Abstract
Purpose of review
To summarize a new form of autoimmune diabetes as an adverse event of specific cancer immunotherapies. Immune checkpoint inhibitors are revolutionary treatments in advanced cancers; however, they can cause type 1 diabetes following treatment with these state-of-the-art therapies.
Recent findings
A review of the literature showed that this new form of autoimmune diabetes has significant similarities with childhood-onset type 1 diabetes but also some distinctions. It frequently presents with severe diabetic ketoacidosis and almost half of the patients have type 1 diabetes-associated antibodies at presentation. Rapid loss of residual beta-cell function with a lack of honeymoon phase is typical. Certain human leukocyte antigen risk genes for prototypical type 1 diabetes that develops in children and young adults are also commonly found in patients with immune checkpoint inhibitor-induced type 1 diabetes.
Summary
Immune checkpoint inhibitor-induced type 1 diabetes presenting with diabetic ketoacidosis is a life-threatening adverse event of cancer immunotherapy. Healthcare providers should be aware of this adverse event to prevent morbidity and mortality related to diabetic ketoacidosis. Developing guidelines to identify and monitor risk groups are of utmost importance.
Keywords: Immune checkpoint inhibitors, immune checkpoint inhibitor-induced diabetes, autoimmune diabetes, type 1 diabetes, cancer, immunotherapy, nivolumab, pembrolizumab
Introduction
Immune checkpoint inhibitors (ICI) are beneficial cancer immunotherapies for many advanced malignancies [1]. These therapies inhibit negative immune regulation allowing an individual’s immune system to target cancer cells. However, immune-related adverse events occur anytime during or after the therapy, mostly within first few weeks to months after initiation of treatment [2,3], including the rapid onset of autoimmune diabetes, which has similarities to type 1 diabetes (T1D) [4]. ICI-induced T1D is a rare but life-threatening side effect as it can present with diabetic ketoacidosis (DKA) [4]. Diabetes is permanent and requires lifelong treatment with insulin therapy. Notably, ICI-induced T1D has been reported with all clinically available PD-1 (nivolumab, pembrolizumab, cemiplimab) and PD-L1 inhibitors (avelumab, durvalumab, atezolizumab) but rarely with the CTLA-4 inhibitor (ipilimumab).
Review
Cancer immunotherapy has been increasing in use and has significant benefits over chemotherapy [5]. ICI therapy has become a gold standard treatment for many advanced stage cancers. Notably, these therapies can also precipitate many immune related adverse events especially affecting endocrine organs. In this review, a serious adverse event, T1D will be discussed in detail.
Incidence
The incidence of ICI-induced T1D comes from large case series at academic medical centers reporting 27 cases out of 2960 patients receiving ICI therapy (0.9%) [6] and 21/1163 (1.8%) [7]. Additionally from the drug information sheets reporting adverse events in registration trials, in patients receiving nivolumab T1D occurred in 0.9% (17/1994) [8], and 0.7% (4/534) for cemiplimab [9]. However, when examining the clinical trials evaluating the efficacy of PD-1 and PD-L1 inhibitors, there is a wide range of reported hyperglycemia and diabetes (Table 1) [10–22]. From these 13 phase 2 or 3 clinical trials spanning many cancer types, hyperglycemia/diabetes is reported in 53 out of 2,394 (2.2%) of treated individuals.
Table 1.
Clinical Trials Reporting Hyperglycemia/Diabetes with ICI use
| Reported cases(n) | Study (n) | Side effect (%) | Side effect | Drug | Cancer Type | Journal | Year | Reference |
|---|---|---|---|---|---|---|---|---|
| 4 | 135 | 2.96 | Hyperglycemia | Lambrolizumab | Melanoma | NEJM | 2013 | 10 |
| 13 | 287 | 4.52 | Hyperglycemia | Nivolumab | Lung | NEJM | 2015 | 11 |
| 9 | 406 | 2.21 | Hyperglycemia | Nivolumab | Renal cell | NEJM | 2015 | 12 |
| 1 | 206 | 0.48 | Diabetes | Nivolumab | Melanoma | NEJM | 2015 | 13 |
| 1 | 154 | 0.64 | Type 1 Diabetes | Pembrolizumab | Lung | NEJM | 2016 | 14 |
| 1 | 88 | 1.13 | Type 1 Diabetes | Avelumab | Merkel-cell | Lancet | 2016 | 15 |
| 1 | 26 | 3.84 | Hyperglycemia | Pembrolizumab | Merkel-cell | NEJM | 2016 | 16 |
| 2 | 452 | 0.44 | Diabetes | Nivolumab | Melanoma | NEJM | 2017 | 17 |
| 3 | 53 | 5.66 | Hyperglycemia | Avelumab | Solid tumors | Lancet | 2017 | 18 |
| 7 | 55 | 12.7 | Hyperglycemia | Avelumab | Renal cell | Lancet | 2018 | 19 |
| 4 | 300 | 1.33 | Hyperglycemia | Pembrolizumab | Squamous cell | Lancet | 2019 | 20 |
| 6 | 78 | 7.7 | Hyperglycemia | Cemiplimab | Cutaneous cell | Lancet | 2020 | 21 |
| 1 | 154 | 0.64 | Hyperglycemia | Pembrolizumab | Childhood tumors | Lancet | 2020 | 22 |
Of note, most of the clinical trials in Table 1 excluded patients with a preexisting autoimmune condition and some even excluded patients with a family history of autoimmunity. As these therapies are now being more widely used in clinical practice, there is an increased reporting of ICI-induced diabetes [23,24]. This is likely due to the increasing use of ICI therapy and differences in patient populations between phase 2/3 clinical trials and clinical practice [25]. Some of the newer cases could also be worsening of type 2 diabetes as this has also been described [6].
To put the incidence of ICI-induced T1D in the context of other adverse events, fatigue and nausea or vomiting are the most common occurring in more than 20% of patients receiving ICI therapy. Endocrine adverse events such as hypothyroidism occur in approximately 8% and adrenal insufficiency occurs less than 1% of treated patients [3].
Taken together, the current incidence of ICI-induced T1D is in the range of 1–2% of all patients receiving anti-PD-1 and anti-PD-L1 therapies. This incidence is much greater than prototypical T1D in children and young adults, which has an incidence of approximately 0.3% (26).
Pathophysiology
The first case series reporting ICI-induced autoimmune diabetes was described in 2015 [27]. In this series of five patients, both humoral and cellular diabetes-associated autoimmunity were described. Several patients had positive T1D associated autoantibodies and diabetes-specific CD8 T-cells in the peripheral blood, consistent with findings from childhood-onset T1D [27]. From an autopsy of a patient that developed ICI-induced diabetes, significant infiltration of T lymphocytes into pancreatic islets was evident with CD8 T-cells more prevalent than CD4 [28]. These studies provide direct evidence for the autoimmune mediated nature of ICI-induced T1D in both the peripheral blood and target organ (pancreatic islets).
The role of the PD-1/PD-L1 pathway in preclinical animal models of T1D has been appreciated for over a decade. Non-obese-diabetic (NOD) mice spontaneously develop autoimmune diabetes so it has been used extensively as an animal model to understand the mechanisms of T1D development [29]. NOD mice with a knockout of either PD-1 or PD-L1 (but not PD-L2) have accelerated onset of diabetes with lymphocytic infiltration of the pancreatic islets (e.g. insulitis) compared to mice with these immune regulatory molecules [30,31]. Furthermore, administration of anti-PD-1 or PD-L1 monoclonal antibodies to NOD mice also accelerated the onset of T1D [32]. When examining the islets in NOD mice, insulin-producing beta-cells express PD-L1 during the progression of autoimmune diabetes [33]. Similar to NOD mice, human islets from T1D organ donors exhibit upregulation of PD-L1, which was strongly associated with insulitis [34]. This likely represents a protective mechanism for beta-cells to lessen their autoimmune destruction. These studies may explain why anti-PD-1/PD-L1 therapies induce T1D, while there is an absence of diabetes with anti-CTLA-4 therapy, whose ligands are CD80 and CD86 on antigen-presenting cells such as B cells, dendritic cells, and macrophages.
Clinical Characteristics
Over the last 4 years, cases have described rapid-onset insulin-dependent diabetes with undetectable C-peptide levels (a measure of residual beta-cell function) and both positive and negative T1D associated autoantibodies at presentation. T1D associated autoantibodies include those directed against insulin and beta cell proteins: glutamic acid decarboxylase (GAD), insulinoma antigen-2 (IA-2), and zinc transporter 8 (ZnT8). Cases of ICI-induced T1D have remained insulin-dependent even upon stopping therapy [35–40]. Steroid treatment has not been able to reverse T1D, and as expected, blood glucose worsens with steroid administration [39,40]. PD-1 and PD-L1 inhibitor prescribing labels include T1D as a possible adverse event with these therapies [8,9,39].
ICI-induced T1D is mostly reported in older patients (50–70 years old) due to the nature of end-stage cancers developing later in life. More cases have been reported with anti-PD-1 therapies (nivolumab and pembrolizumab) as these agents were approved prior to monoclonal antibodies targeting anti-PD-L1 [6,7,38]. Melanoma is the most common cancer in patients that present with ICI-induced T1D, likely due to this being the first approved indication for ICI therapy, and more patients with melanoma have been exposed to ICI therapy compared to other cancer types [6,7,38]. However, with the expanding indications and recent approval of ICI therapy for use in pediatric cancers [42], ICI-induced T1D may increase and present in younger individuals.
Metabolic Features
ICI-induced T1D presents within days to a year after the initiation of anti-PD-1 or PD-L1 therapy [38]. In the largest meta-analysis to date, it was shown that 71% of the cases present in the first 90 days after the initiation of therapy [38]. There is some evidence that T1D presentation is sooner with pembrolizumab compared to nivolumab, however this finding needs to be confirmed in future studies [6,38]. Hemoglobin A1c, which is a measure of the average blood glucose over the preceding three months, is generally lower than 10% at presentation with the majority of patients presenting between 7 to 8% [38, 43]. As these values are mildly elevated, this suggests significant hyperglycemia over a short period rather than a gradual increase in hyperglycemia over a longer time [6,38]. Most of the patients present with severe DKA that can be life-threatening [6,38]. In the majority of cases, C-peptide levels are inappropriately low for the presenting blood glucose or undetectable [38,43]; ‘honeymoon’ periods tend to be absent after diagnosis [25,38]. These observations suggest rapid destruction of beta-cell mass [38,43,44], and a life-long need for exogenous insulin treatment.
In some ICI-induced T1D patients, increased amylase and/or lipase has been reported, suggesting more generalized pancreatic inflammation [43]. The rapid reduction of total pancreas volume has been reported in parallel with endocrine pancreas dysfunction [45]. It has been shown that in patients with established T1D, initiation of ICI therapy did not worsen diabetes management but did worsen other endocrine comorbidities such as autoimmune thyroid disease [46].
Immunologic Features
At least one T1D associated autoantibody, directed against insulin, GAD, IA-2, or ZnT8 has been reported in 40–50% of the cases [6,38,43,47]. Almost all antibody-positive cases had GAD antibodies; however, not all four major autoantibodies were reported or measured in most case series [38]. It is speculated that there is an association between antibody presence and earlier onset of ICI-induced T1D in a subset of patients [6]. Reviewing all reported cases reported to date, it was shown that patients presenting with T1D-associated antibodies had a more rapid onset and higher incidence of ketoacidosis than those without antibodies [38]. In one case, positive conversion of T1D antibodies after ICI therapy was confirmed as the antibodies were not present in a pre-treatment sample [6].
In a case that developed T1D five days after the start of PD-1 inhibitor therapy, GAD antibodies were polyclonal and predominantly of the IgG1 subclass for GAD [46]. Since IgG antibodies are involved in memory immune response and the short time interval from the initiation of anti-PD-1 treatment to T1D onset, these antibodies were likely present before the start of therapy [46]. Based on these findings, a subset of patients developing ICI-induced T1D likely have preexisting T1D associated antibodies which may be an early form of latent autoimmune diabetes of adulthood (LADA); however, prospective studies measuring T1D associated antibodies prior to the start of ICI therapy are needed to evaluate this hypothesis [38].
There is also data to suggest that the cancer response to therapy and survival rates are higher in patients with immune-related adverse events following immune checkpoint inhibitor therapy [48]. In patients that have autoantibodies such as anti-thyroid antibodies, antinuclear antibodies, or rheumatoid factor, the survival rate is higher compared to patients without these autoantibodies [49].
Genetic Risk
Human leukocyte antigen (HLA) genes on chromosome 6 confer genetic risk for many autoimmune disorders including childhood-onset T1D [50]. The polymorphic class II HLA genes (DQ, DR, and DP) confer this risk, especially the DR4-DQ8 and DR3-DQ2 haplotypes for T1D [51,52]. Only a small number of cases with ICI-induced T1D have reported HLA genes but with many having T1D risk alleles [38,43,47]. In one case series, the frequency of HLA-DR4 was found to be enriched in those with ICI-induced T1D compared to rates among Caucasians in the US population [6]. In a larger case series, 62% of the patients expressed HLA-DR4 [43]. In the largest meta-analysis to date, more than half of the cases did not report HLA genes however in reported cases (n=32), 84% had HLA genes with an increased risk for T1D [38]. Further research is necessary to identify HLA and other genetic variants that may confer risk for ICI-induced T1D.
Comparison to Childhood-onset Type 1 Diabetes
We believe it is useful to compare the clinical features and mechanisms of ICI-induced T1D to prototypical spontaneous T1D (Table 2), as this may help provide insights into the mechanisms of conventional T1D development in children and young adults. The age of onset is distinctly different between the two types of diabetes. For ICI-induced T1D, DKA is more common and the onset of diabetes more rapid than traditional T1D. T1D associated autoantibodies are present in ~90% of children and adolescents with T1D compared to half of the reported cases in ICI-induced T1D. There is a predominance of GAD antibodies at the presentation of ICI-induced T1D; however, more research is needed to measure all four major T1D associated autoantibodies in these patients and those directed against post-translationally modified antigens may also reveal insights into the pathogenesis of the disorder. C-peptide levels are low or undetected in those treated with ICI therapy that develop T1D compared to C-peptide levels that vary and gradually go down after the diagnosis of childhood T1D. As a corollary, the honeymoon phase is generally absent in ICI-induced T1D. HbA1c is generally lower than expected as a result of the rapid increase of blood glucose in a short period of time.
Table 2.
Comparison between prototypical and immune checkpoint inhibitor-induced type 1 diabetes
| Prototypical Type 1 Diabetes | Immune Checkpoint Inhibitor-Induced Type 1 Diabetes | |
|---|---|---|
| Age of Onset | Peak in early childhood & adolescence | Later adulthood, 60’s |
| Diabetic ketoacidosis at Onset | Common | Very common |
| Pathophysiology | Autoimmune (years) | Autoimmune (days to months) |
| Autoantibodies | Present in 90–95% | Present in ∼50%* |
| HLA Risk Genes | ∼90% | ∼60–80%+ |
| C-peptide at presentation | Varies | Low/absent |
| Honeymoon phase | Present | Absent |
Predominantly GAD antibodies
Small sample size, as not all cases report HLA alleles; there is an association with HLA-DR4
Screening and Monitoring Recommendations
Currently, there are no clinical guidelines for screening and monitoring of ICI-induced T1D. Discussing the risk of developing T1D with patients and educating them about the signs and symptoms of diabetes and DKA are recommended. Based on the current evidence, patients who have positive T1D associated antibodies and certain HLA alleles may have an increased risk to develop diabetes, so screening for autoantibodies and HLA risk alleles before the initiation of treatment may identify patients with a greater risk.
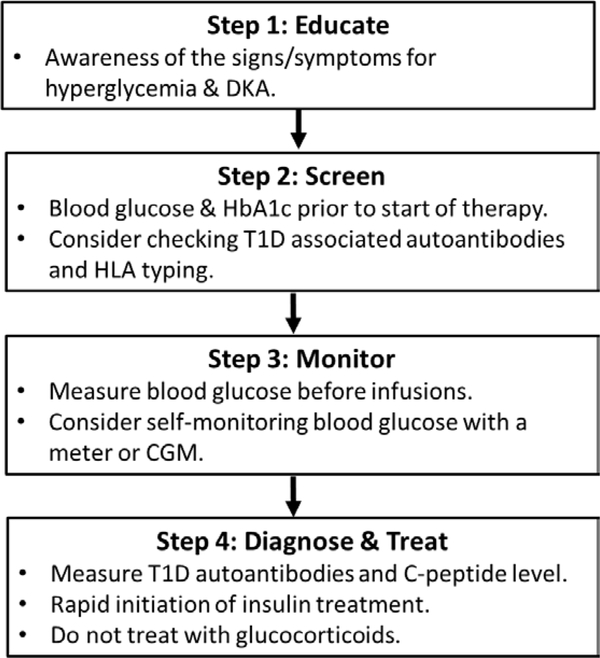
A retrospective study evaluated fasting blood glucose levels of patients receiving ICI treatment during patient visits and showed no detectable upward drift of glycemia before DKA presentation [53]. This is likely due to the rapid onset and progression of ICI-induced T1D. However, we believe monitoring blood glucose and HbA1c levels during patient visits are still necessary. Considering the rapid onset of diabetes, this approach alone may miss a significant amount of hyperglycemia and DKA. We recommend routine self-monitoring of blood glucose by patients and/or using continuous glucose monitoring to recognize hyperglycemia before DKA presentation. Close monitoring of patients with preexisting autoimmunity may also be useful. Our suggested screening and monitoring algorithm is depicted in Figure 1.
Figure 1.
Diagram of an approach to screen and manage patients for ICI-induced T1D. HLA = human leukocyte antigen alleles, CGM = continuous glucose monitor.
Conclusions
Considering the increasing use of immune checkpoint inhibitors in clinical practice, health care providers and patients should be aware of ICI-induced T1D as a serious adverse event. Educating both patients and providers using these state-of-the-art therapies about the signs and symptoms of hyperglycemia/diabetes is critical for an early diagnosis to prevent life-threatening DKA. Developing screening guidelines are essential to identify at-risk patients for close monitoring of this unwanted side effect.
Key Points.
Immune checkpoint inhibitor-induced T1D has a rapid onset of weeks to months following the start of treatment that commonly presents with life-threatening DKA.
Immune checkpoint inhibitor-induced T1D presenting with T1D-associated antibodies have a more rapid onset and higher incidence of ketoacidosis than those without antibodies.
Healthcare providers need to be aware of this adverse event to prevent related morbidity and mortality.
Acknowledgments
Financial support and sponsorship: This work was supported by NIH Grants (DK108868, DK110845, DK099317, and DK032083), the Juvenile Diabetes Research Foundation; and the Children’s Diabetes Foundation.
Footnotes
Conflicts of interest: No potential conflicts of interest relevant to this article are reported.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. The New England journal of medicine 2018;378:158–68. [DOI] [PubMed] [Google Scholar]
- 3.Dougan M, Pietropaolo M. Time to dissect the autoimmune etiology of cancer antibody immunotherapy. J Clin Invest 2020;130(1):51–61■■ Comphrehensive review of immune checkpoint inhibitor mechanisms and autoimmune side effects.
- 4.Akturk HK, Michels AW. Adverse Events Associated with Immune Checkpoint Blockade. The New England journal of medicine 2018;378:1163–4. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman HL, Atkins MB, Subedi P, et al. The promise of Immuno-oncology: implications for defining the value of cancer treatment. J Immunother Cancer 2019;7(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes 2018;67:1471–80.■ First large series of immune checkpoint inhibitor-induced diabetes at two large medical centers.
- 7.Kotwal A, Haddox C, Block M, Kudva YC. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Research & Care 2019;7:e000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nivolumab label information: https://packageinserts.bms.com/pi/pi_opdivo.pdf.
- 9.Cemiplimab label information: http://products.sanofi.ca/en/libtayo.pdf
- 10.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine 2013;369:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. The New England journal of medicine 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. The New England journal of medicine 2015;373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. The New England journal of medicine 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- 14.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology 2016;17:1374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. The New England journal of medicine 2016;374:2542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. The New England journal of medicine 2017;377:1824–35. [DOI] [PubMed] [Google Scholar]
- 18.Heery CR, O’Sullivan-Coyne G, Madan RA, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. The Lancet Oncology 2017;18:587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choueiri TK, Larkin J, Oya M, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. The Lancet Oncology 2018;19:451–60. [DOI] [PubMed] [Google Scholar]
- 20.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019. November 23;394(10212):1915–1928. [DOI] [PubMed] [Google Scholar]
- 21.Migden MR, Khushalani NI, Chang ALS, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020. February;21(2):294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geoerger B, Kang HJ, Yalon-Oren M, et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020. January;21(1):121–133 [DOI] [PubMed] [Google Scholar]
- 23.Wright JJ, Salem JE, Johnson DB, et al. Increased Reporting of Immune Checkpoint Inhibitor-Associated Diabetes. Diabetes care 2018;41:e150–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji HH, Tang XW, Dong Z, et al. Adverse Event Profiles of Anti-CTLA-4 and Anti-PD-1 Monoclonal Antibodies Alone or in Combination: Analysis of Spontaneous Reports Submitted to FAERS. Clinical drug investigation 2019;39:319–30. [DOI] [PubMed] [Google Scholar]
- 25.Akturk HK, Michels AW. Immune checkpoint inhibitor-induced type 1 diabetes: An underestimated risk. Mayo Clin Proc. 2020. March;95(3):614–615. [DOI] [PubMed] [Google Scholar]
- 26.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med 2017;376(15):1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes care 2015;38:e55–7.■ First case series reporting autoimmune diabetes following immune checkpoint inhibitor therapy.
- 28.Yoneda S, Imagawa A, Hosokawa Y, et al. T-Lymphocyte Infiltration to Islets in the Pancreas of a Patient Who Developed Type 1 Diabetes After Administration of Immune Checkpoint Inhibitors. Diabetes Care. 2019. July;42(7):e116–e118 [DOI] [PubMed] [Google Scholar]
- 29.Mullen Y Development of the Nonobese Diabetic Mouse and Contribution of Animal Models for Understanding Type 1 Diabetes. Pancreas 2017;46:455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Yoshida T, Nakaki F, et al. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proceedings of the National Academy of Sciences of the United States of America 2005;102:11823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. The Journal of experimental medicine 2006;203:883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. The Journal of experimental medicine 2003;198:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rui J, Deng S, Arazi A, Perdigoto AL, Liu Z, Herold KC. beta Cells that Resist Immunological Attack Develop during Progression of Autoimmune Diabetes in NOD Mice. Cell metabolism 2017;25:727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osum KC, Burrack AL, Martinov T, et al. Interferon-gamma drives programmed death-ligand 1 expression on islet beta cells to limit T cell function during autoimmune diabetes. Scientific reports 2018;8:8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaudy C, Clevy C, Monestier S, et al. Anti-PD1 Pembrolizumab Can Induce Exceptional Fulminant Type 1 Diabetes. Diabetes care 2015;38:e182–3. [DOI] [PubMed] [Google Scholar]
- 36.Mellati M, Eaton KD, Brooks-Worrell BM, et al. Anti-PD-1 and Anti-PDL-1 Monoclonal Antibodies Causing Type 1 Diabetes. Diabetes care 2015;38:e137–8. [DOI] [PubMed] [Google Scholar]
- 37.Zaied AA, Akturk HK, Joseph RW, Lee AS. New-onset insulin-dependent diabetes due to nivolumab. Endocrinology, diabetes & metabolism case reports 2018:doi: 10.1530/EDM-17-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akturk HK, Kahramangil D, Sarwal A, et al. Immune checkpoint inhibitor-induced Type 1 diabetes: a systematic review and meta-analysis. Diabet Med. 2019. September;36(9):1075–1081.■■ Largest meta-analysis to date of ICI-induced T1D cases (n=71) showing similarities and distinctions between childhood-onset and ICI-induced T1D.
- 39.Fukui A, Sugiyama K, Yamada T, et al. A Case of Nivolumab-Induced Fulminant Type 1 Diabetes with Steroids and Glucagon-Like Peptide 1 Administration during the Early Onset. Journal of Clinical Case Reports 2016;6:doi: 10.4172/2165-7920.1000883. [DOI] [Google Scholar]
- 40.Kapke J, Shaheen Z, Kilari D, Knudson P, Wong S. Immune Checkpoint Inhibitor-Associated Type 1 Diabetes Mellitus: Case Series, Review of the Literature, and Optimal Management. Case reports in oncology 2017;10:897–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durvalumab label information. (https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761069s000lbl.pdf.)
- 42.Wedekind MF, Denton NL, Chen CY, Cripe TP. Pediatric Cancer Immunotherapy: Opportunities and Challenges. Paediatric drugs 2018;20:395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perdigoto AL, Quandt Z, Anderson M, Herold KC. Checkpoint inhibitor-induced insulin-dependent diabetes: an emerging syndrome. Lancet Diabetes Endocrinol. 2019. June;7(6):421–423. [DOI] [PubMed] [Google Scholar]
- 44.Akturk HK, Michels AW: Adverse Events Associated With Immune Checkpoint Inhibitors. JAMA 2019;321:1219. [DOI] [PubMed] [Google Scholar]
- 45.Marchand L, Thivolet A, Saintigny P, et al. Anti-Programmed Death 1 (PD-1) Antibodies and the Pancreas: A Diabetic Storm Ahead? Diabetes Care. 2018. March;41(3):638–639. [DOI] [PubMed] [Google Scholar]
- 46.Akturk HK, Alkanani A, Zhao Z, et al. PD-1 Inhibitor Immune-Related Adverse Events in Patients with Preexisting Endocrine Autoimmunity. The Journal of clinical endocrinology and metabolism 2018;103:3589–92.■ First report of immune checkpoint inhibitor use in a patient with established type 1 diabetes.
- 47.Clotman K, Janssens K, Specenier P, et al. Programmed cell death-1 (PD-1) inhibitor induced type 1 diabetes mellitus: mini-review. The Journal of clinical endocrinology and metabolism 2018;103:3144–54. [DOI] [PubMed] [Google Scholar]
- 48.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small cell lung cancer. Jama Oncol 2018;4(3):374–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toi Y, Sugawara S, Sugisaka J, et al. Profiling preexisting antibodies in patients treated with anti PD-1 therapy for advanced non-small cell lung cancer. Jama Oncol 2019;5(3):376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Current diabetes reports 2011;11:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noble JA, Valdes AM, Varney MD, et al. HLA class I and genetic susceptibility to type 1 diabetes: results from the Type 1 Diabetes Genetics Consortium. Diabetes 2010;59:2972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magis Q, Gaudy-Marqueste C, Basire A, et al. Diabetes and Blood Glucose Disorders Under Anti-PD1. Journal of immunotherapy (Hagerstown, Md : 1997) 2018;41:232–40. [DOI] [PubMed] [Google Scholar]