Abstract
Objectives
A well-documented paradox is that Hispanics tend to live longer than non-Hispanic Whites (NHW), despite structural disadvantages. We evaluate whether the “Hispanic paradox” extends to more comprehensive longitudinal aging classifications and examine how lifecourse factors relate to these groupings.
Methods
We used biennial data (1998–2014) on adults aged 65 years and older at baseline from the Health and Retirement Study. We use joint latent class discrete time and growth curve modeling to identify trajectories of aging, and multinomial logit models to determine whether U.S.-born (USB-H) and Foreign-born (FB-H) Hispanics experience healthier styles of aging than non-Hispanic Whites (NHW), and test how lifecycle factors influence this relationship.
Results
We identify four trajectory classes including, “cognitive unhealthy,” “high morbidity,” “nonaccelerated”, and “healthy.” Compared to NHWs, both USB-H and FB-H have higher relative risk ratios (RRR) of “cognitive unhealthy” and “high morbidity” classifications, relative to “nonaccelerated.” These patterns persist upon controlling for lifecourse factors. Both Hispanic groups, however, also have higher RRRs for “healthy” classification (vs “nonaccelerated”) upon adjusting for adult achievements and health behaviors.
Discussion
Controlling for lifefcourse factors USB-H and FB-H have equal or higher likelihood for “high morbidity” and “cognitive unhealthy” classifications, respectively, relative to NHWs. Yet, both groups are equally likely of being in the “healthy” group compared to NHWs. These segregations into healthy and unhealthy groups require more research and could contribute to explaining the paradoxical patterns produced when population heterogeneity is not taken into account.
Keywords: Aging trajectories, Cognitive aging, Healthy aging, Hispanic paradox, Immigrant health
The U.S. population is aging and increasingly diversifying. By 2060, immigrants are expected to comprise close to 26% of U.S. older adults, and a plurality will be of Hispanic (interchangeably Latino) origins (Colby & Ortman, 2015). Yet, empirical research on aging Hispanics, immigrants in particular, remains underdeveloped, mainly in terms of long-term aging. This paper extends the literature on minority and immigrant health and aging in the United States by examining comprehensive longitudinal trajectories and identifying different patterns of aging among non-Hispanic Whites and U.S.-born (USB-H) and Foreign-born (FB-H) Hispanics.
Aging Models
Epidemiologic evidence points to clusters of risk factors and health conditions. Furthermore, precise biological and mechanistic evidence highlights the complex networks and interconnectedness in pathogenesis of age-related diseases and dysfunctions (e.g., vascular cognitive dysfunctions). In fact, more than a third of Medicare beneficiaries live with more than three chronic conditions (Whitson et al., 2016). These clusters of conditions are anticipated to increase in prevalence due to expected demographic changes, ascendance of brain diseases, longevity gains, and introduction of new treatment technologies. Thus, current measures for population health monitoring and assessment will require upgrading to reflect these changes and facilitate improvement in population health, health care planning, and services delivery (Whitson et al., 2016).
Calls for holistic understandings of aging are becoming more pervasive in the literature (Kok, Aartsen, Deeg, & Huisman, 2017). Most new approaches take conceptual refuge in Rowe and Khan’s proposition of successful aging (Rowe & Kahn, 2015), although their original proposition has been challenged and morphed in recent years (Rowe & Kahn, 2015). Still, existing work adopting these formulations remains limited and includes several conceptual and applied problems (see Kok et al. (2017) for a recent summary). Briefly, most studies continue to treat aging as a binary condition (e.g., optimal vs not based on specific thresholds) leading to skewed representations of population health given the sensitivity of these threshold-based definitions to inclusion of chronic diseases. Furthermore, despite some recent changes, most work continues to focus on cross-sectional data or includes limited longitudinal coverage. This hinders inferences about more complex trajectories of health, function, and disease progression in aging. Additionally, many studies investigate individual domains of aging (e.g., disability or cognition). This limits the predictive validity of the considered risk factors. In addition, studies continue to model aging as a linear process manifesting in equal decrements over certain time metrics. Recent evidence challenges this conceptualization, particularly in less understood and increasingly prevalent brain diseases and their functional correlates (Hochstetler et al., 2016). Most importantly, from the point of view of this study, most work to date has focused on non-Hispanic White populations, and as such can provide limited generalizability to the more diverse aging U.S. population.
Empirical Models
Recent work in aging suggests that the assumption of population homogeneity is not sustainable and that models which account for developmental heterogeneity provide more appropriate approximations of aging realities. This new wave of evidence is facilitated by exceedingly accessible and computationally feasible statistical techniques. Mixture models, in particular, have proven to be well suited for testing updated operationalizations of successful aging (and its conceptual kin). They have been applied to different dimensions and domains of health and aging to examine variations in levels of disease accumulation, physiological and cognitive loss, and emotional and social well-being. The characterization of these aging heterogeneities varies by the investigated health domain, population, and follow-up time but the range of estimates are largely constrained to between three and five groups.
Among studies focusing on single domains, Jonkman et al. (2018), looked at a range of predictors focused on physical performance measures and reported three distinct trajectories of functional decline over 9 years that included no/little, intermediate, and severe decline. Whitson et al. (2016) examined a number of prevalent diseases and reported six classes (ranging from “minimal disease” to a “very sick” class). Other work has focused on cognitive dysfunction trajectories. Hochstetler et al. (2016), for example, examined trajectories of cognitive change and identified three trajectory classes: high baseline impairment and steep decline, low baseline impairment with stable change, and an intermediate pattern. Others who examined cognitive aging, however, offer varying class representations (3–5) depending on follow-up time and sample specifications (Hochstetler et al., 2016).
Work looking at multiple domains of aging show similar variations. Hsu and Jones (2012) used successful aging indicators (chronic diseases, physical function difficulties, depressive symptoms, social support, social participation, and economic satisfaction) and generated a four-group solution including successful aging, usual aging/insecure aging, health declining, and care demanding. Xu, Liang, Bennett, Botoseneanu, and Allore (2015) also identified four distinct patterns of change reflecting a range varying from minimal to severe levels of impairment and deterioration in physical, emotional, and cognitive functioning. More recently, Cosco, Stephan, Muniz, Brayne, and Collaboration (2016) used a successful aging index including social, emotional, cognitive and functional measures to estimate predeath trajectories in a sample of decedents, deriving a three-class model that includes high-functioning/no decline, high-functioning/gradual decline, and low-functioning/steep decline. McClintock, Dale, Laumann, and Waite (2016) included a set of diseases with high death prevalence and several functional domains and found evidence for three major classes labeled robust, intermediate, and vulnerable. Finally, de la Fuente et al. (2018) examined a health metric derived through Item-Response-Theory using physical and cognitive functioning domains and reported five classes in the Health and Retirement Study (HRS) and four in the English Longitudinal Study of Aging.
Interestingly, none of the studies detailed above modeled death directly when extracting the classes, and most relied on linear assumptions in trajectories. Our first aim is to update these models by directly accounting for death and relaxing the parametric assumptions to accommodate a more realistic assumption of nonlinear disease progression. In line with the current range of estimates from the literature, we expect that models extracting between three to five-class solutions will yield appropriate representations of population heterogeneity.
Latino Health
Given the demographic realities of the U.S. population, testing aging models in the sizeable Latino subpopulation is valuable. Latinos present a curious research puzzle because they have complex and paradoxical patterns of mortality, disease, disability, and physical and cognitive function (see Goldman (2016) for a recent synopsis). Briefly, previous research has shown they have better mortality outcomes despite unfavorable socioeconomic conditions and more disadvantageous rates of chronic health conditions, particularly with regards to cardiovascular and cardiometabolic diseases (Daviglus, Pirzada, & Talavera, 2014; Goldman, 2016). Research has also shown higher rates of disability among Latinos, driven largely by higher rates of cardiovascular risk factors (e.g., obesity), higher stress, and more physically demanding occupations (Goldman, 2016). Recent studies of Latino cognitive function also suggest markedly varying and similarly paradoxical patterns of cognitive dysfunction (Mehta & Yeo, 2017). Many of these disadvantageous health outcomes are exacerbated by their disparate access to health care. Latinos, however, benefit from certain group characteristics that help buffer against some of the detrimental effects of disease, including protective cultural practices (Lara, Gamboa, Kahramanian, Morales, & Bautista, 2005; Vega, Rodriguez, & Gruskin, 2009) and lower rates of smoking (Fenelon, 2013).
The paradoxical evidence described above presents a unique opportunity to test whether these seemingly contradictory patterns of outcomes reflect population heterogeneities that are masked when examining unidimensional risks and health domains. As such, our second aim is to evaluate whether the “Hispanic paradox” (Markides & Rote, 2015) of equivalent or better health and increased longevity among Hispanics also extends to better health and functioning longitudinally at older ages. We expect Latinos to be overrepresented in both the healthy and unhealthy aging classes and we posit that this bimodal segregation underlies some of the paradoxical patterns produced when population heterogeneity is not taken into account. Additionally, we consider three different explanations for the “Hispanic paradox.” First, we test whether health advantages, if any, among FB-H are due to “healthy immigrant” factors that stem from favorable health and socioeconomic conditions as children (Jasso, Massey, Rosenzweig, & Smith, 2004; Kennedy, McDonald, & Biddle, 2006). Second, we examine whether and how adult achievement factors (e.g., wealth) among Hispanics influence differences in patterns of aging in concordance with the “acculturation-health” hypothesis (Gonzalez et al., 2009; Gonzalez, Tarraf, & Haan, 2011). This hypothesis posits that positive acculturation, namely socioeconomic success, has the potential to differentially and positively enhance health outcomes among older FB-H. Third, we test whether USB-H and FB-H may benefit differentially from different health habits, such as not smoking or drinking alcohol and being physically active, likely fostered by cultural and acculturative factors (Lara et al., 2005; Vega et al., 2009). The longitudinal nature of our data allows us to control for the possible biasing effects of return migration among FB participants, labeled as “Salmon Bias” (Turra & Elo, 2008).
This study contributes to the debate on minority aging in the United States specifically in the context of the “Hispanic paradox” by focusing on a more comprehensive conceptualization of aging. Nine waves of HRS data are used to identify multiple trajectories describing how older Americans age. We then evaluate whether and how Hispanic ethnicity and U.S. nativity are associated with these patterns of aging and if these associations depend on other possible determinants of health and aging. In line with previous work, we focus on the biological, physical, and cognitive aspects of health since recent research supports distinguishing these from social aspects of aging (McLaughlin, Connell, Heeringa, Li, & Roberts, 2010; McLaughlin, Jette, & Connell, 2012; Pruchno, Wilson-Genderson, & Cartwright, 2010).
Methods
Data
Biennial data spanning 1998–2014 from the ongoing HRS are analyzed. The HRS, as of 1998, is nationally representative of noninstitutionalized adults in the United States, ages 50 years and older. HRS response rates vary from year-to-year and range between 70% and 81%. Interviews were available in English and Spanish. Details regarding the survey’s methodology are available elsewhere (Heeringa & Connor, 1995).
Baseline Sample
We focus on 10,378 HRS participants, ages 65 years and older in 1998. HRS protocol calls for proxy interviews whenever a respondent is unavailable or incapable of completing the survey. Since tests of cognition were not administered during proxy interviews, we exclude 996 individuals who had proxy interviews in 1998. Thus, our final baseline sample consists of 9,382 individuals who represent the weighted equivalent of nearly 30-million older Americans in 1998. Supplementary Figure 1 details our analytic inclusion criteria.
Attrition
Attrition in the HRS is primarily due to mortality, with unweighted per-wave death rates between 4.6% in 2014 and 6.6% in 2002. Our analytic design explicitly accounts for death among respondents by modeling wave-specific mortality using discrete time analyses.
Outcomes
We focus on four domains of health: disability, physical performance, major chronic disease, and cognitive functioning (McLaughlin et al., 2010; McLaughlin et al., 2012). Additionally, we model mortality status as ascertained during each HRS wave.
Disability is measured by the count of difficulty with activities of daily living (ADLs) and instrumental ADLs (IADLs). This measure of disability is consistent with recent work on “successful aging” using HRS data (McLaughlin et al., 2010) and reflects criteria proposed by the MacArthur Studies of Successful Aging (Berkman et al., 1993). Five ADLs (bathing, dressing, eating, walking across a room, and getting in/out of bed) and five instrumental ADLs (IADLs) (using telephone, taking medications, handling money, shopping, and preparing meals) are summed and included.
Physical performance is measured by the number of physical tasks where the respondent reports difficulty. The tasks covered are walking one block, walking several blocks, climbing one flight of stairs, climbing several flights of stairs, lifting/carrying weights of 10-lbs or more, stooping or kneeling/crouching, and pushing/pulling large objects. This measure was originally proposed by the MacArthur Studies of Successful Aging (McLaughlin et al., 2010; Seeman et al., 1994).
Major chronic disease is measured by the number of serious health conditions reported, where the count is for cancer, lung disease, diabetes, heart disease, stroke and depression. These six conditions collectively account for most morbidity and mortality among older Americans. In each wave each respondent was asked whether a doctor has told them they have the condition, except for depression. Depression is measured using a cut-point of 4 on the 8-item Center for Epidemiologic Studies Depression scale (CES-D) (Wallace, 2000).
We assess cognitive functioning using the HRS’ abbreviated version of the Telephone Interview of Cognitive Status (TICS) (Brandt, Spencer, & Folstein, 1988). TICS evaluates memory, attention, concentration, orientation, and language (Herzog & Wallace, 1997). TICS scores range between 0–35 and are reliable and valid (Ofstedal, 2005).
Finally, mortality is measured using the HRS’ respondent tracking indicator that registers whether the respondent is (a) alive and interviewed, (b) alive but not participating, (c) deceased within the past 2-year interval, (d) deceased during previous waves, or (e) dropped from the HRS sample. In line with previous work, at each wave a respondent’s survival status is coded as deceased = 1 if they died within the previous 2 years, deceased = 0 if the respondent was known to be still living, and status set to missing when the respondent is known to have died in a previous wave or has dropped from the sample (Zajacova & Ailshire, 2014).
Primary Predictor
Our primary predictor for trajectories of aging is a 3-category indicator combining respondents’ self-reported ethnicity and place of birth (0 = non-Hispanic Whites [NHW]; 1 = USB-H; and 2 = FB-H). Given the focus of this study we exclude other race/ethnic groups.
Baseline Covariates
Covariates are organized into five sets: demographics, childhood characteristics, adult socioeconomic achievements, and adult health behaviors. In addition to establishing a connection between these covariates and different trajectories of aging, we assess whether these factors explain differences in the patterns observed between NHWs and USB-H and FB-H. Demographics include respondent’s baseline age, gender, and a count of the number of HRS participated waves.
Childhood characteristics (self-reported) include: childhood socioeconomic status, childhood health, mother’s education, and father’s education. Childhood socioeconomic status takes three values: “pretty well off,” “average/varied,” or “poor.” Childhood health is measured using a standard 5-category indicator, (1 = excellent to 5 = poor). Mother’s and father’s education each have three possible values: “missing,” “less than high school,” or “high school or better.” We include a separate indicator for missing to avoid excluding cases unnecessarily.
Adult socioeconomic achievements include own education (“Less than High School”, “General Education Development (GED)”, “High School diploma”, “some college”, and “college or more”), household income, and wealth. Income and wealth are each measured using quintiles of reported dollar values (1 = lower 20th percentile to 5 = highest 20th percentile).
For adult health behaviors we include smoking status (“never,” “former,” or “current”), weekly vigorous physical activity of three times or more (0 = no, 1 = yes), body mass index (BMI) (“underweight (BMI<18),” “normal (18<BMI<25),” “overweight (25<BMI<30),” or “obese (BMI>30”), and alcohol consumption (“does not drink,” “low risk,” and “at risk”), based on National Institute on Alcohol Abuse and Alcoholism (NIAAA) criteria. Alcohol consumption categories are based on self-reported consumption in the past three months. “At risk” reflects NIAAA gender-specific standards: consuming more than 3 drinks in any single day or more than 7 per week for females, and consuming more than 4 drinks in any single day or more than 14 per week for males.(National Institute on Alcohol Abuse and Alcoholism, 2016). Descriptive characteristics for all these variables by ethnicity and nativity are provided in Table 1.
Table 1.
Baseline (1998) Sample Characteristics of NHW and USB-H and FB-H Respondents Aged 65 Years and Older Without Baseline Proxy Interview from the Health and Retirement Study by Ethnic and Nativity Status
| NHW | USB-H | FB-H | Total | p value | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 40.5 | 36.5 | 41.4 | 40.4 | .407 |
| Female | 59.5 | 63.5 | 58.6 | 59.6 | |
| Age (in years) | 74.31 | 72.92 | 73.0 | 74.25 | .004 |
| Number of Waves (range 0–9) | 5.48 | 5.52 | 5.54 | 5.49 | .936 |
| Childhood Health | |||||
| Excellent | 47.2 | 41.7 | 42.7 | 47.0 | .250 |
| Very Good | 27.7 | 24.3 | 26.5 | 27.5 | |
| Good | 19.0 | 25.9 | 23.3 | 19.3 | |
| Fair | 4.5 | 4.8 | 5.6 | 4.5 | |
| Poor | 1.7 | 3.3 | 1.8 | 1.7 | |
| Childhood SES | |||||
| Well Off | 6.1 | 4.8 | 6.2 | 6.0 | <.001 |
| About Average | 61.8 | 53.9 | 46.0 | 61.3 | |
| Poor | 32.1 | 41.3 | 47.8 | 32.7 | |
| Father Education | |||||
| Missing | 10.9 | 16.4 | 17.4 | 11.2 | <.001 |
| Less than High School (LT-HS) | 71.9 | 79.2 | 75.7 | 72.2 | |
| HS or more | 17.2 | 4.3 | 6.9 | 16.6 | |
| Mother Education | |||||
| Missing | 13.7 | 18.4 | 22.4 | 14.0 | <.001 |
| LT-HS | 72.4 | 78.3 | 69.0 | 72.4 | |
| HS or more | 13.9 | 3.3 | 8.5 | 13.5 | |
| Participant Education | |||||
| LT-HS | 25.5 | 64.0 | 70.3 | 27.6 | <.001 |
| GED | 3.5 | 5.1 | 1.8 | 3.5 | |
| High School | 34.1 | 18.3 | 15.1 | 33.3 | |
| Some College | 19.4 | 9.4 | 5.9 | 18.8 | |
| College or more | 17.4 | 3.1 | 6.8 | 16.8 | |
| Household Income (Quintiles) | |||||
| Q1 | 15.1 | 41.2 | 54.0 | 16.7 | <.001 |
| Q2 | 19.3 | 22.7 | 26.1 | 19.6 | |
| Q3 | 20.9 | 16.7 | 10.0 | 20.5 | |
| Q4 | 22.2 | 10.6 | 4.8 | 21.5 | |
| Q5 | 22.6 | 8.8 | 5.1 | 21.8 | |
| Household Wealth (Quintiles) | |||||
| Q1 | 14.8 | 38.5 | 59.0 | 16.5 | <.001 |
| Q2 | 18.3 | 28.8 | 21.8 | 18.6 | |
| Q3 | 21.2 | 10.9 | 11.0 | 20.7 | |
| Q4 | 22.3 | 14.7 | 5.5 | 21.7 | |
| Q5 | 23.5 | 7.0 | 2.7 | 22.5 | |
| Weight | |||||
| Underweight | 2.6 | 3.2 | 3.3 | 2.6 | <.001 |
| Normal | 41.7 | 31.8 | 33.2 | 41.2 | |
| Overweight | 39.2 | 41.0 | 40.7 | 39.2 | |
| Obese | 16.6 | 24.0 | 22.7 | 16.9 | |
| Physical Activity | |||||
| Not Active | 59.0 | 61.3 | 66.6 | 59.2 | .074 |
| Active | 41.0 | 38.7 | 33.4 | 40.8 | |
| Smoking | |||||
| Never | 43.1 | 44.9 | 51.7 | 43.4 | .085 |
| Former | 46.7 | 44.1 | 37.7 | 46.4 | |
| Current | 10.2 | 11.1 | 10.6 | 10.2 | |
| Alcohol Consumption | |||||
| Nondrinker | 70.1 | 80.5 | 83.9 | 70.7 | <.001 |
| Not at Risk | 25.4 | 14.8 | 12.3 | 24.8 | |
| At Risk | 4.5 | 4.6 | 3.8 | 4.5 | |
Note: FB-H = Foreign-born Hispanic; GED = General Education Development; NHW = Non-Hispanic White; SES = Socio-Economic Status; USB-H = U.S.-born Hispanic.
Analytic Approach
We use Latent Class Growth Curve Modeling (GCM) techniques to examine how the four domains of healthy aging change over time (Nagin, 2005), combined with discrete time survival (DTS) analyses to model wave-specific mortality (Allison, 1984). Combining longitudinal growth and survival processes provides a framework for simultaneously modeling change and discrete events such as death (Muthén & Masyn, 2005). Detailed discussions of these techniques are available elsewhere (Muthén & Masyn, 2005; Wickrama, Lee, & O’Neal, 2016).
Modeling Steps
We follow a multistep modeling approach. First, given prior research, we anticipate three-to-five aging trajectories and test for increases in number of classes sequentially (up to 7). We use statistical and substantive theoretical expectations to select the most appropriate model. Statistical criteria included Akaike Information Criteria and sample size Adjusted Bayesian Information Criteria values (and change in values between extracted solutions), and entropy with a minimum threshold set to 0.80 (Supplementary Table 1). In addition, we used visual examination of extracted solutions, clarity of differentiation between the estimated trajectory classes, and theoretical translation of findings for selecting the final model. To ensure final model estimates stability and class replicability once we reached a decision about the appropriate class solution, we conducted a sensitivity analysis by (a) dividing the sample into four random splits and (b) re-estimating the model in each of these subsamples to ensure that the derived classes were consistent across splits (Supplementary Figure 3). We did not impose a functional form on time (e.g., linear) to maintain maximum flexibility when estimating the growth curves (Biesanz, Deeb-Sossa, Papadakis, Bollen, & Curran, 2004). We explicitly accounted for the underlying properties of each outcome variable. Specifically, major disease, disability, and physical function were modeled as count measures and cognitive functioning was modeled as floor-censored at zero (Reinecke & Seddig, 2011). We used years since the baseline wave as the time metric and estimated the models using full information maximum likelihood (FIML) methods on all available data. Supplementary Figure 2 provides a visualization of our conceptual model. The estimated parameters and inference statistics for the best fitting model are provided in Supplementary Table 2.
After estimating the optimal class solution, we assigned participants to their “highest-probability” trajectory class to examine how trajectory classes vary across individuals. We then separately estimated a series of multinomial logit models to predict trajectory class as a function of the covariates described earlier, with “nonaccelerated” aging set as the reference category (Table 2). Model 1 included ethnicity/nativity status only. Model 2 additionally controlled for baseline age, gender, and number of participated waves. Model 3 added in childhood characteristics, Model 4 added in adult achievements, and Model 5 added in adult health behaviors. To guard against bias due to classification precision, the individual probability weights were adjusted to the probability of classification into the trajectory class and all estimates in the multinomial logit and predicted marginal probabilities accounted for this weight. To facilitate the presentation and interpretation of results, we modeled each of these lifecourse periods using factor scores estimated from confirmatory factor models of the observed indicators. Using the observed variables in the model produced very similar estimates for the primary associations of interest. Supplementary Table 4 lists the estimates derived from those models.
Table 2.
Estimated RRR for Trajectories of Aging and Their 95% CIs
| M1 | M2 | M3 | M4 | M5 | |
|---|---|---|---|---|---|
| RRR/95% CI | RRR/95% CI | RRR/95% CI | RRR/95% CI | RRR/95% CI | |
| Cognitive vs Nonaccelerated | |||||
| USB-H (ref: NHW) | 2.40*** | 3.28*** | 3.13*** | 2.63*** | 2.63*** |
| 1.61, 3.60 | 2.10, 5.12 | 2.00, 4.90 | 1.69, 4.1 | 1.69, 4.08 | |
| FB-H (ref: NHW) | 3.14*** | 4.29*** | 4.12*** | 3.15*** | 3.16*** |
| 1.70, 5.81 | 2.34, 7.88 | 2.26, 7.54 | 1.78, 5.59 | 1.78, 5.60 | |
| High morbidity vs Nonaccelerated | |||||
| USB-H (ref: NHW) | 2.48*** | 3.92*** | 3.65*** | 2.49*** | 2.42*** |
| 1.64, 3.76 | 2.41, 6.37 | 2.26, 5.9 | 1.58, 3.91 | 1.52, 3.86 | |
| FB-H (ref: NHW) | 2.21** | 3.6*** | 3.39*** | 1.92** | 1.84* |
| 1.32, 3.72 | 2.27, 5.7 | 2.13, 5.38 | 1.21, 3.05 | 1.15, 2.95 | |
| Healthy vs Nonaccelerated | |||||
| USB-H (ref: NHW) | 1.54* | 1.42 | 1.52* | 1.82** | 1.81** |
| 1.04, 2.28 | 0.96, 2.09 | 1.02, 2.26 | 1.19, 2.78 | 1.18, 2.77 | |
| FB-H (ref: NHW) | 1.39 | 1.21 | 1.29 | 1.77* | 1.78* |
| 0.87, 2.25 | 0.74, 1.99 | 0.79, 2.12 | 1.08, 2.91 | 1.08, 2.94 | |
Note: Results are based on incrementally adjusted multinomial logit models using data from non-Hispanic White (NHW) and U.S.- and Foreign-born Hispanic (USB-H and FB-H) adults aged 65 years and older without a proxy interview at baseline from the Health and Retirement Study (1998–2014).
The reference group for all models is “nonaccelerated.” Model 1 includes ethnicity/nativity status only. Model 2 additionally accounts for age at baseline, gender, and number of participated waves. Model 3 adds in childhood characteristics, Model 4 adds in adult achievements, and Model 5 adds in health habits/behaviors.
CI = Confidence interval; FB-H = Foreign-born Hispanic; NHW = Non-Hispanic White; RRR = Relative risk ratios; USB-H = U.S.-born Hispanic.
*p < .05; **p < .01; ***p < .001.
Analyses were conducted using MPLUS 7.3 (Muthen & Muthen, 2015) and Stata 14.1 software (Stata, College Station, TX, 2011). All models and estimates accounted for the complex design of the HRS, including baseline probability weights, stratification, and clustering (Heeringa, West, & Berglund, 2010).
Sensitivity
To check the robustness of our results, we also estimated the multinomial logit models stratified by age groups: 65–74 years and 75+ years. A primary concern when doing stratified analyses is the loss of power due to smaller sample sizes. That said, the findings from these age-stratified analyses indicated that the estimates and conclusions reached when age groups are combined are qualitatively robust and largely quantitatively unchanged. The estimated relative risk ratios (RRR) for the 65–74 years and 75+ years groups are presented in Supplementary Table 5.
Results
Descriptive Characteristics
Sample characteristics, overall and by ethnicity and nativity, are reported in Table 1. Hispanics more often reported poor childhood socio-economic status (SES) and less educated parents compared to NHWs. Hispanics also much more often reported having less than a high school education and household income and wealth in the lowest 20th percentile. Hispanics were also more often obese and less often physically active, particularly the FB. Finally, all Hispanics more often reported being nondrinkers and FB-H more often reported never having smoked.
Aging Trajectories
Based on statistical criteria, solutions based on four and five extracted trajectory classes provided largely comparable fit. Supplementary Table 4 includes the estimated trajectories within each solution. Visual inspection and theoretical interpretation indicated that the five-class solution did not show clear enough demarcation of additional trajectories relevant to the chosen four-class solution. A three-class solution (also visualized in Supplementary Figures 4 and 4a) was deemed too simple as it failed to extract the critically important “cognitive unhealthy” class described below. Figure 1 presents domain-specific trajectory graphs for the four patterns that best describe how older adults age. Supplementary Table 1 reports goodness-of-fit statistics for all the estimated latent trajectory models and Supplementary Table 2 reports the estimated parameters (intercepts and slopes) for the four-class model deemed most appropriate.
Figure 1.
Estimated trajectories of health domains and mortality. Results are based on data from respondents aged 65 years and older without a proxy interview at baseline from the Health and Retirement Study (1998–2014). Each pattern in the health and mortality subplots represent mean trajectories consistent with a latent class grouping. Four trajectories were derived: “cognitive” (“C”) and its associated dysfunctions, “high morbidity” (“HM”), “nonaccelerated” (“NA”), and “healthy” (“H”) aging. The four trajectory patterns are based on observed measures of activities of daily limitations (ADLs), major chronic disease, physical dysfunction, cognitive function, and wave-specific death. With the exception of cognitive functioning higher values on each of the considered health domains indicates worse health.
One pattern, which we call a “cognitive unhealthy” (henceforth “cognitive”) class, described 21.5% of older adults. It is characterized by a high rate of decline in cognitive function over time, along with initially low but then accelerating (at around 6 years from baseline) physical dysfunction and disability and moderately high initial and accelerating mortality rates (80% by 2014). The prevalence of this class among NHW, USB-H and FB-H was 19%, 25.1%, and 32.3%, respectively.
Another pattern, which we call “high morbidity,” described 28.4% of older adults and is characterized by high initial and increasingly deficient levels of ADLs/IADLs independence, disease, and compromised physical and cognitive functioning, along with a steady worsening in each of these measures and high-accelerated mortality (95% by 2014) over time. The prevalence of this class among NHW, USB-H and FB-H was 28%, 38%, and 33%, respectively.
A “nonaccelerated” aging group described the 24% of older adults who display relatively low and then moderately rising levels of disease and physical and cognitive dysfunction and moderate survival rates (40% by 2014). The prevalence of this class among NHW, USB-H, and FB-H was 25.1%, 13.7%, and 13.5%, respectively.
Finally, a pattern we call “healthy” described 26.1% of older adults and is characterized by initial and consistently low levels of disability, physical dysfunction, and disease, consistently high levels of cognitive function, and high survival rate (66% by 2014). The prevalence of this class among NHW, USB-H, and FB-H was 28%, 23.5%, and 21%, respectively.
A detailed characterization of the four derived classes by the study covariates are presented in Supplementary Table 3. Briefly, relative to the other three groups, the “healthy” were more likely to self-report excellent childhood health, higher parental and personal education, and higher income and wealth levels. They were also more likely to report being physically active and to have moderate alcohol consumption levels, and less likely to be obese and current smokers. The “high morbidity” were more likely to be females, to self-report fair/poor health, to report not being physically active, and to report no alcohol consumption. Both the “cognitive” and “high morbidity” groups had lower parental and personal education and lower household income and wealth.
Correlates of Aging Trajectories
Table 2 reports our key results for how ethnicity and United States nativity relate to patterns of aging, based on the multinomial logit models estimated. For each multinomial logit model “nonaccelerated” aging was the reference category. Adjusting for age, sex, and number of waves completed (Model 2), USB-H and FB-H adults (relative to NHW) had a higher relative risk of cognitive aging (RRRUSB-H = 3.28; 95% CI = 2.10, 5.12; RRRFB-H = 4.29; 95% CI = 2.34, 7.88) and “high morbidity” (RRRUSB-H = 3.92; 95% CI = 2.41, 6.37; RRRFB-H = 3.60; 95% CI = 2.27, 5.70). Neither of the Hispanic groups statistically differed in their relative risk of “healthy” classification compared to NHWs. Incrementally adjusting for childhood characteristics (Model 3), adult achievements (Model 4), and health behaviors (Model 5) partially attenuated U.S.- and FB-Hispanics’ relative risk of cognitive aging (Model5: RRRUSB-H = 2.63; 95% CI = 1.69, 4.08; RRRFB-H = 3.16; 95% CI = 1.78, 5.60) and “high morbidity” (Model5: RRRUSB-H = 2.42; 95% CI = 1.52, 3.86; RRRFB-H = 1.84; 95% CI = 1.15, 2.95) relative to “nonaccelerated” aging, compared to NHWs. The relative risk of “healthy” for both Hispanic groups were slightly accentuated after adjusting for adult achievements (Model 4), and they remained unchanged after adjusting for health behaviors (Model 5: RRRUSB-H = 1.81; 95% CI = 1.18, 2.77; RRRFB-H = 1.78; 95% CI = 1.08, 2.94).
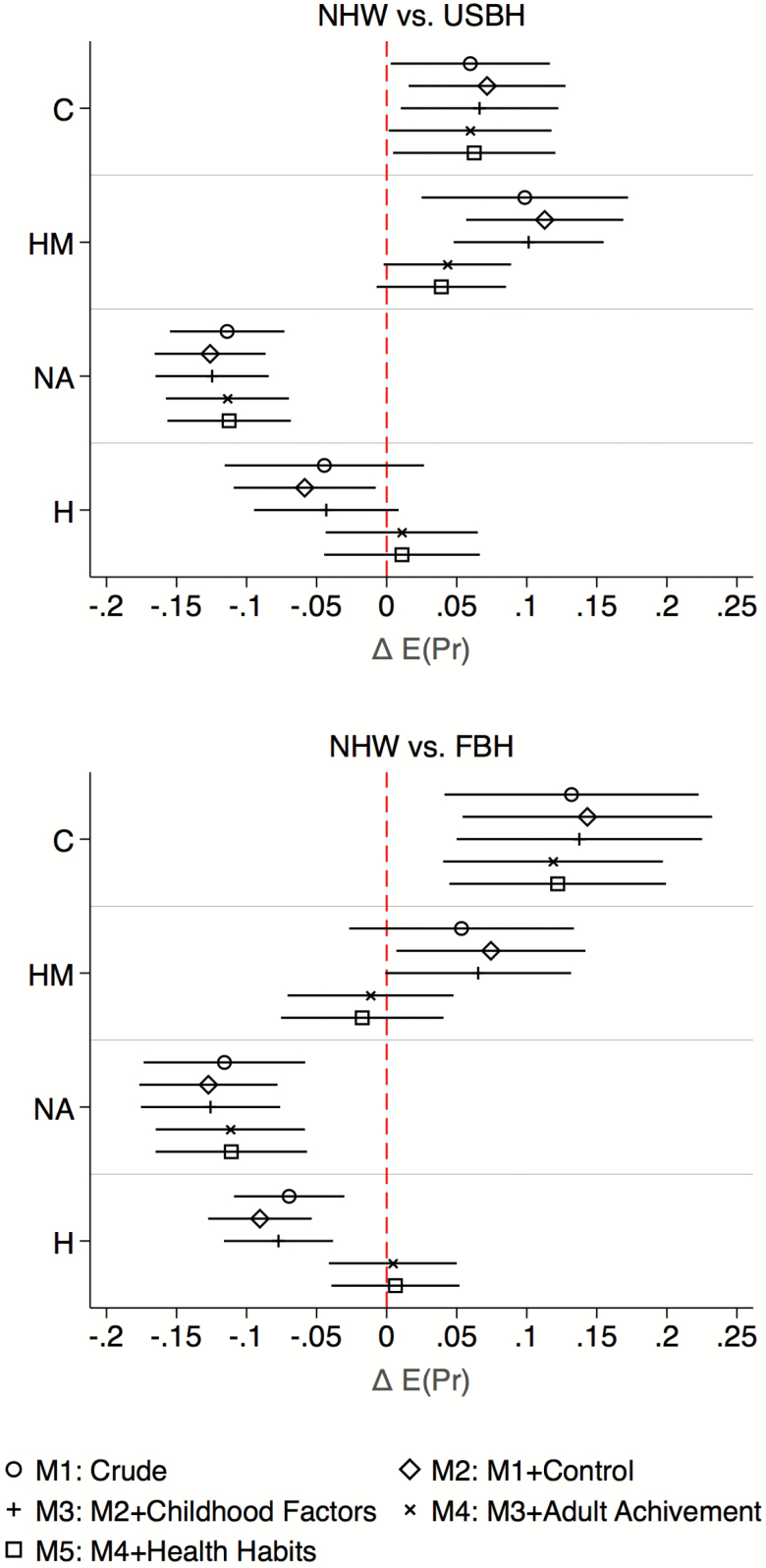
To further facilitate the interpretation and visualization of group differences and attenuation through the inclusion of covariates, Figure 2 plots the difference in predicted probabilities of having each of the aging patterns between the Hispanic groups and the reference group, NHW, along with a 95% confidence interval for each difference. It reveals that differences in the probability of cognitive aging and probability of “nonaccelerated” aging across ethnic and U.S. nativity status varied little with the inclusion of more covariates, whereas differences in the probability of “high morbidity” and probability of “healthy” aging were attenuated after adjustment for adult achievements.
Figure 2.
Estimated average marginal probabilities of classification in health trajectory classes and their 95% confidence intervals. Results are based on incrementally adjusted multinomial logit model using non-Hispanic White (NHW) and U.S.- and Foreign-born Hispanic (USB-H and FB-H) adults aged 65 years and older without a proxy interview at baseline from the Health and Retirement Study (1998–2014). Note 1: Control variables include age at baseline, sex, and number of waves participated. Childhood condition is based on an estimated factor score (mean = 0, SD = 1) using four observed covariates including: childhood health (excellent, very good, good, fair, and poor), childhood socioeconomic status (well off, average, and poor), mother’s education (less than high school, more than high school, and missing), father’s education (less than high school, more than high school, and missing). Adult achievement is based on an estimated factor score (mean = 0, SD = 1) using three observed covariates: respondent’s education (less than high school, General Education Development (GED), high school, some college, and college or more), quintiles of household income, and quintiles of household wealth. Health habits based on an estimated factor score (mean = 0, SD = 1) using four observed covariates including: smoking status (never, former, current), body mass index (BMI) (underweight [BMI < 18], normal [18 < BMI < 25], overweight [25 < BMI < 30], or obese [BMI > 30]), alcohol consumption defined based on National Institute on Alcohol Abuse and Alcoholism criteria (nondrinker, low risk, and at risk), and weekly vigorous physical activity (not active, active). Note 2: “C” stands for “cognitive unhealthy”, “HM” is “high morbidity”, “NA” is “nonaccelerated”, and “H” is “healthy”.
Figure 3 reports the estimated RRR for all covariates in the fully adjusted model (Model 5) along with their confidence intervals. Adult achievements and better health habits were protective against “high morbidity” classification and increased the likelihood of being in the “healthy” group. Adult achievements also protected against cognitive aging and largely explained the associations between childhood factors and the health trajectories.
Figure 3.
Estimated relative risk ratios and their 95% confidence intervals. Results are based on fully adjusted multinomial logit model using non-Hispanic White (NHW) and U.S.- and Foreign-born Hispanic (USB-H and FB-H) adults aged 65 years and older without a proxy interview at baseline from the Health and Retirement Study (1998–2014). Note 1: Control variables include age at baseline, sex, and number of waves participated. Childhood condition is based on an estimated factor score (mean = 0, SD = 1) using four observed covariates including: childhood health (excellent, very good, good, fair, and poor), childhood socioeconomic status (well off, average, and poor), mother’s education (less than high school, more than high school, and missing), father’s education (less than high school, more than high school, and missing). Adult achievement is based on an estimated factor score (mean = 0, SD = 1) using three observed covariates: respondent’s education (less than high school, General Education Development (GED), high school, some college, and college or more), quintiles of household income, and quintiles of household wealth. Health habits based on an estimated factor score (mean = 0, SD = 1) using four observed covariates including: smoking status (never, former, current), body mass index (BMI) (underweight [BMI < 18], normal [18 < BMI < 25], overweight [25 < BMI < 30], or obese [BMI > 30]), alcohol consumption defined based on National Institute on Alcohol Abuse and Alcoholism criteria (nondrinker, low risk, and at risk), and weekly vigorous physical activity (not active, active). Note 2: ***p < .001; **p < .01; *p < .05. Relative risk ratios and confidence intervals in light grey are not statistically significant in the final adjusted model. Note 3: “C” stands for “cognitive unhealthy”, “HM” is “high morbidity”, “NA” is “nonaccelerated”, and “H” is “healthy”.
Discussion
We uncovered important heterogeneities in how older U.S. adults age. Analyzing longitudinal HRS data, we found evidence to support four classes of aging, each with a distinct combination of trajectories for need-with-ADLs/IADLs, major chronic disease, level of physical dysfunction, cognitive change, and mortality.
First, we derived four groups describing (a) a classical physiological loss class driven primarily by high prevalence and increasing incidence of chronic conditions and leading to accelerated rates of mortality; (b) a cognitive aging class reflecting recent work on brain disease progression and showing that cognitive decline is typically slower in the early stages and then increases more rapidly triggering other functional losses as the patient progresses into more severe disease stages (e.g., dementia); (c) a “nonaccelerated” group that depicts incremental but manageable accumulation of organ-system diseases and concomitant functional loss with moderate mortality rates; and finally (d), a high resilience (or “healthy” aging) class that ages with minimal chronic disease accumulation and cognitive and functional loss and low levels of mortality. We argue that these patterns are consistent with “high morbidity”, “cognitive”, “nonaccelerated”, and “healthy” aging.
Second, we reported that the prevalence of each derived trajectory class varies depending on ethnic background and nativity status and found mixed support for extending the “Hispanic paradox” to more comprehensive longitudinal assessments of health and aging. Adjusting for adult achievements and health habits, when compared to non-Hispanic Whites, USB-H and FB-H were equally likely to be in the “healthy” class. These results provide evidence for resilience among some older Hispanics, regardless of nativity, compared to non-Hispanic Whites. However, USB-H and FB-H were also equally or more likely to exhibit patterns of aging indicative of “high morbidity” and insidious cognitive decline and its associated physical dysfunctions and activities limitations. These findings indicate that many aging Hispanics, irrespective of nativity status, have excess morbidity relative to Whites. These results provide support for published evidence on disparities among older Hispanics that are more prominent in functional and cognitive health trajectories (Gurland et al., 1999; Liang, Xu, Bennett, Ye, & Quinones, 2010; Lines, Sherif, & Wiener, 2014).
In terms of early lifecourse, we observed that childhood factors were positively associated with healthier patterns of aging and inversely so with “high morbidity” and “cognitive” aging. These factors, however, did not attenuate the differences in aging trajectories classification among Hispanics relative to NHW. The effects of advantageous childhood conditions were also explained by adult achievements and better health behaviors in old age. These findings are in line with previously published studies (Hayward & Gorman, 2004). Targeted policies to improve children’s health are long-term public health investments, but deficits in childhood environments are not insurmountable when attempting to change and improve the health trajectories of older adults.
Our results provide support for strong positive associations between adult achievements and “healthy” classification and inverse associations with “cognitive” patterns of aging. These results parallel findings in the literature and suggest that education, income and wealth may be protective (Hayward & Gorman, 2004). Health differences between non-Hispanic Whites and Hispanics, particularly the FB, were partially but not completely confounded by socioeconomic differences and as such could lend limited support for a positive acculturation hypothesis. Available evidence shows the importance of socioeconomic conditions as correlates of health stratification (Xu et al., 2015) and indicate that race does not constrain the ability of individuals to transform socioeconomic achievement into better health (Hayward, Miles, Crimmins, & Yang, 2000; Louie & Ward, 2011). Unlike race-based differences in health and mortality, where structural disadvantages that hinder socioeconomic advancement play a critical role in disparities (Woolf & Braveman, 2011), these mechanisms might play a less prominent role in explaining differences between Hispanics and non-Hispanic Whites. However, others have argued that some of the evidence for better health functioning among Hispanic immigrants may derive from greater socioeconomic resources (Gonzalez et al., 2009).
Finally, health behaviors had independent and nonconfounding effects on aging patterns. The independent effects of health behaviors mirror published work that points to the importance of not smoking and having a healthy weight for healthful aging (Britton, Shipley, Singh-Manoux, & Marmot, 2008) and the protective role that absence of behavioral cardiovascular risk factors such as smoking, obesity, and a inactivity play for cognitive impairment and decline (Anstey et al., 2014). Our finding that health behaviors did not confound the associations between Hispanic ethnicity and nativity and health trajectories supports the negative acculturation hypothesis. That is, advantageous health behaviors among FB-H regress over time and converge to U.S. levels as they acculturate to U.S. society, thus conferring no additional benefit relative to NHW and USB-H (Abraido-Lanza, Chao, & Florez, 2005; Antecol & Bedard, 2006). This is a reasonable argument given that close to 94% of the HRS older immigrant sample, used in this study, reported being in the United States for more than 15 years. This finding provides additional evidence that healthy immigrant characteristics and advantageous health habits, found among younger immigrants, might contribute to better health at younger ages but do not carry over into older age.
Limitations
First, self-reported data is subject to group and individual biases. Some work suggests that Hispanics have lower awareness and less frequently report chronic diseases such as hypertension and diabetes (Schneiderman et al., 2014; Sorlie et al., 2014). Additionally, we used aggregated Hispanics and were unable to determine if results differed by Hispanic background (e.g., Puerto-Ricans). Second, the proxy participants that we excluded were sicker and had higher levels of disability and physical performance limitations. Hispanics were also more likely to have proxy interviews at baseline relative to non-Hispanic Whites. This could have introduced bias to the estimated classes. Third, biennial nonmortality missingness in the sample varied between 3% and 5.5% over the study period. Although we used FIML to fit our models, this missingness could have introduced some bias into our estimates. Fourth, the cognitive measure we used might be biased by unknown cultural factors. Fifth, researchers have cautioned against focusing on mostly physiologic aspects of aging, pointing to the compensatory role that psychosocial factors can play (Pruchno et al., 2010; Young, Frick, & Phelan, 2009). Sixth, the identified groups are putative and the patterns uncovered by analyzing these groups, as Nagin (2005) argues (Nagin, 2005), represent approximations of shared trajectories.(p.62) These approximations simplify much more “complex realities” in order to link clusters of individuals with shared health features to substantive clinical and policy measures.(p.173) Finally, we focused on individual characteristics and did not stratify by sex. Future studies should look at how contextual and social characteristics and subjective assessments interact with sex and ethnic and nativity specific cultural factors in determining trajectories of aging.
Conclusion
Healthy aging has the potential to reduce societal and individual burdens in a graying and increasingly diverse U.S. population. We uncovered four distinct patterns of aging among older adults. We found significant variation in their distribution based on ethnicity and nativity and mixed evidence to support the “healthy migrant”, “positive acculturation”, or “healthy habits” hypotheses as explanations for Hispanic and NHW differences. Ignoring population heterogeneity in aging can lead to erroneous conclusions that leave vulnerable groups shielded from necessary policy interventions, particularly in the realm of cognitive aging. We argue that recognizing these population patterns is crucial for maintaining the integrity of the public health system as the older U.S. population increases in size and diversifies. Doing so can help clinicians, policy makers, and researchers devise more targeted interventions to increase the prevalence of optimal aging in these groups and reduce individual and societal burdens associated with unhealthy patterns of aging. Further investigations of the underlying causes of such differences are warranted.
Supplementary Material
Acknowledgments
W. Tarraf conceived the work, and G. A. Jensen, H. E. Dillaway, P. Vásquez, and H. M. González provided advice on the development, writing, and revision of the manuscript. W. Tarraf acquired and analyzed the data and drafted the manuscript. All coauthors edited and shaped the final draft.
Funding
W. Tarraf, H. M. González, and P. Vásquez receive support from NIH/NIA grant R01AG48642, and W. Tarraf receives training support from grant P30AG053760. The content of this manuscript is the responsibility of the authors alone and does not necessarily reflect the views of the sponsors.
Conflict of Interest
None reported.
References
- Abraido-Lanza A. F., Chao M. T., & Florez K. R (2005). Do healthy behaviors decline with greater acculturation? Implications for the Latino mortality paradox. Social Science & Medicine, 61, 1243–1255. doi:10.1016/j.socscimed.2005.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison P. D. (1984). Event history analysis regression for longitudinal event data. Beverly Hills, CA: Sage Publications. [Google Scholar]
- Anstey K. J., Kingston A., Kiely K. M., Luszcz M. A., Mitchell P., & Jagger C (2014). The influence of smoking, sedentary lifestyle and obesity on cognitive impairment-free life expectancy. International Journal of Epidemiology, 43, 1874–1883. doi:10.1093/ije/dyu170 [DOI] [PubMed] [Google Scholar]
- Antecol H., & Bedard K (2006). Unhealthy assimilation: Why do immigrants converge to American health status levels?Demography, 43, 337–360. doi:10.1353/dem.2006.0011 [DOI] [PubMed] [Google Scholar]
- Berkman L. F., Seeman T. E., Albert M., Blazer D., Kahn R., Mohs R., Finch C., Schneider E., Cotman C., McClearn G. and Nesselroade J, 1993. High, usual and impaired functioning in community-dwelling older men and women: Findings from the MacArthur Foundation research network on successful aging. Journal of Clinical Epidemiology, 46, 1129–1140. doi:10.1016/0895-4356(93)90112-E [DOI] [PubMed] [Google Scholar]
- Biesanz J. C., Deeb-Sossa N., Papadakis A. A., Bollen K. A., & Curran P. J (2004). The role of coding time in estimating and interpreting growth curve models. Psychological Methods, 9, 30–52. doi:10.1037/1082-989X.9.1.30 [DOI] [PubMed] [Google Scholar]
- Brandt J., Spencer M., & Folstein M (1988). The telephone interview for cognitive status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 1, 111–117. [Google Scholar]
- Britton A., Shipley M., Singh-Manoux A., & Marmot M. G (2008). Successful aging: The contribution of early-life and midlife risk factors. Journal of the American Geriatrics Society, 56, 1098–1105. doi:10.1111/j.1532-5415.2008.01740.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby L. S., & Ortman M. J (2015). Projections of the size and composition of the US population: 2014 to 2060, Current Population Reports, P25-1143. Washington, DC: U.S. Census Bureau. [Google Scholar]
- Cosco T. D., Stephan B. C., Muniz G., & Brayne C; CC75C Study Collaboration (2016). A novel examination of successful aging trajectories at the end of life. Canadian Journal on Aging, 35, 533–540. doi:10.1017/S0714980816000519 [DOI] [PubMed] [Google Scholar]
- Daviglus M. L., Pirzada A., & Talavera G. A (2014). Cardiovascular disease risk factors in the Hispanic/Latino population: Lessons from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Progress in Cardiovascular Diseases, 57, 230–236. doi:10.1016/j.pcad.2014.07.006 [DOI] [PubMed] [Google Scholar]
- Fenelon A. (2013). Revisiting the Hispanic mortality advantage in the United States: The role of smoking. Social Science & Medicine, 82, 1–9. doi:10.1016/j.socscimed.2012.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier de la Fuente, Caballero Francisco Félix, Sánchez-Niubó Albert, Panagiotakos Demosthenes B, Prina A Matthew, Holger Arndt, Haro Josep Maria, Chatterji Somnath, Ayuso-Mateos José Luis; Determinants of Health Trajectories in England and the United States: An Approach to Identify Different Patterns of Healthy Aging, The Journals of Gerontology: Series A, , gly006. doi:10.1093/gerona/gly006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N. (2016). Will the Latino mortality advantage endure?Research on Aging, 38, 263–282. doi:10.1177/ 0164027515620242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H. M., Ceballos M., Tarraf W., West B. T., Bowen M. E., & Vega W. A (2009). The health of older Mexican Americans in the long run. American Journal of Public Health, 99, 1879–1885. doi:10.2105/AJPH.2008.133744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H. M., Tarraf W., & Haan M. N (2011). The metabolic syndrome, biomarkers, and the acculturation-health relationship among older Mexican Americans. Journal of Aging and Health, 23, 1101–1115. doi:10.1177/0898264311421371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurland B. J., Wilder D. E., Lantigua R., Stern Y., Chen J., Killeffer E. H., & Mayeux R (1999). Rates of dementia in three ethnoracial groups. International Journal of Geriatric Psychiatry, 14, 481–493. doi:10.1002/(SICI)1099-1166(199906)14:6%3C481::AID-GPS959%3E3.0.CO;2-5 [PubMed] [Google Scholar]
- Hayward M. D., & Gorman B. K (2004). The long arm of childhood: The influence of early-life social conditions on men’s mortality. Demography, 41, 87–107. [DOI] [PubMed] [Google Scholar]
- Hayward M. D., Miles T. P., Crimmins E. M., & Yang Y (2000). The significance of socioeconomic status in explaining the racial gap in chronic health conditions. American Sociological Review, 65, 910–930. doi:10.2307/2657519 [Google Scholar]
- Heeringa S. G., & Connor J (1995). Technical description of the health and retirement study sample design: HRS/AHEAD documentation report DR-002. http://hrsonline.isr.umich.edu/ sitedocs/userg/HRSSAMP.pdf [Google Scholar]
- Heeringa S. G., West B. T., & Berglund P. A (2010). Applied survey data analysis. Boca Raton, FL: Chapman & Hall/CRC. [Google Scholar]
- Herzog A. R., & Wallace R. B (1997). Measures of cognitive functioning in the AHEAD study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 52, 37–48. [DOI] [PubMed] [Google Scholar]
- Hochstetler H., Trzepacz P. T., Wang S., Yu P., Case M., Henley D. B.,…Lyketsos C. G (2016). Empirically defining trajectories of late-life cognitive and functional decline. Journal of Alzheimer’s Disease, 50, 271–282. doi:10.3233/JAD-150563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. C., & Jones B. L (2012). Multiple trajectories of successful aging of older and younger cohorts. The Gerontologist, 52, 843–856. doi:10.1093/geront/gns005 [DOI] [PubMed] [Google Scholar]
- Jasso G., Massey D. S., Rosenzweig M. & Smith J (2004). Immigrant health: selectivity and acculturation, IFS Working Papers W04/23, Institute for Fiscal Studies; http://econwpa.repec.org/eps/lab/papers/0412/0412002.pdf [Google Scholar]
- Jonkman N. H., Del Panta V., Hoekstra T., Colpo M., van Schoor N. M., Bandinelli S.,…Maier A. B (2018). Predicting trajectories of functional decline in 60- to 70-year-old people. Gerontology, 64, 212–221. doi:10.1159/000485135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S., McDonald J. T., & Biddle N (2006). The healthy immigrant effect and immigrant selection: Evidence from four countries. Social and Economic Dimensions of an Aging Population Research Papers 164, McMaster University; https://socialsciences.mcmaster.ca/sedap/p/sedap164.pdf [Google Scholar]
- Kok A. A., Aartsen M. J., Deeg D. J., & Huisman M (2017). Capturing the diversity of successful aging: An operational definition based on 16-year trajectories of functioning. The Gerontologist, 57, 240–251. doi:10.1093/geront/gnv127 [DOI] [PubMed] [Google Scholar]
- Lara M., Gamboa C., Kahramanian M. I., Morales L. S., & Bautista D. E (2005). Acculturation and Latino health in the United States: A review of the literature and its sociopolitical context. Annual Review of Public Health, 26, 367–397. doi:10.1146/annurev.publhealth.26.021304.144615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Xu X., Bennett J. M., Ye W., & Quinones A. R (2010). Ethnicity and changing functional health in middle and late life: A person-centered approach. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65, 470–481. doi:10.1093/geronb/gbp114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines L. M., Sherif N. A., & Wiener J. M (2014). Racial and ethnic disparities among individuals with Alzheimer’s disease in the United States: A literature review (RTI Press publication No. RR-0024-1412). Research Triangle Park (NC): RTI Press; Retrieved from http://www.rti.org/rtipress [Google Scholar]
- Louie G. H., & Ward M. M (2011). Socioeconomic and ethnic differences in disease burden and disparities in physical function in older adults. American Journal of Public Health, 101, 1322–1329. doi:10.2105/AJPH.2010.199455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markides K. S. and Rote S (2015). Immigrant Health Paradox. In Scott R. A. & Kosslyn S. M. (Eds.), Emerging trends in the social and behavioral sciences. doi:10.1002/9781118900772.etrds0174 [Google Scholar]
- McClintock M. K., Dale W., Laumann E. O., & Waite L (2016). Empirical redefinition of comprehensive health and well-being in the older adults of the United States. Proceedings of the National Academy of Sciences of the United States of America, 113, E3071–E3080. doi:10.1073/pnas.1514968113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. J., Connell C. M., Heeringa S. G., Li L. W., & Roberts J. S (2010). Successful aging in the United States: Prevalence estimates from a national sample of older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65B, 216–226. doi:10.1093/geronb/gbp101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. J., Jette A. M., & Connell C. M (2012). An examination of healthy aging across a conceptual continuum: Prevalence estimates, demographic patterns, and validity. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 67, 783–789. doi:10.1093/gerona/glr234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta K. M., & Yeo G. W (2017). Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimer’s & Dementia, 13, 72–83. doi:10.1016/j.jalz.2016.06.2360 [DOI] [PubMed] [Google Scholar]
- Muthén L. K., and Muthén B. O (1998-2015). Mplus User’s Guide. Eighth Edition Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Muthén B., & Masyn K (2005). Discrete-time survival mixture analysis. Journal of Educational and Behavioral statistics, 30, 27–58. doi:10.3102/10769986030001027 [Google Scholar]
- Nagin D. (2005). Group-based modeling of development. Cambridge, MA: Harvard University Press. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (2016). Drinking levels defined Retrieved March 7th, 2016, from http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
- Ofstedal M. B., Fisher G. G., & Herzog R. A (2005). Documentation of cognitive functioning measures in the Health and Retirement Study. Ann Arbor, MI: Survey Research Center, University of Michigan. [Google Scholar]
- Pruchno R. A., Wilson-Genderson M., & Cartwright F (2010). A two-factor model of successful aging. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65, 671–679. doi:10.1093/geronb/gbq051 [DOI] [PubMed] [Google Scholar]
- Reinecke J., & Seddig D (2011). Growth mixture models in longitudinal research. AStA Advances in Statistical Analysis, 95, 415–434. doi:10.1007/s10182-011-0171-4 [Google Scholar]
- Rowe J. W., & Kahn R. L (2015). Successful aging 2.0: Conceptual expansions for the 21st century. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 70, 593–596. doi:10.1093/geronb/gbv025 [DOI] [PubMed] [Google Scholar]
- Schneiderman N., Llabre M., Cowie C. C., Barnhart J., Carnethon M., Gallo L. C.,…Avilés-Santa M. L (2014). Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care, 37, 2233–2239. doi:10.2337/dc13-2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T. E., Charpentier P. A., Berkman L. F., Tinetti M. E., Guralnik J. M., Albert M.,…Rowe J. W (1994). Predicting changes in physical performance in a high-functioning elderly cohort: MacArthur studies of successful aging. Journal of Gerontology, 49, M97–M108. doi:10.1093/geronj/49.3.M97 [DOI] [PubMed] [Google Scholar]
- Sorlie P. D., Allison M. A., Avilés-Santa M. L., Cai J., Daviglus M. L., Howard A. G.,…Talavera G. A (2014). Prevalence of hypertension, awareness, treatment, and control in the Hispanic Community Health Study/Study of Latinos. American Journal of Hypertension, 27, 793–800. doi:10.1093/ajh/hpu003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turra C. M., & Elo I. T (2008). The impact of Salmon bias on the Hispanic mortality advantage: New evidence from social security data. Population Research and Policy Review, 27, 515–530. doi:10.1007/s11113-008-9087-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega W. A., Rodriguez M. A., & Gruskin E (2009). Health disparities in the Latino population. Epidemiologic Reviews, 31, 99–112. doi:10.1093/epirev/mxp008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Herzog A. R., Ofstedal M. B., Steffick D., Fonda S., & Langa K (2000). Documentation of affective functioning measures in the health and retirement study. Ann Arbor, MI: University of Michigan. [Google Scholar]
- Whitson H. E., Johnson K. S., Sloane R., Cigolle C. T., Pieper C. F., Landerman L., & Hastings S. N (2016). Identifying patterns of multimorbidity in older Americans: Application of latent class analysis. Journal of the American Geriatrics Society, 64, 1668–1673. doi:10.1111/jgs.14201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickrama K., Lee T., & O’Neal C. W (2016). Higher-order growth curves and mixture modeling with mplus: A practical guide. New York, NY: Routledge. [Google Scholar]
- Woolf S. H., & Braveman P (2011). Where health disparities begin: The role of social and economic determinants–and why current policies may make matters worse. Health Affairs (Project Hope), 30, 1852–1859. doi:10.1377/hlthaff.2011.0685 [DOI] [PubMed] [Google Scholar]
- Xu X., Liang J., Bennett J. M., Botoseneanu A., & Allore H. G (2015). Socioeconomic stratification and multidimensional health trajectories: Evidence of convergence in later old age. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 70, 661–671. doi:10.1093/geronb/gbu095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young Y., Frick K. D., & Phelan E. A (2009). Can successful aging and chronic illness coexist in the same individual? A multidimensional concept of successful aging. American Medical Directors Association, 10, 87–92. doi:10.1016/j.jamda.2008.11.003 [DOI] [PubMed] [Google Scholar]
- Zajacova A., & Ailshire J (2014). Body mass trajectories and mortality among older adults: A joint growth mixture–discrete-time survival analysis. The Gerontologist, 54, 221–231. doi:10.1093/geront/gns164 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.