Abstract
Objective
This study examined the relation of electrically evoked compound action potential thresholds obtained using neural response telemetry (NRT) to T- and C-levels in children’s speech processor programs optimized for recognition of very soft to loud sounds while ensuring tolerance of very loud sounds.
Design
Forty-one children (age 2 to 14 yr) with stable electrical hearing participated. All children were Nucleus 24 System recipients and attended one of three auditory-oral schools that have on-site pediatric audiologists experienced at cochlear implant programming. Speech processor MAPs were created and adjusted over a period of months until aided warble-tone thresholds were between 10 and 30 dB HL at octave frequencies between 250 and 4000 Hz, and understanding of speech was maximized for many listening situations. At least 1 yr postactivation, visual (vNRT) and predicted (tNRT) thresholds were obtained on 9 to 11 electrodes and compared to each child’s T- and C-level values on these electrodes in their MAPs. Test-retest stability of NRT thresholds was compared for two test sessions 1 mo apart.
Results
NRT-based evoked compound action potential thresholds could be obtained from 36 of the 41 children. vNRT and tNRT test-retest reliability was high; average correlation coefficients (r) across subjects were 0.90 (range: 0.64 to 0.99) and 0.88 (range: 0.31 to 1.00), respectively. Group average correlation coefficients between vNRT and T-level, vNRT and C-level, tNRT and T-level, and tNRT and C-level were low (0.18, 0.21, 0.24, and 0.26, respectively). Group mean tNRT thresholds were four current levels lower than the group mean vNRT thresholds. Subsequent analysis was performed with the vNRT thresholds because the range of test-retest correlation coefficients for individual subjects was narrower than with tNRT. Hierarchical linear modeling was used to determine if vNRT could be used to predict T- and C-levels. This analysis indicated a significant average relation between vNRT and T-levels and between vNRT and C-levels, but significant heterogeneity in the individual-level estimates of those relations. In other words, subjects varied significantly in the size of the relation between their individual vNRT values and both T- and C-levels. Attempts to account for that heterogeneity did not identify any subject characteristics that were significantly related to the individual-level parameters.
Conclusions
The position of the group average vNRT and tNRT thresholds in the upper half of the dynamic range between Ts and Cs agrees with previous studies. The fact that the profile of vNRT thresholds did not parallel the profiles of Ts and Cs across electrodes for most children suggests that simply shifting the NRT profile to select Tand C-levels in initial MAPs is likely to result in a loudness imbalance for certain speech frequencies and/or tolerance issues for many children. This was verified by the hierarchical linear modeling analysis, which showed substantial and significant heterogeneity in the relations between vNRT and T-levels and between vNRT and C-levels. In summary, vNRT is not related to T- or C-levels in a simple and uniform way that would allow it to guide MAP fine tuning with any precision. Consequently, it is recommended that MAP fine tuning be based on the child’s behavioral responses on individual electrodes.
INTRODUCTION
The electrically evoked compound action potential (ECAP), obtained through neural response telemetry (NRT) or neural response imaging, is used by many clinicians to obtain ECAP thresholds as a basis for programming the T- and C-levels or M-levels on individual electrodes in speech processor MAPs. This application is particularly useful with infants, young children, and difficult-to-test patients from whom it is difficult or impossible to obtain behavioral responses.
The application of ECAP measures for the nucleus cochlear implant system (i.e., NRT software versions 2.0 and 3.0) has been described by Abbas et al. (1999). Dillier et al. (2002) validated the measurement of ECAP via the NRT system, and Brown (2003) further described the basis for optimizing parameters to be used during NRT testing.
A key clinical question addressed in many studies has been how well the NRT thresholds predict behaviorally obtained Tand C-levels used to create Nucleus speech processor MAPs. These T- and C-levels have been obtained at the MAP stimulation rate each subject used in everyday life (e.g., between 250 and 1800 pps). For most of these studies, T-level corresponded to threshold or a barely heard level and C-level corresponded to maximum (Brown, et al., 2000; Cafarelli Dees, et al., 2005; Gordon, et al., 2004; Smoorenburg, et al., 2002; Willeboer & Smoorenburg, 2006). In a few, the C-levels reflected the adjustment for equal loudness level across electrodes studied (Seyle & Brown, 2002; Smoorenburg, et al., 2002; Willeboer & Smoorenburg, 2006). However, no other adjustments of T- and C-levels were made.
The T- and C-levels for adults in a recent NRT study (Potts, et al., 2007) differ from those used in other studies. The T- and C-levels reflect substantial changes from conventionally set, barely heard thresholds and maximum acceptable levels, respectively, being those in use at the time of the NRT study (4 mo to 3 yr after initial stimulation). Most MAP adjustments were made during the initial 16 wk of programming and aural rehabilitation sessions. During rehabilitation, subjects were encouraged to listen to sounds at louder levels than they had been used to with their hearing aids. Based on the findings of James et al. (2003), the subject’s C-levels were adjusted until a sensitivity control setting of approximately 12 on the SPrint processor was acceptable when listening to very loud sounds. In addition, T-levels often were adjusted higher than counted thresholds (Skinner, et al., 1995, 1999) so that sound field thresholds for frequency-modulated (FM) tones between 250 and 6000 Hz were at similar levels that approximated 20 dB HL whenever possible. The rationale for this fitting target is based on the articulation index (Pavlovic, et al., 1985; Pavlovic, 1988) which has been simplified for clinical use (Mueller & Killion, 1990). The acoustic cues of normal conversational speech are audible if aided sound field FM thresholds are 20 dB HL between 500 and 4000 Hz and closer to 10 dB HL at 250 and 6000 Hz (i.e., an articulation index value of 1). The major goal of the MAP fitting process used in the Potts et al. (2007) study was fluent communication by the subjects (e.g., talking with someone across the room, understanding when not directly spoken to, and expending less effort throughout the day to understand speech).
Smoorenburg et al. (2002) identified two dimensions in which the profile of NRT thresholds differed from the profiles of T- and C-levels through principal component analysis. The first dimension is the “shift” or difference in overall level between the NRT threshold profile and T- and C- profiles. The second dimension is the difference in slopes of the profiles across the electrode array. Investigators have routinely described the shift dimension. For example, Brown et al. (2000) and Hughes et al. (2000) found that NRT thresholds were typically obtained at 91% of the dynamic range across electrodes (near C-level) for adults and at 53% of the dynamic range across electrodes for children. They noted, however, that great variability existed within and across patients. For some, NRT thresholds occurred at levels greater than C-levels. Other studies also noted this substantial variability within and across patients (Cafarelli Dees, et al., 2005).
To improve the predictability of the overall level of T- and C-level profiles from the NRT threshold profile, Brown et al. (2000) and Hughes et al. (2000) both used a behaviorally determined threshold and maximum acceptable level on one electrode (electrode 10) for which an NRT threshold had been obtained. The NRT threshold profile was shifted to the behavioral threshold to set the T-level profile and to the behavioral maximum level to set the C-level profile. They found that this change improved the correlation between NRT and T- and C-level across subjects. When Cafarelli Dees et al. (2005) applied this overall level correction to subjects in their study, they found root mean square errors of 15 and 14 clinical units in predictions of T- and C-levels; these errors were large from a clinical perspective.
Performance with NRT-based MAPs compared to behavioral MAPs has been evaluated by Seyle and Brown (2002), Smoorenburg et al. (2002), and Willeboer & Smoorenburg (2006). In the Seyle and Brown study, three MAPs created using different methods were compared to simulate conditions that may apply to children. One was created using standard programming techniques (adaptive balancing procedure) and a second was created by setting T-levels 20 current levels (CL) below and C-levels 10 CL above NRT thresholds. The third MAP was created with a combination of NRT and behavioral measures. The profile of NRT thresholds included values on untested electrodes that were linearly interpolated from tested electrode values. After obtaining a behavioral threshold on electrode 10 as described above (Brown, et al., 2000), T- and C-levels in the MAP were set by shifting the NRT profile so they coincided with behavioral threshold and maximum audible loudness, respectively. When sentences were presented at 70 dBA, recognition scores were significantly better with the behaviorally fit MAP compared to the two NRT-based MAPs. Smoorenburg et al. (2002) evaluated adults with two MAPs. On each electrode, the conventional MAP had T-levels that were set at a very soft, but consistent hearing sensation, and C-levels set at the maximum level that was comfortably loud; C-levels were then balanced across electrodes. The ECAP MAP was based on the profile of NRT thresholds obtained on 20 electrodes (i.e., 22–3). This profile was adjusted so that live-voice speech was just detectable at T-levels and for maximum comfort at C-levels. The sensitivity was set at 8, which is substantially lower than that used by Potts et al. (2007). Group mean scores on consonant vowel consonant (CVC) words were 9% lower with the ECAP MAP. This lower score may have been related to the higher C-level settings based on ECAP responses. Individual subjects had higher or lower scores with the ECAP-based MAP compared to the conventional MAP. During the 2 wk of home use, five of the 11 subjects used the ECAP MAPs for only 0.5 to 5 hr/day because sound perception was unsatisfactory. By using a balanced crossover design over a 12-wk period, Willeboer & Smoorenburg (2006) examined CVC word recognition by 18 adults using conventional and ECAP MAPs created the same as those described by Smoorenburg et al. (2002). Specifically, they compared recognition of CVC words in quiet at 65 and 55 dBA and CVC words at 65 dBA in a 55-dBA continuous speech-shaped spectrum noise. They did not find significant group mean differences in word or phoneme recognition for the conditions tested. In general, adult patient performance was unchanged or they preferred and performed better with behavioral MAPs compared to ECAPbased MAPs. Performance was improved in only a few individual cases using the ECAP-based MAP.
The relation between ECAP and the T- and C-level has been studied for a range of MAP stimulation rates. Cafarelli Dees et al. (2005) studied 147 adult implant recipients, of whom a subset of 85 were tested on five electrodes; 12 used stimulation rates of 700 pps or higher, the rest used 250 pps. The correlation between ECAP thresholds and T- and C-levels for subjects who used 250 pps was slightly higher (r = 0.44 to 0.58) than those for the group who used higher stimulation rates (r = 0.40 to 0.51). Both correlations were weak. Zimmerling and Hochmair (2002) evaluated the correlation between ECAP thresholds obtained using an 80 pps stimulation rate and MAP levels obtained using an 80 pps or 2020 pps MAP stimulation rate in Ineraid patients. Results indicated a strong correlation (r = 0.89) between the 80 pps ECAP response and MAP stimulation rates; however, a weak correlation (r = 0.5 to 0.6) was found between the 80 pps ECAP stimulation rate and the 2020 pps MAP stimulation rate. The results of these studies reflect the effects of temporal integration, as greater differences between behavioral T- and C-levels and ECAP thresholds are found with increasing MAP stimulation rates.
Several studies have focused on the use of NRT at initial stimulation and over time (up to 1 or 2 yr postinitial stimulation). In research by Gordon et al. (2004), a combination of objective measures was investigated to determine the relation between these and useful T- and C-levels in MAPs. A battery of measures including the electrically evoked stapedius reflex, the electrically evoked auditory brain stem response, and the ECAP response were recorded at regular intervals beginning in the operating room and over the first year of implant use in 19 patients. Although a significant change in threshold was not seen for evoked auditory brain stem response and ECAP responses over the first 6 and 12 mo, behavioral T-levels decreased and C-levels increased in MAPs over time, confounding comparisons. Hughes et al. (2001) demonstrated that significant increases occurred in electrode impedance, ECAP thresholds, slope of the ECAP growth function, and T-levels in MAPs beyond the 1- to 2-mo visit. ECAP thresholds in children stabilized by the 3- to 8-mo visit, yet the slope of the ECAP growth function and T- and C-levels in MAPs did not stabilize until 1 yr postinitial stimulation. The observed changes were expected because of changes within the ear as a result of electrode placement and electrical stimulation. Consequently, applying these findings to children or adults when the ear has stabilized may be problematic.
The method used to define the NRT response may also contribute to the variation in reported correlations between NRT thresholds and behavioral measures. The clinician may judge the threshold visually (vNRT) or it may be predicted (tNRT) using an amplitude growth function (AGF) calculation within the NRT software. The predicted NRT corresponds to the x-intercept of the AGF based on three or more suprathreshold NRT data points. This assumes that ECAP growth is linear; however, this may not be true in all individuals (Brown, 2003). This method is most often used by clinicians as it is the quickest method and requires no clinical judgment. Consequently, it also is most frequently reported in studies. The vNRT threshold is based on clinician-optimized parameters, scale, and CL step size to search out and confirm threshold. It is more time intensive, although quickness may be achieved with experience. Precise results depend on clinician expertise. The vNRT typically occurs at a higher CL than the tNRT, because the Nucleus 24 noise floor of 20 to 40 µv precludes observing a response below 20 µv.
Although previous studies investigated the relation between NRT and behavioral levels used to create MAPs, methods varied substantially and results have been inconsistent making application difficult. Variables included the method used to define NRT threshold (tNRT versus vNRT), the method used to set MAP T- and C-levels behaviorally (T-level set at threshold or barely heard, C-level set at maximum comfort— some balanced, no other adjustments), the method used to set MAP T- and C-levels based on NRT (shift, slope, 1 behavioral T- and C-level, live voice), MAP parameters, and time postactivation (initial—2 yr). Given the variability in results and methods, there is no clear guidance for the use of NRT in creating effective MAPs for children.
Currently, NRT is used with very young and difficult-to-test patients as they are less able to provide behavioral responses early on. Because it is a time-efficient procedure that does not require participation on the part of the child, clinicians have expanded their use of NRT beyond the initial activation period, especially when an assistant is not available. For some, this is the sole method used to set children’s MAP levels. It is critical to ensure early and continued optimal auditory input to support development of speech and language in children. Consequently, it is important to determine whether NRT can provide reasonable estimates of T-levels based on consistent detection of soft sounds and C-levels ensuring comfort of loud sounds in most pediatric subjects, as found by Potts et al. for adults. The purpose of our study was to determine if the profile of NRT thresholds could predict the profile of T- and C-levels in children’s well-established MAPs fit according to a protocol and criteria that maximized their ability to hear at school, during audiological testing, at home, and in the outside world.
MATERIALS AND METHODS
Subjects
Participants were forty-one pre/perilinguistically deaf children, who were recipients of the Nucleus 24 cochlear implant system. They had been implanted at least 1 yr before inclusion in the study and had no fluctuation in electrical hearing. In addition, they were selected only if their MAPs were giving them as much benefit as possible according to reports of teachers, parents, and audiologists over a period of months. The children consistently wore their devices during all waking hours, and device malfunction was addressed the same day if possible.
Subject information including gender, etiology, age at NRT, age at implantation, ear of implantation, Nucleus 24 electrode array (CI24M: straight array; CI24R: contour array), and information about the speech processor, speech coding strategy, rate of stimulation, and pulse duration are shown in Table 1. Subjects’ ages at the time of NRT testing ranged from 2 yr 10 mo to 15 yr 3 mo. All were enrolled at one of three auditory-oral schools in St. Louis (Central Institute for the Deaf, The Moog Center for Deaf Education, and St. Joseph Institute for the Deaf).
TABLE 1.
Subjects
| No. | G | Etiology | Age NRT | Age CI | Ear | Array | Processor | Strategy | Rate | MAP PW |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Ototoxic drug | 4;10 | 2;3 | L | Straight | SPrint | ACE | 900 | 25 |
| 2 | F | Unknown | 5;8 | 1;7 | R | Straight | SPrint | ACE | 900 | 25 |
| 3 | F | Ectodermal dysplasia | 6;3 | 1;6 | L | Straight | SPrint | ACE | 900 | 25 |
| 4 | M | Genetic | 6;7 | 1;10 | L | Straight | ESPrit 3G | SPEAK | 250 | 37 |
| 5 | F | Unknown | 6;8 | 3;1 | R | Straight | ESPrit 3G | ACE | 900 | 25 |
| 6 | M | Genetic-Waardenburg | 7;1 | 2;1 | R | Straight | SPrint | ACE | 900 | 25 |
| 7 | F | Unknown | 7;1 | 2;10 | R | Straight | Sprint | ACE | 900 | 25 |
| 8 | F | Unknown | 7;9 | 2;3 | L | Straight | ESPrit 3G | ACE | 900 | 25 |
| 9 | F | Unknown | 7;11 | 3;0 | R | Straight | ESPrit 3G | ACE | 900 | 25 |
| 10 | M | Genetic-Waardenburg | 7;11 | 4;5 | L | Straight | Sprint | ACE | 900 | 25 |
| 11 | M | Unknown | 8;0 | 2;11 | R | Straight | ESPrit 3G | ACE | 900 | 25 |
| 12 | F | Unknown | 8;1 | 3;2 | L | Straight | ESPrit 3G | ACE | 900 | 37 |
| 13 | F | Unknown | 8;9 | 2;2 | R | Straight | Sprint | ACE | 900 | 25 |
| 14 | M | Unknown | 8;9 | 4;1 | L | Straight | ESPrit 3G | ACE | 900 | 25 |
| 15 | M | Unknown | 9;0 | 3;9 | L | Straight | Sprint | ACE | 720 | 37 |
| 16 | F | Unknown | 9;7 | 4;10 | L | Straight | ESPrit 3G | ACE | 900 | 25 |
| 17 | F | Unknown | 10;9 | 6;0 | L | Straight | ESPrit 3G | ACE | 900 | 37 |
| 18 | M | Genetic | 11;6 | 5;11 | R | Straight | ESPrit 3G | ACE | 900 | 25 |
| 19 | F | Meningitis | 13;7 | 9;7 | R | Straight | Sprint | ACE | 900 | 25 |
| 20 | M | Genetic-Connexin 26 | 2;10 | 1;5 | R | Contour | SPrint | ACE | 900 | 25 |
| 21 | M | Genetic-Waardenburg | 3;0 | 1;9 | R | Contour | SPrint | ACE | 900 | 25 |
| 22 | M | Usher, type I | 4;2 | 2;4 | R | Contour | Sprint | ACE | 1200 | 25 |
| 23 | F | Prematurity | 4;6 | 3;6 | L | Contour | SPrint | ACE | 900 | 25 |
| 24 | M | Unknown | 4;6 | 2;2 | L | Contour | Sprint | ACE | 1200 | 25 |
| 25 | M | Unknown | 4;8 | 1;6 | L | Contour | SPrint | ACE | 900 | 25 |
| 26 | F | CMV/maternal virus | 4;11 | 3;1 | L | Contour | SPrint | ACE | 900 | 25 |
| 27 | M | Genetic-Connexin 26 | 4;11 | 1;9 | R | Contour | SPrint | ACE | 900 | 25 |
| 28 | M | Genetic-Connexin 26 | 4;11 | 2;5 | R | Contour | ESPrit 3G | ACE | 900 | 25 |
| 29 | F | Prematurity | 5;0 | 1;4 | L | Contour | SPrint | ACE | 900 | 25 |
| 30 | M | Genetic-Connexin 26 | 5;3 | 2;9 | R | Contour | SPrint | ACE | 900 | 37 |
| 31 | M | Prematurity | 5;10 | 2;7 | L | Contour | Sprint | ACE | 900 | 25 |
| 32 | M | Unknown | 7;3 | 4;6 | R | Contour | ESPrit 3G | ACE | 900 | 25 |
| 33 | F | Unknown | 8;3 | 6;9 | L | Contour | SPrint | ACE | 900 | 25 |
| 34 | F | Unknown | 8;11 | 6;5 | R | Contour | ESPrit 3G | ACE | 1200 | 25 |
| 35 | M | CMV/maternal virus | 12;7 | 2;7 | R | Contour | ESPrit 3G | SPEAK | 250 | 25 |
| 36 | M | CMV/maternal virus | 14;11 | 13;8 | L | Contour | ESPrit 3G | ACE | 900 | 25 |
| 37 | F | Genetic-unknown | 8;9 | 4;4 | R | Straight | Sprint | ACE | 1200 | 25 |
| 38 | F | Genetic-unknown | 10;0 | 5;8 | R | Straight | SPrint | ACE | 900 | 25 |
| 39 | F | Unknown | 12;1 | 6;9 | R | Straight | Esprit 3G | ACE | 1200 | 25 |
| 40 | F | Unknown | 12;3 | 7;4 | R | Straight | Esprit 3G | ACE | 900 | 37 |
| 41 | F | Unknown | 15;3 | 2;0 | L | Straight | Esprit 3G | ACE | 1000 | 25 |
NRT thresholds, obtained at least 1 yr postactivation, could not be obtained from five of the 41 children (subjects 37 to 41 in Table 1) despite the fact that they all received significant benefit from their devices. Of the remaining 36 children, 34 used the Advanced Combination Encoder (ACE) speech coding strategy (30 with a rate of 900 pps, 2 with a rate of 720 pps, and 2 with a rate of 1200 pps on each channel). Two children used the Spectral Peak (SPEAK) strategy with a rate of 250 pps. This distribution of rates reflects those used by Nucleus 24 recipients at the three schools; that is, there is a preponderance of those using a 900-pps rate (over 80%).
Speech Processor Programming
The pediatric audiologists at each school had considerable expertise programming cochlear implants and evaluating the children’s hearing. Many of the children had received all of their device programming since initial activation from these audiologists. Criteria for creating MAPs were strongly influenced by clinical research at nearby Washington University (James, et al., 2003; Potts, et al., 2007; Skinner, et al., 1999). Between initial activation and participation in this study, each child’s speech processor MAP was evaluated as frequently as needed, but typically at least twice yearly. As part of this evaluation, MAPs were created and adjusted as follows. Using a task and terminology appropriate for the child’s ability, T-levels were set where the child responded confidently 100% of the time (above first hearing). When possible, counted thresholds (100% correct) were obtained. For older children, the final T-level was set based on confidence of response, accurate counting, and/or a loudness level of “soft.” C-levels were set using ability-appropriate play techniques or verbal reports and modified loudness scaling tasks. The target sensitivity control setting was equivalent to “12” on the SPrint processor (James, et al., 2003). Further adjustments to T- and C-levels or other MAP parameters were made to improve audibility and discrimination of very soft sounds and to achieve appropriate loudness of conversational and loud sounds. Testing to verify an appropriate fitting included (1) detection or identification of Ling 6 sounds and/or open-set words/ sentences spoken with normal vocal effort live voice at minimum distance of 6 ft without visual cues; (2) detection of sound field, FM tones at levels between 10 and 30 dB HL for octave frequencies between 250 and 4000 Hz; and (3) confirmation of loud but comfortable sounds (e.g., shouting; door slamming). If sound field thresholds were not within the desired range, increases in T-levels, sensitivity control, and/or channel gain were selectively implemented to improve detection. When this goal was reached, the C-levels sometimes needed to be reduced a few levels to prevent sound from being too loud. Further adjustments were made if needed, based on reports from the teacher and/or the child’s parents on his or her hearing in school or in everyday life. The T- and C-levels in this study reflect all of these adjustments in the MAPs.
Equipment
At all three schools, an IBM-compatible computer installed with Nucleus R125 V2.0 programming software and NRT V3.0 software was coupled to a programming interface with the SPrint processor connector. The programming software was used for creating children’s maps, and the NRT software was used for testing with the school’s SPrint speech processor coupled to each child’s own headset.
NRT Measurements
Children were seen twice, approximately 1 mo apart, for NRT measurement. This was performed at their respective schools by the audiologist responsible for their care. Equipment, impedance, and MAPs were checked before NRT testing to address any concerns. During the sessions, the children played games, read a book, or watched a video for entertainment.
The NRT stimulus is not a fixed-rate/fixed-amplitude pulse train. It cycles through four conditions in each sweep (probe, masker + probe, masker, and no stimulus). For the 80 pps NRT stimulus, this group of four stimuli repeats every 1/20 of a second with 10 sweeps in every stimulus presentation. Therefore, the NRT stimulus has a duration of 500 msec (10 sweeps × 1/20 sec = 500 msec). The amplitude varies between the masker and probe stimuli, with the masker stimulus being 10 CL stronger than the probe stimulus.
For this study, the threshold of the ECAP response (characterized by a negative peak [N1] followed by a positive peak [labeled either P2 or P1 in the literature]) was judged both visually (vNRT) and predicted from an AGF (tNRT). Both types of thresholds were obtained at least 1 yr postactivation on 11 active electrodes in each child’s MAP (every other electrode starting with electrode 21 unless an electrode was deactivated, in which case an adjacent electrode was used). The NRT software (v3.0) default stimulus and recording parameters recommended by Abbas et al. (1999) were used unless a response could not be obtained or morphology was poor. If an acceptable response was not obtained, then an optimization series was performed. The parameters used were as follows. A default gain of 60 was used for all electrodes for 25 children. A gain of 40 on all electrodes was used with four children, and a combination of 60 and 40 gain was used for some electrodes for seven children. Recording delays varied from 40 to 120 µsec. Pulse durations were always those used in a child’s MAP (either 25 or 37 µsec/phase) as shown in Table 1. If an acceptable waveform was not found, additional parameter changes were implemented. NRT stimulation rate was the default of 80 Hz for 32 of the children; however, a slower rate of 35 Hz was required for four children on all electrodes. The recording electrode was changed from the default of two electrodes away to three electrodes away from the probe electrode for nine children. This change was made on one, two, or three electrodes for eight children; for one child, this change was made for 10 electrodes. An optimization series was then repeated to determine the best gain and recording delay setting for the new parameter(s).
Once the optimal parameters were determined, an AGF series was performed using two CL steps and the visual NRT threshold (vNRT) identified (see Fig. 1A). The first vertical line marked on the waveform was the N1 amplitude; the second vertical line was the P2 amplitude. Criteria for judging the vNRT response included (1) N1 P2 amplitude > 20 µV; (2) amplifier saturation absent as viewed in the D-buffer; (3) pre-N1 low-resolution waveforms roughly parallel; and (4) for the two low-resolution components, lower waveform N1 precedes upper waveform N1 (see Fig. 1B). NRT responses were used to obtain the predicted NRT threshold (tNRT) using AGF slope for each electrode (see Fig. 1C). If there were fewer than three amplitude estimates for an electrode, then it was not possible to predict tNRT. This occurred for 32 of 397 electrodes tested in this study.
Fig. 1.
Example of NRT waveforms on Electrode 11. A, An AGF series of waveforms for stimulation between 221 and 207 current levels [S3; probe and masker on electrode 11; recording from electrode 13]. The first vertical bar denotes N1; the second one denotes P2. The vNRT was judged to be at 211 current level. B, The two low-resolution waveforms for stimulation at 211 current level [38.4 μV], the level judged to be vNRT. The lower and upper waveforms were associated with slightly earlier and later stimulation, respectively. C, The amplitude of the neural response in μV as a function of the probe stimulus level in current level with six responses from maximal audible loudness (221) to vNRT (211); tNRT and slope calculated at the top of the graph. tNRT is the current level at which the linear regression line crosses the 0 μV axis—209 current level.
Data Analysis
Test-retest stability of the vNRT and tNRT thresholds was determined by calculating for each subject the correlation coefficient for each of these measures for the 9 to 11 electrodes tested approximately 1 mo apart. The group correlation coefficient for vNRT and tNRT was estimated by calculating the arithmetic mean of the individual subject’s correlations for each of these measures. A group correlation coefficient was calculated between vNRT and tNRT, between vNRT and T-level, and between vNRT and C-level to determine the strength of these relations. Given that the range of individual subject’s test-retest correlation coefficients were substantially smaller for vNRT (r = 0.64 to 0.99) than for tNRT (r = 0.31 to 1.00), vNRT values were chosen for further statistical analysis. This choice ensured that NRT measures used in the analyses had more similar and generally higher reliabilities.
The goal of this analysis was to determine if a statistical fit relating vNRT to T- and C-levels for individual children could be used in the future to predict T- and C-levels from vNRT thresholds in the absence of a patient’s behavioral response. The ability of vNRT to adequately predict T- and C-levels was examined using hierarchical linear modeling (HLM; Raudenbush & Bryk, 2002). This approach separates variability in the outcome variable (e.g., C-levels) into within-person and between-person sources. In the present application, the relationship of vNRT to T- or C-levels is estimated at the individual level. Then, the individual-level parameters that define the relationship between vNRT and T- or C-level are examined to determine if the relationship is general to all participants, or demonstrates heterogeneity. The key is not whether there is variability in the individual-level parameters (there will be), but whether that variability represents expected sampling variability or whether it is greater than would be expected and potentially due to betweenperson variables. If significant heterogeneity of parameters is present, specific between-person moderators (e.g., subject age, processor used, etc.) can be tested to determine if they account for parameter variability. In summary, the HLM approach comprises three major steps: (1) estimate the individual-level parameters that define the relation between vNRT and T- or C-level; (2) determine if the variability in those parameters suggests homogeneity (implying a simple and single equation for using vNRT to predict Tor C-level) or heterogeneity (implying that the equations are specific to individuals); and (3) in the event of heterogeneity, test the ability of between-subject variables to account for the diversity of individual-level parameters. Because of the nested nature of the data, maximum likelihood estimation is most appropriate and was used for these analyses (Raudenbush & Bryk, 2002).
HLM begins by regressing T- or C-level on vNRT for each subject. This is known as the level 1 model and represents within-person sources of variability
Cei is the C-level for subject i for electrode e. vNRTei refers to the vNRT value for the same electrode and subject. The vNRT variable was centered so that the regression coefficients then represent the average C-level for a particular person (π0i) and the linear relationship between vNRT and C-level for a particular person (π1i, i.e., the slope). The final component in the equation, errorei, represents the error in prediction of C-level by vNRT for subject i and electrode e. Homogeneity of error variance was assumed for each individual and confirmed by analysis of residuals.
The level 1 parameters (π0i, π1i) are then used as outcomes at the next highest level, called the level 2 model in HLM. This model, in its most basic form, is represented as
The dependent variables at this level are the intercept (π0i) and linear (slope) coefficient (π1i) from the first level. The means of these parameters are estimated by β00 and β10, respectively. When divided by their standard errors, tests of significance result, which indicate if the average C-level (β00) is significantly different from zero (it must be, so this is not an interesting test) and whether the average relation between vNRT and C-level (β10) is different from zero. The variance of the residuals (r0i and r1i), τ00 and τ11, represent the variability of the level 1 parameters and can be tested for homogeneity. If homogeneity is rejected, the implication is that the individuallevel parameters (π0i, π1i) show meaningful (not random) variation and a single and simple equation linking vNRT to C-level cannot be used to represent all subjects’ data. Instead, equations adjusted for subject characteristics are necessary, if they can be identified. In HLM, these more complex relations can be explored by expanding the level 2 model to include subject characteristics
The additional predictor, Si, represents a particular subject’s score on a particular subject characteristic (e.g., age). If the corresponding parameter (e.g., β11), is found to be significant, that would indicate that the level 1 parameter (e.g., π1i) is linearly related to the subject characteristic. The two parameters, β01 and β11, have different implications. A significant β01 would indicate that a subject’s overall C-level (estimated by π0i) depends on a subject’s level on the subject characteristic (Si). A significant β11, however, would indicate that the magnitude of the relation between vNRT and C-level for a particular subject (estimated by π1i) depends on the subject’s level of the subject characteristic (Si). With the new predictor (S) in the equations, the residuals, r0i and r1i, are now reduced because a source of systematic variability has been removed. They can be tested to determine if heterogeneity still exists and additional subject characteristics should be included to account for that variability.
The HLM analysis proceeded by first estimating an intercepts-only model. Then vNRT was added to the level 1 equation and the overall slope was tested for significance. Homogeneity of the level 2 variances was then tested. If heterogeneity was detected, subject characteristics were explored as possible moderators. The HLM approach resembles the principal components approach used by Smoorenburg et al. (2002). Like that approach, HLM identifies parameters that indicate overall magnitude and slope. The advantage to the HLM approach is that it provides a formal test for the heterogeneity of these parameters, and, if heterogeneity is present, it provides a formal way to determine the potential sources of that heterogeneity. In sum, it replaces a largely exploratory approach with one based on strong statistical theory and formal procedures for significance testing.
RESULTS
A total of 41 children participated. For 36 of the 41 children, vNRT was obtained on at least 9 of the 11 electrodes (e.g., subjects 1 to 36 in Table 1). As shown in Table 2, vNRT ECAP thresholds were obtained on all 11 electrodes (21, 19, 17, 15, 13, 11, 9, 7, 5, 3, and 1) for 22 of the 36 children (61%). For 11 of the 36 children (30.5%), vNRT thresholds were obtained on 10 of the 11 electrodes; and for 3 of the 36 (8.4%) children, vNRT thresholds were obtained on 9 of the 11 electrodes. The primary reason vNRT could not be obtained on an electrode was because it was not included in the child’s MAP either because of abnormal electrical impedance, poor sound quality, or facial nerve stimulation. For three children (S11, S21, and S29), an even-numbered probe electrode was substituted for an odd-numbered electrode for which a vNRT could not be obtained. One child (S1) had poor waveform morphology that made determination of vNRT impossible on two electrodes despite various manipulations of parameters. For children with vNRT thresholds that were at or just below a loudest acceptable presentation level (LAPL), it was not possible to obtain waveforms at two higher levels because the stimuli would have been perceived as too loud. For this reason, the voltage associated with three CL was not available to plot the linear regression line. When this occurred, tNRT threshold could not be predicted.
TABLE 2.
Subject’s NRT threshold values, their reliability and relation to MAP T- and C-levels
| Subject | vNRT Mean | vNRT SD | vNRT Minimum | vNRT Maximum | vNRT Count | tNRT mean | Correlation v-tNRT | Average T | Average C | Reliability of vNRT | Reliability of tNRT | Correlation vNRT-T | Correlation vNRT-C | Correlation tNRT-T | Correlation tNRT-C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 216.2 | 10.1 | 200 | 234 | 9 | 206.6 | 0.81 | 161.9 | 193.5 | 0.91 | 0.94 | −0.57 | −0.38 | −0.67 | −0.48 |
| 2 | 191.0 | 4.4 | 183 | 197 | 11 | 189.0 | 0.87 | 167.6 | 208.5 | 0.85 | 0.91 | −0.06 | 0.08 | 0.21 | 0.44 |
| 3 | 207.3 | 4.8 | 199 | 213 | 11 | 205.6 | 0.97 | 182.4 | 226.9 | 0.94 | 0.95 | 0.82 | 0.95 | 0.83 | 0.97 |
| 4 | 197.6 | 5.7 | 188 | 209 | 10 | None | 0.99 | 170.1 | 203.4 | 0.98 | − | −0.65 | −0.36 | −0.63 | −0.30 |
| 5 | 177.0 | 9.1 | 165 | 192 | 10 | 172.4 | 0.97 | 128.0 | 163.0 | 0.98 | 0.94 | − | 0.66 | − | 0.69 |
| 6 | 196.4 | 6.0 | 187 | 208 | 10 | 189.1 | 0.89 | 169.1 | 207.3 | 0.77 | 0.98 | 0.87 | 0.74 | 0.99 | 0.92 |
| 7 | 182.2 | 3.3 | 177 | 187 | 10 | 181.2 | 0.99 | 164.6 | 205.0 | 0.91 | 0.91 | 0.55 | 0.03 | 0.49 | 0.07 |
| 8 | 212.4 | 5.2 | 206 | 226 | 10 | 207.6 | 0.87 | 192.3 | 224.7 | 0.88 | 0.85 | 0.35 | 0.83 | 0.60 | 0.70 |
| 9 | 186.0 | 5.5 | 176 | 192 | 10 | 182.0 | 0.96 | 139.6 | 208.8 | 0.96 | 0.99 | 0.10 | 0.91 | 0.02 | 0.90 |
| 10 | 186.3 | 8.6 | 174 | 207 | 11 | 184.9 | 0.99 | 142.6 | 182.5 | 0.99 | 0.99 | 0.99 | 0.96 | 0.97 | 0.96 |
| 11 | 196.9 | 7.0 | 186 | 207 | 10 | 189.5 | 0.94 | 143.3 | 195.0 | 0.87 | 0.91 | 0.45 | 0.09 | 0.39 | 0.22 |
| 12 | 186.4 | 2.7 | 182 | 190 | 11 | 185.6 | 0.88 | 188.2 | 215.2 | 0.86 | 0.93 | 0.36 | 0.33 | 0.24 | 0.11 |
| 13 | 194.3 | 5.2 | 188 | 203 | 11 | 190.1 | 0.98 | 151.8 | 193.7 | 0.97 | 0.87 | 0.41 | 0.41 | 0.35 | 0.36 |
| 14 | 192.5 | 8.0 | 184 | 206 | 11 | 188.2 | 0.93 | 162.2 | 188.2 | 0.97 | 0.91 | −0.70 | −0.70 | −0.66 | −0.63 |
| 15 | 217.2 | 3.7 | 212 | 225 | 11 | 213.9 | 0.97 | 196.7 | 225.3 | 0.81 | 0.47 | 0.49 | 0.61 | 0.48 | 0.54 |
| 16 | 177.6 | 7.1 | 164 | 187 | 9 | 175.6 | 0.98 | 143.2 | 177.2 | 0.97 | 0.97 | −0.31 | −0.31 | −0.37 | −0.37 |
| 17 | 180.6 | 4.2 | 173 | 188 | 11 | 177.7 | 0.91 | 152.1 | 187.1 | 0.95 | 0.86 | −0.35 | −0.08 | −0.01 | 0.30 |
| 18 | 184.7 | 4.3 | 178 | 191 | 11 | 180.8 | 0.95 | 158.9 | 203.5 | 0.95 | 0.86 | −0.12 | 0.52 | 0.14 | 0.70 |
| 19 | 189.2 | 3.5 | 185 | 197 | 10 | 182.4 | 0.87 | 157.2 | 196.5 | 0.72 | 0.32 | −0.26 | 0.20 | −0.36 | 0.29 |
| 20 | 187.6 | 6.0 | 179 | 198 | 11 | 182.8 | 0.92 | 157.7 | 194.5 | 0.92 | 0.96 | −0.19 | 0.02 | −0.12 | 0.12 |
| 21 | 203.8 | 5.0 | 195 | 213 | 10 | 202.4 | 0.86 | 168.4 | 186.2 | 0.64 | 0.31 | 0.02 | −0.33 | 0.00 | −0.34 |
| 22 | 184.6 | 5.7 | 178 | 192 | 9 | 181.5 | 0.87 | 148.7 | 188.8 | 0.85 | 0.91 | −0.12 | −0.44 | 0.28 | −0.21 |
| 23 | 180.3 | 14.6 | 158 | 200 | 11 | 176.3 | 0.99 | 148.8 | 187.4 | 0.99 | 1.00 | 0.76 | 0.44 | 0.79 | 0.47 |
| 24 | 179.8 | 4.7 | 170 | 186 | 11 | 178.4 | 0.92 | 165.1 | 205.1 | 0.92 | 0.95 | −0.83 | −0.83 | −0.61 | −0.61 |
| 25 | 181.2 | 5.9 | 168 | 188 | 11 | 175.6 | 0.9 | 126.6 | 180.8 | 0.64 | 0.75 | −0.41 | −0.28 | −0.58 | −0.39 |
| 26 | 176.7 | 4.3 | 166 | 180 | 11 | 174.1 | 0.97 | 125.1 | 160.5 | 0.88 | 0.92 | 0.45 | 0.34 | 0.54 | 0.44 |
| 27 | 182.5 | 6.7 | 170 | 195 | 11 | 177.4 | 0.89 | 147.7 | 196.5 | 0.75 | 0.96 | 0.04 | 0.39 | 0.07 | 0.26 |
| 28 | 171.5 | 9.5 | 156 | 184 | 11 | 168.7 | 0.98 | 136.5 | 174.4 | 0.95 | 0.95 | −0.17 | −0.17 | −0.10 | −0.10 |
| 29 | 196.6 | 11.7 | 181 | 211 | 10 | 191.9 | 0.95 | 168.3 | 213.0 | 0.95 | 0.94 | 0.22 | −0.03 | 0.37 | 0.05 |
| 30 | 185.9 | 6.1 | 174 | 195 | 10 | 183.0 | 0.94 | 159.5 | 192.1 | 0.88 | 0.99 | 0.63 | 0.67 | 0.79 | 0.83 |
| 31 | 171.5 | 10.6 | 154 | 186 | 11 | 167.5 | 0.98 | 126.9 | 155.3 | 0.99 | 0.97 | 0.80 | 0.64 | 0.73 | 0.54 |
| 32 | 180.5 | 10.1 | 166 | 198 | 11 | 178.2 | 0.99 | 135.7 | 190.4 | 0.99 | 0.98 | 0.61 | 0.04 | 0.58 | 0.08 |
| 33 | 186.5 | 23.0 | 154 | 220 | 11 | 180.0 | 1 | 145.5 | 191.5 | 0.95 | 0.96 | 0.84 | 0.92 | 0.84 | 0.92 |
| 34 | 188.4 | 6.1 | 180 | 196 | 11 | 183.2 | 0.95 | 167.2 | 198.5 | 0.96 | 0.95 | 0.20 | 0.49 | 0.24 | 0.53 |
| 35 | 191.1 | 6.1 | 178 | 198 | 11 | 187.1 | 0.83 | 177.2 | 209.3 | 0.94 | 0.95 | 0.39 | 0.25 | 0.80 | 0.70 |
| 36 | 177.7 | 6.6 | 166 | 186 | 11 | 175.3 | 0.99 | 171.2 | 198.3 | 0.93 | 0.86 | 0.75 | −0.18 | 0.67 | −0.17 |
| Mean | 188.8 | 7 | 178 | 200 | 11 | 184.7 | 0.93 | 157 | 195 | 0.90 | 0.88 | 0.18 | 0.21 | 0.24 | 0.26 |
| Minimum | 171.5 | 2.7 | 154 | 180 | 9 | 167.5 | 0.81 | 125 | 155 | 0.64 | 0.31 | −0.83 | −0.83 | −0.67 | −0.63 |
| Maximum | 217.2 | 23.0 | 212 | 226 | 11 | 213.9 | 1.00 | 197 | 227 | 0.99 | 1.00 | 0.99 | 0.96 | 0.99 | 0.97 |
The five children (subjects 37 to 41; Table 1) for whom a vNRT could not be obtained on any electrode tested, ranged in age from 8 to 15 yr. Four of the five children were implanted fairly late (between ages 5 and 7 yr). The other (S41), who was originally implanted with the Nucleus 22 at 2 yr, was reimplanted with the Nucleus 24 at 13 yr. Subject 39 had LAPLs for the 80-Hz rate, 25 μsec/phase stimulus that were very close to her C-levels; even at slower rates, longer pulse durations, and optimized recording parameters, vNRT could not be obtained. Subjects 37 and 38 had LAPLs that were substantially above the C-levels in their MAPs, but not sufficiently high enough to obtain a vNRT. Subject 40 had facial nerve stimulation for stimuli just above the C-levels in her MAP; consequently, the loudness of the maximum level that could be used for NRT testing was only “soft to medium.”
Test-Retest Reliability
The reliability of the vNRT and tNRT measures was calculated for each child from data on the tested electrodes obtained approximately 1 mo apart. These correlation coefficients for each child are shown in Table 2. The average correlation coefficient across subjects was high for vNRT (r = 0.90, range = 0.64 to 0.99) and tNRT (r = 0.88, range = 0.31 to 1.00).
Relation of vNRT and tNRT Thresholds
The mean vNRT and tNRT thresholds across electrodes for each child are shown in Table 2. The group mean tNRT was 4.0 CL less than for vNRT. This likely occurred because vNRT threshold is based on an N1P2 amplitude of no less than 20 μV, whereas the tNRT N1P2 amplitude is 0 μV. The correlation coefficients between vNRT and tNRT across electrodes for each child are shown in Table 2. The average correlation between vNRT and tNRT across subjects was high (0.93) and quite uniform across subjects (range 0.81 to 1.00).
Relation of NRT Thresholds with T- and C-Levels
The correlation coefficients between vNRT and T-level, vNRT and C-level, tNRT and T-level, and tNRT and C-level are shown for individual children and the group in Table 2. The range of r values for each of these four pairs of values for the 36 children was substantial and nearly spanned the possible range for correlations (r values ranging from 0.99 to 0.83, 0.96 to 0.83, 0.99 to 0.67, and 0.97 to 0.63, respectively). For the high positive correlations, the profiles of the NRT and T- or C-levels correspond in the sense that increasing levels on one are closely associated with increasing levels on the other. For the high negative correlations, however, these profiles move in opposite ways, with increasing levels for one profile being closely associated with decreasing levels for the other. High correlations of either sign can, in principle, provide useful prediction, but the more troublesome feature of these data is the wide variability in the correlations across subjects indicating that consistent prediction of behavioral measures from NRT thresholds is not possible (the HLM analysis examines this more systematically). The group average correlations between vNRT and T-level, vNRT and C-level, tNRT and T-level, and tNRT and C-level were low (r values of 0.18, 0.21, 0.24, and 0.26, respectively).
Figure 2 shows group mean vNRT and tNRT thresholds in comparison to group mean T- and C-levels for individual electrodes across the array. For apical electrodes 21, 19, and 17, vNRT and tNRT thresholds are closer to the middle of the MAP dynamic range between T- and C-level, whereas for electrodes 13, 11, 9 and 7 they are close to C-level.
Fig. 2.
Group mean vNRT and tNRT plotted within the MAP dynamic range from T-level to C-level on electrodes 21 through 5.
Relation of NRT Thresholds to T- and C-Levels in Individual Children
The comparison of group mean vNRT and tNRT to T-level and to C-level as a function of electrode shown in Figure 2 does not convey the large variation in these measures for individual children. In Figure 3, CL for vNRT, T- and C-level on each tested electrode have been plotted for six children. For each child, the fitted third-order polynomials are shown as a way to highlight the major trends in these data. The profile for vNRT for subject 12 follows profiles for T- and C-level. The vNRT profile intertwines with that for the T-levels. To select C-levels in the MAP for this subject, it would be possible to use the same CL as at vNRT and increase this level equally on all electrodes (global change in the software) in the live-voice mode to determine an appropriate loudness level at which to set C-levels across electrodes. For subject 13, the vNRT profile intertwines with the C-level profile, and fairly closely approximates the T-level profile. To select the T-levels based on the vNRT profile, it would be essential to start at a substantially lower level and globally increase the levels across electrodes to determine where to set T-levels and C-levels in the MAP. Because one cannot predict the loudness of stimulation at vNRT threshold, it is essential to set T-levels using behavioral responses to stimuli below vNRT threshold. Subjects 30 and 10 (whose data are shown in the middle panel of Fig. 3) have vNRT profiles that follow the T-level but not the C-level profiles. Subjects 8 and 19 (whose data are shown in the lower panel of Fig. 3) have NRT thresholds that follow the C-level profiles but not the T-level profiles. For these four subjects, the vNRT profile would be appropriate for the T- or C-level profile but not both. Therefore, fine tuning of Ts and Cs in the MAP would have to be done based on behavioral responses.
Fig. 3.
Profiles of vNRT, MAP T-levels and MAP C-levels across electrodes plotted for six subjects (Ss 12, 13, 30, 10, 8, and 19). Third-order polynomials have been fitted to relate electrode number to vNRT, T- and C-levels for each subject.
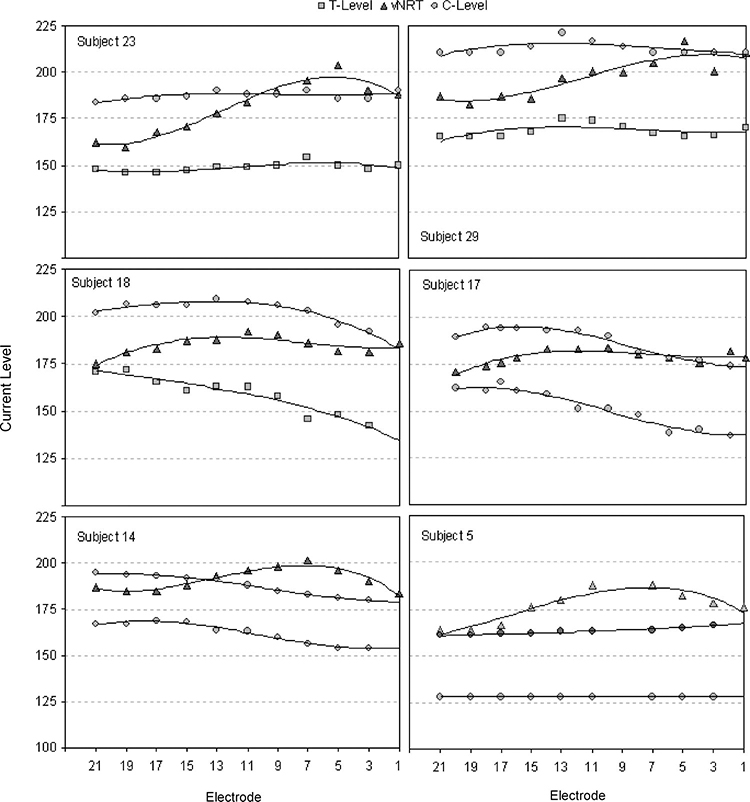
Figures 4 and 5 show examples of children for whom the vNRT profiles do not approximate either the T- or the C-level profiles. Most of the children in the study fall into this category. For these children, using a behavioral estimate of the relation of T- and C-levels to vNRT on one electrode and then applying the shift in CL between vNRT and T- and C-level on this electrode to all other electrodes would not provide a MAP that approximates the T- or C-profiles in their MAPs.
Fig. 4.
Profiles of vNRT, MAP T-levels and MAP C-levels across electrodes plotted for six subjects (Ss 23, 29, 18, 17, 14, and 5). Third-order polynomials have been fitted to relate electrode number to vNRT, T- and C-levels for each subject.
Fig. 5.
Profiles of vNRT, MAP T-levels and MAP C-levels across electrodes plotted for six subjects (Ss 1, 33, 11, 28, 2, and 24). Third-order polynomials have been fitted to relate electrode number to vNRT, T- and C-levels for each subject.
In the group of 36 children, vNRT thresholds fell below C-levels for 70% of electrodes tested, and there was considerable variability in the position of this vNRT threshold within the MAP range from T- to C-level. Four children had vNRT thresholds above C-level across all 11 electrodes, and 11 children had vNRT thresholds below C-level across all 11 electrodes. Twenty-five of the 36 children (69%) had vNRT that was above C-level on at least one electrode.
Statistical Analysis of the Relation of Individual Subjects’ vNRT, MAP T-, and MAP C-Level
As shown in Table 2, and illustrated in Figures 3–5, there is a wide range of correlations for individual subjects between both vNRT and T-level and vNRT and C-level. This variation seems substantial, but some sampling variation would be expected and it is important to determine if the variation is expected random variability or suggestive of underlying systematic moderator variables. In the former case, a single prediction equation linking vNRT and C-levels (or T-levels) is implicated, although the variability suggests it might not be very precise. On the other hand, if the variability is greater than would be expected by simple sampling variation, then a search for moderators is indicated and an improved prediction equation could be possible by taking subject characteristics into account.
Table 3 summarizes the results of the HLM analysis of Tand C-levels. For both outcomes, vNRT was a significant predictor (i.e., β10 was significantly greater than 0). Furthermore, the level 2 residual variances for both level 2 outcomes were significant. These results indicate that for both T-and C-levels, vNRT was a significant overall predictor, but the degree to which vNRT predicted T- and C-levels varied significantly across subjects. For example, the upper panel of Table 3 shows that the average T-level was 156.12 and the average slope relating vNRT to T-levels was 0.18. But, both the mean T-level and the vNRT slope had significant variation. Especially important, the variance component for the vNRT slope (τ11 = 0.15) indicates that the individual-level slopes have a 95% confidence interval that extends from 0.61 to 0.97. Clearly, a single equation cannot be used to describe a simple relation between vNRT and T-levels across subjects. A similar conclusion is indicated by the lower panel in Table 3. The overall C-level was 193.96 and the average slope relating vNRT to C-levels was 0.24. Here too, however, both parameters had significant variability, with the variance component for vNRT slope (τ11 = 0.17) indicating a 95% confidence interval that extends from −0.60 to 1.08.
Table 3.
Hierarchical linear model analysis summary for T-levels and C-levels predicted from vNRT.
| T-Levels | ||||
| Fixed Effect | Coefficient | SE | t | p |
| Mean T | ||||
| β00 | 156.12 | 2.71 | 57.61 | <.001 |
| vNRT | ||||
| β10 | 0.18 | 0.08 | 2.32 | 0.026 |
| Variance | ||||
| Random Effect | Component | df | χ2 | p |
| Mean T | ||||
| r0i | 263.65 | 35 | 1299.63 | <.001 |
| vNRT | ||||
| r1i | 0.15 | 35 | 126.66 | <.001 |
| C-Levels | ||||
| Fixed Effect | Coefficient | SE | t | p |
| Mean C | ||||
| β00 | 193.96 | 2.36 | 82.23 | <.001 |
| vNRT | ||||
| β10 | 0.24 | 0.08 | 3.08 | 0.004 |
| Variance | ||||
| Random Effect | Component | df | χ2 | p |
| Mean C | ||||
| r0i | 200.06 | 35 | 1356.98 | <.001 |
| vNRT | ||||
| r1i | 0.17 | 35 | 156.73 | <.001 |
The heterogeneity of the level 2 residuals indicated significant variability in the level 1 parameters. To determine if this variation could be accounted for by subject variables, the level 2 models were modified to allow each of the measured subject variables to be tested as an additional predictor. The subject variables tested in these analyses were gender, ethnicity (white or non-white), processor (SPrint or ESPrit 3G), speech coding strategy (ACE or SPEAK), internal device (CI24M or CI24R), array (straight or contour), ear implanted, rate of stimulation, pulse duration, and age at NRT. Each was tested in a separate analysis and because of the large number of tests conducted and their exploratory nature, a Bonferroni-corrected significance level (p = 0.005) was used. None of the subject variables was found to be a significant predictor of either the mean Tor C-level or of the vNRT slopes. In other words, subjects varied substantially and significantly in their vNRT slopes, but none of this variability could be accounted for by the subject characteristics measures in this study. Clearly, single and simple equations for using vNRT to predict T- and C-levels are not viable and subject-adjusted equations could not be identified using the subject characteristics measured in this study.
DISCUSSION
The results of this study provide important information about the relation between the T- and C-level from everyday MAPs, and thresholds obtained for vNRT and tNRT as a function of individual electrodes for children implanted with the Nucleus 24 electrode array. Although NRT has typically been related to initial MAP generation, this study sought to determine whether NRT thresholds could provide reasonable estimates of stable T- and C-levels as found in everyday MAPs. Because of the typical need to adjust MAPs to improve sound quality and to optimize perception of soft and loud sounds, it was valuable to assess this relation in our population.
The positions of vNRT and/or tNRT thresholds in the upper half of the dynamic range between T- and C-levels for the group of children in this study agrees with those of previous studies with children (Hughes, et al., 2000) and adults (Brown, et al., 2000; Cafarelli Dees, et al., 2005; Smoorenburg, et al., 2002). In addition, all of these studies have found large intersubject variability in the position of NRT threshold in relation to T- and C-levels as well as intrasubject variability in NRT threshold, T- and C-levels across electrodes. This variability is documented for the present study in Table 2 and in Figures 3–5. As shown in these figures for 18 children, the position of the vNRT profile in relation to the profiles for Tand C-levels may be shifted, so that it superimposes on the T-level profile (S12), superimposes on C-level profile (S13), is above the C-level profile (S11) or is between the T- and C-level profiles (S8). In addition, the tilt of the vNRT profile may slope upward in relation to the T- and C-level profiles (S18), slope downward in relation to the T- and C-level profiles (S 24), or be parallel to them (S12). Similar patterns are shown for the 12 adults in the study by Potts et al. (2007). However, there is one important difference between the two studies. In the Potts et al. study, only one of 12 adults had a negative correlation between vNRT and T- or C-level, whereas in the present study, negative correlations occurred for 13 of 36 children between vNRT and T-level for 12 of 36 children between vNRT and C-level.
The same protocol was used for obtaining vNRT thresholds in the present study and that of Potts et al. (2007). In the present study, 16 children implanted with the Nucleus contour electrode array used either 900 or 1200 Hz stimulation rates in their MAPs; the group mean vNRT thresholds across electrodes ranged from ~179 to 190 CL. In the Potts et al. study, in which all subjects were implanted with the Nucleus contour array, the group mean vNRT thresholds for the four adults who used 900 or 1200 Hz stimulation rates in their MAPs ranged from 171 to 195 CL. These ranges for adults and children are relatively close and overlapping.
During data collection for this study, vNRT thresholds were obtained on most of the electrodes tested. Nevertheless, vNRT thresholds could not be obtained on 12 percent (5 of 41) of the children, some of whom have substantial open-set speech recognition. It is not known whether the lack of measurable thresholds was due to the function of the telemetry circuit of the internal receiver/stimulator or due to the lack of a physiological response from the child. Given this result, the lack of vNRT threshold response does not necessarily mean that the device is not working properly or that the child has no auditory response with the implant. For 39% (14 of 36) of the children, vNRT thresholds could not be obtained on one or two electrodes out of the 11 tested because of poor waveform morphology. Clinically, none of the electrodes for which vNRT threshold could not be obtained had been deactivated in the children’s MAPs. It is clear that further research is needed to determine whether or not the absence of a measurable vNRT threshold is associated with stimulation of spiral ganglion cells that is aberrant compared to electrodes for which a measurable vNRT threshold is obtained.
At a number of children’s cochlear implant centers, ECAP NRT thresholds are obtained during the programming of speech processors for very young children and recipients who are unable or not expected to give appropriate feedback using behavioral fitting methods alone. The profile of NRT thresholds is used in conjunction with live-voice testing and shifting levels globally from well below estimated behavioral threshold to a level at which a behavioral response is first seen and then to a level estimated to be appropriate for the C-level profile. Given the large variability of the overall level and tilt of the NRT profile in relation to those of T- and C-level files from individuals’ MAPs described in this study and by Potts et al. (2007), it is important to progress beyond the NRT-based MAP and to individualize and optimize the MAP using behavioral measures as quickly as possible. Because CL corresponding to NRT thresholds produce audible sounds, these thresholds can be used within the programming software to train the child to perform visual reinforcement audiometry and conditioned play audiometry tasks. The fine tuning of a young child’s MAP will require frequent visits early in programming as additional information is gathered on electrodes over time, and the child becomes more consistent in responding. Maximum benefit from the cochlear implant may not be achieved if this optimization is replaced by time-saving estimation methods.
As Abbas et al. (2006) have indicated, ECAP NRT thresholds obtained during the first few months of stimulation can serve as a valuable baseline with which to monitor the child’s neural responsiveness at subsequent intervals. If a child is not receiving as much benefit as expected, it is important that this information be coupled with measurements that reflect functioning of the device, that is, electrical impedance, average electrode voltages (Hughes, et al., 2004), and an integrity test, to make every effort for a child to receive appropriate information from the implant.
In summary, the present study suggests that when programming cochlear implant devices in children, ECAP measures should be supplemented with careful and frequent behavioral measures to assist in the optimization of speech processor programs for a given child.
ACKNOWLEDGMENTS
We are grateful to the 41 children and their parents who participated in this study and to the Directors, Principals, and staff at Central Institute for the Deaf, The Moog Center for Deaf Education and St. Joseph Institute for the Deaf for their support. We also thank Carolyn J. Brown, Jill Firszt, Ruth Reeder, Gail Donaldson, and two anonymous reviewers for their comments on an earlier draft of this manuscript. The protocol for this study was approved by the Human Research Protection Committee at Washington University School of Medicine.
This research was funded by grant #R01 DC000581 from the National Institute on Deafness and Other Communication Disorders.
REFERENCES
- Abbas PJ, Brown CJ, & Etler CP (2006). Electrophysiology and device telemetry In Waltzman SB & Cohen NL (Eds.), Cochlear implants (2nd ed., pp. 96–109). New York, NY: Thieme Medical Publisher. [Google Scholar]
- Abbas PJ, Brown CJ, Shallop J, et al. (1999). Summary of results using the Nucleus CI24M implant to record the electrically evoked compound action potential. Ear Hear, 20, 45–59. [DOI] [PubMed] [Google Scholar]
- Brown CJ (2003). The electrically evoked whole nerve action potential In Cullington HE (Ed.), Cochlear implants: objective measures (pp. 96–129). London: Whurr Publishers. [Google Scholar]
- Brown CJ, Hughes ML, Luk B, et al. (2000). The relationship between EAP and EABR thresholds and levels used to program the Nucleus 24 speech processor: data from adults. Ear Hear, 21, 151–163. [DOI] [PubMed] [Google Scholar]
- Cafarelli Dees D, Dillier N, Lai WK, et al. (2005). Normative findings of electrically evoked compound action potential measurements using the neural response telemetry of the Nucleus CI24M cochlear implant system. Audiol Neurootol, 10, 105–116. [DOI] [PubMed] [Google Scholar]
- Dillier N, Lai WK, Almqvist B, et al. (2002). Measurement of the electrically evoked compound action potential (ECAP) via a neural response telemetry (NRT) system. Ann Otol Rhinol Laryngol, 111, 407–414. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Papsin BC, & Harrison TV (2004). Toward a battery of behavioral and objective measures to achieve optimal cochlear implant stimulation levels in children. Ear Hear, 25, 447–463. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Brown CJ, & Abbas PJ (2004). Sensitivity and specificity of averaged electrode voltage (AEV) measures in cochlear implant recipients. Ear Hear, 25, 431–446. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Brown CJ, Abbas PJ, et al. (2000). Comparison of EAP thresholds with MAP levels in the Nucleus 24 cochlear implant: data from children. Ear Hear, 21, 164–174. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Vander Werff KR, Brown CJ, et al. (2001). A longitudinal study of electrode impedance, the electrically evoked compound action potential, and behavioral measures in Nucleus 24 cochlear implant users. Ear Hear, 22, 471–486. [DOI] [PubMed] [Google Scholar]
- James CJ, Skinner MW, Martin LF, et al. (2003). An investigation of input level range of the Nucleus 24 cochlear implant system: speech perception performance, program preference, and loudness comfort ratings. Ear Hear, 24, 157–174. [DOI] [PubMed] [Google Scholar]
- Mueller HG, & Killion MC (1990). An easy method for calculating the articulation index. Hear J, 43, 14–17. [Google Scholar]
- Pavlovic CV, Studebaker GA, & Sherbecoe RL (1985). An articulation index based procedure for predicting the speech recognition performance of hearing-impaired individuals. J Acoust Soc Am, 80, 50–57. [DOI] [PubMed] [Google Scholar]
- Pavlovic CV (1988). Articulation index predictions of speech intelligibility in hearing aid selection. ASHA, 30, 63–65. [PubMed] [Google Scholar]
- Potts LG, Skinner MW, Gotter BD, et al. (2007). Relation between neural response telemetry thresholds, MAP T and C levels, and loudness judgments in 12 adult Nucleus 24 cochlear implant recipients. Ear Hear, 28, 495–511. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, & Bryk AS Hierarchical linear models: applications and data analysis methods (2nd ed.). Thousand Oaks: SAGE. [Google Scholar]
- Seyle K, & Brown CJ (2002). Speech perception using maps based on neural response telemetry measures. Ear Hear, 23, 72S–79S. [DOI] [PubMed] [Google Scholar]
- Skinner MW, Holden LK, Demorest ME, et al. (1995). Comparison for obtaining thresholds and maximum acceptable loudness levels with the nucleus cochlear implant system. J Speech Hear Res, 38, 677–689. [DOI] [PubMed] [Google Scholar]
- Skinner MW, Holden TA, Holden LK, et al. (1999). Comparison of two methods for selecting minimum stimulation levels used in programming the Nucleus 22 cochlear implant. J Speech Lang Hear Res, 42, 814–828. [DOI] [PubMed] [Google Scholar]
- Smoorenburg GF, Willeboer C, & van Dijk JE (2002). Speech perception in Nucleus CI24M cochlear implant users with processor settings based on electrically evoked compound action potential thresholds. Audiol Neurootol, 7, 335–347. [DOI] [PubMed] [Google Scholar]
- Willeboer C, & Smoorenburg GF (2006). Comparing cochlear implant users’ speech performance with processor fittings based on conventionally determined T and C levels or compound action potential thresholds and live-voice speech in a prospective balanced crossover study. Ear Hear, 27, 789–798. [DOI] [PubMed] [Google Scholar]
- Zimmerling MJ, & Hochmair ES (2002). EAP recordings in Ineraid patients—correlations with psychophysical measures and possible implications for patient fitting. Ear Hear, 23, 81–91. [DOI] [PubMed] [Google Scholar]