Abstract
Background:
depression is common among people living with HIV, but rarely diagnosed and treated in sub-Saharan Africa, in part due to the paucity of mental health professionals. Task-shifting approaches have been used to address this barrier. We compared the effects of two task-shifting models of depression care on depression alleviation and antidepressant response.
Methods:
we conducted a cluster randomized controlled trial of two task-shifting models to facilitating depression care delivered by trained medical providers, one that utilized a structured protocol (protocolized) and one that relied on the judgment of trained providers (clinical acumen), in 10 HIV clinics in Uganda. A sample of 1252 clients (640 at protocolized clinics, 612 at clinical acumen clinics) who had screened positive for potential depression on the 2-item Patient Health Questionnaire (PHQ-2) were enrolled and followed for 12 months. Interviewer-administered 9-item PHQ (PHQ-9) data from the research surveys, and provider administrations to clients treated with antidepressant therapy, were examined. Linear probability regression analyses were conducted using a wild cluster bootstrap to control for clustering.
Results:
among the whole sample (regardless of treatment status), rates of depression alleviation (PHQ-9<5) at month 12 were equivalent in the protocolized (75%) and clinical acumen (77%) arms, in an intention-to-treat analysis. Similarly, among the 415 participants who received antidepressant care, rates of treatment response (PHQ-9<5) at the last provider administered PHQ-9 (average of 8 months into treatment) were equivalent between the protocolized (65%) and clinical acumen (69%) arms; rate of improvement over the course of treatment was also equivalent.
Conclusions:
nurses can provide quality depression care to HIV clients, regardless of whether treatment is guided by a structured protocol or clinical acumen, in the context of appropriate training and ongoing supervision support.
Keywords: depression, task-shifting, HIV, Uganda, cluster randomized controlled trial, depression alleviation, anti-depressant response
Introduction
Depression is common among people living with HIV (PLWHIV) in sub-Saharan Africa (SSA) and Uganda specifically, with rates of clinical depression ranging from 10–20%, and an additional 20–30% having elevated depressive symptoms (1,2), rates of which are approximately three times that of the general population (2). Depression is a medical condition characterized by both cognitive/affective (e.g., sadness, loss of interest in normally pleasurable activities) and somatic (e.g., difficult sleeping, loss of appetite, fatigue) symptoms, and for PLWHIV can result from numerous challenges ranging from coping with the HIV diagnosis, dealing with the biological effects of HIV or unwanted effects of taking daily medication, social stigma and rejection, and lost income, among others. In the context of HIV, depression has been associated with a greater likelihood of mortality (3), worse immunologic and virologic response (4) and adherence (5) to antiretroviral therapy (ART), as well as less consistent condom use (6). Despite the availability of effective treatments for depression in PLWHIV (7), including antidepressants, and the benefits of depression treatment for ART use and adherence (8), depression treatment is rarely integrated into HIV care programs across SSA, in part because of the severe shortage of mental health professionals (9).
Task-shifting models of care, in which lower trained cadres take on duties traditionally performed by more highly trained providers, present a solution to deficits in highly trained health care providers. Task-shifting, nurse-driven care has been shown to result in equivalent levels of quality of care in the context of HIV care and scale-up of antiretroviral therapy (ART) in SSA (10), and is prominent in Uganda where nurses often assume the role of primary care provider and a clinical or medical officer (physician) provides oversight and manages more complicated cases. “Collaborative care” models of depression treatment, which combine task-shifting, use of a structured, algorithm-based protocol, and supervision by a specialist, have rarely been studied in HIV-infected populations, but have been successfully implemented with non-HIV patients in the US (11,12), as well as resource constrained settings such as India and Asia (13). Controlled studies have demonstrated the effectiveness of collaborative care models and that algorithm-based, protocol driven care is associated with better quality of care and treatment outcomes by ensuring adequate treatment dosage and duration (11–13).
While task-shifting models of depression care have been shown to be effective, how to best implement such models or what mechanisms are key to their success, are less clear. INDEPTH (INtegration of DEPression Treatment in HIV care) Uganda is a cluster randomized trial that compared two task-shifting implementation models of depression care: a protocolized model in which care was provided largely by trained nurses using a systematic, structured protocol, and a model that relied on the clinical acumen of trained primary care providers (most of whom were nurses as well). Ongoing, on-site supervision from study psychiatrists was provided at all study sites in both models. Therefore, the study evaluated the value of a protocolized approach to depression care in the context of supervision from a specialist.
A prior analysis revealed that the protocolized model was statistically superior to clinical acumen in ensuring that patients who screened positive for potential depression at triage were further evaluated for clinical depression by their medical provider (83 vs 42%), but only marginally better with regard to clinically depressed patients receiving antidepressant treatment (67 vs 55%) (14). In this paper we report on analyses that compare the two depression care models on depression alleviation and antidepressant treatment response. Our hypothesis was that the protocolized model would result in better overall alleviation of depression and treatment response compared to clinical acumen.
Methods
Study design
INDEPTH-Uganda was a comparative trial that compared two active task-shifting implementation models for integrating antidepressant treatment into HIV care within 10 health care facilities in Uganda. The selection of 10 sites (clusters) was based on feasibility given the funding level of the grant. Using a cluster randomization, 5 clinics were assigned to implement a protocolized model, and 5 others relied on the clinical acumen of trained providers. To ensure the two study arms were balanced on clinic size, separate randomization drawings were conducted within the 6 larger clinics and the 4 small clinics. At a meeting attended by representatives from all study sites, names of the sites were placed on pieces of paper that were folded and placed into a hat, from which one of the study investigators drew the assignments to the two study arms in alternating order.
The models were implemented over 24 months starting in January 2013. To evaluate the models, data was collected from documentation mechanisms integrated into routine care, and a cohort comprised of adult clients who screened positive for potential depression at triage. A more detailed description of the study protocol has been published elsewhere (15), and the trial has been registered with the National Institutes of Health sponsored clinical trials registry (NCT02056106). The study protocol was approved by Institutional Review Boards at the RAND Corporation and Makerere University School of Medicine Ethical Research Committee, as well as the Uganda National Council for Science and Technology.
Study setting
The study was conducted in collaboration with Mild-may Uganda, a non-government organization that provides holistic outpatient HIV care at its own clinics, as well as technical assistance in HIV care to healthcare facilities across Uganda. Of the 10 healthcare facilities that participated in the study, eight were run by the Ministry of Health and two were private, faith-based, not-for-profit healthcare facilities; the clinics were located in the districts of Mpigi, Mityana, Luweero, and Wakiso. Each facility is a hospital that operates an HIV clinic on specific designated days of the week, and it is in these clinics that depression care was integrated as part of this study. The six larger clinics operate 2–3 days per week and generally have one clinical or medical officer and 3 to 5 nurses to provide primary HIV care to 1500–3000 clients. The four smaller clinics operate one day per week and are manned by one clinical or medical officer and 2–3 nurses; the number of clients ranges from 350–1000. At all clinics, nurses serve as primary care providers (along with the clinical/medical officers) and manage the prescription and monitoring of ART and other common HIV medications; more complex conditions or complications that arise are typically managed by the clinical/medical officer. All clinics have expert clients (volunteer, experienced HIV clients who display exemplary HIV care adherence and retention, many of whom are also village health team workers) who volunteer to take on tasks such as triage assessments, and filing and retrieving charts. No psychiatric or depression care services were being provided at these clinics prior to the study; usual care did not include any depression screening, but rather it relied on primary care providers to identify and treat depressed clients, or refer to external specialists at the nearest district or regional hospital - which resulted in depression rarely being assessed or treated.
Participants
Between January and December of 2013, clients who screened positive for potential depression on the 2- item Patient Health Questionnaire (PHQ-2)(16) at triage were eligible for enrollment into the cohort if they were at least 18 years of age, medically stable (not about to start [or recently started] ART or treatment for an acute opportunistic infection), and the research coordinator confirmed their depression status after re-administering the PHQ-2. All clients who screened positive for potential depression on the days of recruitment (one day per week at each site) were informed of the study and asked to provide written informed consent. Records on refusals were not kept, but research coordinators indicate that the vast majority (> 95%) of eligible clients agreed to participate. Participants were followed for 12 months with assessments at baseline and months 6 and 12. Participants received 10,000 Ush (~$4 USD) for completing each assessment.
Depression care models
A more detailed description of these models is available elsewhere (15), but we briefly describe here the components of the models, with particular focus on the monitoring of treatment component given the aims of this paper’s analyses. Implementation of the models began after a one-day training workshop for clinic staff, followed by on-site mentorship and supervision from the study psychiatrist that continued throughout the two-year implementation of the program.
Protocolized model
The model’s gateway to all downstream depression care processes was the routine depression screening of all adult patients at each clinic visit using the PHQ- 2 administered at the triage station by trained expert clients. For patients who screened positive for potential depression (PHQ-2 ≥ 3) and were medically stable, the depression care nurse was to conduct a depression diagnostic evaluation using the 9-item PHQ (PHQ-9) (16) and Mini International Neuropsychiatric Interview (MINI) (17). When warranted, antidepressant treatment consisted of either fluoxetine or imipramine depending on the client’s presenting symptoms and psychiatric history. Fluoxetine was started at 20 mg/day, while imipramine was started at 50 mg/day and increased to 75 mg after one week. Psychoeducation was provided to patients regarding depression as a disease and what to expect from treatment.
Monitoring of treatment response and side effects:
once antidepressants were prescribed, patients were asked to return two weeks later for the prescribing nurse to monitor side effects, treatment response and any need for change in dosage or medication. The visit schedule then became monthly until the patient was in remission (defined as PHQ-9 < 5 and tolerating any side effects) for one month, at which time the visit schedule was modified to match the patient’s HIV care visit schedule (typically every 2–3 months), but no less than every 3 months; if the patient experienced a relapse, visits returned to monthly until in remission. At each visit, the provider was to assess depressive symptoms (using the PHQ-9), presence of side effects and medication adherence. Dose increases in increments of 20 mg and 25 mg for fluoxetine and imipramine, respectively, or medication changes were considered starting at the second follow-up visit, depending on treatment response and side effects. Psychoeducation regarding depression treatment was to continue as needed in order to support patient adherence. Once the patient was in remission for at least 6 months, treatment was to be discontinued, unless the patient had a history of multiple episodes of Major Depression, in which case treatment was to be maintained for two years. A Depression Treatment Registry was instituted at each site in which the above listed parameters were recorded for each visit, as well as medication and dosage prescribed. These data were reviewed during supervision and used for reference in future follow-up visits, as well as for tracking fidelity to the treatment protocol.
Ongoing supervision:
training and ongoing supervision are critical for ensuring quality of care and fidelity to the depression care models, and to provide technical and emotional support to the providers. Following the initial start-up training, supervision took place monthly at each site. During these visits, supervisors conducted one-on-one sessions with each nurse to discuss challenging or non-responsive cases and any problematic areas of performance highlighted by the supervisor’s review of patient charts and the Depression Treatment Registry. At each supervision visit, supervisors reviewed the charts of all patients prescribed antidepressants within the past month, as well as 10 randomly selected charts of patients receiving ongoing antidepressant treatment monitoring. At each supervision visit, supervisors also had group meetings with all clinic staff involved with implementation of depression care so that organizational barriers to care could be addressed as a team and peer support could be fostered. Supervisors were available 24 hours a day, 7 days a week, for emergency or suicide crisis consultations.
Clinical acumen model
Like the protocolized model, the clinical acumen arm also included routine screening of all adult clients with the PHQ-2 by trained expert clients at triage, the information from which was relayed to the primary care provider; however, this model relied on the clinical judgment of the primary care provider to decide whether to further evaluate and treat clients who screen positive for possible depression (PHQ-2 ≥ 3), as opposed to following a structured protocol. For patients prescribed antidepressants, providers in the clinical acumen arm were trained to monitor treatment in the same way as providers in the protocolized model. The structure of supervision provided to the clinical acumen sites was identical to that in the protocolized arm.
Measures
Depression
Depressive symptoms were assessed using the 9- item Patient Health Questionnaire (PHQ-9), in both the survey (administered by the study coordinator, who was a trained psychologist) and by medical providers when diagnosing depression and monitoring antidepressant treatment. Each item of the PHQ-9 corresponds to the 9 symptoms assessed in the depression module of the Diagnostic Statistical Manual, Fifth Edition (18). The total score ranges from 0–27, as each item is scored on a 0 ‘never’ to 3 ‘every day’ scale of symptom frequency over the past two weeks; total scores of 0–4 represent ‘none/minimal’ depressive symptoms, 5–9 ‘mild’, 10–14 ‘moderate’, 15–19 ‘moderately severe’, and 20+ ‘severe’. Total scores greater than 9 signify clinical depression, and have been shown to correspond highly with Major Depression as determined by a diagnostic interview (16). A PHQ-9 score less than 5 represents full remission of depression (19), and response to treatment for those prescribed antidepressants.
Client characteristics
Demographic variables in the analysis included gender, age, and education (whether or not the participant had any secondary education). CD4 count at study entry, date of HIV diagnosis, and whether or not the participant was on antiretroviral therapy were abstracted from the participant’s clinical chart. Physical health functioning was measured with the 6-item subscale of the Uganda-adapted Medical Outcomes Study HIV Health Survey (20).
Antidepressant therapy characteristics
Participants who were on antidepressants at the time of the follow-up assessments at Months 6 and 12 were asked about medication adherence and perceived side effects. Adherence to antidepressant medication was assessed by asking respondents to estimate their adherence over the past month on a 0–100% scale. Perceived side effects of antidepressant therapy was assessed by asking respondents to report whether or not they experienced any side effects from antidepressant therapy over the past week, and if so, how bothersome the side effects were on a scale of 0 ‘not at all bothersome’ to 3 ‘very bother-some’.
Analysis
Bivariate tests (two-tailed, independent t-test; chi square test) were used to examine whether baseline characteristics of participants differed between the two study arms, or between study completers and those who prematurely discontinued their participation. Subsequent analyses were divided into two sets of parallel analyses, one with the full sample (n=1252) to assess depression alleviation, and the other with the subgroup of participants who received antidepressant treatment (n=415) to assess treatment response. First, an Analysis of Covariance (ANCOVA) model was used to test for significant differences between the two study arms regarding (1) the average month 12 survey PHQ-9 score using data from the whole sample, and (2) the score from the final PHQ-9 administered by the provider to those treated with antidepressants, controlling for PHQ-9 scores at study entry orinitiation of antidepressants, respectively.
Next, chi square tests were used to compare rates of depression alleviation (PHQ-9 < 5 at Month 12) and treatment response (PHQ-9 < 5 at last provider administered assessment) in these two analyses. These comparisons employed an intention-to-treat approach, such that those without follow-up data were classified as not achieving depression alleviation or not responding to treatment; in secondary analyses, these comparisons were run using data only from those with follow-up survey data or who had completed the course of treatment. In order to account for the repeated assessments of each client over time, we examined the two outcomes using multivariate, longitudinal regression models. Each model adjusted for baseline client characteristics (age, sex, CD4 count, physical health functioning, and depression severity [PHQ-9]) and clinic size. To account for potential clustering of client-level outcomes within clinic, we derived standard errors by estimating the models using the wild bootstrap-t approach (21), which is valid for a study design with a small number of clusters. We did not use standard clustering adjustments, which can lead to substantially underestimated standard errors when the number of clusters is small. This approach requires a linear specification, even when the outcome measure is binary; therefore, we supplemented these analyses with robustness tests to compare the estimated coefficients from the linear models with average marginal effects from logit regression models, and found similar results (data not reported in this paper).
To examine the rate of alleviation of depression over time among those treated with antidepressants, we used a piecewise regression approach that estimated the linear reduction in PHQ-9 scores between each of the first four follow-up visits after antidepressant baseline. We used a multi-level model to account for clinic-level clustering along with patient fixed effects over time. This model provides estimates of depression alleviation by depression care model over time, controlling for differences in client characteristics across clinics and across months.
For those respondents who stopped treatment prior to four visits, we imputed missing PHQ9 scores using survey data captured six months post-baseline and adjusted our estimates with nonresponse weights to account for any remaining attrition. We found that our results are robust to alternative imputation and weighting schemes.
Results
Sample characteristics
A sample of 1252 clients (range of 82 to 182 clients at each site) was enrolled in the longitudinal cohort, including 640 and 612 clients from sites in the protocolized and clinical acumen arms, respectively. Table 1 lists the baseline characteristics of the study participants in terms of demographics, HIV disease characteristics and depression. Among the whole sample, the mean survey PHQ-9 at study entry was 8.2 (SD = 4.3), and 82% had at least mild depression (PHQ-9 ≥ 5), including 30% with clinical depression (PHQ-9 > 9). The two study arms did not differ at baseline on any of the client characteristics. Study retention was high, with 85% of the overall sample completing a month 12 assessment, including 86 and 84% of the protocolized and clinical acumen arms, respectively. Participants who completed the study did not differ from the dropouts on client characteristics with the exception of mean age (40.3 years vs 38.1; t= −2.4; p=.02).
Table 1.
Baseline characteristics of the full sample and by study arm.
| Total (N=1252) | Protocolized (N= 640) | Clinical Acumen (N=612) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Mean (SD) age (years) | 40.0 (11.2) | 40.6 (11.0) | 39.3 (11.3) | .057 |
| Male gender | 23.2% | 74.7% | 78.9% | .076 |
| Any secondary education | 18.6% | 17.3% | 19.9% | .239 |
| HIV characteristics | ||||
| Mean (SD) CD4 count (cells/mm3) | 430 (270) | 429 (260) | 426 (248) | .838 |
| On ART | 73.7% | 72.7% | 74.8% | .381 |
| Mean (SD) years since HIV diagnosis | 3.7 (3.4) | 3.8 (3.0) | 3.5 (3.6) | .104 |
| Depression characteristics | ||||
| Mean (SD) PHQ-9 total score | 8.2 (4.34) | 8.2 (4.24) | 8.2 (4.44) | .992 |
| None/minimal: < 5 | 17.7% | 16.6% | 18.8% | |
| Mild: 5–9 | 52.8% | 53.6% | 51.9% | |
| Moderate: 10–14 | 19.5% | 20.2% | 18.7% | |
| Moderately severe: 15–19 | 7.9% | 7.6% | 8.3% | |
| Severe: 20–27 | 2.2% | 2.0% | 2.3% | |
| Clinically depressed (PHQ-9>9) | 29.5% | 29.8% | 29.3% | .851 |
Alleviation of depression
Depression levels declined significantly over the course of the study, as mean PHQ-9 at month 12 was 2.7 and 2.1 in the protocolized and clinical acumen arms, respectively. This difference was statistically significant in a regression model controlling for baseline PHQ-9 [F(1,1058)=6.4, p=.007]. At month 12, 82% of each of the protocolized and clinical acumen arm had no/minimal depressive symptoms (PHQ-9 < 5) or complete depression alleviation (Tab. 2); 6% of the protocolized arm remained with clinical depression, as did 3% of the clinical acumen arm. For those without month 12 data, month 6 data were used in the analysis, but those with only baseline data (8% of sample) were excluded from this analysis. Using an intent-to-treat approach (ITT), those with only baseline data (n=93) were classified as not achieving depression alleviation, resulting in 75% of the protocolized arm achieving complete depression alleviation, compared to 77% of the clinical acumen arm (Tab. 2). In the multivariate regression analysis (Tab. 3), complete depression alleviation (using the ITT definition) was not associated with the depression care model, but was associated with having any secondary education (p = .006), higher CD4 count (p = .046), lower work functioning (p = .022), and greater food security (p = .008). The analysis that excluded those with only baseline data resulted in similar results with secondary education (p=.032), higher CD4 count (p = .038), greater food security (p=.002), and better physical health functioning (p=.020) being predictive of complete depression alleviation.
Table 2.
Comparison of measures of depression alleviation, antidepressant response, and quality of antidepressant care between protocolized and clinical acumen arms.
| Protocolized | Clinical Acumen | P value | |
|---|---|---|---|
| Depression Alleviation (whole sample) | |||
| PHQ-9 at baseline survey [M(SD)] | 8.17 (4.24) | 8.17 (4.44) | .992 |
| PHQ-9 at month 12 survey [M(SD)] among completers, controlling for baseline PHQ-9 | 2.70 (3.87) | 2.13 (3.00) | .007 |
| Clinically depressed (PHQ-9>) at baseline survey | 30% | 29% | .851 |
| Clinically depressed at month 12 survey, among completers | 6% | 3% | .033 |
| No depression (PHQ-9<5) at month 12 survey, among completers | 82% | 82% | .984 |
| No depression (PHQ-9<5) at month 12 survey (ITT definition) | 75% | 76.5% | .545 |
| Antidepressant Response (treated subgroup) | |||
| Number treated with antidepressants | 218 | 197 | |
| PHQ-9 at treatment baseline [M(SD)] | 13.32 (3.70) | 14.38 (3.86) | .005 |
| PHQ-9 at treatment (or study)1 endpoint2 [M(SD)], controlling for treatment baseline PHQ-9 | 3.07 (3.92) | 2.34 (4.31) | .057 |
| Depression in remission (PHQ-9 < 5) at treatment (or study)1 endpoint2 among completers | 75.0% | 82.0% | .120 |
| Depression in remission (PHQ-9 < 5) at treatment (or study)1 endpoint2 (ITT definition) | 64.4% | 68.9% | .335 |
| Quality of Antidepressant Care | |||
| Number of chart reviews of newly treated patients (and range across sites) | 401 (46–132) | 343 (34–100) | |
| Correct diagnosis (% and range across sites) | 98.3 (90.8–100) | 99.1 (98.4–100) | .448 |
| Correct selection of antidepressant (%; range) | 97.2 (88.5–100) | 97.5 (93.6–100) | .890 |
| Correct initial antidepressant dose (%; range) | 96.6 (88.5–100) | 94.6 (76.4–100) | .439 |
| Number of chart reviews of patient in ongoing treatment (and range across sites) | 1055 (180–229) | 958 (174–213) | |
| Depression symptoms assessed (%; range) | 91.0 (77.8–96.7) | 86.7 (58.1–99.6) | .193 |
| Treatment side effects assessed (%; range) | 88.2 (72.6–96.7) | 84.1 (53.2–99.6) | .327 |
| Correct antidepressant dose (%; range) | 88.4 (75.3–96.7) | 78.8 (48.2–99.6) | .056 |
| Client attended the session (%; range) | 88.6 (77.8–93.7) | 84.2 (60.5–95.3) | .220 |
M = mean; SD = standard deviation
For those who remained on antidepressants at study endpoint, the last PHQ-9 administered by the provider at the end of the client’s 12-month participation in the study was used in analysis.
Treatment/study endpoint was after a mean of 8.6 months in the protocolized arm and 8.2 months in the clinical acumen arm.
Table 3.
Multivariate regression analysis of baseline predictors of depression alleviation and antidepressant response.
| Depression Alleviation | Antidepressant Response1 | Antidepressant Response2 | |
|---|---|---|---|
|
| |||
| Beta | Beta | Beta | |
| Protocolized depression care model | −0.009 | −0.053 | −0.048 |
| Secondary education | 0.101** | −0.019 | −0.006 |
| Age | 0.001 | −0.002 | −0.001 |
| Female | −0.016 | −0.085 | −0.050 |
| CD4 count | 0.0001** | 0.0001 | 0.0001 |
| On ART | 0.062* | −0.109* | −0.060 |
| PHQ-9 at study entry | −0.006* | −0.009 | −0.006 |
| Work functioning | −0.001** | 0.0002 | 0.0002 |
| Physical health functioning | 0.002 | 0.0000 | 0.0003 |
| General social support | −0.008 | −0.025 | −0.015 |
| Internalized HIV stigma | −0.016 | −0.016 | −0.039 |
| Food Security | 0.036** | 0.009 | 0.007 |
| Perfect antidepressant adherence | – | 0.227** | – |
| Presence of antidepressant side effects | – | 0.029 | – |
Regression model includes antidepressant adherence and side effect variables
Regression model excludes antidepressant adherence and side effect variables
p < .10
p < .05
Response to antidepressant therapy
A total of 415 participants received antidepressant therapy during their participation in the study, 218 in the protocolized arm and 197 in the clinical acumen arm. Among those who received treatment, 10% had started treatment prior to study enrollment (but after depression care implementation had started as part of the project), 78% started treatment at or within three months following study enrollment, and the remaining 12% started treatment between 3 to 6 months following enrollment. Just over a third (38%) were treated with fluoxetine alone, 49% were treated with imipramine alone, and 12% had either started with fluoxetine or imipramine and switched to the other medication at some point during the course of treatment due to side effects or nonresponse; only two patients were treated with amitriptyline.
Course of treatment
At the onset of antidepressant therapy, mean PHQ-9 as administered by the provider was 13.9 (SD = 3.7), with a slightly higher score found among those in the clinical acumen arm compared to the protocolized arm (14.4 vs 13.3; t = 2.9, p = .01). At month 12, 66% (n=272) of all treated participants had completed their course of antidepressant therapy after a mean of 9.1 months of treatment (SD = 2.7); 20% (n=82) remained on treatment (having been on treatment for an average of 10.0 months), and 15% (n=61) had prematurely discontinued treatment. At the last study survey in which the respondent was on antidepressant treatment (for which we have 320 participants with data, as the others were not currently on antidepressants at the time of the month 6 or month 12 survey or did not complete those surveys), mean self-reported adherence to antidepressant therapy over the past month of treatment was 87.2% (SD = 22.4), with 56% reporting perfect (100%) adherence; also at this assessment, 20% reported any treatment side effects in the prior month, of whom 24% reported these side effects to be ‘quite a lot’ or ‘very’ bothersome.
Antidepressant response
The mean final recorded PHQ-9 administered by providers to those who had completed the course of treatment or who were still on treatment at study endpoint (all of whom had been on treatment for at least 6 months) was 3.1 (SD = 3.9) in the protocolized arm (after an average of 8.6 months of treatment) and 2.3 (SD = 4.3) in the clinical acumen arm (after an average of 8.2 months of treatment) (Tab. 1); this difference was not significantly different after controlling for the baseline PHQ-9 score [F(1,341)=3.6; p=.06]. The vast majority of those in both the protocolized arm (75%) and clinical acumen arm (82%) were in full remission (PHQ-9<5) at this time point, a difference that is also non-significant (Chi square= 2.5, p = .12). In an intent-to-treat analysis in which those who had prematurely discontinued treatment were classified as non-responders, the response rates in the protocolized and clinical acumen arms dropped to 64 and 69%, respectively (Chi square = 0.9, p=.33) (Tab. 2).
In the multivariate regression analysis with the ITT sample, none of the variables in the model (depression care model, demographics and other client characteristics) were predictive of treatment response (Tab. 3). When the binary indicators of perfect treatment adherence and presence of treatment side effects were included in the model (which we ran separately due to 23% of the treated sample not having adherence and side effect data), having perfect adherence (p = .042) was the sole predictor of response. The multivariate regression analysis that excluded those who had prematurely discontinued treatment produced similar results: perfect treatment adherence predicted treatment response (p=.012), but being female was predictive of not responding (p=.044).
Rate of depression alleviation over the course of treatment
Figure 1 depicts the rate of significant improvement in depression (based on provider administered PHQ-9 data) over the first four follow-up visits (average of 26.2 weeks) after antidepressant baseline in the whole treated sample as well as by study arm; data is included only from the 354 participants who completed their course of antidepressant therapy or remained on treatment at the time of the month-12 study visit (note that 10 participants were not included because of absence of PHQ-9 data at the follow-up visits). Multivariate regression analysis (Tab. 4) revealed that clients in both study arms showed a similar trajectory of improvement (p = .15); only at the first follow-up visit was there a marginal advantage for the protocolized arm (61% reduction from baseline vs. 39%; p = .09). In the combined sample, mean PHQ-9 was reduced by an average of 7.0 points at the first treatment follow-up visit (a mean of 5.6 weeks post treatment baseline), followed by an added mean reduction of 3.1 points by the third follow-up visit (a mean of 20.1 weeks after treatment baseline), with scores leveling off thereafter. Clients with moderately severe to severe depression (PHQ-9 ≥15) at treatment baseline were more likely to experience a greater reduction in depressive symptoms overall (p < .001), and from second through fourth follow-up visits in particular (Tab. 4).
Figure 1.
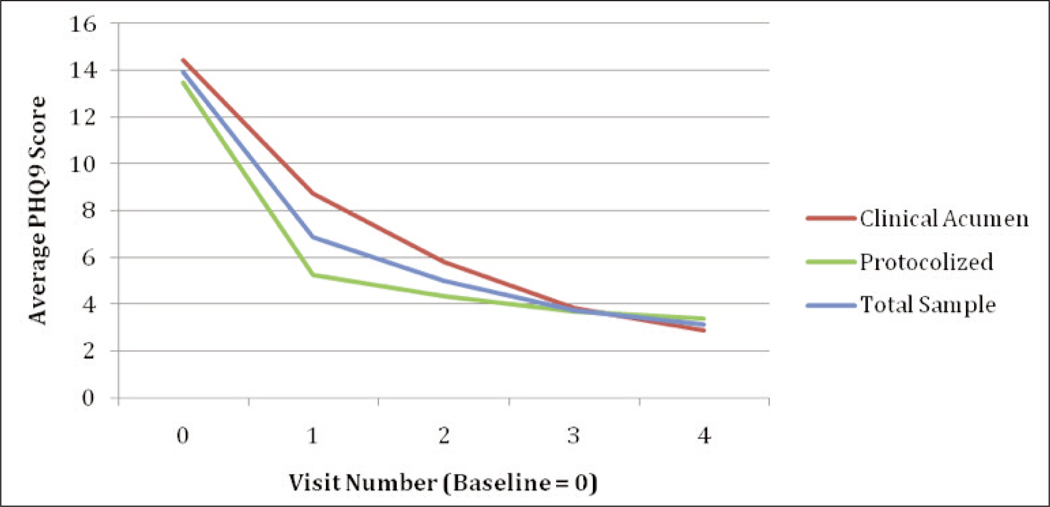
Rate of change in depression over course of antidepressant therapy in whole treated sample and by depression care model received.
Table 4.
Multivariate regression analysis of the rate of change in depression over course of antidepressant treatment.
| Change in PHQ-9 | |
|---|---|
|
| |
| Beta | |
| Protocolized depression care model | −0.794 |
| First treatment follow-up visit | −5.427* |
| Second treatment follow-up visit | −8.065* |
| Third treatment follow-up visit | −10.06* |
| Fourth treatment follow-up visit | −10.87* |
| First follow-up visit X depression care model | −2.456 |
| Second follow-up visit X depression care model | −0.440 |
| Third follow-up visit X depression care model | 0.905 |
| Fourth follow-up visit X depression care model | 1.580 |
| Baseline PHQ-9 ≥ 15 | 3.752* |
| First follow-up visit X Baseline PHQ-9 ≥ 15 | −1.371 |
| Second follow-up visit X Baseline PHQ-9 ≥ 15 | −2.633* |
| Third follow-up visit X Baseline PHQ-9 ≥ 15 | −2.680* |
| Fourth follow-up visit X Baseline PHQ-9 ≥ 15 | −3.284* |
p < .05
Discussion
This study may be the first to publish data comparing two task-shifting approaches to integrating depression care into mostly public HIV clinics in SSA. Our findings provide evidence for the fact that quality depression care, as represented by rates of depression alleviation and treatment response, can be implemented by medical providers (mostly nurses) in low resourced clinics using either a structured protocol model or one that relies on clinical acumen, with appropriate training and ongoing supervision of providers.
Our prior analyses showed that the protocolized depression care model was superior to reliance on clinical acumen in the early stages of the depression care process, as it helps to ensure a depression diagnostic evaluation for those who screen positive for potential depression; however, the models did not differ significantly with regards to clinically depressed clients receiving antidepressant therapy, in part because of the shortcomings of the PHQ-2 as a screening tool (14). The analyses reported here show that both depression care models result in strong mental health improvement as rates of depression alleviation (regardless of treatment status) and treatment response (among depressed patients receiving antidepressant therapy) were equally impressive in both models of care. About 2/3 of treated patients responded to treatment and experienced a full remission of their depression, which is consistent with other studies of antidepressants in the context of HIV (22, 23), though this study is one of few such studies conducted in SSA. Our data also revealed good patient adherence to antidepressant treatment and sustained engagement in care.
Other studies have suggested that protocolized approaches to facilitating task-shifted depression care results in superior quality of care compared to clinical acumen (11–13), as protocols help to ensure appropriate medication and doses are prescribed. The success of the clinical acumen model in this study may be a result of the ongoing supervision that was provided to providers in both models. This in-person supervision was provided monthly and focused on helping providers feel comfortable and confident in their newly acquired depression care skills, making appropriate treatment changes, addressing challenging clinical cases, and facilitating implementation concerns. Ongoing supervision and feedback has been found to promote adoption and quality implementation of evidence-based mental health care (24) and may have enabled providers in the clinical acumen to provide quality care in the absence of a treatment protocol. Supervision in this study was provided by hired study psychiatrists; for these models to be scalable and sustainable, existing mental health professionals (e.g., psychiatrists, psychiatric clinical officers, and psychiatric nurses) will need to be available to provide this supervision, which could be a significant challenge in some settings due to the scarcity of mental health professionals. Evidence of the importance of supervision requires a study design that compares varying doses or forms of supervision, including minimal or sparse supervision, which is both common in resourced constrained settings and likely more advantageous than sole reliance on a protocolized approach, particularly after initiation of antidepressant treatment.
There is a number of limitations to this study. We opted to evaluate two models of depression care that we believed to be viable models for successful integration of depression care into public HIV clinics, but the absence of a control condition that offered provider training in depression care but minimal or no supervision (which is closer to what the Ministry of Health would typically do when implementing new services into routine care) precluded us from being able to evaluate the effect of ongoing supervision support. However, discussions with our community partners when planning the study revealed a great need and desire for clinics to have supervision support, which outweighed the research goal of a cleaner design that would have allowed for more clear differences between the two implementation models. Our comparisons between the two models, and generalizability of the findings, are hampered by the small number of clinics in the study, which lowered the statistical power given the need to control for clustering effects. Unfortunately, the costs associated with including more study sites were prohibitive. Furthermore, our data regarding depression diagnoses were limited by our reliance on the PHQ-9, rather than a more rigorous diagnostic interview, although the PHQ-9 has been found to have high correspondence with diagnostic interviews (16).
Together with findings from other studies that have shown similar results in other regions of the world and with different patient populations, these data lend further credence to the feasibility of non-mental health professionals providing quality depression care in the context of task-shifting models of care. Even in public health facilities with scarce resources, medical providers are able to provide quality depression care with proper training and in-person, ongoing supervision and mentorship. These findings provide hope for integration of depression care into routine HIV care management in public health facilities in developing regions such as SSA. Lack of mental health specialists remains a key barrier, in terms of direct service provision but also provision of training and supervision services, but should not be viewed as a valid reason for the current relative absence of depression care in HIV and primary care settings.
Acknowledgement
Funding for this research is provided by a grant from the National Institute of Mental Health (5R01M H098996). The funding source played no role in the conduct of this study.
References
- 1.Nakimuli-Mpungu E, Bass JK, Alexandre P, Mills EJ, Musisi S, Ram M, et al. Depression, alcohol use and adherence to antiretroviral therapy in sub-Saharan Africa: a systematic review. AIDS Behav. 2012;16(8):2101–18. [DOI] [PubMed] [Google Scholar]
- 2.Maling S, Todd J, Van der Paal L, Grosskurth H, Kinyanda E. HIV-1 seroprevalence and risk factors for HIV infection among first-time psychiatric admissions in Uganda. AIDS Care. 2011;23(2):171–8. [DOI] [PubMed] [Google Scholar]
- 3.Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21:1175–1183. [DOI] [PubMed] [Google Scholar]
- 4.Cook JA, Grey D, Burke J, Gurtman AC, Richardson JL, Wilson TE, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94:1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ammassari A, Trotta MP, Murri R, Bartoli L, Monforte AD, Wu AW, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosom. 2004;45:394–402. [DOI] [PubMed] [Google Scholar]
- 6.Wagner GJ, Ghosh-Dastidar B, Slaughter M, Akena D, Naka- sujja N, Okello E, et al. Changes in condom use during the first year of HIV treatment in Uganda and the relationship to depression. Ann of Behav Med, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olatunji B, Mimisga MJ, O’Clerigh C, Safren SA. A review of treatment studies of depression in HIV.Top HIV Med. 2006;14: 116–128. [PubMed] [Google Scholar]
- 8.Yun LW, Maravi MBS, Kobayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients. J Acquir Immune Defic Syndr. 2005;38:432–8. [DOI] [PubMed] [Google Scholar]
- 9.Collins PY, Homan AR, Freeman MC, Patel V. What is the relevance of mental health to HIV/AIDS care and treatment programs in developing countries? A systematic review. AIDS. 2006;20:1571–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callaghan M, Ford N, Schneider H. A systematic review of task-shifting for HIV treatment and care in Africa. Hum Resour Health. 2010;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaynes BN, Rush AJ, Trivedi MH, Wisniewski SR, Balasubramani GK, McGrath PJ, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells K, Sherbourne C, Schoenbaum M, Duan N, Meredith L, Unützer J, et al. Impact of disseminating quality improvement programs for depression in managed primary care: a randomized controlled study. JAMA. 2000;283:212–220. [DOI] [PubMed] [Google Scholar]
- 13.Patel V, Weiss HA, Chowdhary N, Naik S, Pednekar S, Chatterjee S, De Silva MJ, et al. Effectiveness of an intervention led by lay health counselors for depressive and anxiety disorders 19. in primary care in Goa, India (MANAS): a cluster randomized controlled trial. Lancet. 2010;376:2086–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner GJ, Ngo V, Goutam P, Glick P, Musisi S, Akena D. A protocolized model of depression care versus clinical acumen: A cluster randomized trial of the effects on depression screening, diagnostic evaluation, and treatment uptake in Ugandan HIV clinics. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner GJ, Ngo V, Glick P, Obuku E, Musisi S, Akena D. IN- 21. tegration of DEPression Treatment into HIV care in Uganda (INDEPTH-Uganda): study protocol for a randomized controlled trial. Trials. 2014;15:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001; 23. 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheehan D, Lecrubier Y, Sheehan K, Janavs J, Weiller E, Keskiner A, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. European Psychiatr. 1997;12:232–241. [Google Scholar]
- 18.Patel V, Araya R, Bolton P. Treating depression in the developing world.Trop Med Intl Health. 2004;9:539–541. [DOI] [PubMed] [Google Scholar]
- 19.Rush A, Fava M, Wisniewski S, Lavori PW, Trivedi MH, Sack- eim HA, et al. Sequenced Treatment Alternatives to Relieve Depression (STAR*D): Rationale and design. Control Clin Trials. 2004;25:119–142. [DOI] [PubMed] [Google Scholar]
- 20.Mast TC, Kigozi G, Wabwire-Mangen F, Black R, Sewankambo N, Serwadda D, et al. Measuring quality of life among HIV-infected women using a culturally adapted questionnaire in Rakai district, Uganda. AIDS Care. 2004;16:81–94. [DOI] [PubMed] [Google Scholar]
- 21.Cameron AC, Gelbach JB, Miller DL. Bootstrap-based improvements for inference with clustered errors. The Review of Economics and Statistics. 2008;90:414–427. [Google Scholar]
- 22.Ferrando SJ. Psychopharmacologic treatment of patients with HIV and AIDS. Curr Psychiatry Rep. 2009;11:235–42. [DOI] [PubMed] [Google Scholar]
- 23.Wagner GJ, Rabkin JG, Rabkin R. A comparative analysis of standard and alternative antidepressants in the treatment of human immunodeficiency virus patients. Compr Psychiatry. 1996;37:402–8. [DOI] [PubMed] [Google Scholar]
- 24.Dorsey S, Pullmann MD, Deblinger E, Berliner L, Kerns SE, Thompson K, et al. Improving practice in community-based settings: a randomized trial of supervision - study protocol. Implement Sci. 2013;8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]


