Abstract
Partitioning-defective 1 (PAR-1), a conserved cell polarity regulator, plays an important role in synaptic development, and its mutation affects the formation of synaptic boutons and localization of postsynaptic density protein Discs large (Dlg) at the neuromuscular junction (NMJ) in Drosophila. Drosophila PAR-1 and its human homolog, Microtubule affinity-regulating kinases (MARK), are also known to be implicated in Alzheimer’s disease (AD) by controlling tau-mediated Aβ toxicity. However, the molecular mechanisms of PAR-1 function remain incompletely understood. Here we identified Pod-1, an actin-microtubule crosslinker, which functionally and physically interacts with PAR-1 in Drosophila. Pod-1 prominently co-localizes with PAR-1 in the postsynaptic region and regulates PAR-1 activity at the NMJ. Synaptic defects, including the reduction of boutons and delocalization of Dlg caused by PAR-1 overexpression, were rescued by Pod-1 knockdown. Conversely, the reduction of synaptic boutons in PAR-1 overexpressed NMJ was synergistically enhanced by the overexpression of Pod-1. Furthermore, Pod-1 increases the PAR-1 dependent S262 phosphorylation of tau, which is known to contribute to tau-mediated Aβ toxicity. In line with the change of tau phosphorylation, Pod-1 knockdown rescued tau-mediated synaptic toxicity at the NMJ. Our results suggest that Pod-1 may act as a modulator of PAR-1 in synaptic development and tau-mediated toxicity.
Keywords: PAR-1, Pod-1, Synaptic defect, Tau-mediated synaptic toxicity, NMJ, Drosophila
1. Introduction
Precise organization of cytoskeleton required for diverse cellular functions, such as cell division, migration, membrane trafficking, and morphogenesis, is essential for establishing functional neural networks (Kapitein and Hoogenraad, 2015). In the nervous system, cytoskeletons determine the neuronal shape and polarity and provide physical support to build up the architecture. Moreover, the cytoskeleton undergoes dynamic remodeling to regulate exploratory movement through molecular rearrangement underlying axon guidance or synapse formation (Lasser et al., 2018). Thus, to understand the role of the cytoskeleton in the development and function of the nervous system, a range of cytoskeleton binding proteins and regulators have been extensively studied, revealing that the dysfunctions of cytoskeleton-related proteins are tightly linked to developmental defects and neurodegenerative diseases (Lasser et al., 2018).
Coronins are an evolutionarily conserved family of actin cytoskeleton regulators whose functions have been studied extensively in the nervous system (de Hostos et al.,1991). All coronin homologs share the basic N terminal motif and WD repeats. They also contain a unique region of variable length conferring specific features for each coronin protein (Liu et al., 2016) (Rybakin and Clemen, 2005). They play important roles in actin-dependent processes, including cell motility, morphogenesis, and trafficking (Uetrecht and Bear, 2006) (Chan et al., 2011). In mammals, 7 isoforms of Coronin have been reported, most of which show tissue-specific expression mainly in the nervous system (Chen et al., 2014) (Liu et al., 2016). In mouse neurons, Coronin 1 prevents the fusion of the retrograde signaling endosome with lysosomes and instead facilitates the recycling of the signaling endosome (Suo et al., 2014). In addition, deletion or mutation of coronin 1 leads to neurobehavioral disabilities, including social deficits, increased aggression, and learning defects, in both mice and humans (Jayachandran et al., 2014). Coronin 6 is localized at the neuromuscular junction (NMJ) and regulates acetylcholine receptor clustering by modulating the interaction between receptors and the actin cytoskeleton in mice (Chen et al., 2014). In C.elegans and Drosophila, Coronins were also known to be involved in the control of neurodevelopment. The C.elegans Coronin regulates cell morphology by coordinating the actin organization in postembryonic neuroblast migration (Chen et al., 2014). The Drosophila homolog of Coronin, Pod-1, which can crosslink actin and microtubule cytoskeletons, is localized to the tips of growing axons and is required for targeting of axons (Rothenberg et al., 2003). Pod-1 is also known to function in glutamate receptor cluster formation at the Drosophila NMJ (Liebl and Featherstone, 2008).
PAR-1, the Drosophila homolog of mammalian MARK, is a conserved serine/threonine kinase with multiple domains, including catalytic and protein-protein interaction domains, and was initially identified as a regulator of the microtubule cytoskeleton. The cellular functions of PAR-1 and its homologs include establishing cell polarity and asymmetric cell division in multiple cell types and organisms (Guo and Kemphues, 1995) (Penton et al., 2002). Especially in the NMJ of Drosophila, PAR-1 was known to affect the formation and function of the synapse (Zhang et al., 2007). PAR-1 regulates the postsynaptic scaffold protein Disc large (Dlg), the Drosophila homolog of mammalian PSD-95, which recruits synaptic proteins and assembles them into postsynaptic complexes. It directly phosphorylates the GUK domain of Dlg and negatively regulates the postsynaptic targeting of Dlg (Zhang et al., 2007). Additionally, PAR-1 phosphorylates tau at the S262 and S356 residues, thereby implicating dysregulation of PAR-1 in Alzheimer’s disease (AD)-related tau hyperphosphorylation and toxicity (Nishimura et al., 2004) (Ando et al., 2016). The phosphorylation of T408 residue of PAR-1 by the tumor suppressor protein LKB1 is required for PAR-1 activation in vivo. Previous studies have shown that overexpression of LKB1 strongly promotes PAR-1 phosphorylation at T408 residue and enhances PAR-1-induced neurodegeneration, whereas inhibition of LKB1 has the opposite effect (Wang et al., 2007). Moreover, activated phospho-PAR-1 is a common substrate for both Skp-Cullin-F-box (SCF)-containing E3 ligase, Slimb, and the deubiquitinating enzyme, Fat facets (FAF), in synaptic development. The control of PAR-1 kinase activity through Slimb and FAF also affects tau-induced synaptic toxicity (Lee et al., 2012).
Here, we reveal another layer of regulation for PAR-1 activity. We identify Pod-1, the actin/MT crosslinker, as a novel regulator of PAR-1 in synaptic development and tau-mediated synaptic toxicity. Pod-1 physically interacts with PAR-1 and its downregulation rescues the synaptic defects caused by PAR-1 overexpression. Furthermore, we provide evidence that the downregulation of PAR-1 activity through pod-1 knockdown influences tau-induced synaptic toxicity. Our findings suggest that the modulation of PAR-1 activity through the inhibition of actin/MT crosslinker Pod-1 could be a potential target for AD therapies.
2. Materials and methods
2.1. Drosophila strains
The w1118 strain was used as the wild-type control. The UAS-PAR-1-WT, UAS-PAR-1-T408 A, and UAS-htauM were described previously (Lee et al., 2012). The Mhc-GAL4 fly line was provided by Dr. T Littleton. The FAFEP381, UAS-Pod-1-WT, UAS-Pod-1-RNAi, UAS-Slimb-RNAi, and GMR-GAL4 lines were obtained from Bloomington Drosophila Stock Center. The flies were maintained at 25 °C on standard Drosophila food.
2.2. Immunohistochemistry imaging
For immunostaining, third instar larvae were dissected in PBS, fixed in 4% formaldehyde (Ted Pella) in PBS for 15 minutes and washed 4 times with 0.1% Triton X-100 in PBS. The fixed samples were incubated with primary antibodies at 4 °C overnight. The primary antibodies used were as follows: anti-PAR-1 (1:10,000), anti-Pod-1 (1:1000), and anti-Dlg (4F3) (1:50, Developmental Studies Hybridoma Bank). All secondary antibodies (Molecular Probes) and FITC-conjugated anti-HRP (Jackson ImmunoResearch Laboratories) were incubated for 2 hours at room temperature. Sample preparations were mounted in SlowFade Antifade kit (Invitrogen). Confocal images were collected from Leica TCS SP5 AOBS confocal microscopes equipped with 40 × or 100 × inverted NX oil lens, located at Gwangju Center, Korea Basic Science Institute. For analysis of the neuromuscular junction, we used the procedure described in the previous study (Lee et al., 2010).
2.3. Immunoprecipitation and western blot analysis
For coimmunoprecipitation experiments, 200 fly heads expressing UAS-PAR-1-WT-Myc transgene driven by GMR-GAL4 were homogenized in lysis buffer (50 mM Tris-HCl pH 8.0%, 1% Triton X-100, 150 mM NaCl, 2 mM Na3VO4,10 mM NaF, 10% glycerol, protease inhibitors), and the supernatant of lysate was obtained after centrifugation at 12,000 × g for 30 minutes at 4 °C. The supernatant was pre-cleared by incubation with protein A/G agarose (Pierce) for 1 hour at 4 °C. The supernatant was incubated with antiPAR-1 antibodies for 2 hours at 4 °C and then incubated with protein A/G agarose for 12 hours at 4 °C. Beads were washed 5 times with lysis buffer or PBS and boiled in SDS sample buffer. The samples were separated by gel electrophoresis and analyzed by western blot analysis. For western blot analysis, the following primary antibodies were used: anti-PAR-1 (1:8000), anti-phospho-PAR-1 (1:1000); anti-GFP (1:2,000, Abcam); anti-Myc (1:1,000, Millipore); anti-tau (pS262) (1:2,000, Life technology); total tau (1:2,000, Dako); and anti-GAPDH (1:2,000, Abclone). All primary antibodies were diluted in 0.5% BSA in TBST (tris-buffered saline, 0.1% tween 20) and incubated with the membrane at 4 °C overnight. All secondary antibodies (Jackson ImmunoResearch Laboratories) were incubated for 1 hour at room temperature.
3. Results
3.1. Pod-1 interacts with PAR-1
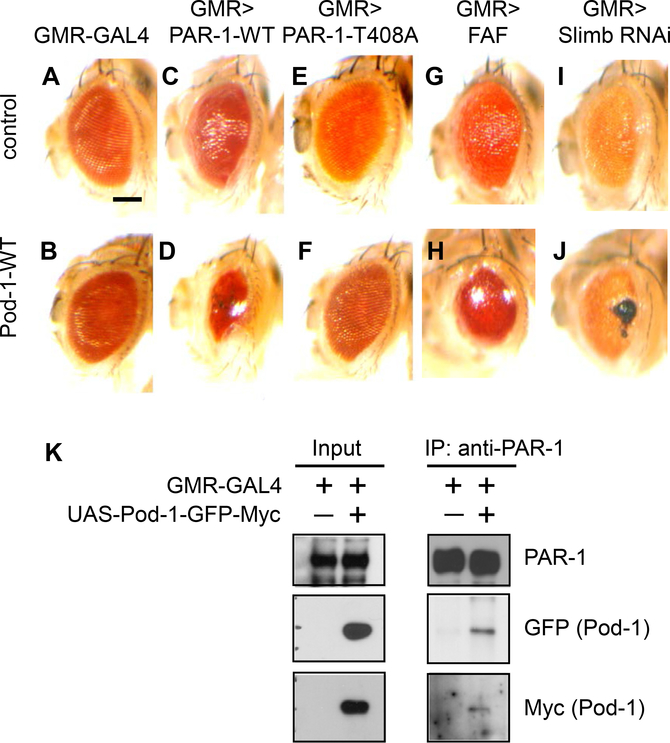
PAR-1 is a critical regulator of synapse development and tau-induced synaptic toxicity. To identify potential regulators of PAR-1, we performed genetic modifier screening in the Drosophila eye. We found Pod-1, which encodes an actin/microtubule crosslinker, as a strong interactor of PAR-1. While overexpression of Pod-1 or PAR-1-WT alone had a mild effect on eye morphology (Fig. 1A–C), coexpression of Pod-1 and PAR-1-WT (Fig. 1D), but not PAR-1-T408A mutant (Fig. 1E and F), in which the LKB1-dependent phosphorylation is abolished (Wang et al., 2007), significantly reduced the size of the adult eye. The ubiquitin E3 ligase, Slimb, and deubiquitinating enzyme, FAF, have been known to control the stabilization of PAR-1 (Lee et al., 2012). When we induced the stabilization of PAR-1 by knockdown of Slimb or overexpression of FAF in the genetic background of Pod-1 overexpression, severe eye phenotypes were clearly observed (Fig. 1G–J). Collectively, these data suggest that Pod-1 functionally interacts with PAR-1, as well as the PAR-1-modifying Slimb/FAF axis.
Fig. 1.
Pod-1 functionally and physically interacts with PAR-1. (A–J) The interaction between PAR-1 and Pod-1 in the Drosophila eye. Note the severely reduced eye size in coexpressing PAR-1 and Pod-1 animal (1D). The genotypes are as follows: GMR-GAL4/+ (A), GMR-GAL4>UAS-Pod-1-WT (B), GMR-GAL4>UAS-PAR-1-WT (C), GMR-GAL4>UAS-PAR-1-WT+UAS-Pod-1-WT (D), GMR-GAL4>UAS-PAR-1-T408A (E), GMR-GAL4>UAS-PAR-1-T408A+UAS-Pod-1-WT (F), GMR-GAL4> FAFEP381 (G), GMR-GAL4>FAFEP381+UAS-Pod-1-WT (H), GMR-GAL4>UAS-Slimb RNAi (I) and GMR-GAL4>UAS-Slimb RNAi+UAS-Pod-1-WT (J). Scale bar (A–J), 100 μm. (K) Coimmunoprecipitation of Pod-1 and PAR-1 in fly head samples. Fly head extracts from GMR–GAL4>Pod-1-GFP-Myc and control animals were immunoprecipitated with anti-PAR-1 antibody against PAR-1, and the presence of Pod-1-GFP-Myc in the immunoprecipitate was detected by western blot analysis with anti-Myc or GFP.
Next, to examine the molecular basis of the functional interaction between PAR-1 and Pod-1, we tested for possible physical association between them. Coimmunoprecipitation analysis was performed using fly head extracts overexpressing both PAR-1 and Pod-1-GFP-Myc. Pod-1-GFP-Myc was detected in the immunoprecipitate of PAR-1 by western blot analysis with anti-Myc or GFP antibody (Fig. 1K). These genetic and biochemical analyses provide clear evidence that Pod-1 functionally and physically interacts with PAR-1.
3.2. Pod-1 colocalizes with PAR-1
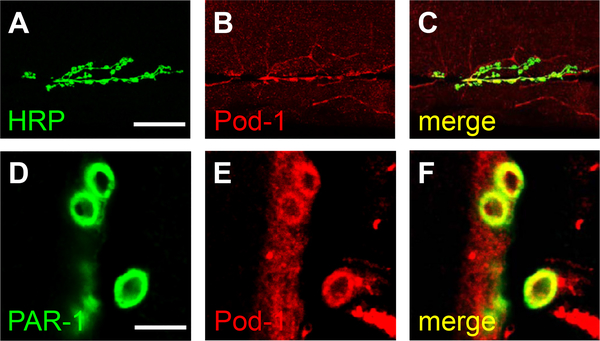
Since the role of PAR-1 has been well established for synaptic development at the NMJ (Zhang et al., 2007) (Lee et al., 2012), we sought to investigate the interaction between PAR-1 and Pod-1 at the larval NMJ in Drosophila. Prior to this analysis, we examined the localization of Pod-1 at the larval NMJ. Pod-1 is localized to the NMJ marked by HRP, which was consistent with the findings of a previous study (Liebl and Featherstone, 2008) (Fig. 2A–C). Next, to determine the colocalization of Pod-1 with PAR-1 in the NMJ, we carried out double-labeling immunohistochemistry experiments. Pod-1 appears largely overlapping with PAR-1 at the postsynaptic region (Fig. 2D–F), raising the possibility that Pod-1 may cooperate with PAR-1 to regulate NMJ development.
Fig. 2.
Pod-1 is expressed at the Drosophila neuromuscular junction. (A–C) wild-type larvae stained with anti-HRP (A) and anti-Pod-1 (B) antibodies. Merged image is shown in (C). Scale bar (A–C), 50 μm. (D–F) Pod-1 colocalizes with PAR-1. Immunostaining of the third instar larval NMJ stained with anti-PAR-1 (D) and anti-Pod-1 (E) antibodies. Merged image is shown in (F). Scale bar (D–F), 10 μm.
3.3. Pod-1 regulates PAR-1 function
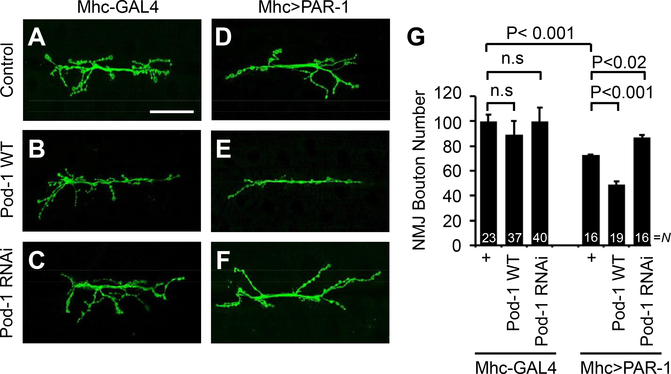
As reported previously (Zhang et al., 2007), postsynaptic overexpression of PAR-1 results in the 27% loss of synaptic bouton number compared to control (Fig. 3A, D, and 3G). Although the overexpression or knockdown of Pod-1 alone in the postsynaptic region had no discernable effect on NMJ morphology, cooverexpression of postsynaptic Pod-1 and PAR-1 enhanced the NMJ morphological defects caused by the overexpression of PAR-1, showing the 51% reduction of bouton number compared to the control (Fig. 3B, C, E, and G). Moreover, the postsynaptic expression of Pod-1 RNAi was able to largely rescue the bouton-loss phenotype caused by PAR-1 overexpression (Fig. 3F and G). It is noted that no significant difference was found in muscle size in either genotype. In the presynaptic region where the overexpression of PAR-1 does not alter the NMJ morphology (Zhang et al., 2007), the overexpression or knockdown of Pod-1 did not show any abnormality and had no synergistic effect when combined with PAR-1 overexpression (Supplementary Fig.1).
Fig. 3.
Phenotypic interaction between PAR-1 and Pod-1 in Drosophila larval NMJ. (A–F) Representative NMJ terminals of the indicated genotypes revealed by anti-HRP immunostaining. The genotypes are Mhc-GAL4/+ control (A), Mhc-GAL4>UAS-Pod-1-WT (B), Mhc-GAL4>UAS-Pod-1-RNAi (C), Mhc-GAL4>UAS-PAR-1-WT (D), Mhc-GAL4>UAS-PAR-1-WT+UAS-Pod-1-WT (E) and Mhc-GAL4>UAS-PAR-1-WT+UAS-Pod-1 RNAi (F). Scale bar (A–F), 50 μm. (G) Quantification of data showing phenotypic interaction between PAR-1 and Pod-1 on the total number of boutons per muscle area on muscle 6/7 of A3. N indicates the number of animals analyzed in each group. Statistical significance was determined pair-wise using a two-tailed Student’s t-test. n.s, not significant.
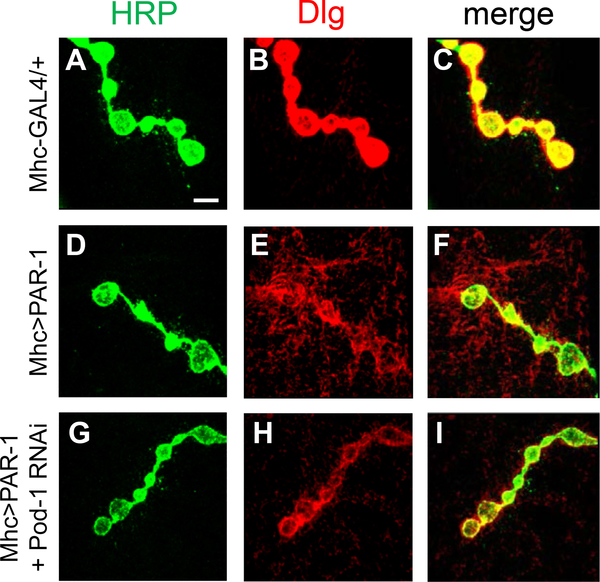
Previous studies showed that Dlg was diffuse and less concentrated by PAR-1 overexpression, which, in turn, led to aberrant synaptic structure (Zhang et al., 2007). Since the PAR-1 overexpression effect is suppressed by Pod-1 knockdown, PAR-1-induced delocalization of Dlg might be restored by Pod-1 knockdown. To test this possibility, we examined the effect of Pod-1 knockdown on Dlg localization in a PAR-1 overexpression background. In Mhc-GAL4>UAS-PAR-1 larval NMJ, Dlg signals were diffused at the postsynaptic compartments (Fig. 4D–F) compared to the control (Fig. 4A–C). This PAR-1-induced delocalization of Dlg was rescued by Pod-1 knockdown using Pod-1 RNAi (Fig. 4G–I) but not Pod-1-WT overexpression (data not shown). These results suggest that Pod-1 plays an important role in the postsynaptic region of the NMJ by regulating the synaptic function of PAR-1.
Fig. 4.
Pod-1 knockdown rescues the Dlg delocalization caused by postsynaptic overexpressing PAR-1. Double-labeling at the third instar larval NMJ with anti-HRP in green, anti-Dlg in red, and merged images. The genotypes are as follows: Mhc-GAL4/+ (A–C), Mhc-GAL4>UAS-PAR-1-WT (D–F), and Mhc-GAL4>UAS-PAR-1-WT+UAS-Pod-1 RNAi (G–I), Scale bar, 5 μm.
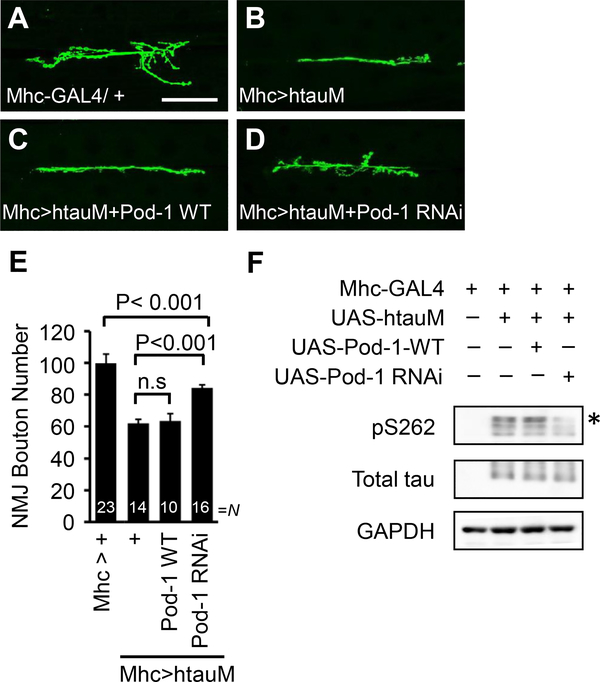
3.4. Pod-1 knockdown attenuates tau-induced synaptic toxicity
Next, we examined whether Pod-1 knockdown can ameliorate tau toxicity induced by PAR-1 activity. First, we examined whether Pod-1 regulates the tau-mediated synaptic toxicity in NMJ. Postsynaptic overexpression of human tau R406W mutation (htauM) leads to a strong reduction in the total number of boutons compared to the control (Lee et al., 2012) (Fig. 5A and B). Interestingly, this synaptic defect was blocked by Pod-1 knockdown (Fig. 5D and E), while overexpression of Pod-1-WT did not have an obvious effect (Fig. 5C and E). Next, to test whether the rescue by Pod-1 knockdown results from its effect on PAR-1 activity, we analyzed PAR-1 dependent phosphorylation of htau at Serine 262. The phosphorylation of htau was markedly attenuated by the knockdown of Pod-1 but increased by the overexpression of Pod-1 (Fig. 5F). The data, taken together, indicate that the downregulation of PAR-1 activity by Pod-1 knockdown may be responsible for the regulation of tau phosphorylation.
Fig. 5.
Pod-1 knockdown reduces synaptic toxicity caused by htauM. (A–D) The interaction between Pod-1 and htauM in the Drosophila NMJ. Representative NMJ terminals of the indicated genotypes were shown by anti-HRP immunostaining. The genotypes are Mhc-Gal4>+ (A), Mhc-Gal4>UAS-htauM (B), Mhc-Gal4>UAS-htauM+UAS-Pod-1-WT (C) and Mhc-Gal4>UAS-htauM+UAS-Pod-1 RNAi (D). Scale bar (AeD), 50 μm. (E) Quantification of data showing phenotypic interaction between htauM and Pod-1 on the total number of boutons per muscle area on muscle 6/7 of A3. N indicates the number of animals analyzed in each group. Statistical significance was determined pair-wise using a two-tailed Student’s t-test. n.s, not significant. (F) Western blot analysis of pTau S262 and total tau after coexpression of Pod-1-WT and Pod-1 RNAi in an Mhc-GAL4>UAS-htauM background. The pTau S262 level in larval muscle wall extracts was increased by Pod-1-WT, whereas it was decreased by Pod-1 RNAi compared to control. pTau S262 proteins marked with an asterisk. GAPDH serves as a loading control.
4. Discussion
The conserved kinase PAR-1/MARK has been known as a key regulator of cell polarity in diverse organisms. In Drosophila, PAR-1 was initially reported to regulate microtubule dynamics and/or stability during oocyte determination and differentiation (Cox et al., 2001)
(Shulman et al., 2000). More recently, PAR-1 has also been found to be involved in the synaptic development of the NMJ and dendritic pruning (Zhang et al., 2007) (Herzmann et al., 2017). Moreover, PAR-1 mediates the phosphorylation of tau, and its abnormal regulation can lead to neurodegeneration (Ando et al., 2016; Wang et al., 2007). This is consistent with the role of MARK, the mammalian homolog of PAR-1 (Yu et al., 2012). In light of the functional importance of PAR-1, regulators of PAR-1 have been pursued, and several proteins, including LKB1, FAF, and Slimb have been identified (Lee et al., 2012).
In this study, we found Pod-1, an actin-microtubule crosslinker, to be a novel regulator of PAR-1 through modifier screening. Pod-1 physically associates with PAR-1 and regulates the activity of PAR-1 in the targeting of the postsynaptic scaffold protein, Dlg, and in tau-mediated synaptic toxicity. Drosophila Pod-1 belongs to the conserved family of Coronin proteins involved in cell motility, morphogenesis, and trafficking. Most Coronin homologs are known to orchestrate the actin filament network by Arp2/3 based branching assembly and ADF/cofilin based disassembly. In mice, Coronin 1 and Coronin 6 were reported to interact with actin cytoskeletons or affect vesicle trafficking in synapses (Chen et al., 2014) (Suo et al., 2014). The Drosophila Pod-1 has the ability to directly crosslink actin and microtubule cytoskeletons (Rothenberg et al., 2003). Taken together with this molecular function of Pod-1, our data suggest that Drosophila Pod-1 modulates PAR-1 activity through the coordination of actin and microtubule cytoskeletons in synaptic development and toxicity. Further studies on the underlying molecular mechanisms are required to understand how Pod-1-mediated regulations of actin and/or microtubule cytoskeletons affect the activity of PAR-1, which may help reveal the conserved molecular mechanism of Coronin homologs in synaptic development and function.
The dysregulation of PAR-1 is implicated in the pathogenesis of AD and related tauopathies. The pathogenic beta-amyloid, Aβ42, induced tau phosphorylation mediated by PAR-1/MARK, stabilizes microtubule-unbound tau and contributes to sequential phosphorylation and tau toxicity (Nishimura et al., 2004) (Ando et al., 2016). Our study revealed a novel regulatory mechanism of PAR-1-mediated tau toxicity by Pod-1. The knockdown of Pod-1 reduced PAR-1-mediated phosphorylation of tau and relieved the synaptic abnormality caused by pathogenic tau overexpression. Tau is a microtubule-associated protein, which stabilizes and promotes the assembly of microtubules, the dysregulation of which is related to neurotoxicity (Ittner and Gotz, 2011) (Burnouf et al., 2016). Meanwhile, the association of actin with tau-induced neurotoxicity has been recently unveiled, and tau has been shown to crosslink actin and microtubules (Cabrales Fontela et al., 2017). Our data support the possibility that the coordination of actin and microtubule could be critical to AD-related tau toxicity and suggest the investigation of Pod-1 in degenerative neurological diseases, including tauopathies.
Neuronal cytoskeletal impairment is strongly linked to neurodegenerative disease. Disturbance of the microtubule network accompany axonal retraction and affect axonal transport, which affects neurodegeneration processes. Indeed, alterations of microtubule modification and dysregulation of microtubule-associated proteins are involved in Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis. Currently, several drugs that target cytoskeleton dysfunction, including a microtubule stabilizer, compounds targeting tubulin modification, and compounds targeting regulatory proteins of cytoskeleton dynamics, are being tested. Pod-1 could be another therapeutic target of AD as it augments tau-induced synaptic toxicity.
Supplementary Material
Acknowledgements
We are grateful to Dr. Y. Jan for the anti-Pod-1 antibody; Dr. Peter Seubert for the 12E8 antibody; Drs. T. Littleton, M. Feany, and the VDRC and Bloomington Stock Center for fly stocks. We thank the members of the Lee laboratory for discussions and help. This work was supported by the Korea Health Industry Development Institute (Grant HI18C1241 to S.L.), National Research Foundation of Korea (Grant 2017R1C1B1008825 to K.K.), and Korea Brain Research Institute (KBRI) Research Initiative Program (19-BR-02-03 to H.K. and 19-BR-03-02 to J.K.).
Footnotes
Disclosure statement
All authors declare no conflict of interest that might potentially bias this work.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurobiolaging.2020.02.005.
References
- Ando K, Maruko-Otake A, Ohtake Y, Hayashishita M, Sekiya M, Iijima KM, 2016. Stabilization of microtubule-unbound tau via tau phosphorylation at Ser262/356 by par-1/MARK contributes to augmentation of AD-related phosphorylation and abeta42-induced tau toxicity. PLoS Genet. 12, e1005917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnouf S, Gronke S, Augustin H, Dols J, Gorsky MK, Werner J, Kerr F, Alic N, Martinez P, Partridge L, 2016. Deletion of endogenous Tau proteins is not detrimental in Drosophila. Sci. Rep. 6, 23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrales Fontela Y, Kadavath H, Biernat J, Riedel D, Mandelkow E, Zweckstetter M, 2017. Multivalent cross-linking of actin filaments and microtubules through the microtubule-associated protein Tau. Nat. Commun. 8, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KT, Creed SJ, Bear JE, 2011. Unraveling the enigma: progress towards understanding the coronin family of actin regulators. Trends Cell Biol. 21, 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ip FC, Shi L, Zhang Z, Tang H, Ng YP, Ye WC, Fu AK, Ip NY, 2014. Coronin 6 regulates acetylcholine receptor clustering through modulating receptor anchorage to actin cytoskeleton. J. Neurosci. 34, 2413–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DN, Lu B, Sun TQ, Williams LT, Jan YN, 2001. Drosophila par-1 is required for oocyte differentiation and microtubule organization. Curr. Biol. 11, 75–87. [DOI] [PubMed] [Google Scholar]
- de Hostos EL, Bradtke B, Lottspeich F, Guggenheim R, Gerisch G, 1991. Coronin, an actin binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein beta subunits. EMBO J. 10, 4097–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Kemphues KJ, 1995. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 81, 611–620. [DOI] [PubMed] [Google Scholar]
- Herzmann S, Krumkamp R, Rode S, Kintrup C, Rumpf S, 2017. PAR-1 promotes microtubule breakdown during dendrite pruning in Drosophila. EMBO J. 36, 1981–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner LM, Gotz J, 2011. Amyloid-beta and tau–a toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 12, 65–72. [DOI] [PubMed] [Google Scholar]
- Jayachandran R, Liu X, Bosedasgupta S, Muller P, Zhang CL, Moshous D, Studer V, Schneider J, Genoud C, Fossoud C, Gambino F, Khelfaoui M, Muller C, Bartholdi D, Rossez H, Stiess M, Houbaert X, Jaussi R, Frey D, Kammerer RA, Deupi X, de Villartay JP, Luthi A, Humeau Y, Pieters J, 2014. Coronin 1 regulates cognition and behavior through modulation of cAMP/protein kinase A signaling. PLoS Biol. 12, e1001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Hoogenraad CC, 2015. Building the neuronal microtubule cytoskeleton. Neuron 87, 492–506. [DOI] [PubMed] [Google Scholar]
- Lasser M, Tiber J, Lowery LA, 2018. The role of the microtubule cytoskeleton in neurodevelopmental disorders. Front. Cell. Neurosci. 12, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S,Liu HP,Lin WY,Guo H,Lu B,2010LRRK2kinaseregulatessynapticmorphology through distinct substrates at the presynaptic and postsynaptic compartments of the Drosophila neuromuscular junction. J. Neurosci. 30, 16959–16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Wang JW, Yu W, Lu B, 2012. Phospho-dependent ubiquitination and degradation of PAR-1 regulates synaptic morphology and tau-mediated Abeta toxicity in Drosophila. Nat. Commun. 3, 1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl FL, Featherstone DE, 2008. Identification and investigation of Drosophila postsynaptic density homologs. Bioinform. Biol. Insights 2, 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gao Y, Lin X, Li L, Han X, Liu J, 2016. The coronin family and human disease. Curr. Protein Pept. Sci. 17, 603–611. [DOI] [PubMed] [Google Scholar]
- Nishimura I, Yang Y, Lu B, 2004. PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell 116, 671–682. [DOI] [PubMed] [Google Scholar]
- Penton A, Wodarz A, Nusse R, 2002. A mutational analysis of dishevelled in Drosophila defines novel domains in the dishevelled protein as well as novel suppressing alleles of axin. Genetics 161, 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg ME, Rogers SL, Vale RD, Jan LY, Jan Y-N, 2003. Drosophila pod-1 crosslinks both actin and microtubules and controls the targeting of axons. Neuron 39, 779–791. [DOI] [PubMed] [Google Scholar]
- Rybakin V, Clemen CS, 2005. Coronin proteins as multifunctional regulators of the cytoskeleton and membrane trafficking. Bioessays 27, 625–632. [DOI] [PubMed] [Google Scholar]
- Shulman JM, Benton R, Johnston St D, 2000. The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell 101, 377–388. [DOI] [PubMed] [Google Scholar]
- Suo D, Park J, Harrington AW, Zweifel LS, Mihalas S, Deppmann CD, 2014. Coronin-1 is a neurotrophin endosomal effector that is required for developmental competition for survival. Nat. Neurosci. 17, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetrecht AC, Bear JE, 2006. Coronins: the return of the crown. Trends Cell Biol 16, 421–426. [DOI] [PubMed] [Google Scholar]
- Wang JW, Imai Y, Lu B, 2007. Activation of PAR-1 kinase and stimulation of tau phosphorylation by diverse signals require the tumor suppressor protein LKB1. J. Neurosci. 27, 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Polepalli J, Wagh D, Rajadas J, Malenka R, Lu B, 2012. A critical role for the PAR-1/MARK-tau axis in mediating the toxic effects of Abeta on synapses and dendritic spines. Hum. Mol. Genet. 21, 1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guo H, Kwan H, Wang JW, Kosek J, Lu B, 2007. PAR-1 kinase phosphorylates Dlg and regulates its postsynaptic targeting at the Drosophila neuromuscular junction. Neuron 53, 201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.