Abstract
Objectives
Prenatal detection of congenital heart disease (CHD) with obstetrical screening remains <50% in most population studies, far from is thought to be achievable. We sought to identify barriers/facilitators to screening from the perspective of interpreting physicians and to understand how these barriers/facilitators may be associated with interpretation of screening images.
Methods
Our mixed-methods studies included 4 focus groups in centers across the U.S. with obstetric, maternal-fetal medicine, and radiology providers who interpret obstetric ultrasound. Themes around barriers/facilitators to fetal heart screening were coded from transcripts. A national web-based survey was then conducted which 1) quantitatively measured reported barriers/facilitators and 2) measured physician ability to interpret fetal heart screening images. Multivariable generalized linear random effect models assessed the association between barriers/facilitators and accuracy of image interpretation at the image level.
Results
Three main themes were identified in focus groups: intrinsic barriers (i.e. comfort with screening), external barriers (i.e. lack of feedback), and organizational barriers (i.e. study volumes). Among 180 physician respondents, 104 interpreted ultrasounds. Perception of barriers varied by practice setting with non-tertiary providers having lower self-efficacy and perceived usefulness around cardiac screening. Facilitators associated with odds of accurate interpretation of screening images were knowledge (OR 2.54, p=0.002) and volume of scans per week (OR 1.01 for every additional scan, p=0.04).
Conclusion
Some of the main barriers to cardiac screening identified and prioritized by physicians across the United States were knowledge around screening and minimal volumes of scans. Targeting these barriers will aid in improving prenatal detection of CHD.
Keywords: congenital heart defect, screening, prenatal diagnosis, ultrasound, survey, barriers
Introduction
Since >80% of congenital heart disease (CHD) occurs in mothers who have no identifiable risk factors for delivering an affected child, prenatal detection is challenging and relies on population-level screening.1 The sensitivity of obstetric ultrasound can be as high as 85% in efficacy studies when a four chamber (4C) and outflow tract views are included in routine sonographic screening. However, though >90% of pregnant women in developed countries have an obstetric ultrasound, population-based studies show that only 30–50% of CHD cases are detected in actual day-to-day clinical practice settings.2–4
Regional and institutional variation in the procedure for and interpretation of obstetrical ultrasound screening for CHD may be a critical determinant of lower detection rates. For example, higher sensitivity of obstetric ultrasound screening has been achieved when both 4C and outflow tract views are obtained, although both views may not be effectively performed in all settings, largely because outflow views are more challenging.5 Interventions aimed at improving prenatal CHD detection have not had widespread impact on detection rates. Since most interventions were studied at tertiary or academic centers 6, where detection rates are already higher 3,4,7, the impact on detection may be improved by looking at barriers/ facilitators in community settings where >75% of prenatal screening for CHD actually occurs.3,4
The factors that influence health professional practice and behaviors are complex.8,9 Because of this, most experts agree that multifaceted interventions targeting identified barriers/facilitators are more likely to be effective.10 A well–established framework for understanding physician behaviors is that knowledge influences attitudes which then influences behavior.11 Several studies have identified core beliefs, perceived usefulness, organizational barriers, self-efficacy, and inertia of current practice as important for understanding physician implementation of guidelines and use of screening tests in all areas of medicine.8,12,13
We sought to 1) explicitly engage physicians who interpret fetal cardiac screening images in identifying determinants of successful CHD detection, 2) explore how these determinants vary among practice settings so future interventions can be effectively targeted, and 3) engage physicians in prioritizing potential targets for modifying knowledge, skills, practice standards, organizational structures, and policy.
Materials and Methods
This mixed-methods study employed focus groups to inform the development and implementation of a quantitative cross-sectional national survey. In the first part of the study we conducted focus groups with local providers who interpret obstetric ultrasounds at four geographic sites across the United States. For the second part of the study we conducted a national survey targeting the same type of providers. Institutional Review Board approval was obtained at all participating institutions for the focus group part of the study and at the University of Utah for the survey study.
Focus Groups
Focus Group Study Population and Recruitment
Focus groups were conducted at the University of Utah, Stanford University, Northwestern University and Duke University from January to May of 2014. These four sites were selected to obtain representation from across the country and because they offered collaboration with established investigators from both the Pediatric Heart Network and the Maternal Fetal Medicine Units Network. The target population for the focus groups were obstetricians [including maternal fetal medicine (MFM) specialists] and radiologists who interpret second trimester obstetric ultrasound (fetal anomaly scans). We mailed letters to potential participants using addresses obtained from the National Provider Identifier (NPI) file. We also sent out fliers through American College of Obstetrics and Gynecology state chapters to member and local institutional email listservs to recruit participants from each state.
Focus Group Methods
Using recommended techniques,14 focus groups were designed and conducted using a scripted moderator guide and face-to-face meetings while also allowing for participation by teleconference. Participants were asked open-ended questions regarding the importance of cardiac screening, their perception of its difficulty, barriers/facilitators to screening, and suggestions for improvement. Participants were then asked the relevance of candidate factors (compiled from our sonographer study15 and a literature review of key determinants of physician practice 8,9,11,16,17) that might influence cardiac screening. They were also encouraged to contribute factors not previously identified. Participants reviewed the draft survey instrument and provided suggestions for refinement. All sessions were audio recorded and transcribed. Observational notes captured non-verbal nuances. Informed consent was obtained from all participants.
Focus Group Analysis
Transcripts were analyzed using a five-stage method: 1) familiarization with data, 2) identifying thematic framework, 3) indexing data, 4) charting, and 5) mapping and interpretation.18 Transcripts were first reviewed to determine themes and investigators met to discuss face validity and reach consensus regarding the thematic designation. The content of the audio files was coded using TAMS Analyzer software (Version 4.14b1h).
National Survey
The second part of this study consisted of a cross-sectional national survey of providers involved in ultrasound interpretation for pregnant women. The web-based survey was designed and refined using input from the focus groups discussed above.
Survey Study Population and Recruitment
The target population for the survey was primarily physician providers who interpret general obstetrical ultrasound studies. The comprehensive NPI file for taxonomy codes specific for Obstetrics (207V00000X) and Diagnostic Ultrasound in Radiology (2085U001X) was used as the sampling frame. Estimates of obstetricians and radiologists practicing in different regions of the United States were determined using the Area Resource File of the Health Resources and Services Administration19 and the 2010 geocoded NPI file from the North American Association of Central Cancer Registries.20 We employed probability sampling stratified by 1) disproportionate sampling by practitioner (2:1 obstetrician to radiologist, since 10–45% of obstetric ultrasounds are interpreted by radiologists),3,4 2) proportionate sampling by census region, and 3) disproportionate sampling of 1:1 rural and urban addresses utilizing Rural Urban Commuting Area (RUCA) codes21,22 to create a sample of 2000 physician mailing addresses. These addresses were then matched to the American Medical Association resource email file to create a final list of 1300 physicians that could be reached by mail and email. To enhance response rates, we included a $5 pre-incentive gift card mailed with the invitation letter, since advance participant support of even small monetary amounts has proven to be more successful than larger sums promised upon survey completion with professional groups such as physicians.23,24 To further increase participation, we used a multi-phase mixed mode recruitment strategy including a flyer, pre-notification letter, letter with a survey link, and two additional electronic reminders.25
Survey Methods
The draft survey was reviewed by survey experts from the University of Utah Center for Clinical and Translational Science’s Biostatistics and Study Design Survey Core for instrument design, question format, question order and wording. It was programmed online using Checkbox v 6.0 and pretested on physicians who had participated in the focus groups. Refinements were made before was the survey was launched.
Initial survey questions determined if physicians qualified (provided prenatal care and/or interpreted 2nd trimester obstetric ultrasound in the last six months). The web survey had two main parts. Part 1 assessed barriers and facilitators to prenatal cardiac screening using validated scales obtained from the literature on common behavioral domains used to understand physician behavior and their adoption of evidence-based recommendations 8–11 (Table 1). The main behavioral determinants assessed included 1) knowledge, 2) perceived usefulness of fetal ultrasound cardiac screening, 3) physician self-efficacy or confidence in his/her ability to interpret fetal cardiac images, 4) perceived ease of use or thoughts on ease of interpreting cardiac screening, 5) professional expectations or beliefs about whether cardiac screening is expected by peers, supervising physicians or guidelines, 6) feedback which measured importance of some assessment of cardiac screening performance and how much feedback was received, and 7) organizational barriers/facilitating conditions of the infrastructure support to perform fetal cardiac screening. Respondents were also asked to assess their own ability to interpret cardiac screening images and estimate the time to perform interpretation.
Table 1.
Selected Survey Measures
| Definition | Scale/Item | Cronbach’s Alpha | |
|---|---|---|---|
| Self-Assessment | |||
| Time | Average time to interpret fetal heart images | 1-item | NA |
| Ability- 4-chamber | Ability to interpret four chamber views | 1-item | NA |
| Ability - outflow | Ability to interpret outflow tract views | 1-item | NA |
| Determinants of Practice | |||
| Knowledge | Familiarity with screening guidelines, efficacy of fetal heart screening and current detection rates | 5-item | TBD |
| Perceived Usefulness | Usefulness of obstetric cardiac screening | 2-item | 0.66 |
| Self-efficacy | Belief about own ability to interpret screening images | 6-item | 0.82 |
| Social/Professional Expectancies | Belief about whether screening is expected by peers, superiors or professional guidelines | 3-item | 0.82 |
| Perceived Ease of Use | Degree to which screening is free of effort | 4-item | 0.89 |
| Organizational Barriers | Infrastructure to allow for screening | 3-item | 0.70 |
Part 2 of the survey provided 20 video clips of typical fetal heart screening views. The first set asked physicians to interpret the technical adequacy of 4C fetal heart images provided for screening purposes. The second set of images asked them to interpret the 4C view images provided as either “normal” or “abnormal”. The remaining two sets repeated the same for outflow tract images. To attempt to replicate real-life situations, images included those affected by maternal obesity, suboptimal fetal position, and early versus late gestational ages. Images were independently reviewed by two fetal cardiologists and a fetal cardiac sonographer and only those with 100% inter-rater reliability were included. To decrease the impact of the image itself on physician performance, there were 3 sets of 20 images and the image set (test number) was randomly selected for each participant.
Survey analysis
We reported descriptive statistics as mean ± standard deviation for continuous variables or as frequency (percentage) for categorical variables. We compared characteristics and outcomes between tertiary/academic or high-risk clinics versus low-risk or community clinics by using a t-test and chi-squared test as appropriate. Image items were classified as correct or incorrect and a total score was calculated for all images. Raw and mean scores with standard deviations were assessed for construct scales used to measure the factors identified as perceived barriers/facilitators. In addition to the validated scales, practitioner type (radiologist, obstetrician or MFM), age, experience, and gender were assessed. We analyzed the association of correctness and potential barriers/facilitators in univariate and multivariable analysis using general and generalized linear mixed effects models. The mixed effect model allowed identification of factors that influence imaging ability in a manner similar to logistic regression, but had the added advantage of allowing us to consider correlation of responses by physician (that each physician will perform similarly on questions). Variables were included in initial models if p <0.1 in univariate models and their Pearson correlation coefficients with other variables was <0.7. Final multivariable model covariates were selected using a backward stepwise technique.
We repeated subgroup analyses for physicians who practice in tertiary/academic or high-risk clinics and physicians who practice in low-risk or community clinics using the analytic strategy described above. These subgroup analyses allowed us to assess differences in the manner in which candidate factors were associated with physician screening abilities. We tested for interaction between subgroups (academic versus community practice subgroup) and the association of potential barriers with accuracy of image interpretation. All statistical tests were two-sided with a significance level of 0.05, and all analyses were conducted using Stata/MP 15 (College Station, TX).
RESULTS
Focus Groups
Four focus groups (n=43 participants) were held across the U.S. in Salt Lake City, UT (1/2014 with three MFMs, two obstetricians and one radiologist), Durham, NC (2/2014 with six MFMs, two obstetricians and one radiologist), Palo Alto, CA (3/2014 with five MFMs and one obstetrician) and Chicago, IL (5/2014 with four MFMs and six obstetricians). Three main themes around barriers/facilitators to ultrasound screening for CHD emerged: 1) individual level barriers including comfort/confidence with cardiac imaging, training, and experience, 2) external barriers such as lack of feedback and incongruent patient expectations of anomaly scans; and 3) system-level barriers including varying volume, access to expertise, and the lack of formal standards/quality control. Exemplar quotes for themes are shown in Table 2. Provider knowledge about current detection rates of CHD, attitudes regarding the usefulness of prenatal detection, and current screening protocols (i.e. recommended screening images) varied among participants. Additional common threads in the discussions were 1) variation in these barriers by practice setting and 2) tension between increased expertise/volume versus conserving resources and ensuring access. When coding by practitioner type, MFMs and obstetricians more frequently discussed system-level and individual level barriers such as ability and training (Figure 1). Among the few participating radiologists, more discussion focused on the usefulness of ultrasound prenatal screening for heart defects.
Table 2.
Themes/Sub-themes and Representative Quotes from Focus Group Participants
| Theme | Exemplar Quotes |
|---|---|
| Intrinsic Barriers | |
| Ability/Self-Efficacy | “to be perfectly honest, I never feel horribly comfortable with heart screening” “I feel reasonably comfortable, though I’m always in pursuit of learning more” |
| Training | “Radiologists that just come out of practice are not comfortable with fetal hearts.” “All you really do during residency is try biometry and assess how big is the baby..” |
| Attitude/Usefulness |
“look at the huge debate over mammography …stating that screening really isn’t changing outcomes. Has anybody looked at screening in fetal echoes?” “It’s never been a lack of awareness that doing a screen on the heart was important” “it might be that it we are just stuck at the moment with a crummy screening test or one that has several significant deficits” |
| Extrinsic Barriers | |
| Expectations | “a lot of patients think their ultrasound is to see their baby and get the gender. But they need to go to high-quality places, and they don’t …demand better quality.” |
| Feedback |
“I’ve been doing this for a long time, but I have really no sense of how many I’ve missed. I could be..missing a lot” “I would also mention that getting the feedback from my referrals would increase my knowledge, … hearing back, really was valuable. So that communication between us… and those we refer to is really important” |
| Organizational Barriers | |
| System-level | “with initial prenatal detection, ultrasound is done in thousands of different offices, by physicians doing low volume and low risk… “locations with much less resources you’re not going to have the access to the specialists, to have the resources to refer to them.” “I do not think you get the same ability to look at something that you want to see better … reading a scan from home.” “I did rely on the ultrasonographer, you know, I just looked at the pictures. She was the one that told me if something was weird” |
| Process Implementation | “So how do we get that implemented as a requirement? How do we get that implemented across the nation?” “..at the end of the day financial incentives and disincentives at the level of the organization will be what ultimately pushes change across the board” |
| Policy/Quality Control | “Standardize the terminology and make sure everybody uses the same thing to prevent this kind of hedgy-ness“ “We are taking on certification for nuchal translucencies and cervical length, but yet for cardiac images, a basic 4-chamber and outflow tracts views, there is practically no accreditation system” |
Figure 1.
Frequency of discussion of themes in physician focus groups by physician specialty.
Survey Results
From 1300 survey invitations, 1267 were delivered. Initially 282 physicians responded to the web survey (response rate 22%) between June 2015 and May 2016. Among these only 190 met qualifying criteria. An additional, 86 did not interpret obstetric ultrasounds (or had not in the last six months), leaving 104 qualified respondents. As demographic information was not a required response, only 61 respondents gave their zip code. Among these, 28% resided in the Northeast, 31% in the South, 13% in the Midwest and 28% in the West regions of the United States.
Among 104 physicians who completed the survey, only 82 completed all the image questions. Their average age was 46 years with 22±10 years of experience and 37% were female (Table 3). The majority (58%) were MFMs, another quarter obstetricians, and the remainder radiologists; 61% practiced in a tertiary center. Table 3 shows the average reading volume and domain measures for the overall cohort and by primary practice setting. Compared to those practicing in tertiary centers, those practicing in non-tertiary settings were similar in experience and age, but differed in the volume of ultrasounds read per week. Average scores for all measured domains were higher for physicians in tertiary settings. For example, physicians in tertiary practices rated their self-efficacy in interpreting outflow images on average 0.8 points higher on a 5-point Likert response scale compared to those in non-tertiary settings (p=<0.001). The differences between average scores by setting was larger for measures of outflow tract barriers compared to 4C domains.
Table 3.
Characteristics of Participants and Univariate Analysis.
| Factor | Overall | Non-tertiary | Tertiary | P value* | |||
|---|---|---|---|---|---|---|---|
| N | Mean/N (STD/%) | N | Mean/N (STD/%) | N | Mean/N (STD/%) | ||
| Female | 68 | 25 (36.76%) | 24 | 7 (29.17%) | 44 | 18 (40.91%) | 0.34 |
| Experience (years) | 103 | 21.60 ± 10.27 | 32 | 21.03 ± 10.23 | 50 | 22.34 ± 9.84 | 0.56 |
| Volume (obstetric scans/week) | 102 | 35.27 ± 28.29 | 32 | 14.47 ± 10.37 | 50 | 49.84 ± 27.73 | <0.001 |
| Age (years) | 67 | 55.94 ± 9.51 | 24 | 54.79 ± 8.30 | 43 | 56.58 ± 10.16 | 0.46 |
| Additional training in cardiac | 71 | 48 (67.61%) | 25 | 13 (52.00%) | 46 | 35 (76.09%) | 0.038 |
| Feedback | 93 | 4.44 ± 0.83 | 32 | 4.13 ± 1.05 | 50 | 4.66 ± 0.58 | 0.005 |
| Facilitating conditions | 93 | 4.28 ± 0.88 | 32 | 3.81 ± 0.98 | 50 | 4.58 ± 0.68 | <0.001 |
| Self-efficacy 4Cr | 94 | 3.98 ± 0.65 | 32 | 3.68 ± 0.67 | 50 | 4.18 ± 0.54 | <0.001 |
| Self-efficacy outflow | 94 | 3.62 ± 0.90 | 32 | 3.15 ± 0.93 | 50 | 3.98 ± 0.71 | <0.001 |
| Outcome expectancy 4C | 95 | 4.56 ± 0.76 | 32 | 4.36 ± 0.90 | 50 | 4.65 ± 0.71 | 0.11 |
| Outcome expectancy outflow | 95 | 4.59 ± 0.75 | 32 | 4.25 ± 0.88 | 50 | 4.73 ± 0.68 | 0.007 |
| Perceived ease of use 4C | 95 | 4.39 ± 0.86 | 32 | 4.00 ± 0.91 | 50 | 4.63 ± 0.78 | 0.001 |
| Perceived ease of use outflow | 95 | 4.20 ± 0.83 | 32 | 3.78 ± 0.90 | 50 | 4.48 ± 0.73 | <0.001 |
| Knowledge | 71 | 0.50 ± 0.26 | 25 | 0.37 ± 0.24 | 46 | 0.58 ± 0.25 | <0.001 |
| Outcomes | |||||||
| Time to interpret 4C images (min) | 82 | 2.78 ± 2.89 | 32 | 3.25 ± 3.65 | 50 | 2.48 ± 2.27 | 0.24 |
| Time to interpret outflow images (min) | 81 | 3.57 ± 3.27 | 31 | 3.74 ± 3.90 | 50 | 3.47 ± 2.86 | 0.72 |
| Overall accuracy (%) | 82 | 0.67 ± 0.14 | 32 | 0.62 ± 0.13 | 50 | 0.70 ± 0.15 | 0.02 |
| Accuracy 4C (%) | 82 | 0.71 ± 0.18 | 32 | 0.66 ± 0.16 | 50 | 0.75 ± 0.19 | 0.02 |
| Accuracy outflow (%) | 72 | 0.61 ± 0.15 | 26 | 0.59 ± 0.17 | 46 | 0.63 ± 0.14 | 0.32 |
| Accuracy adequate (%) | 82 | 0.59 ± 0.16 | 32 | 0.55 ± 0.15 | 50 | 0.61 ± 0.17 | 0.12 |
| Accuracy normal (%) | 80 | 0.76 ± 0.18 | 30 | 0.70 ± 0.19 | 50 | 0.79 ± 0.17 | 0.04 |
Data are presented as mean ± SD for continuous measures and frequency (percentage) for categorical measures. Comparisons between non-tertiary and tertiary use Chi-square test for the categorical variables and T-test for continues variables 4C – four chamber
A quarter of physician respondents indicated that they spoke with the imaging sonographer “less than half of the time” or “never” when reading obstetric ultrasounds. About half of physician respondents reported having access to a pediatric cardiologist. About 60% of obstetricians and radiologists had access to an MFM specialist. A small but notable number of physicians (4%) reported that cardiac imaging was not a required part of a 2nd trimester obstetric ultrasound. An additional 10% reported that imaging of the outflow tracts was not required. The majority interpreted both cine and still clips of the heart. About half (53%) of reading physicians indicated their training did not adequately prepare them to interpret fetal cardiac images and the majority (67%) had completed additional training in cardiac screening.
Image interpretation
The overall accuracy in interpretation of the 1420 fetal cardiac screening images was 67±14% (Table 3). Accuracy for outflows was lower (61%) than for 4C views (71%) and lower for adequacy of the screening image than for interpretation of normal versus abnormal images (59% and 76% accuracy respectively). Most measured factors were significantly associated with odds of correct interpretation of all fetal heart screening images as well as subsets of image types (4C, outflow, adequacy and normal vs abnormal) on univariate analysis (Table 4).
Table 4.
Univariate Associations of Factors with Accuracy of Fetal Heart Image Interpretation
| Barriers | Overall (N=1420) | 4C (N=710) | Outflow (N-710) | Adequacy (N=710) | Normality (N=710) |
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI | Odds Ratio (95% CI) | Odds Ratio (95% CI) | |
| Self-efficacy 4C | 1.30 (1.07, 1.58) | 1.45 (1.11, 1.91) | 1.17 (0.88, 1.56) | 1.14 (0.86, 1.50) | 1.56 (1.17, 2.08) |
| Self-efficacy outflow | 1.20 (1.05, 1.37) | 1.26 (1.05, 1.52) | 1.15 (0.95, 1.40) | 1.14 (0.94, 1.37) | 1.30 (1.07, 1.58) |
| Outcome expectancy 4C | 1.28 (1.11, 1.48) | 1.35 (1.10, 1.66) | 1.22 (0.98, 1.51) | 1.26 (1.02, 1.56) | 1.33 (1.08, 1.64) |
| Outcome expectancy outflow | 1.28 (1.11, 1.48) | 1.40 (1.15, 1.72) | 1.17 (0.95, 1.46) | 1.19 (0.96, 1.46) | 1.43 (1.15, 1.76) |
| Perceived ease of use 4C | 1.20 (1.05, 1.37) | 1.24 (1.03, 1.49) | 1.17 (0.96, 1.42) | 1.15 (0.95, 1.39) | 1.27 (1.05, 1.55) |
| Perceived ease of use outflow | 1.32 (1.16, 1.52) | 1.43 (1.18, 1.72) | 1.24 (1.01, 1.51) | 1.27 (1.04, 1.54) | 1.42 (1.17, 1.73) |
| Time to interpret 4C images (min) | 1.00 (0.95, 1.04) | 1.01 (0.95, 1.07) | 0.97 (0.90, 1.06) | 1.03 (0.96, 1.10) | 0.97 (0.90, 1.03) |
| Time to interpret outflow images (min) | 1.01 (0.99, 1.03) | 1.02 (0.99, 1.05) | 1.00 (0.98, 1.03) | 1.01 (0.99, 1.04) | 1.01 (0.98, 1.04) |
| Experience (years) | 1.00 (0.99, 1.01) | 1.01 (0.99, 1.02) | 1.00 (0.98, 1.02) | 1.01 (0.99, 1.03) | 0.99 (0.98, 1.01) |
| Volume (obstetric scans/week) | 1.01 (1.00, 1.01) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.01) | 1.01 (1.00, 1.01) | 1.01 (1.00, 1.02) |
| Feedback | 1.32 (1.15, 1.52) | 1.49 (1.22, 1.82) | 1.17 (0.95, 1.44) | 1.26 (1.02, 1.54) | 1.44 (1.17, 1.77) |
| Knowledge | 3.10 (1.86, 5.17) | 4.63 (2.20, 9.76) | 1.16 (0.79, 1.69) | 3.57 (1.73, 7.37) | 2.94 (1.39, 6.26) |
| Facilitating conditions | 1.29 (1.13, 1.48) | 1.33 (1.10, 1.60) | 1.27 (1.04, 1.55) | 1.19 (0.98, 1.44) | 1.46 (1.20, 1.78) |
| Tertiary practice | 1.37 (1.06, 1.76) | 1.59 (1.13, 2.24) | 7.06 (2.23, 22.38) | 1.35 (0.94, 1.92) | 1.43 (0.98, 2.08) |
| Defect Image | 5.25 (2.86, 9.65) | 3.77 (1.84, 7.73) | 1.17 (0.88, 1.56) | 5.23 (1.90, 14.41) | 1.46 (0.60, 3.55) |
| Specialty | |||||
| MFM | Ref | Ref | Ref | Ref | Ref |
| Ob/Gyn | 0.60 (0.45,0.80) | 0.51 (0.35, 0.75) | 0.73 (0.47,1.13) | 0.73 (0.48, 1.09) | 0.47 (0.31, 0.72) |
| Radiology | 0.83 (0.59, 1.17) | 0.76 (0.47, 1.23) | 0.90 (0.54, 1.50) | 0.81 (0.50, 1.31) | 0.84 (0.50, 1.41) |
Odds ratios are for each 1 score increasing in above barriers or each 10% increasing in Knowledge, Tertiary practice vs. Non-tertiary practice, and Abnormal or Inadequate image vs. Normal or Adequate image.
4C – four chamber, MFM – maternal fetal medicine, Ob/Gyn – obstetrics and gynecology
Multivariate analysis
In multivariable analysis, overall accuracy in image interpretation was associated with physician knowledge (increasing odds of accurate interpretation by 2.2 if the knowledge score increased to 100%, 95% CI 1.2–3.8), practice setting (non-tertiary OR 0.7, CI 0.4–0.99) and volume of ultrasounds read per week (OR 1.01 for every additional scan, p=0.04, Table 5). While volume remained associated with accurate interpretation of outflow tract screening images, it was not associated with accuracy for 4C screening images. Perceived feedback was independently associated with accuracy for both outflow and 4C imaging (OR 1.5, CI 1.1–2.1). The interactions between measured barriers and type of screening image (4C versus outflow) were not significant (Supplementary Table 1), but interactions between barriers and practice setting (tertiary versus non-tertiary) were significant.
Table 5.
Multivariable Regression Model of Factors Associated with Accuracy in Fetal Heart Image Interpretation
| Barrier | Overall (n=1420) | 4C (N=710) | Outflow (N=710) | Adequacy (N=710) | Normality (N=710) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio (CI) | P value | Odds Ratio (CI) | P value | Odds Ratio (CI) | P value | Odds Ratio (CI) | P value | Odds Ratio (CI) | P value | |
| Self-efficacy outflow | 1.02 (08,1.3) | 0.840 | 0.96 (0.7,1.3) | 0.783 | 1.08 (0.8, 1.4) | 0.586 | 1.06 (0.8, 1.4) | 0.678 | 0.97 (0.7,1.3) | 0.841 |
| Outcome expectancy 4C | 1.08 (0.8, 1.5) | 0.648 | 0.89 (0.6,1.4) | 0.61 | 1.29 (0.8, 2.0) | 0.254 | 1.28 (0.8, 1.9) | 0.269 | 0.88 (0.5,1.4) | 0.593 |
| Outcome expectancy outflow | 0.99 (0.7, 1.5) | 0.945 | 1.34 (0.8, 2.3) | 0.307 | 0.73 (0.4, 1.3) | 0.262 | 0.77 (0.4, 1.3) | 0.357 | 1.29 0.7,2.3) | 0.384 |
| Perceived ease of use 4C | 0.89 (0.7, 1.1) | 0.328 | 0.79 (0.6, 1.1) | 0.182 | 1.00 (0.7, 1.4) | 0.992 | 0.99 (0.7, 1.4) | 0.972 | 0.77 (0.5,1.1) | 0.149 |
| Volume (obstetric scans/week) | 1.01 (1.0, 1.01) | 0.038 | 1.00 (0.99,1.01) | 0.349 | 1.01 (1.0, 1.02) | 0.043 | 1.00 (0.99,1.01) | 0.412 | 1.01 (1.0,1.02) | 0.028 |
| Feedback | 1.15 (0.9, 1.5) | 0.233 | 1.48 (1.1, 2.1) | 0.023 | 0.89 (0.6,1.3) | 0.521 | 1.21 (0.9, 1.7) | 0.256 | 1.11 (0.8,4.5) | 0.536 |
| Facilitating conditions | 1.19 (0.9, 1.5) | 0.175 | 1.06 (0.7, 1.5) | 0.768 | 1.35 (0.9, 1.9) | 0.102 | 0.97 (0.7, 1.4) | 0.865 | 1.52 (1.04,2.2) | 0.031 |
| Knowledge | 2.54 (1.4, 4.6) | 0.002 | 3.66 (1.6,8.6) | 0.003 | 1.83 (0.8, 4.2) | 0.151 | 3.35 (1.4, 7.6) | 0.004 | 1.90 (0.8,4.5) | 0.149 |
| Non-tertiary practice | 0.67 (0.5, 0.99) | 0.045 | 0.71 (0.4, 1.3) | 0.248 | 0.61 (0.3, 1.1) | 0.086 | 0.73 (0.4,1.3) | 0.269 | 0.60 (0.3,1.1) | 0.082 |
| Image type | 6.87 (3.5,13.5) | <0.001 | 5.51 (2.4, 12.9) | <0.001 | 7.56 (2.3,24.6) | 0.001 | 6.51 (2.3,18.7) | 0.001 | 1.75 (0.6,4.9) | 0.29 |
Multivariable models were also adjusted for image test version and view (outflow vs. 4C). Odds ratios are for each 1 score increasing in above barriers, each additional ultrasound per week for volume, or increase in knowledge to 100%, and abnormal/inadequate image vs. normal/adequate image
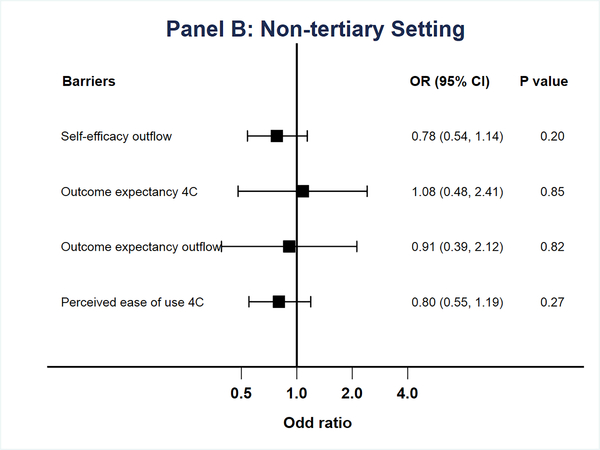
In subgroup analysis of physicians practicing in tertiary or high-risk settings, knowledge was the only factor associated with accuracy of image interpretation (OR 2.9, CI 1.3–6.7, Figure 2a). For physicians who practiced in non-tertiary settings, facilitating conditions were associated with improved accuracy (OR 1.5, CI 1.02–2.3, Figure 2b).
Figure 2.
Multivariable models of odds of accurate image interpretation by practice setting. A. Tertiary Practice. B. Non-tertiary practice. Factors with p>0.05 on univariate analysis or correlation ≥0.7 were excluded from multivariable models.
Discussion
Our study assessing barriers/facilitators for detection of CHD using fetal ultrasound is among the first to identity physician cited determinants of performance. These may serve as intervention targets for improving the effectiveness of prenatal screening. Physicians who interpret these ultrasounds identified several barriers including not only knowledge, training and ability as expected, but also lack of feedback, quality control and issues with consistent volumes. Of these determinants, knowledge about screening and volume of studies appeared most pertinent to measured ability to interpret fetal heart screening images on our survey. Feedback received on screening and facilitating conditions also influenced accuracy of interpretation in certain aspects.
The gap between what ultrasound screening for heart defects can achieve and what is actually accomplished in practice has been the subject of many studies and editorials.26–29 While training and experience are the targets of many interventions,6,30–34 they are largely focused on sonographers. Expanding interventions to include physicians who interpret these images may be warranted since lack of training and familiarity with cardiac imaging were cited as key barriers. Even physicians practicing in high risk settings have little knowledge about fetal cardiac screening guidelines 35 and sensitivity of screening views. Since physicians who interpret ultrasounds represent multiple specialty and subspecialty fields with variation in training in cardiac imaging in the U.S., the level of familiarity likely depends on individual interest.36 If the expected baseline of knowledge for image interpretation is standardized with well-accepted guidelines for training and maintenance of expertise in obstetric ultrasound, detection should increase.37 As some have suggested, providing focused training for the cardiac portion of the exam, thought to be the most complex part of the fetal ultrasound, may be effective.15
Systematic feedback has been useful for improving detection of heart defects.38 While providers in tertiary settings typically have ready access to expertise, feedback may not be formalized. Feedback is even more challenging for the lone radiologist or obstetrician practicing in low-risk community settings. Despite this, single centers have developed supportive data structures with formalized feedback.39 Participants in the survey also cited certification similar to that required for nuchal translucency and cervical length assessments as a high priority to improve screening. Indeed, regular audits for maintenance of certification or quality control have been successful 38,40–42 and could be implemented on a wider scale by either a governing body such as American College of Obstetrics and Gynecology or the American Institute of Ultrasound Medicine.
The impact of perceived facilitating conditions on image interpretation in community settings where detection rates are low is intriguing, 3,4,7,43 since equipment, age at referral and maternal body habitus have been previously cited as common barriers.7 The lack of supportive structures and equipment in these settings may influence ability and skill compared to tertiary settings with sufficient technology and assistance. Stakeholders (MFMs and pediatric cardiologists) should identify the minimal level of infrastructure needed to effectively perform screening ultrasounds and advocate for inclusion of these elements into health systems.
Physicians cited volume as an important factor in accurately interpreting cardiac images consistent with studies in other areas.44 The guidelines for maintenance of practice and accreditation/certification services already recognize that a minimum number of obstetric ultrasounds is needed to maintain skills/expertise.37 Possible ways to address volume issues include restricting reimbursements to centers that read a minimum number of studies, but maybe more realistically, providing support within referring regions for telemedicine services, consults and over reads. Measures that improve practice in even low volume settings have the advantage of allowing continued local access for pregnant mothers.39,45
National screening programs in countries with more standardized protocols for training, feedback, and minimum study volumes have led to increased detection of CHD. 46 Determining how to implement similar multifaceted programs in the highly variable screening settings in the U.S. will be crucial to improving care for CHD families.
Limitations
This study is limited by the possibility of selection bias in the focus group participants and survey respondents who may have been more interested in the topic of cardiac imaging than non-respondents. The results may not be generalizable to all geographic regions particularly for the Northeast which was not represented in our focus groups and the Midwest which had lower representation in the survey. Additionally, like all surveys, there is a risk of nonresponse bias. This risk may be accentuated by our small sample size. While response bias is also a possibility, the survey instrument and the individual self-administration were designed to minimize this bias. While we examined differences in barriers to screening by practice setting, there are likely additional variabilities in settings and providers which were not represented. Though we analyzed barriers to screening within a behavioral framework, our measure of image interpretation on a web survey theory was a surrogate outcome for true detection of CHD on obstetric ultrasound. Given the anonymity of the survey, we were unable to relate barriers/facilitators measured to true detection rates of individual physicians or practices.
Conclusion
In this national survey of physicians, we identified and prioritized barriers/facilitators to effective cardiac screening that are affecting physicians in practice across the United States. These novel results can be combined with the previously identified barriers for sonographers15 to inform targeted interventions based on the perspectives of those who are at the forefront of population screening prenatally for CHD.
Supplementary Material
Acknowledgments
This investigation was funded by the Pediatric Heart Network Scholars Program within the National Heart, Lung, and Blood Institute of the NIH under Award Number U10HL068270. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The investigation was also supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institute of Health, through Grant 5UL1TR001067-02.
References
- 1.Stumpflen I, Stumpflen A, Wimmer M, Bernaschek G. Effect of detailed fetal echocardiography as part of routine prenatal ultrasonographic screening on detection of congenital heart disease. Lancet. 1996;348(9031):854–857. doi:S0140–6736(96)04069-X [pii] 10.1016/S0140-6736(96)04069-X. [DOI] [PubMed] [Google Scholar]
- 2.Khoo NS, Van Essen P, Richardson M, Robertson T. Effectiveness of prenatal diagnosis of congenital heart defects in South Australia: a population analysis 1999–2003. Aust N Z J Obs Gynaecol. 2008;48(6):559–563. doi:AJO915 [pii] 10.1111/j.1479-828X.2008.00915.x. [DOI] [PubMed] [Google Scholar]
- 3.Pinto NM, Keenan HT, Minich LL, Puchalski MD, Heywood M, Botto LD. Barriers to prenatal detection of congenital heart disease: A population-based study. Ultrasound Obstet Gynecol. 2012;40(4):418–425. doi: 10.1002/uog.10116. [DOI] [PubMed] [Google Scholar]
- 4.Friedberg MK, Silverman NH, Moon-Grady AJ, Tong E, et al. Prenatal detection of congenital heart disease. J Pediatr. 2009;155(1):26–31, 31 e1. doi: 10.1016/j.jpeds.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 5.Sklansky MS, Berman DP, Pruetz JD, Chang R-KR. Prenatal Screening for Major Congenital Heart Disease. J Ultrasound Med. 2009;28(7):889–899. doi: 10.7863/jum.2009.28.7.889. [DOI] [PubMed] [Google Scholar]
- 6.Tegnander E, Eik-Nes SH. The examiner’s ultrasound experience has a significant impact on the detection rate of congenital heart defects at the second-trimester fetal examination. Ultrasound Obs Gynecol. 2006;28(1):8–14. doi: 10.1002/uog.2804. [DOI] [PubMed] [Google Scholar]
- 7.Wong SF, Chan FY, Cincotta RB, Lee-Tannock A, Ward C. Factors influencing the prenatal detection of structural congenital heart diseases. Ultrasound Obs Gynecol. 2003;21(1):19–25. doi: 10.1002/uog.7. [DOI] [PubMed] [Google Scholar]
- 8.Smith WR. Evidence for the effectiveness of techniques To change physician behavior. Chest. 2000;118(2 Suppl):8S–17S. http://www.ncbi.nlm.nih.gov/pubmed/10939994. [DOI] [PubMed] [Google Scholar]
- 9.Grimshaw JM, Shirran L, Thomas R, Mowatt G, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001;39(8 Suppl 2):II2–45. http://www.ncbi.nlm.nih.gov/pubmed/11583120. [PubMed] [Google Scholar]
- 10.Chaillet N, Dumont A. Evidence-based strategies for reducing cesarean section rates: a meta-analysis. Birth. 2007;34(1):53–64. doi: 10.1111/j.1523-536X.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 11.Cabana MD, Rand CS, Powe NR, Wu AW, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. http://www.ncbi.nlm.nih.gov/pubmed/10535437. [DOI] [PubMed] [Google Scholar]
- 12.Maue SK, Segal R, Kimberlin CL, Lipowski EE. Predicting physician guideline compliance: an assessment of motivators and perceived barriers. Am J Manag Care. 2004;10(6):383–391. doi:2607 [pii]. [PubMed] [Google Scholar]
- 13.Honda K, Gorin SS. A model of stage of change to recommend colonoscopy among urban primary care physicians. Heal Psychol. 2006;25(1):65–73. doi:2006–01035-009 [pii] 10.1037/0278-6133.25.1.65. [DOI] [PubMed] [Google Scholar]
- 14.Krueger RA, Casey MA, Mary AW. Focus Groups : A Practical Guide for Applied Research. 4th ed. Thousand Oaks, CA: SAGE; 2009. [Google Scholar]
- 15.Pinto N, Sheng X, Keenan HT, Byrne JLB, Stanton B, Kinney AY. Sonographer-Identified Barriers and Facilitators to Prenatal Screening for Congenital Heart Disease: A Mixed Methods Study. J Diagnostic Med Sonogr. 2017;33(1):3–12. doi: 10.1177/8756479316677019. [DOI] [Google Scholar]
- 16.Cabana MD, Rand C, Slish K, Nan B, Davis MM, Clark N. Pediatrician self-efficacy for counseling parents of asthmatic children to quit smoking. Pediatrics. 2004;113(1 Pt 1):78–81. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14702452. [DOI] [PubMed] [Google Scholar]
- 17.Cabana MD, Ebel BE, Cooper-Patrick L, Powe NR, Rubin HR, Rand CS. Barriers pediatricians face when using asthma practice guidelines. Arch Pediatr Adolesc Med. 2000;154(7):685–693. http://www.ncbi.nlm.nih.gov/pubmed/10891020. [DOI] [PubMed] [Google Scholar]
- 18.Rabiee F Focus-group interview and data analysis. Proc Nutr Soc. 2004;(63):655–660. doi: 10.1079/pns2004399. [DOI] [PubMed] [Google Scholar]
- 19.Health Resources and Services Administration. Area Health Resources Files. Health Workforce HRSA; https://data.hrsa.gov/topics/health-workforce/ahrf. Published 2013. Accessed September 1, 2013. [Google Scholar]
- 20.Centers for Medicare and Medicaid Serves. National Plan and Provider Enumeration System (NPPES). https://www.naaccr.org/gis-resources/#NATIONALPROVIDER. Published 2010. Accessed July 1, 2013.
- 21.United States Department of Agriculture ERS. USDA ERS - Rural-Urban Commuting Area Codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/. Published 2013. Accessed September 5, 2018.
- 22.UW RHRC Rural Urban Commuting Area Codes - RUCA. http://depts.washington.edu/uwruca/. Published 2010. Accessed February 7, 2019.
- 23.VanGeest JB, Johnson TP, Welch VL. Methodologies for improving response rates in surveys of physicians: a systematic review. Eval Heal Prof. 2007;30(4):303–321. doi:30/4/303 [pii] 10.1177/0163278707307899. [DOI] [PubMed] [Google Scholar]
- 24.Kellerman SE, Herold J. Physician response to surveys. A review of the literature. Am J Prev Med. 2001;20(1):61–67. doi:S0749–3797(00)00258–0 [pii]. [DOI] [PubMed] [Google Scholar]
- 25.Dillman Smyth JD, and Christian LMDA. The Tailored Design Method. In: Internet, Mail and Mixed-Mode Surveys: The Tailored Design Method. 3rd ed. Hoboken, NJ: John Wiley and Sons; 2009:15–39. [Google Scholar]
- 26.Sharland G Routine fetal cardiac screening: what are we doing and what should we do? Prenat Diagn. 2004;24(13):1123–1129. doi: 10.1002/pd.1069. [DOI] [PubMed] [Google Scholar]
- 27.Chaoui R The four-chamber view: four reasons why it seems to fail in screening for cardiac abnormalities and suggestions to improve detection rate. Ultrasound Obstet Gynecol. 2003;22(1):3–10. doi: 10.1002/uog.187. [DOI] [PubMed] [Google Scholar]
- 28.Sklansky MS, Berman DP, Pruetz JD, Chang RK. Prenatal screening for major congenital heart disease: superiority of outflow tracts over the 4-chamber view. J Ultrasound Med. 2009;28(7):889–899. http://www.ncbi.nlm.nih.gov/pubmed/19546331. [DOI] [PubMed] [Google Scholar]
- 29.Bishop KC, Kuller JA, Boyd BK, Rhee EH, Miller S, Barker P. Ultrasound Examination of the Fetal Heart. Obstet Gyneological Surv. 2017;72(1). https://ovidsp-tx-ovid-com.ezproxy.lib.utah.edu/sp-3.32.1b/ovidweb.cgi?WebLinkFrameset=1&S=KECDFPGBAIDDOKBINCDKHFIBAHEPAA00&returnUrl=ovidweb.cgi%3F%26Full%2BText%3DL%257cS.sh.39.40%257c0%257c00006254-201701000-00020%26S%3DKECDFPGBAIDDOKBINCDKHFIBAHEPAA00. Accessed January 30, 2019. [DOI] [PubMed] [Google Scholar]
- 30.Carvalho JS, Mavrides E, Shinebourne EA, Campbell S, Thilaganathan B. Improving the effectiveness of routine prenatal screening for major congenital heart defects. Heart. 2002;88(4):387–391. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12231598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogge G, Gaglioti P, Maccanti S, Faggiano F, Todros T. Prenatal screening for congenital heart disease with four-chamber and outflow-tract views: a multicenter study. Ultrasound Obs Gynecol. 2006;28(6):779–784. doi: 10.1002/uog.3830. [DOI] [PubMed] [Google Scholar]
- 32.Dudley D, Schneider D. Prenatal detection of congenital heart disease: the case for improved training. BJOG An Int J Obstet Gynaecol. 2016;123(3):408–408. doi: 10.1111/1471-0528.13349. [DOI] [PubMed] [Google Scholar]
- 33.McBrien A, Sands A, Craig B, Dornan J, Casey F. Impact of a regional training program in fetal echocardiography for sonographers on the antenatal detection of major congenital heart disease. Ultrasound Obs Gynecol. 2010;36(3):279–284. doi: 10.1002/uog.7616. [DOI] [PubMed] [Google Scholar]
- 34.Pézard P, Bonnemains L, Boussion F, Sentilhes L, et al. Influence of ultrasonographers training on prenatal diagnosis of congenital heart diseases: a 12-year population-based study. Prenat Diagn. 2008;28(11):1016–1022. doi: 10.1002/pd.2113. [DOI] [PubMed] [Google Scholar]
- 35.AIUM Practice Guideline for the Performance of Obstetric Ultrasound Examinations. J Ultrasound Med. 2013;32(6):1083–1101. doi: 10.7863/ultra.32.6.1083. [DOI] [PubMed] [Google Scholar]
- 36.Asplin N, Dellgren A, Conner P. Education in obstetrical ultrasound--an important factor for increasing the prenatal detection of congenital heart disease. Acta Obs Gynecol Scand. 2013;92(7):804–808. doi: 10.1111/aogs.12140. [DOI] [PubMed] [Google Scholar]
- 37.American Institute of Ultrasound in Medicine. Training Guidelines for Physicians Who Evaluate and Interpret Diagnostic Obstetric Ultrasound Examinations. https://www.aium.org/resources/viewStatement.aspx?id=59. Accessed June 2019.
- 38.Jaudi S, Granger B, Herpin CN, Fries N, Montcel ST Du, Dommergues M. Online audit and feedback improve fetal second-trimester four-chamber view images: a randomised controlled trial. Prenat Diagn. 2013;33(10):1–6. doi: 10.1002/pd.4173. [DOI] [PubMed] [Google Scholar]
- 39.Gardiner HM, Kovacevic A, van der Heijden LB, Pfeiffer PW, et al. Prenatal screening for major congenital heart disease: assessing performance by combining national cardiac audit with maternity data. Heart. 2014;100(5):375–382. doi: 10.1136/heartjnl-2013-304640. [DOI] [PubMed] [Google Scholar]
- 40.Jaudi S, Tezenas Du Montcel S, Fries N, et al. Online evaluation of fetal second-trimester four-chamber view images: a comparison of six evaluation methods. Ultrasound Obstet Gynecol. 2011;38(2):185–190. doi: 10.1002/uog.8941. [DOI] [PubMed] [Google Scholar]
- 41.Sairam S, Awadh AMA, Cook K, Papageorghiou AT, Carvalho JS. Impact of audit of routine second-trimester cardiac images using a novel image-scoring method. Ultrasound Obstet Gynecol. 2009;33(5):545–551. doi: 10.1002/uog.6323. [DOI] [PubMed] [Google Scholar]
- 42.Ursem NTC, Peters IA, Kraan-van der Est MN, Reijerink-Verheij JCIY, Knapen MFCM, Cohen-Overbeek TE. An Audit of Second-Trimester Fetal Anomaly Scans Based on a Novel Image-Scoring Method in the Southwest Region of the Netherlands. J Ultrasound Med. 2017;36(6):1171–1179. doi: 10.7863/ultra.16.06055. [DOI] [PubMed] [Google Scholar]
- 43.Sekar P, Heydarian HC, Cnota JF, Hornberger LK, Michelfelder EC, Sekar P. Diagnosis of congenital heart disease in an era of universal prenatal ultrasound screening in southwest Ohio. Cardiol Young. 2015;25:35–41. doi: 10.1017/S1047951113001467. [DOI] [PubMed] [Google Scholar]
- 44.Hertzberg BS, Kliewer MA, Bowie JD, Carroll BA, DeLong DH, Gray L, Nelson RC. Physician Training Requirements in Sonography. Am J Roentgenol. 2000;174(5):1221–1227. doi: 10.2214/ajr.174.5.1741221. [DOI] [PubMed] [Google Scholar]
- 45.McCrossan BA, Sands AJ, Kileen T, Cardwell CR, Casey FA. Fetal diagnosis of congenital heart disease by telemedicine. Arch Dis Child Fetal Neonatal Ed. 2011;96(6):F394–7. doi: 10.1136/adc.2010.197202. [DOI] [PubMed] [Google Scholar]
- 46.van Velzen C, Clur S, Rijlaarsdam M, Bax C, et al. Prenatal detection of congenital heart disease-results of a national screening programme. BJOG An Int J Obstet Gynaecol. 2016;123(3):400–407. doi: 10.1111/1471-0528.13274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.