Abstract
Background
Individuals with Down syndrome (DS) are at high risk for autism spectrum disorder (ASD) with ~20% of individuals meeting diagnostic criteria for ASD. Despite the high risk, there is no research documenting early signs of ASD in infants with DS or potential prodromal ASD-associated behaviors.
Aim
This preliminary case-control study described ASD-associated behaviors in infants with DS contrasted to typically developing (TD) infants.
Patients and Methods
The Autism Observation Scale for Infants (AOSI) was used to describe ASD-related behaviors in 18 infants with DS (7–18 months) and 18 TD infants (9–14 months).
Results
Thirty nine percent (7 out of 18) of infants with DS in our sample were designated “at risk” for ASD on the AOSI with 100% of infants with DS demonstrating at least one feature of ASD. In contrast, only 11% (2 out of 18) of TD infants were designated “at risk” for ASD on the AOSI. Social and communication impairments appear to represent early signs of elevated ASD-associated behavior in infants with DS.
Conclusions
Early signs of ASD-associated behavior appear present and detectable in infants with DS. These early signs mirror findings of other populations at risk for ASD with social communication as the primary behavioral impairment to signal elevated risk for the emergence of ASD. This study contributes to the refinement of the DS behavioral phenotype and identifies important next steps to help improve the identification, diagnosis, and treatment of ASD in DS.
Keywords: Down syndrome, Autism Spectrum Disorder, Infants
Background
Autism spectrum disorder (ASD) is a common and lifelong disorder affecting 1:59 in the general population [1]. Early treatment of ASD leads to improved outcomes [2, 3]; thus, early detection is critical. Examining ASD in neurogenetic syndromes at high risk for ASD, such as Down syndrome (DS), helps establish the shared phenomenology between disorders [4] and advance our understanding of the specific needs of children with neurogenetic syndromes.
DS is caused by the presence of an overexpression of genes on chromosome 21 and is the most common genetic (chromosomal) abnormality associated with intellectual disability (affecting 1 in 691 individuals [5]). While not extensively researched, existing work suggests that ~20% (7–42%) of children with DS meet diagnostic criteria for ASD [6–8]. As such, ASD is highly expressed in DS at a rate much higher than the general population. The limited research is likely due to diagnostic challenges given the presence of intellectual disability because clinicians need to differentiate ASD-like behaviors from similar behaviors associated with intellectual disability [9, 10] and a historical bias that the “social strengths” in DS represent a protective factor against ASD [11]. Also, many children with DS may not be eligible for ASD diagnostic tools depending on the level of their motor skills (e.g., the toddler module of the Autism Diagnostic Observation Schedule-2 requires a minimum mental age of 12 months and that the child is walking or “cruising” [12]). These challenges have led to reluctance in making a diagnosis of ASD in DS resulting in delays or misdiagnoses [10, 11, 13]. Whereas, the average age of diagnosis in non-syndromic ASD (nsASD) is 3–4 years [1].
Research identifying early signs of nsASD has primarily focused on developmental trajectories of infants with older siblings with nsASD suggesting that early behavioral signs include impaired visual attention [14], delays in language and gesture [15], and motor difficulties [16]. To date, no research has described signs of ASD in children with DS younger than 2 years-of-age with only a handful of studies conducted at preschool-age and older [17]. In a longitudinal study with 20 2-to-3-year-olds with DS, 15% (3 children) met ASD diagnostic criteria (mean age 34 months [17]). Diagnostic stability was high over time, and all three children with DS+ASD demonstrated impairments in communication and social skills with more severe difficulties in communication than social skills and had greater developmental delays than those with DS without ASD. These data provide initial evidence that ASD symptoms emerge within the second year of life and remain stable in DS.
Despite the high prevalence of ASD in DS and the importance of early identification, no research has examined behavioral signs of ASD in infants with DS.
Aim
This preliminary case-control study aims to describe ASD-associated behaviors in infants with DS contrasted to typically developing (TD) infants. This is accomplished by reporting those who score above risk cut-offs on the Autism Observation Scale for Infants [18, 19], identifying potential ASD risk markers, and describing the relationship of ASD-associated behavior to chronological age and developmental level.
Patients and Methods
Participants
Participants were 18 infants with DS (14 males, 4 females) between 7–18 months and 18 TD infants (14 males, 4 females) between 9–14 months (see Table 1). TD infants were included to represent typical variation in development (i.e., the control group). Infants with DS were from three pilot studies examining the infant phenotype in neurogenetic syndromes from the University of South Carolina (USC; n = 11), the University of Illinois (UIUC; n = 3), and Purdue University (PU; n = 4). Across all three sites, infants with DS were recruited through flyers shared with local parent groups, DS clinics, and ongoing research studies in Georgia, Illinois, Indiana, Missouri, and South Carolina. TD infants were recruited as part of a study on the emergence of ASD in fragile X syndrome at USC. As part of this larger study, TD infants were followed until they were 3 years of age and were determined not to have ASD or another developmental disability. TD infants were matched to infants with DS on sex at an individual level and age at a group level, no significant differences between groups (see Table 2 for matching statistics).
Table 1.
Participant Characteristics
| Characteristic | Down syndrome | Typically developing | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| M/% | SD | Range | M/% | SD | Range | |
| Child | ||||||
| Chronological Age (in months) | 11.67 | 3.29 | 7–18 | 11.54 | 1.71 | 9–14 |
| AOSI Total Score | 9.28 | 4.91 | 3–20 | 4.72 | 3.41 | 1–15 |
| AOSI Markers Score | 5.89 | 2.49 | 3–10 | 3.33 | 1.97 | 1–8 |
| Early Learning Composite | 68.31 | 12.40 | 49–93 | 96.44 | 13.01 | 71–115 |
| Overall Mental Age (in months) | 8.03 | 2.41 | 4.25–13.00 | 11.79 | 3.39 | 7–21.75 |
| Nonverbal Abilities Composite | 32.25 | 9.45 | 20–48.50 | 51.22 | 10.02 | 32.50–65.50 |
| Verbal Abilities Composite | 33.09 | 7.24 | 20.50–46.50 | 44.92 | 6.51 | 36–57.50 |
| Expressive Language T-Score | 34.81 | 8.07 | 20–45 | 47.22 | 9.88 | 29–66 |
| Receptive Language T-Score | 31.38 | 8.28 | 20–49 | 42.61 | 7.37 | 34–58 |
| Child Race/Ethnicity (%) | ||||||
| White | 55.5 | 77.8 | ||||
| African American | 5.6 | 16.7 | ||||
| Hispanic | 5.6 | - | ||||
| More than One Race/Ethnicity | 5.6 | 5.6 | ||||
| Unknown/Choose Not to Respond | 27.8 | - | ||||
Table 2.
Independent Samples t-test Comparing Age and AOSI Markers Score between Groups
| Down Syndrome | Typically Developing | t(34) | p | Cohen’s d | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Age | 11.67 | 3.29 | 11.54 | 1.71 | −0.51 | 0.88 | 0.05 |
| AOSI Markers Score | 5.89 | 2.49 | 3.33 | 1.97 | −3.41 | 0.002 | 1.14 |
Tools
Autism Observation Scale for Infants (AOSI)
The AOSI is a semi-structured, play-based observation to identify early signs of ASD from 6–18 months [18, 19]. The infant’s behavior is ranked on 19 items as either “typical” (score 0), “inconsistent, partial or questionable” (score 1), or “atypical” (score 2–3). ASD risk is defined as the Number of Markers Score (tally of the number of items scored ≥1), with a score of >7 indicating ASD risk [20]. Test-retest reliability at 12 months is .61 for the Total Score [19]. The AOSI has been used with other neurogenetic syndromes, but not with DS [21, 22]. All examiners were research reliable on the AOSI.
Mullen Scales of Early Learning (MSEL)
The MSEL is a standardized assessment of development from birth to 68 months [23]. We used the Early Learning Composite and calculated a nonverbal (visual reception + fine motor), and verbal (expressive language + receptive language) composite using the average T-scores, which account for age effects. Several infants with DS scored at the floor on the different domains of the MSEL (expressive language = 1, receptive language = 2, fine motor = 4, visual reception = 3). No TD infants in our sample scored at the floor. The MSEL has well-established validity and reliability [23].
Ethical Considerations
All study procedures were approved by the Institutional Review Board (IRB) for each site (UIUC, USC, PU). In addition, each IRB had approved a data sharing plan, which allowed for data from USC and PU to be shared with the lead author (Hahn) at UIUC. Participant’s mothers consented for their child to participate. Family-friendly procedures were used to reduce stress and promote a positive experience (e.g., taking frequent breaks, scheduling visits at a time that is best for the family, asking mothers for input, etc.).
Procedure
Informed consent was provided by the parents of all infants. As part of a larger assessment battery, the MSEL and AOSI were administered to all infants at either their home (UIUC, USC) or the lab (USC, PU). Two infants with DS were not administered the MSEL because the Bayley Scales of Infant Development [24] was used instead.
Analytical Plan
Given the descriptive nature of this study, results include descriptive results and a report of the proportion of infants with DS who met criteria for ASD-risk using the AOSI Number of Markers Score. The proportion of individual AOSI items scored for infants with DS is reported to reflect potential discreet ASD-associated behaviors that might signal risk. In addition, an independent samples t-test was conducted to examine differences between groups on the AOSI Number of Markers Score. Pearson correlations were conducted to assess the relationship between the AOSI Number of Markers Score to age and developmental variables for infants with DS and TD infants. A partial correlation was conducted as a follow up analysis to examine whether the effect between the AOSI Number of Markers Score and expressive language remained when covarying age and Early Learning Composite. For all inferential statistics, our p-value was set at .05.
Results and Discussion
Results
ASD Risk
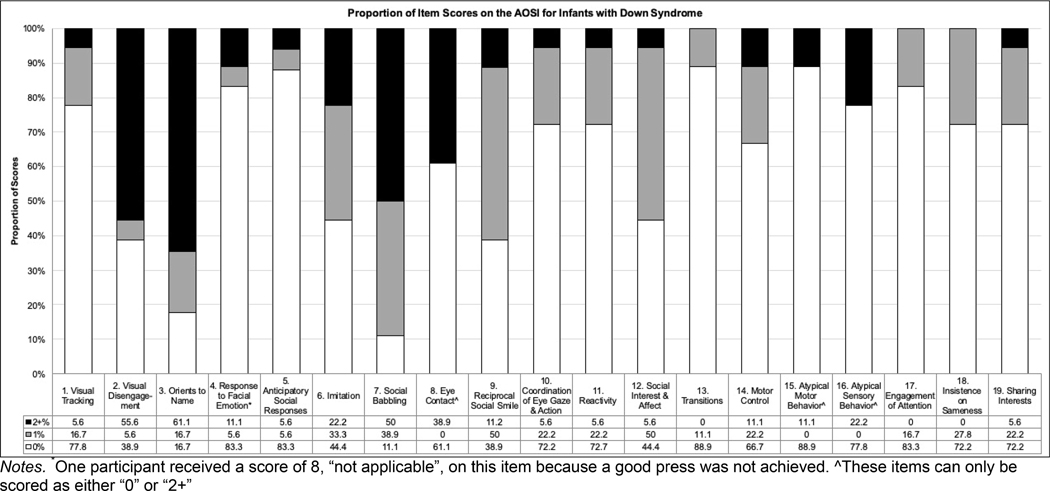
Of the 18 infants with DS, 7 (39%) met criteria for ASD-risk. Of note, 2 infants with DS had a total of 6 markers indicating a sub-threshold score, while no infants with DS had an AOSI score of 0 (see Table 1). For each site, a range of AOSI scores were observed (USC = 3–17, UIUC = 9–13, PU = 3–20); no significant differences between sites on AOSI scores existed, F[2,15] = .29, p = .75). The proportion of AOSI item scores for infants with DS (i.e., 0, 1, 2+) are displayed in Figure 1.
In contrast, only 2 TD infants (11%) met criteria for ASD-risk. As a follow-up analysis, we compared AOSI scores between infants with DS and TD. Results indicated that infants with DS had significantly higher AOSI scores than TD infants (see Table 2).
Relationship Between AOSI Number of Marker Scores and Developmental Variables
For infants with DS, a large and statistically significant effect was found between the AOSI and expressive language (See Table 3 for correlation matrix). While not significant, a small to medium effect was observed between the AOSI and age, overall verbal abilities, overall nonverbal abilities, Early Learning Composite, and receptive language for infants with DS. For both the verbal abilities composite and the Early Learning Composite, these relationships may be driven by the relationship between the AOSI and expressive language. To explore this possibility, we conducted a follow-up analysis to investigate if the relationship between the AOSI and expressive language was still observed when covarying age and Early Learning Composite. Results indicated that the large, and statistically significant, effect between the AOSI and expressive language remained when covarying age and Early Learning Composite (rp = −.62, p = .02).
Table 3.
Correlations between AOSI Number of Marker Scores and Developmental Variables for the DS group
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. AOSI Markers Score | - | −0.08 | −0.28 | −0.12 | −0.42 | −0.59* | −0.17 |
| 2. Age | - | - | −0.53* | −0.40 | −0.59* | −0.33 | −0.71** |
| 3. Early Learning Composite | - | - | - | 0.92*** | 0.85*** | 0.68** | 0.83*** |
| 4. Nonverbal Abilities Composite | - | - | - | - | 0.58* | 0.40 | 0.63** |
| 5. Verbal Abilities Composite | - | - | - | - | - | 0.88*** | 0.89*** |
| 6. Expressive Language T-Score | - | - | - | - | - | - | 0.57* |
| 7. Receptive Language T-Score | - | - | - | - | - | - | - |
p < 0.10
p < 0.05
p < 0.01
p < 0.001
For TD infants, no statistically significant relationships were evident between the AOSI and developmental variables (See Table 4 for correlation matrix); however, several small to medium effects were observed for age, verbal abilities, expressive language, and receptive language. For the Early Learning Composite and nonverbal abilities, the correlations with the AOSI were very small.
Table 4.
Correlations between AOSI Number of Marker Scores and Developmental Variables for the TD group
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. AOSI Markers Score | - | −0.23 | −0.06 | 0.02 | −0.13 | −0.35 | 0.24 |
| 2. Age | - | - | −0.08 | −0.08 | 0.27 | 0.09 | 0.35 |
| 3. Early Learning Composite | - | - | - | 0.88** | 0.68** | 0.56* | 0.45^ |
| 4. Nonverbal Abilities Composite | - | - | - | - | 0.25 | 0.20 | 0.18 |
| 5. Verbal Abilities Composite | - | - | - | - | - | 0.83*** | 0.66** |
| 6. Expressive Language T-Score | - | - | - | - | - | - | 0.12 |
| 7. Receptive Language T-Score | - | - | - | - | - | - | - |
p < 0.10
p < 0.05
p < 0.01
p < 0.001
Discussion
Despite being at an increased risk for ASD, no reports of early signs or prodromal features of ASD in infants with DS exist. To address this gap, we present descriptive findings from our preliminary case-control pilot study on ASD-associated behaviors in infants with DS. Our results indicate that whereas only 2 of our TD infants (11%) were designated as “at risk,” 39% of our DS sample (7 of 20 infants) was designated “at risk” for ASD. This is somewhat higher than the consensus of ~20% but falls within the range of existing prevalence studies in DS (7–42% [5–7]). The somewhat higher incidence of DS infants “at risk” for ASD likely reflects that the AOSI is a screener, and elevated false-positive rates are expected19. It is also possible that children with neurogenetic syndromes, such as DS, are “over-identified”, and, a higher “risk” cut-off score may provide better sensitivity and specificity for later ASD outcomes, as has been suggested for other neurogenetic samples [21, 22]. Answers to these questions require empirical study with longitudinal studies that track the trajectories of infant development through to diagnoses, which is beyond the scope of this study.
None of our sample with DS received an AOSI Markers or Total score of 0, indicating that all infants demonstrated either inconsistent/questionable or atypical behavior on one or more AOSI items. While these items are designed to detect ASD risk, they may also be sensitive to areas of difficulty associated with the broader DS phenotype. The two items scored with the highest rating (item score of 2+) for infants with DS were Visual Disengagement (55%) and Orients to Name (61%; see Figure 1). Difficulties with visual attention have been noted in DS starting in infancy [25]. Also, the ability to orient to name involves disengaging from the current attentional focus and shifting attention to the person speaking to indicate a response. If infants with DS are having difficulty with visual disengagement, they may be less likely to shift their attention when their name is called. Thus, visual disengagement may not be an early indicator of ASD, but instead, capture early impairments associated with the DS behavioral phenotype. This highlights the difficulty of identifying ASD in infants as some early behavioral signs are present in those who are not later diagnosed with ASD, and simply represent variability in early development, or who are at risk for other neurodevelopmental disorders (i.e., developmental delay, language disorders, etc. [26, 27]).
The majority of infants with DS received atypical scores (item score of 2+) in key areas of social functioning—Orients to Name (61%), Social Babbling (50%), Eye Contact (39%), Imitation (22%)—despite social function being an area of noted strength in infants and young children [28]. In preschoolers with DS, elevated severity of social and communication impairments was associated with an ASD diagnosis [17]. However, there are also social and communication AOSI items that many infants with DS received typical behavior scores on (item scores of 0; see Figure 1), such as Response to Facial Emotion (83%), Coordination of Eye Gaze & Action (72%), Engagement of Attention (83%), and Sharing Interest (72%). Within the context of our preliminary findings, it seems that infants with DS are showing subtle impairments in select social and communication skills. These subtle impairments may represent a typical pattern for infants with DS depending on their level of developmental delay or they may be an early indicator of ASD in DS, similar to other populations at risk for ASD [17, 29]. More research is needed to explore the potentially nuanced social and communication profiles of those with DS and ASD.
Due to our small sample size, correlations between ASD risk and most of the developmental variables did not reach statistical significance, but the strength of these associations range from small to medium in infants with DS. A significant and large effect was observed indicating that those with higher AOSI Marker Scores had lower expressive language abilities. This pattern is similar to previous studies suggesting that higher ASD risk is related to lower verbal abilities in DS [17, 26, 29], and this pattern was also observed in our sample of TD infants.
Limitations of the Study
While the AOSI is designed to detect early signs of ASD in infants, this is the first study to use this measure with infants with DS; therefore, the validity of the AOSI indicators for detecting ASD in DS is not yet clear. However, our results provide initial evidence that ASD-associated behaviors can be identified in DS using the AOSI. Our sample was primarily male, and we were not able to examine sex effects. Future research is needed to examine sex differences in ASD-associated behavior in DS. Further examination of the developmental impact of ASD on DS is important because those with DS and ASD may require different approaches and unique supports than those with DS only.
Conclusion
In summary, 39% of infants with DS displayed a high degree of ASD-associated behaviors with 100% displaying at least one feature of ASD-associated behavior. The diagnostic relevance of these early features of ASD in predicting either intellectual disability, ASD, or both is unclear, and further research is needed to examine these important developmental trajectories. While we are the first to describe ASD-associated behavior in infants with DS using the AOSI, the small size of our sample and our lack of data on outcomes are limitations. The results of this study contribute to the refinement of the DS behavioral phenotype and identify important next steps to help improve the identification, diagnosis, and treatment of ASD in DS.
Acknowledgments
This research was supported by grants from the National Institute of Mental Health (R01 MH090194 [Roberts]; L40 MH108014 [Hahn], K23MH111955 [Kelleher]), the ASPIRE grant from the Office of the Vice President for Research at the University of South Carolina (Hahn), and the Campus Research Board grant from the Office of the Vice Chancellor for Research at the University of Illinois Urbana-Champaign (Hahn). This manuscript was presented as a poster at the 50th Annual Gatlinburg Conference on Research and Theory in Intellectual and Developmental Disabilities, San Antonio, Texas. We would like to thank Jessica Scherr for her assistance in data collection.
Abbreviations
- DS
Down syndrome
- ASD
autism spectrum disorder
- TD
typically developing
- nsASD
non-syndromic ASD
- AOSI
Autism Observation Scale for Infants
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ. 2018;67(6):1–23. doi: 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landa RJ, Kalb LG. Long-term outcomes of toddlers with autism spectrum disorders exposed to short-term intervention. Pediatrics. 2012;130(Supplement 2):S186–S190. doi: 10.1542/peds.2012-0900q [DOI] [PubMed] [Google Scholar]
- 3.Wetherby AM, Guthrie W, Woods J, et al. Parent-implemented social intervention for toddlers with autism: An RCT. Pediatrics. 2014;134(6):1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glennon JM, Karmiloff-Smith A, Thomas MSC. Syndromic autism: Progressing beyond current levels of description. Rev J Autism Dev Disord. 2017;4(4):321–327. doi: 10.1007/s40489-017-0116-2 [DOI] [Google Scholar]
- 5.Parker SE, Mai CT, Canfield MA, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88(12):1008–1016. doi: 10.1002/bdra.20735 [DOI] [PubMed] [Google Scholar]
- 6.Diguiseppi C, Hepburn SL, Davis JM, et al. Screening for autism spectrum disorders in children with Down syndrome. Jounral Dev Behav Pediatr. 2010;31(3):181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowenthal R, Paula CS, Schwartzman JS, Brunoni D, Mercadante MT. Prevalence of pervasive developmental disorder in Down’s syndrome. J Autism Dev Disord. 2007;37(7):1394–1395. doi: 10.1007/s10803-007-0374-4 [DOI] [PubMed] [Google Scholar]
- 8.Oxelgren UW, Myrelid Å, Annerén G, et al. Prevalence of autism and attention-deficit–hyperactivity disorder in Down syndrome: A population-based study. Dev Med Child Neurol. 2017;59(3):276–283. doi: 10.1111/dmcn.13217 [DOI] [PubMed] [Google Scholar]
- 9.Capone GT, Grados M a, Kaufmann WE, Bernad-Ripoll S, Jewell A. Down syndrome and comorbid autism-spectrum disorder: Characterization using the aberrant behavior checklist. Am J Med Genet A. 2005;134(4):373–380. doi: 10.1002/ajmg.a.30622 [DOI] [PubMed] [Google Scholar]
- 10.Howlin P, Wing L, Gould J. The recognition of autism in children with Down syndrome-Implications for intervention and some spectulations about pathology. Dev Med Child Neurol. 1995;37:398–414. [DOI] [PubMed] [Google Scholar]
- 11.Reilly C Autism spectrum disorders in Down syndrome: A review. Res Autism Spectr Disord. 2009;3(4):829–839. doi: 10.1016/j.rasd.2009.01.012 [DOI] [Google Scholar]
- 12.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, 2nd Edition (ADOS-2) Manual (Part 1): Modules 1–4. Los Angeles, CA: Western Psychological Services; 2012. [Google Scholar]
- 13.Ji NY, Capone GT, Kaufmann WE, Kaufmann WE, Krieger K. Autism spectrum disorder in Down syndrome: Cluster analysis of Aberrant Behaviour Checklist data supports diagnosisj. J Intellect Disabil Res. 2011;55(11):1064–1077. doi: 10.1111/j.1365-2788.2011.01465.x [DOI] [PubMed] [Google Scholar]
- 14.Sacrey LR, Armstrong VL, Bryson SE, Zwaigenbaum L. Impairments to visual disengagement in autism spectrum disorder: A review of experimental studies from infancy to adulthood. Neurosci Biobehav Rev. 2014;47:559–577. doi: 10.1016/j.neubiorev.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 15.Zwaigenbaum L, Bryson S, Garon N. Early identification of autism spectrum disorders. Behav Brain Res. 2013;251:133–146. doi: 10.1016/j.bbr.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 16.Flanagan JE, Landa R, Bhat A, Bauman M. Head lag in infants at risk for autism: A preliminary study. Am J Occup Ther. 2012;66(5):577–585. doi: 10.5014/ajot.2012.004192 [DOI] [PubMed] [Google Scholar]
- 17.Hepburn SL, Philofsky A, Fidler DJ, Rogers S. Autism symptoms in toddlers with Down syndrome : A descriptive study. J Appl Res Intellect Disabil. 2008;21:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryson SE, Zwaigenbaum L. Autism Observation Scale for Infants. In: Patel VB, Preedy VR, Martin CR, eds. Comprehensive Guide to Autism. New York, NY; 2014:299–310. doi: 10.1007/978-1-4614-4788-7 [DOI] [Google Scholar]
- 19.Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. The Autism Observation Scale for Infants: Scale development and reliability data. J Autism Dev Disord. 2008;38(4):731–738. doi: 10.1007/s10803-007-0440-y [DOI] [PubMed] [Google Scholar]
- 20.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 21.Capal JK, Horn PS, Murray DS, et al. Utility of the Autism Observation Scale for infants in early identification of autism in tuberous sclerosis complex. Pediatr Neurol. 2017;75:80–86. doi: 10.1016/j.pediatrneurol.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts JE, Tonnsen BL, McCary LM, Caravella K, Shinkareva S V. Autism symptoms in infants with fragile X syndrome. J Autism Dev Disord. 2016:1–8. doi: 10.1007/s10803-016-2903-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullen E Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 24.Bayley N Bayley Scales of Infant Development (3rd Edition). San Antonio, TX: Harcourt Assessment; 2005. [Google Scholar]
- 25.Breckenridge K, Braddick O, Anker S, Woodhouse M, Atkinson J. Attention in Williams syndrome and Down’s syndrome: Performance on the new early childhood attention battery. Br J Dev Psychol. 2013;31(2):257–269. doi: 10.1111/bjdp.12003 [DOI] [PubMed] [Google Scholar]
- 26.Landa RJ, Gross AL, Stuart EA, Bauman M. Latent class analysis of early developmental trajectory in baby siblings of children with autism. J Child Psychol Psychiatry. 2012;53(9):986–996. doi: 10.1111/j.1469-7610.2012.02558.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landa RJ, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x [DOI] [PubMed] [Google Scholar]
- 28.Fidler DJ, Most DE, Booth-laforce C, Kelly JF. Emerging social strengths in young children with Down syndrome. Infants Young Child. 2008;21(3):207–220. doi: 10.1097/01.IYC.0000324550.39446.1f [DOI] [Google Scholar]
- 29.Molloy CA, Murray DS, Kinsman A, et al. Differences in the clinical presentation of Trisomy 21 with and without autism. J Intellect Disabil Res. 2009;53(2):143–151. doi: 10.1111/j.1365-2788.2008.01138.x [DOI] [PubMed] [Google Scholar]