-Acebes M, et al. , Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell, 2013. 153(5): p. 1025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendez-Ferrer S, et al. , Haematopoietic stem cell release is regulated by circadian oscillations. Nature, 2008. 452(7186): p. 442–7. [DOI] [PubMed] [Google Scholar]
- 28.Haspel JA, et al. , Circadian rhythm reprogramming during lung inflammation. Nat Commun, 2014. 5: p. 4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibbs J, et al. , An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med, 2014. 20(8): p. 919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. ●.Shimba A, et al. , Glucocorticoids Drive Diurnal Oscillations in T Cell Distribution and Responses by Inducing Interleukin-7 Receptor and CXCR4. Immunity, 2018. 48(2): p. 286–298 e6. [DOI] [PubMed] [Google Scholar]; This study reveals how circadian oscillations in glucocortoids influence T cell survival and tissue distribution.
- 31. ●●.He W, et al. , Circadian Expression of Migratory Factors Establishes Lineage-Specific Signatures that Guide the Homing of Leukocyte Subsets to Tissues. Immunity, 2018. 49(6): p. 1175–1190 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]; A powerful circadian screen of adhesion and migratory receptors on leukocytes and endothelial cells reveals mechanisms driving time-of-day changes in leukocytes frequencies in various tissues.
- 32. ●.Druzd D, et al. , Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity, 2017. 46(1): p. 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals rhythmic exression of migratory factors and associated oscillations in lymphocyte transit between blood and lymphoid tissues.
- 33. ●.Adrover JM, et al. , A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity, 2019. 50(2): p. 390–402 e10. [DOI] [PubMed] [Google Scholar]; This article identifies a cell-intrinsic clock in neutrophils dictating egress from blood to control infections and prevent vascular injury.
- 34.Christ P, et al. , The Circadian Clock Drives Mast Cell Functions in Allergic Reactions. Front Immunol, 2018. 9: p. 1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silver AC, et al. , The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity, 2012. 36(2): p. 251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellet MM, et al. , Circadian clock regulates the host response to Salmonella. Proc Natl Acad Sci U S A, 2013. 110(24): p. 9897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Logan RW, et al. , Altered circadian expression of cytokines and cytolytic factors in splenic natural killer cells of Per1(−/−) mutant mice. J Interferon Cytokine Res, 2013. 33(3): p. 108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nussbaum JC, et al. , Type 2 innate lymphoid cells control eosinophil homeostasis. Nature, 2013. 502(7470): p. 245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam MT, et al. , Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature, 2013. 498(7455): p. 511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibbs JE, et al. , The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A, 2012. 109(2): p. 582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. ●.Allen NC, et al. , Desynchronization of the molecular clock contributes to the heterogeneity of the inflammatory response. Sci Signal, 2019. 12(571). [DOI] [PubMed] [Google Scholar]; This work reveals a mechanism of inflammatory heterogeneity driven by desynchronization of the molecular clock in myeloid cells.
- 42.Kiessling S, et al. , The circadian clock in immune cells controls the magnitude of Leishmania parasite infection. Sci Rep, 2017. 7(1): p. 10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hopwood TW, et al. , The circadian regulator BMAL1 programmes responses to parasitic worm infection via a dendritic cell clock. Sci Rep, 2018. 8(1): p. 3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Early JO, et al. , Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc Natl Acad Sci U S A, 2018. 115(36): p. E8460–E8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majumdar T, et al. , Circadian transcription factor BMAL1 regulates innate immunity against select RNA viruses. Innate Immun, 2017. 23(2): p. 147–154. [DOI] [PubMed] [Google Scholar]
- 46.Zhuang X, et al. , The circadian clock components BMAL1 and REV-ERBalpha regulate flavivirus replication. Nat Commun, 2019. 10(1): p. 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. ●.Ehlers A, et al. , BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunol, 2018. 11(1): p. 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstarte circadian control of immune responses against respiratory pathogens that dictate severity of lung inflammation and pathogenesis of asthma like disease.
- 48.Edgar RS, et al. , Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc Natl Acad Sci U S A, 2016. 113(36): p. 10085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. ●●.Sengupta S, et al. , Circadian control of lung inflammation in influenza infection. Nat Commun, 2019. 10(1): p. 4107. [DOI] [PMC free article] [PubMed] [Google Scholar]; Exciting study revealing that time-of-day susceptibility to morbidity and mortality during influenza infection is attributable to differences in number and function of innate lymphocytes as well as inflammatory monocytes in the lung.
- 50.Mukherji A, et al. , Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell, 2013. 153(4): p. 812–27. [DOI] [PubMed] [Google Scholar]
- 51. ●.Wang Y, et al. , The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science, 2017. 357(6354): p. 912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals crosstalk between circadian proteins REV-ERBα and NFIL3 critical for proper regulation of Th17 differentiation and lipid metabolism.
- 52. ●●.Godinho-Silva C, et al. , Light-entrained and brain-tuned circadian circuits regulate ILC3s and gut homeostasis. Nature, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrates circadian circuit in ILC3 integrating light and microbial cues to shape intestinal health and proper metabolism.
- 53. ●●.Kuang Z, et al. , The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science, 2019. 365(6460): p. 1428–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show that microbiota program circadian rhythms in intestinal epithelium via activation of HDAC3.
- 54.Fortier EE, et al. , Circadian variation of the response of T cells to antigen. J Immunol, 2011. 187(12): p. 6291–300. [DOI] [PubMed] [Google Scholar]
- 55. ●●.Sutton CE, et al. , Loss of the molecular clock in myeloid cells exacerbates T cell-mediated CNS autoimmune disease. Nat Commun, 2017. 8(1): p. 1923. [DOI] [PMC free article] [PubMed] [Google Scholar]; Crosstalk between T cells and APC is under stringent clock control to limit development of autoimmune-disease causing Th1 and Th17 cells.
- 56. ●.Nobis CC, et al. , The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proc Natl Acad Sci U S A, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; Circadian rhythms in DCs determine amplitude of CD8 T cell responses after immunization.
- 57. ●.Suzuki K, et al. , Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J Exp Med, 2016. 213(12): p. 2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration of adrenergic control of lymphocyte trafficking and resulting humoral immune responses.
- 58.Long JE, et al. , Corrigendum to ‘Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial’ [Vaccine 34 (2016) 2679–2685]. Vaccine, 2016. 34(40): p. 4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. ●.Cao Q, et al. , Circadian clock cryptochrome proteins regulate autoimmunity. Proc Natl Acad Sci U S A, 2017. 114(47): p. 12548–12553. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals that CRY genes are critical for prevention of exaggerated responses of autoreactive B cells like those observed in human lupus.
- 60.Fu L and Lee CC, The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer, 2003. 3(5): p. 350–61. [DOI] [PubMed] [Google Scholar]
- 61.Masri S, et al. , Lung Adenocarcinoma Distally Rewires Hepatic Circadian Homeostasis. Cell, 2016. 165(4): p. 896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. ●.Sulli G, et al. , Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature, 2018. 553(7688): p. 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]; Agonism of REV-ERBs is lethal to cancer cells iva inhibition of autophagy and lipid metabolism.
- 63.Deng W, et al. , The Circadian Clock Controls Immune Checkpoint Pathway in Sepsis. Cell Rep, 2018. 24(2): p. 366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. ●.Nakao A, Clockwork allergy: How the circadian clock underpins allergic reactions. J Allergy Clin Immunol, 2018. 142(4): p. 1021–1031. [DOI] [PubMed] [Google Scholar]; Comprehensive review of the intersections between circadian biology and clinical presentation of allergic and asthmatic diseases.
- 65.Zaslona Z, et al. , The circadian protein BMAL1 in myeloid cells is a negative regulator of allergic asthma. Am J Physiol Lung Cell Mol Physiol, 2017. 312(6): p. L855–L860. [DOI] [PubMed] [Google Scholar]
- 66. ●.Kawauchi T, et al. , Clock-dependent temporal regulation of IL-33/ST2-mediated mast cell response. Allergol Int, 2017. 66(3): p. 472–478. [DOI] [PubMed] [Google Scholar]; This study shows that CLOCK regulates IL-33-mast cell axis via contraint of cytokines and cytokine receptor expression.
- 67.Cederroth CR, et al. , Medicine in the Fourth Dimension. Cell Metab, 2019. 30(2): p. 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sitaula S, et al. , Suppression of atherosclerosis by synthetic REV-ERB agonist. Biochem Biophys Res Commun, 2015. 460(3): p. 566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hand LE, et al. , The circadian clock regulates inflammatory arthritis. FASEB J, 2016. 30(11): p. 3759–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scheiermann C, et al. , Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity, 2012. 37(2): p. 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borniger JC, et al. , Time-of-Day Dictates Transcriptional Inflammatory Responses to Cytotoxic Chemotherapy. Sci Rep, 2017. 7: p. 41220. [DOI] [PMC free article] [PubMed] [Google Scholar]


