Abstract
Tumor immunology is undergoing a renaissance due to the recent profound clinical successes of tumor immunotherapy. These advances have coincided with an exponential growth in the development of –omics technologies. Armed with these technologies and their associated computational and modeling toolsets, systems biologists have turned their attention to tumor immunology in an effort to understand the precise nature and consequences of interactions between tumors and the immune system. Such interactions are inherently multivariate, spanning multiple time and size scales, cell types, and organ systems, rendering systems biology approaches particularly amenable to their interrogation. While in its infancy, the field of ‘Cancer Systems Immunology’ has already influenced our understanding of tumor immunology and immunotherapy. As the field matures, studies will move beyond descriptive characterizations toward functional investigations of the emergent behavior that govern tumor-immune responses. Thus, Cancer Systems Immunology holds incredible promise to advance our ability to fight this disease.
Introduction
Systems Biology is an interdisciplinary field that aims to interrogate and predict complex behaviors of multivariate biological systems. It employs quantitative approaches to understand the integrated behaviors of multiple biological components. In contrast to reductionist approaches, which seek to identify how individual components affect particular phenotypes, systems biology attempts to query the simultaneous responses of many elements to uncover how they work in concert to elicit a given response. It is predicated upon the belief that many biological processes cannot be comprehensively understood by analyses of individual components alone (e.g. a single molecule, cell, etc.), but rather require a holistic appreciation of entire networks and systems (e.g. signaling networks, heterotypic cell-cell interactions, physiologic interplay between organs, etc.). By combining mathematical modeling and computation with experimental and clinical data, systems biologists can construct a framework for understanding the multiscale and temporal elements regulating biological responses and elucidate emergent behaviors.
While the discipline of systems biology became well established around 2000 (Ideker et al., 2001), its underlying concepts have been appreciated for over half a century (Waterman and Theory, 1968; Kitano, 2002). Indeed, some have suggested that the study of medicine, which requires an understanding of the complex interactions between multiple molecules, cell types, and organ systems in response to different treatments over time, represents an original implementation of Systems Biology (Germain, 2018). Nonetheless, recent advances in technologies and computational approaches have enabled researchers to query systems-level dynamics at scales not possible in previous decades (Hood et al., 2004).
Recently, researchers in the fields of both cancer biology and immunology have embraced systems approaches to advance their disciplines. In cancer biology, genomics and proteomics approaches have been implemented to identify the effects of defects in signaling networks on malignant transformation and progression (Sanchez-Vega et al., 2018; Mertins et al., 2016). Next-generation sequencing (NGS) has enabled studies of tumor heterogeneity and clonal evolution (Jacoby et al., 2015). In the United States, the National Cancer Institute formed the Cancer Systems Biology Consortium to promote applications of systems approaches to cancer.
Immunology represents a field that is readily amenable to systems level approaches. Deciphering the immune system requires an understanding of the interactions between numerous cell types, immune receptors, and cytokines as they traverse multiple anatomical locations and organ systems in order to orchestrate effective immune responses. While the multivariate components governing an immune response have been slowly elucidated through reductionist approaches, they have recently become subject to a much more comprehensive characterization through advances in modeling and high-throughput technologies (Davis et al., 2017).
Although the study of tumor immunology can be traced back at least to the advent of Coley’s toxins at the turn of the twentieth century (Starnes, 1992), the recent clinical successes of immunotherapies in the treatment of advanced stage cancers have catalyzed renewed interest in the field. Consequently, cancer systems immunology represents a new avenue of interrogation for understanding how the immune system interacts with tumors during tumorigenesis, progression, and treatment. Cancer systems biology and systems immunology have been reviewed elsewhere (Davis et al., 2017; Faratian, 2010; Suhail et al., 2019; Germain et al., 2011; Vera, 2015; Werner et al., 2014; Korsunsky et al., 2014; Kreeger and Lauffenburger, 2010; Chuang et al., 2010). In this review, we will discuss approaches to the nascent field of cancer systems immunology as well as their potential applications and current limitations.
Applying systems biology to overcome challenges and discrepancies with animal models
Traditionally, animal models have served as critical tools to cancer biologists and immunologists as they try to decipher how tumors affect the host organism or how the immune response is orchestrated across multiple tissues, respectively. Nonetheless, animal models are frequently imperfect surrogates for human biology. While orthologous genes typically elicit similar functions across species, there are many instances where there exists a stark divergence in phenotypes for orthologs of different species (Gharib and Robinson-Rechavi, 2011; Koonin, 2005). Furthermore, there are even greater discrepancies between gene products that elicit the same functions, often reflecting a high degree of convergent evolution (Koonin, 2003). For example, inhibitory signaling in natural killer (NK) cells following recognition of major histocompatibility (MHC) class Ia molecules is achieved by Ly49 family members in mice but killer immunoglobulin-like receptors (KIRs) in humans (Lanier, 2005; Karlhofer et al., 1992; Moretta et al., 1990). In addition to differences in orthology, the cellular immune repertoires and the very existence of their associated effector molecules can vary significantly between species (Mestas and Hughes, 2004). All these factors frequently conspire to yield failed translation of therapeutic approaches when moving from preclinical models to human clinical trials (Denayer et al., 2014). As discussed throughout our report, systems biology offers potential solutions to this otherwise vexing problem through its ability to bridge data sets and models across species. Indeed, systems biology approaches have already provided predictive insights into human responses where preclinical models alone may be insufficient or inaccurate.
Computational biologists have developed a variety of tools to translate findings from preclinical models to humans when simple matching of orthologs is insufficient for predicting responses (Brubaker and Lauffenburger, 2020). To overcome differences in gene-to-function relationships and predict human responses from rodent data, researchers used Bayesian analysis of gene expression to define ‘functional orthologs’ across species (Chikina and Troyanskaya, 2011), and others have applied unsupervised and semi-supervised machine learning approaches to transcriptomic and proteomic data generated in rodent models to predict human responses (Brubaker et al., 2019). On collaborative initiatiative, termed SBV-IMPROVER (Systems Biology Verification for Industrial Methodology for PROcess VErification in Research), sought to develop computational methods capable of cross-species translation using multimodal datasets including transcriptomics, phosphoproteomics, and cytokine data (Poussin et al., 2014; Rhrissorrakrai et al., 2015). Solutions ranged from approaches using support vector machines, neural networks, random forest trees, and more, with no one algorithm outperforming the others across all datasets. These types of cross-species comparisons have been extended to single-cell analyses, wherein cell types can be defined separately in the different organisms and matched manually, through correlation analyses, or through the use of random forest machine learning (Tosches et al., 2018; Butler et al., 2018; Shafer, 2019; Elyada et al., 2019). Nonetheless, the choice of preclinical model is critical as a given model may not be as informative as another (Olson et al., 2018). For example, transplantable pancreatic cancer models did not predict the limited efficacy of gemcitabine as accurately as autochthonous models due to a lack of desmoplastic stroma in the transplantable setting (Olive et al., 2009).
Even with improved animal models, however, there are many instances where they fail to predict clinical responses, and only 8% of drugs entering clinical trials succeed in Phase I (Mak et al., 2014). Thus, the use of systems approaches, informed by existing data sets, to accurately predict human responses in the absence of accurate animal models represents an important opportunity to improve translation. Mathematical models have proven effective at accurately predicting many aspects of cancer biology ranging from growth kinetics and tumor evolution to responses to therapy (Altrock et al., 2015). For example, such models have tracked the development of resistance in CML (Michor et al., 2005) or have served as the basis for clinical trials altering dosing strategies (Norton and Simon, 1977; Citron et al., 2003). Critically, mathematical models have been used to inform clinical decisions. In an approach they term ‘adaptive therapy’, one group used an evolutionary game theory model to predict patient-specific treatment responses in patients with castration-resistant prostate cancer, modifying their treatment accordingly, and in doing so, extended the time to progression in these patients (Zhang et al., 2017a). This study highlights the profound impact systems-level mathematical modeling can impart on clinical decision-making. Systems immunology has been applied particularly in the field of vaccinology to reduce the reliance upon animal models (Davis et al., 2017), and these types of modeling approaches have recently been extended to cancer immunology to understand how treatment regimens can be tailored to improve immune responses (Park et al., 2019). By extending these approaches further, cancer systems immunology holds the potential to inform clinical approaches when animal models are insufficient.
Technologies
The multivariate nature of systems biology has rendered it particularly applicable to comprehensive datasets derived from quantification of systems-level parameters (e.g. genomics, transcriptomics, metabolomics, proteomics, etc.). Indeed, the beginning of modern-era systems biology largely coincides with the Human Genome Project (Lander et al., 2001; Venter et al., 2001; International Human Genome Sequencing Consortium, 2004), which enabled researchers to interrogate the genome-wide contributions of mutations to diseases. It is worth noting that systems biology does not require the use of any of these advanced technologies, and many mathematical and computational models were derived from experimental evidence collected with conventional assays. Similarly, the use of these next-generation technologies for a particular study does not, in and of itself, constitute a systems biology approach. Frequently, such technologies are used to screen for targets that are subsequently subjected to conventional reductionist analyses (e.g. differential gene expression (DGE) analyses of RNA-seq data to identify a gene of interest). Such approaches alone do not provide a systems-level understanding of a particular phenomenon, as they do not describe emergent behavior that could not be uncovered with reductionist approaches. Nonetheless, a wide range of new technologies has enabled researchers to examine the breath and dynamics of entire systems in order to better understand the interplay of multiple elements and networks (Figure 1; Hood et al., 2004; Ideker, 2001). Here, we describe some of the major technologies adopted by cancer systems immunologists to uncover new biology. Strengths and weaknesses for the various genomic and epigenomic profiling technologies are highlighted in Tables 1–3.
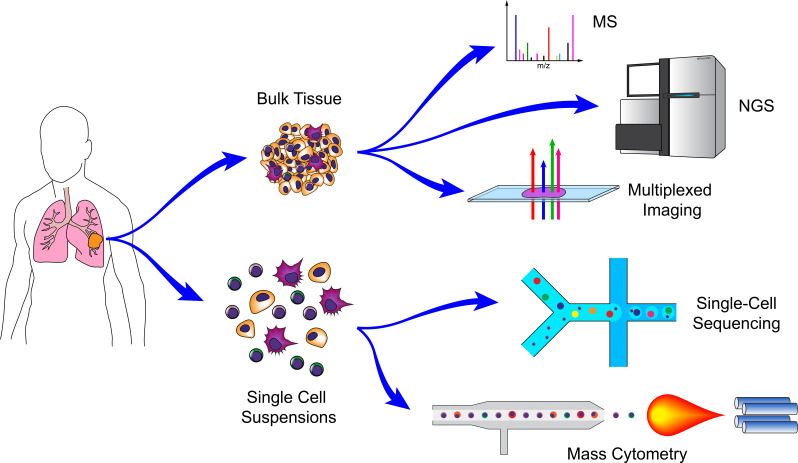
Figure 1. Technologies for cancer systems immunology.
Technologies used in cancer systems immunology operate either on bulk tissue samples or single-cell suspensions. Conventional MS, NGS, and imaging platforms do not require tissue dissociation (although histology provides single-cell resolution). Droplet-based microfluidics and mass cytometry, in contrast, require cell suspensions, but generate high-dimensional data for individual cells.
Table 1. Genomic and transcriptomic profiling technologies.
| Measurement | DNA | DNA | DRNA/RNA | RNA | RNA |
|---|---|---|---|---|---|
| Technology | WGS | WES | Amplicon (e.g. TCR, BCR, specific loci) | RNA-seq | Microarray |
| Strengths | • Captures coding and non-coding regions • may be more accurate in some exons as well • better coverage in low-complexity regions • no PCR step required |
• Reduced cost of sequencing since restricted to 2% of genome | • Lower cost • greater sequencing depth |
• Not limited to known genes with probes • can identify splice variants • can include ncRNA • can identify sequence variations (e.g. mutations) |
• Can theoretically detect very low abundance transcripts at no additional cost |
| Weaknesses | • High cost | • Does not capture non-coding regions • may fail to capture some coding regions depending on probe hybridization • GC bias can be introduced due to PCR • hybridization bias can occur in regions with heterozygous SNVs |
• Limited to specific regions (not genome-wide) | • Sequencing depth can limit the ability to detect low-abundance transcripts | • Probe bias • inability to compare relative abundance across genes • limited to known transcripts (for which there are probes) |
| Single-cell Version? | Y | Y | Y | Y (see Table 3) | Y* (very uncommon, Esumi et al., 2008) |
Table 3. scRNA-seq Technologies.
| Strengths and weaknesses of the ever-evolving compendium of scRNA-seq technologies and analysis packages have been evaluated reviewed extensively in Ziegenhain et al., 2017; Chen et al., 2019a; Haque et al., 2017. Here, we provide a basic overview of the strengths of the general approaches. | |||
|---|---|---|---|
| Technology | Plate-based (e.g. Smart-seq2, MARS-seq) | Microfluidic capture (e.g. C1, Seq-well, CEL-seq2/C1) | Droplet (e.g. 10X, Drop-Seq) |
| Strengths | • Highest sensitivity (number of genes detected) • fewer multiplets • full-length transcripts possible |
• High sensitivity (number of genes detected) • fewer multiplets • no sorting required |
• Inexpensive (per cell) • profile high numbers of cells • can identify less frequent cell types • no sorting required • Can use UMIs |
| Weaknesses | • Requires sorting • low throughput • high cost per cell • not strand specific |
• 3' Only • limited cell numbers • (typically) not strand-specific |
• 3' Only • fewer genes/UMIs • more dropout |
Bulk sequencing technologies
The promise of the Human Genome Project and whole genome sequencing (WGS) has inspired the development of –omics technologies capable of characterizing the entirety of a particular attribute within a sample. Such approaches include, but are not limited to, genomics, transcriptomics, epigenomics, proteomics, metabolomics, lipidomics (Yang and Han, 2016), and glycomics (Bennun et al., 2016; Cummings and Pierce, 2014; Bertozzi and Sasisekharan, 2009), and their current manifestations typically utilize variations of next-generation sequencing (NGS) or mass spectrometry (MS). The bulk implementations of these technologies (i.e. those that require multiple cells as inputs – frequently, thousands to millions) were the precursors of many of the single-cell versions that have recently gained popularity. While these technologies on their own do not provide single-cell resolution, they frequently provide a degree of sensitivity that cannot yet be achieved at the single-cell level (e.g. most glycomics). Furthermore, the cost of these approaches is typically considerably lower than their single-cell counterparts. Complex mixtures of cells can be purified by fluorescence activated cell sorting (FACS), magnetic purification, or microfluidic systems prior to subjecting them to these technologies, and recent computational approaches provide the means of deconvolving mixed populations when purification is not feasible or gene expression data from such mixed populations have already been collected (Racle et al., 2017; Newman et al., 2015; Newman et al., 2019; Ahn et al., 2013; Yoshihara et al., 2013; Gong and Szustakowski, 2013; Li et al., 2016b; Becht et al., 2016; Shen-Orr et al., 2010; Zhong et al., 2013; Aran et al., 2017; Quon et al., 2013; Shen-Orr and Gaujoux, 2013; Vallania et al., 2018; Du et al., 2019).
The cornerstone technologies underlying both cancer systems biology and systems immunology are genomic sequencing (WGS and whole exome sequencing, WES) and transcriptomic sequencing (RNA sequencing (RNA-seq)) (Table 1). At its core, cancer is a genetic disease; malignant transformation is the consequence of mutations in tumor suppressor genes and oncogenes (Stratton et al., 2009). WGS and WES have shed light on the contributions of multiple mutations or copy number variations in such genes, and RNA-seq has revealed pathways and signaling networks involved in tumor progression (Creixell et al., 2015; Lawrence et al., 2014; Garraway and Lander, 2013). While the genomes of leukocytes exhibit considerably less variance than those of malignant populations, there are notable exceptions such as the B cell receptor (BCR) and T cell receptor (TCR) present on B and T lymphocytes, respectively. The genomic loci for these receptors undergo rearrangement in order to generate diversity of the antigen-recognition domains in a manner that confers specific immunity against an enormous range of pathogens (Hozumi and Tonegawa, 1976). Elevated expression of a variety of normal proteins, expression of embryonic proteins and antigens, and expression of mutated proteins (neoantigens) all represent targets on tumor cells that can be recognized by BCRs and TCRs to elicit antitumor responses by the immune system. Consequently, cancer systems immunologists have employed targeted amplicon sequencing (typically, of cDNA derived from amplified TCR or BCR mRNA) to evaluate the BCR and TCR repertoires, providing insight into how lymphocytes respond to tumors (Han et al., 2016; Page et al., 2016; Woodsworth et al., 2013; Sims et al., 2016; Linnemann et al., 2013; Jiang et al., 2019; Liu et al., 2018; Chaudhary and Wesemann, 2018; Zhang et al., 2017b). Furthermore, researchers have used WES, frequently in combination with RNA-seq, to identify the range of potential neoantigens expressed by tumor cells as a consequence of their high mutation rates (Garcia-Garijo et al., 2019). Finally, RNA-seq has enabled researchers to identify gene networks and transcriptional programs exploited by tumors to evade anti-tumor immunity, as well as changes in the states of immune cells as they interact with tumors.
While genomic and transcriptomic analyses have been mainstays for Systems Biologists, both cancer biology and immunology have benefitted from epigenetic studies (Egger et al., 2004; Flavahan et al., 2017; Esteller, 2008; Suvà et al., 2013; Li et al., 2013; Schmidl et al., 2018; Busslinger and Tarakhovsky, 2014; Peng et al., 2015; Berdasco and Esteller, 2010; Feinberg and Vogelstein, 1983; Henning et al., 2018). A number of technologies have enabled investigations into the epigenetic control of gene regulation, and by combining these methods with NGS approaches capable of querying the entire genome, researchers have been able to apply systems-level analyses to epigenetics (Table 2). Methylation represents one of the most common epigenetic modifications for silencing transcription (Jones and Takai, 2001), and can be surveyed at the genome level through the use of Whole Genome Bisulfite Sequencing (WGBS) or Reduced Representation Bisulfite Sequencing (RRBS), which use sodium bisulfite to convert unmethylated cytosine residues to uracil while leaving their methylated counterparts (5-methylcytosine) intact (Frommer et al., 1992; Lister et al., 2009; Meissner et al., 2005; Booth et al., 2012; Booth et al., 2014; Yu et al., 2012). To interrogate how specific proteins (e.g. transcription factors) interact with DNA, researchers often use Chromatin Immunoprecipitation (ChIP). In this approach, DNA is crosslinked to proteins with which it is interacting, sheared, and precipitated through the use of antibodies against the protein of interest (Gilmour and Lis, 1985). Combining ChIP with NGS (ChIP-seq), enables the generation of genome-wide maps of DNA binding to proteins of interest (Johnson et al., 2007; Barski et al., 2007). An improved version, known as CUT and RUN, also enables a similar approach to be performed in situ with considerably lower background (Skene and Henikoff, 2017). In immunology, Bisulfite-Seq and ChIP-seq have proven effective tools for uncovering the underpinning epigenetic modifications driving fate decisions and activation states of leukocytes (Henning et al., 2018; Northrup and Zhao, 2011; Zhang et al., 2012; Russ et al., 2014; Abdelsamed et al., 2017). Histone modifications represent some of the most important regulators of cell states (Strahl and Allis, 2000), and ChIP-seq has proven to be one of the most effective technologies for querying such changes. For example, ChIP-seq has been used to define the super-enhancer (SE) landscape in CD4+ T cells and identify how polymorphisms in these regions can potentiate risk of autoimmune disease (Vahedi et al., 2015). Similarly, using ChIP-seq to profile a variety of methylation and acetylation patterns of Histone H3, researchers have uncovered the regulatory epigenetic signatures that distinguish the naïve, effector, central memory, and effector memory CD8+ T cell subsets (He et al., 2016; Rodriguez et al., 2017; Araki et al., 2009). In cancer biology, ChIP-seq has proven effective for defining differential enhancer signatures in tumor cells (Akhtar-Zaidi et al., 2012). Mutation-independent epigenetic control of tumor suppressors through trimethylation of histone H3 at lysine 4 (H3K4me3) has been uncovered using ChIP-seq approaches (Chen et al., 2015b). Similarly, ChIP-seq has revealed patterns of promoter and enhancer invasion by Myc to drive widespread RNA biogenesis in both tumors and immune cells (Sabò et al., 2014; Lin et al., 2012; Nie et al., 2012).
Table 2. Epigenetic profiling technologies.
| Measurement | Technology | Strengths | Weaknesses | Single-cell version? |
|---|---|---|---|---|
| Methylation | WGBS | • No a priori sequence selection | • High cost and may require higher coverage • cannot distinguish type of modification at cytosines |
Y |
| Methylation | RRBS | • Lower cost | • Limited mainly to CpG islands • cannot distinguish type of modification at cytosines |
Y |
| Protein Localization | ChIP-seq | • Genome-wide profiling of histone modifications and DNA-protein interactions (Histone H3 acetylation/methylation, TF binding site identification, SE identification) | • Survey only one type of interaction (protein) at once • lots of sources of noise/bias • requires good antibodies • requires input DNA and isotype controls • requires large input of cells |
Y |
| Protein Localization | CUT&RUN | • Fewer input cells required than ChIP • less noise • fewer sequencing reads required • no cross-linking required |
• Requires good antibody • potential for overdigesting DNA |
Y (CUT&Tag, uliCUT&RUN) |
| Chromatin Accessibility | DNAse-seq | • Identify a range of cis and trans regulatory elements including TF binding sites | • High input cells requirement • more time-consuming that ATAC • sequence bias |
Y |
| Chromatin Accessibility | ATAC-seq | • Identify a range of cis and trans regulatory elements including TF binding sites • minimal input cells required • increased sensitivity over DNAse-seq • simple protocol |
• Footprint profiles can be less well-defined than DNAse-seq • potential mitochondrial DNA contamination |
Y |
| Chromatin Accessibility | MNAse-seq | • Nucleosome occupancy and positioning • can be used to predict higher-order structure (e.g. 3D) |
• Requires crosslinking • highly dependent on enzyme concentration • some sequence bias |
Y |
| Chromatin Accessibility | FAIRE-seq | • No sequence bias • simple protocol • no enzymes required |
• Requires crosslinking • lower resolution (crosslinking binds chromatin but also TFs) • large input cell requirement |
N |
| 3D Conformation | 3C (Chromosome Conformation Capture) | • Identify single chromosomal interaction (one vs. one) | • limited resolution (by 6bp cutters) • laborious • PCR biases • high library complexity • single viewpoint |
N |
| 3D Conformation | 4C (Circular 3C) | • Improved resolution over 3C • can identify very long range interactions • can identify all contacts for a locus (one vs. all) |
• Biases from circularization • PCR biases • high input cell requirements • single viewpoint |
N |
| 3D Conformation | 5C (3C Carbon Copy) | • Can identify many contacts for multiple loci (many vs. many) | • Bias introduced by probe ligation efficiencies • not all fragments can bind probes • all vs. all prohibitively expensive |
N |
| 3D Conformation | NG Capture-C | • Analyze hundreds of viewpoints • can identify PCR duplicates (low bias) • highest sensitivity and resolution • fewer input cells required |
• Occasional non-specific interactions | N |
| 3D Conformation | Hi-C | • Maps contacts across whole genome (all vs. all) • kilobase resolution |
• Fewer contacts per fragment than 4C or Capture-C • higher resolution versions require extremely high sequencing depths |
Y |
| 3D Conformation and Protein Localization | ChIA-PET | • Combines 3D interactions with protein interactions | • Interactions defined by few reads • high input requirements • bias toward interactions with targeted protein |
Y* (ChIA-Drop: single molecule, Zheng et al., 2019) |
| 3D Conformation and Protein Localization | Hi-ChIP (and PLAC-seq) | • Lower input required • higher yield than ChIA-PET • higher signal to noise over Hi-C |
• Bias toward interactions with targeted protein | N |
In addition to approaches that evaluate acetylation, methylation, and protein binding to various loci in the genome, tools to interrogate higher order structure of the genome have recently been developed. Techniques such as DNAse-seq and ATAC-seq (Assay for Transposase Accessible Chromatin) can identify regions of open and closed chromatin (i.e. chromatin accessibility) across the genome (Buenrostro et al., 2013; Boyle et al., 2008; Thurman et al., 2012). These approaches use enzymes to cleave regions of DNA that are not tightly wrapped around nucleosomes, presumably due to active transcription or their occupancy by DNA-binding proteins (e.g. transcription factors). They also enable transcription factor footprinting to identify transcription factor binding sites. Furthermore, by combining these methods with computational approaches, the effects of cis- and trans-regulatory elements upon gene function can be analyzed. A modified version of these techniques can simultaneously enable Bisulfite-seq on the same sample (methyl-ATAC-seq) (Spektor et al., 2019). Chromatin accessibility analyses have enabled genome-wide characterization and determination of functional implications of such changes in a range of cancers (Corces et al., 2018; Denny et al., 2016), leukocytes (Buenrostro et al., 2018; Shih et al., 2016; Scharer et al., 2017; Sen et al., 2016), and tumor immunology studies (Satpathy et al., 2019; Corces et al., 2016; Philip et al., 2017; Benci et al., 2016). Such approaches have revealed how widespread increases in chromatin accessibility enable transcriptional programs that drive tumor progression and metastasis (Denny et al., 2016). Other studies found that dysfunctional tumor-specific CD8+ T cells enter one of two distinct chromatin states that determine whether they can be reprogrammed (Philip et al., 2017).
While regions of open chromatin reveal evidence of transcriptional regulation, higher order chromatin structures also play critical roles in controlling gene expression. Long-range distal elements, such as enhancers, affect gene expression even at distances greater than 1 Mb in linear genome space (Lettice et al., 2003; Bulger and Groudine, 2011; Dekker, 2008). To assess how three-dimensional conformations affect regulation, a variety of technologies have been developed that are capable of querying chromosomal interactions at the genome scale (Davies et al., 2017). These include derivatives of chromosome conformation capture (3C) (Dekker et al., 2002) such as circular chromosome conformation capture (4C) (Zhao et al., 2006; Simonis et al., 2006), chromosome conformation capture carbon copy (5C) (Dostie et al., 2006), NG Capture-C (Hughes et al., 2014; Davies et al., 2016), Hi-C (Lieberman-Aiden et al., 2009), and methods that combine 3C with ChIP such as chromatin interaction analysis by paired-end tag sequencing (ChIA-PET) (Fullwood et al., 2009) and HiChIP (Mumbach et al., 2016; Mumbach et al., 2017). These 3C methodologies are variants of protocols wherein DNA is crosslinked, digested by restriction endonucleases, ligated together, and amplified by PCR to identify regions in close proximity. In particular, Hi-C has enabled mapping of all interactions within the genome at kilobase resolution (Rao et al., 2014). These techniques have informed a variety of studies in both tumor biology and immunology. Earlier studies using fluorescence in situ hybridization (FISH) and 3C originally suggested that interchromosomal interactions between promoters and enhancers on distinct chromosomes can drive immune cell development (Hewitt et al., 2008; Ling et al., 2006), but subsequent high-resolution genome-wide studies using Hi-C failed to confirm the existence of such interactions (Johanson et al., 2018). Recently, as part of the Cancer Genome Atlas (TCGA) studies, enhancer activity has been mapped across nearly 9000 cancer patients combining RNA-seq and Hi-C data to characterize enhancer-gene interactions, and this effort identified a key enhancer of the immunomodulatory protein programmed death ligand 1 (PD-L1) (Chen et al., 2018). Integrating these multiple types of epigenetic signatures with transcriptional datasets should enable the generation of systems level models for transcriptional regulation in both malignant and immune populations during tumor progression. For example, how do genome-wide changes in chromosomal confirmations within immune or malignant cells alter their states of differentiation, and how do heterotypic cellular interactions drive these changes?
Single-cell sequencing technologies
While light and electron microscopy endowed scientists with the ability to survey biology at the resolution of single cells, perhaps the most influential single-cell technological advance in immunology was the invention of flow cytometry and fluorescence activated cell sorting (FACS) (Fulwyler, 1965; Hulett et al., 1969). These technologies enable scientists to identify and enumerate phenotypically and functionally distinct immune cells, and query the activation states of individual cells in a suspension based on excitation of fluorescent probes (e.g. fluorescently labeled antibodies, fluorescent protein-based genetic reporters, fluorescent DNA intercalating dyes, etc.). In addition to representing a fundamental tool for investigating and identifying the cellular components of the immune system, flow cytometry has been useful clinically. For example, prior to the discovery of the Human Immunodeficiency Virus, it served as the primary means for identifying individuals at risk for AIDS and monitoring their course and response to therapy, based on the relative frequency of circulating CD4 T cells (Lifson and Engleman, 1989). Today, in addition to its use in monitoring the frequencies of various immune cell types in patients with cancers and recipients of organ transplants who are receiving immunosuppressive drugs, flow cytometry is used to characterize malignant cells in blood for clinical diagnosis. By multiplexing these probes, scientists can simultaneously measure tens of markers on individual cells at rates of thousands of cells per second, and recent advances (discussed later) have increased this multiplexing to above 40 markers (Bendall et al., 2012). Due to its ability to survey the states of mixed populations at a single-cell resolution, flow cytometry has facilitated the majority of major discoveries in the field of immunology.
Adaptation of these bulk NGS technologies to single cells has recently led to insights at the single-cell level previously thought to be unattainable. These new technologies were largely enabled by advances in microfluidics and are predicated upon one of three general approaches: (1) single cells are sorted into individual wells of a plate by using conventional FACS, (2) single cells are captured in capture sites of a microfluidic chip, or (3) single cells are captured in emulsion droplets generated in a microfluidic chip. Both microfluidic approaches were derivatives of technologies developed at the turn of the century (Unger et al., 2000; Thorsen et al., 2001; Anna et al., 2003; Thorsen et al., 2002; Tice et al., 2003), wherein precise control of picoliter volumes, aided by advances in soft lithography, is utilized to isolate individual cells and subject them to chemical and enzymatic reactions. These approaches are particularly amenable to DNA sequencing approaches, as reactions involving endonucleases, transposases, and reverse transcriptases as well as PCR can all be performed with exquisite control in these volumes and formats.
The vast majority of bulk NGS approaches have been adapted to these single-cell formats (see Table 1). The primary tradeoff is that while these approaches provide insights into the distinct profiles of individual cells, far fewer sequencing reads can be gathered for a given cell than bulk approaches due to the low abundance of transcripts or DNA within a cell as well as the high costs of sequencing hundreds to tens of thousands of cells at high read depths. Stochasticity, transcriptional bursting, and dropout also add challenges to interpreting single-cell data. Nonetheless, a variety of computational tools have been developed to help account for some of these effects, and the resultant analyses have been transformative for the field of tumor immunology. Some of the single-cell NGS approaches include RNA-seq (scRNA-seq) (Hashimshony et al., 2012; Islam et al., 2011; Shalek et al., 2013; Ramsköld et al., 2012; Tang et al., 2009; Wu et al., 2014; Tang et al., 2010; Tariq et al., 2011; Klein et al., 2015; Macosko et al., 2015; Gierahn et al., 2017; Ziegenhain et al., 2017; Ding et al., 2020; Table 3), genome and exome sequencing (Gawad et al., 2016; Wang et al., 2014a; Navin et al., 2011; Baslan et al., 2012; Xu et al., 2012), nucleus sequencing for RNA or DNA (scNuc-seq) (Wang et al., 2014a; Habib et al., 2016; Habib et al., 2017; Lacar et al., 2016; Hu et al., 2017a), WGBS or RRBS (scBS-seq) (Smallwood et al., 2014; Clark et al., 2017; Guo et al., 2013; Farlik et al., 2015; Angermueller et al., 2016), ChIP-seq (scChIP-seq) (Grosselin et al., 2019; Rotem et al., 2015), ATAC-seq (scATAC-seq) (Satpathy et al., 2019; Cao et al., 2018; Cusanovich et al., 2018; Cusanovich et al., 2015; Buenrostro et al., 2015; Satpathy et al., 2018), and Hi-C (scHi-C) (Ramani et al., 2017; Nagano et al., 2013; Stevens et al., 2017).
The immune system is comprised of a myriad of cell types with distinct functions working in concert to elicit responses against a pathogenic insult. The multitude and diversity of these cell types render bulk sequencing approaches challenging in that the aggregation of reads from the pool of different cell types often masks differences exhibited by particular subsets. Furthermore, changes in the frequencies of cell types are difficult to distinguish from changes in gene expression within those cells. For example, an increase in IFNG within a tumor may reflect increased IFN-γ production by T cells already within tumors or an increase in T cell infiltration into the tumor. Recent computational approaches have helped deconvolve immune subsets from bulk RNA-seq data (discussed below), but single-cell sequencing presents an opportunity to accurately quantitate genome-wide changes at the resolution immunologists have become accustomed to from flow cytometry. Applied to tumor immunology, these studies have revealed heterogeneity in both the malignant and hematopoietic compartments, identified novel subsets, aided in reclassification of existing subsets, revealed activation, exhaustion, and suppression states of multiple immune types within tumors, shed light on responses to immunotherapy, and much more.
Single-cell proteomics
Long before the advent of NGS, immunologists and cancer biologists have been performing low- to moderate-dimensional single cell protein analyses using a variety of platforms such as ELISPOTs, flow cytometry, and various forms of microscopy. More recently, scientists have developed new technologies to achieve higher dimensionality with theoretical ranges reaching the entire proteome. A recent study used confocal immunofluorescence (IF) to image and characterize the subcellular localization of over 12,000 human proteins at the single-cell level and presented the results in an interactive database known as the Cell Atlas (Thul et al., 2017). Using an approach based off of Edman Sequencing and total internal refraction microscopy (TIRF), scientists have demonstrated the ability to identify proteins at zeptomolar concentrations in parallel in a manner that should enable single-cell proteomics (Swaminathan et al., 2018). As single-cell genomic approaches cannot always replace protein level analyses (Latonen et al., 2018), such approaches could greatly enhance our understanding of individual cells.
To assess heterogeneity in leukocyte populations, bioengineers have developed technologies to quantify secreted cytokines at the single-cell level. Using oligo-barcoded antibodies in microfluidic devices, researchers characterized heterogeneity in the secretome of tumor antigen-specific T cells from melanoma patients (Ma et al., 2011). Similarly, a technology based upon single-cell microwells and antibody-coated slides has enabled profiling of the temporal dynamics of T cell cytokine responses at the single-cell level (Han et al., 2012). These researchers also adapted their platform to enable low-cost single-cell RNA sequencing (Gierahn et al., 2017).
One of the most widely adopted technologies used by systems immunologists is mass cytometry (CyTOF) (Bendall et al., 2012). This approach replaces fluorescent tags on antibodies with transition element metal isotopes, to enable measurement of single cells labeled with such antibodies using time-of-flight mass spectrometry (Bandura et al., 2009; Bendall et al., 2011). This technology enables researchers to measure the expression of more than 40 proteins simultaneously on single cells, by eliminating limitations derived from spectral overlap of fluorescent probes. This added dimensionality facilitates a more comprehensive characterization of cellular states or population frequencies, particularly among hematopoietic cells, and has been used to evaluate a wide range of complex processes ranging from hematopoiesis and maturation (Bendall et al., 2014; Good et al., 2019), to antigen-specific responses and vaccine responses (Newell et al., 2013; Newell and Davis, 2014; Swadling et al., 2014), to immune responses to cancer (Hartmann et al., 2019; Good et al., 2018; Lavin et al., 2017; Irish and Doxie, 2014; Chevrier et al., 2017; Simoni et al., 2018; Mistry et al., 2019; Spitzer et al., 2017). By combining immunolabeling with mass tags, mass cytometry has enabled Systems Immunologists to acquire high-dimensional single-cell data for parameters that cannot be measured by single-cell sequencing approaches.
High-dimensional imaging modalities
With the exception of the peripheral blood, regulation of the immune response relies upon tissue architecture to facilitate homotypic and heterotypic interactions between cells. Secondary lymphoid organs (SLOs, e.g. spleen and lymph nodes) are notable examples wherein local chemokine gradients and tissue architecture enable lymphocytes and APCs to efficiently interact in a manner that orchestrates adaptive immunity (Cyster, 1999; Drayton et al., 2006). Similarly, immune cell locations and interactions within and surrounding tumors are typically not random, and many tumors contain organized lymphoid structures known as tertiary lymphoid structures that are believed to impact clinical outcome (Jones et al., 2016; Joshi et al., 2015; Goc et al., 2013). While the aforementioned single-cell technologies provide the requisite resolution for evaluating cell-cell interactions, they rely upon tissue dissociation and the generation of cell suspensions, which prohibit the evaluation of interactions within the native tissue architecture. Furthermore, the tissue dissociation process itself may affect the states of the cells results of the analyses (van den Brink et al., 2017). Microscopy approaches including light microscopy, fluorescence microscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and variations on these technologies have all enabled clinicians and researchers to investigate tumor-immune interactions in their natural environments, but have been limited in the number of simultaneous parameters that can be measured. As with conventional flow cytometry, spectral overlap limits the number of probes that can be used with fluorescence microscopy. Nonetheless, researchers have used deconvolution approaches with fluorescence confocal imaging to increase the degree multiplexing (Gerner et al., 2012).
Recently, new imaging approaches have been developed that enable increased multiplexing capacity. Following a similar approach as employed with mass cytometry, researchers have combined heavy metal isotope-labeled antibodies to image over 40 parameters simultaneously on histology slides (Angelo et al., 2014; Giesen et al., 2014). In one approach, histology slides are labeled with metal-conjugated antibodies and a layer of tissue is subsequently removed by laser ablation allowing for evaluation using a mass cytometer (Giesen et al., 2014). Another technology, termed multiplexed ion beam imaging (MIBI), uses a focused ion beam to release secondary ions from the antibody-labeled tissue, which are subsequently detected using a magnetic sector mass spectrometer (Angelo et al., 2014). The group that developed MIBI has used it to interrogate tumor-immune interactions in triple-negative breast cancer (Keren et al., 2018).
While mass spectrometry-based approaches enable multiplexing and simultaneous quantitation of all of the parameters at subcellular resolution, they require conjugation of heavy metal isotopes to antibodies as well as access to large and expensive instrumentation. One alternative is to use cycles of conventional immunohistochemistry (IHC) by inactivating dyes or stripping antibodies after imaging and restaining with additional antibodies in a cyclical process. A number of variations of this strategy exist (Gerdes et al., 2013; Schubert et al., 2006; Lin et al., 2015; Tsujikawa et al., 2017; Glass et al., 2009; Wählby et al., 2002), but approaches aimed at minimizing target degradation typically either photobleach the samples or exploit alkaline oxidation to bleach the fluorescence of cyanine dyes after each round of imaging. One drawback to these approaches is that they require repeated rounds of staining. A modified version of this approach, termed CODEX, has been developed wherein oligo-conjugated antibodies are used to stain all antigens at once. Subsequently, fluorophore-labeled complementary oligos to two or three of the antigens are hybridized to the probes, imaged, and removed. Additional cycles are performed enabling highly multiplexed imaging without requiring antibody staining between each cycle (Goltsev et al., 2018).
In addition to immunohistochemistry (IHC) approaches, single molecule RNA fluorescence in situ hybridization (FISH) approaches have been adapted to enable multiplexed imaging of tens to thousands of RNA probes in cells and tissues (Chen et al., 2015a; Lubeck and Cai, 2012; Lubeck et al., 2014; Coskun and Cai, 2016; Levesque and Raj, 2013). In particular, multiplexed error-robust FISH (MERFISH) uses combinatorial barcodes spaced with a Hamming distance of four to enable a high degree of multiplexing while maintaining minimal chances of calling errors (Chen et al., 2015a). Using rolling circle amplification, other groups have developed in situ sequencing approaches capable of evaluating spatial transcript patterns in tissues (Ke et al., 2013; Lee et al., 2014). All these highly multiplexed imaging modalities are designed to enable cancer systems immunologists to investigate how tissue architecture and cell interactions shape immune responses to tumors, and these methods will likely facilitate the development of novel imaging biomarkers for clinical applications in oncology.
Additional systems-level technologies
While sequencing, cytometry, and imaging technologies are currently the predominant tools employed by systems biologists, a number of other highly multiplexed tools have enhanced systems-level studies of tumor-immune interactions. Alterations in metabolic pathways is a key feature of many cancers (Ward and Thompson, 2012; Warburg et al., 1927). Similarly, immune function is highly impacted by the local metabolic state, and many studies have uncovered important metabolism-mediated tumor-immune interactions that affect tumor progression (Renner et al., 2017). The Human Metabolome Database provides a comprehensive collection of human metabolism data enabling systems-level analyses (Wishart et al., 2007). While most metabolic profiling is performed by conventional forms of MS or nuclear magnetic resonance (NMR) (Holmes et al., 2008; Nicholson et al., 2002), recent advances in imaging mass spectrometry have enabled researchers to profile these pathways directly in tissues (Caprioli, 2016; Kompauer et al., 2017; Sun et al., 2019). Metabolomics has been adopted by systems biologists, who have coined the term ‘Metabonomics’ to refer to the combined outputs of the various metabolomic influences in a multicellular system (Nicholson et al., 2002; Nicholson et al., 2008). Similarly, the field of Glycomics has been transformed by advances in MS and NMR as well as technologies such as glycan microarrays (Song et al., 2011). These technologies have enabled systems-level studies into tumor-immune interactions, and in particular the ability of the immune system to recognize tumor-specific carbohydrate antigens (Satomaa et al., 2009; Liau et al., 2017).
Both tumor cells and immune cells rely heavily upon a variety of soluble proteins for their growth, development, and migration. These collections of secreted proteins, sometimes referred to as the ‘Secretome’ (Tjalsma et al., 2000), have been profiled using a variety of MS-based techniques (Makridakis and Vlahou, 2010; Naba et al., 2012; Meissner et al., 2013). Approaches using antibody arrays and microfluidics have also enabled secretome profiling (Mustafa et al., 2011; Kshitiz et al., 2019). Cytokines and chemokines, in particular, mediate crosstalk between tumors and immune cells and orchestrate immune responses to tumors. To profile these molecules, a number of companies now offer bead-based platforms wherein antibody-labeled beads are incubated with samples and analytes are detected with a secondary antibody, akin to a sandwich ELISA, and quantified on a flow cytometer or dedicated instrument. By performing these assays in solution, multiple analytes can be assayed simultaneously in the same sample, enabling high degrees of multiplexing.
Finally, it is worth noting that while the majority of the technologies discussed above enable characterization of endogenous states, the implementation of genetic screens, particularly those employing the CRISPR/Cas9 system, have made it possible to determine the genetic underpinnings of pathways and phenotypes in a variety of settings (Wang et al., 2014b; Shalem et al., 2014; Doench et al., 2016). These screens have been pioneered in both cancer and immune cells and have revealed previously unappreciated underlying regulatory networks beneath common signaling molecules (Berger et al., 2016; Parnas et al., 2015; Shifrut et al., 2018). In particular, these approaches have been used to interrogate lymphocyte responses to tumors, including in the context of immunotherapy (Shifrut et al., 2018; Dong et al., 2019; Zhou et al., 2014; Pech et al., 2019). A recent study used CRISPR activation (CRISPRa) (Konermann et al., 2015) to activate endogenous genes within tumors as potential neoantigens in a multiplexed fashion that renders the tumors susceptible to immunotherapy (Wang et al., 2019). A number of approaches enable these screens to be applied to single cells (Dixit et al., 2016; Datlinger et al., 2017; Jaitin et al., 2016). Among these approaches is Perturb-seq, which combines multi-locus CRISPR screens with scRNA-seq (Dixit et al., 2016), making it possible to interrogate the effects of higher order interactions that cannot be predicted by the responses to single-gene knockouts. In addition to simply identifying new gene targets, these genome-wide screens should enable systems biologists to discover emergent behaviors of tumor-immune interactions by combining multiple genetic perturbations, cell types, or by performing these screens in vivo.
Modeling approaches for cancer systems immunology
While often disparate fields, mathematical modeling, computational modeling, informatics tools, and statistical analyses are often inextricably linked, as mathematical modeling (e.g. differential equations) can be aided by computational approaches and bioinformatics tools often rely upon novel or existing mathematical analyses for their implementation. The breadth of modeling approaches and informatics tools in the field of Systems Biology (applied to cancer, the immune system, or both) is too large to review here and has been discussed elsewhere (Germain et al., 2011; Altrock et al., 2015; Meyer and Heiser, 2019; Machado et al., 2011; Norton et al., 2019; Woelke et al., 2010; Stuart and Satija, 2019; Materi and Wishart, 2007). Many modeling approaches, such as Petri Nets (Petri, 1962; Sackmann et al., 2006; Koch, 2015) and Boolean Networks (Thomas, 1973; Saez-Rodriguez et al., 2007; Mai and Liu, 2009; Stoll et al., 2012), have been used extensively to model regulatory and signaling networks within cells (Vieira and Vera-Licona, 2019). While such networks play important roles in governing tumor-immune interactions, the multiscale nature and complexity of immune responses to tumors often renders these approaches challenging to scale up for many applications in cancer systems immunology. Here, we focus on some general principles and tools that are particularly germane to the field of tumor immunology.
Both malignant and immune cells respond to their microenvironments. Factors such as the mechanics of the extracellular matrix (ECM), gradients in cytokines and chemokines, availability of nutrients, and much more drive cells to alter their behavior. Frequently, tumor cells and immune cells influence their own behavior and that of each other by inducing changes in these factors (e.g. immune cells secreting cytokines to recruit and activate more immune cells or cancer cells invading along stiff ECM). To model these elements, mathematicians have frequently turned to systems of ordinary differential equations (ODEs, e.g. for mechanics or chemical reactions) (Ideker, 2001; Bangasser and Odde, 2013) and partial differential equations (PDEs, e.g. for reaction-diffusion analyses) (Turing, 1952; Matzavinos et al., 2004; Khain and Sander, 2006). These models are both intuitive and adaptable and can be solved under many conditions with the assistance of computational approaches. Indeed, such deterministic models have been employed to evaluate tumor-immune interactions (Bellomo et al., 1999; Matzavinos et al., 2004; Owen and Sherratt, 1998). With increasing complexity (e.g. inclusion of many cell types, all producing gradients of multiple soluble factors and responding to them outside the assumptions of steady state conditions), however, analysis of ODEs and PDEs can become cumbersome and computationally impractical, and in such instances, alternate approaches can be more computationally feasible.
A variety of approaches have been used to model competition between immune cells and different clones within tumors. Probabilistic models that utilize evolutionary game theory (EGT) can be amenable to such modeling (Basanta et al., 2012; Pacheco et al., 2014; Stanková et al., 2019; Nowak and Sigmund, 2004; Archetti, 2013; Bellomo and Delitala, 2008). Rule-based approaches have gained popularity for modeling complex systems. Cellular automaton (CA) models can be used to study effects of the microenvironment on tumor growth (Jiao and Torquato, 2011; Gerlee and Anderson, 2007) and immune system homeostasis (Seiden and Celada, 1992; Kaufman et al., 1985). These models place cells within lattices and rely on defined rules to simulate their interactions. Stochasticity (e.g. Brownian motion) can be incorporated into such models as well as the ability to evaluate temporal responses. Perhaps, one of the most powerful modeling approaches in systems biology is agent-based modeling (ABM) (Soheilypour and Mofrad, 2018; Holcombe et al., 2012; Pogson et al., 2008; Odell and Foe, 2008; Drasdo and Höhme, 2005; Ghaffarizadeh et al., 2018; Poleszczuk et al., 2016; An, 2008; Chiacchio et al., 2014). ABM is based upon the interactions of agents, which can be anything from molecules, to cells, to organisms. Their interactions and behaviors are governed by a set of rules, but unlike CA, they are not restricted to a lattice. The agents are autonomous; they can evolve, and their interactions can reveal collective emergent behavior. The ability to simulate interactions between thousands of agents, which could represent a myriad of cell types or molecules, renders this approach particularly amenable to the study of tumor immune interactions (Norton et al., 2019). A number of studies have used ABM to model these interactions during tumor progression (Enderling et al., 2012; Alfonso et al., 2016; Pourhasanzade et al., 2017; Dréau, 2009) or response to immunotherapy (Pappalardo et al., 2011; Gong et al., 2017). PhysiCell is an ABM tool that has been used to interrogate adaptive immune responses to tumors (Ghaffarizadeh et al., 2018; Ozik et al., 2018). In this model, oxygen consumption by cells can result in necrosis and is determined by a normally-distributed parameter representing ‘oncoprotein’ expression. This parameter also affects immunogenicity of a given cell (as an approximation for neoantigen burden) and affects the degree to which immune cells attack the tumor. PhysiCell analyses have revealed how gradients of immunogenicity can ‘trap’ immune cells at the tumor center and allow tumor cells at the outside to escape immune attack and reestablish a tumor (Ozik et al., 2018). Another recent study combined EGT with ABM to model tumor invasion and show how subpopulations within tumors can co-opt distinct macrophage populations to both degrade stroma and suppress immune responses (Gatenbee, 2019).
One of the most promising aspects of systems biology analyses is their potential to integrate observations across multiple physical and temporal scales to discover emergent behavior that is not discernable from analysis of the individual components. This potential is especially attractive in the tumor setting where orchestration of immune responses occurs across all these levels (Figure 2A). To address these types of problems, systems biologists often use multi-level or hybrid analyses (Norton et al., 2019; Gerlee and Anderson, 2007; Letort et al., 2019; Jeon et al., 2010; Deisboeck et al., 2011; Chamseddine and Rejniak, 2019). Such modeling approaches combine continuous or deterministic models (e.g. ODEs and PDEs) with discrete models such as agent-based models (ABMs). By combining discrete and continuum models, researchers can bridge scales and combine bottom-up approaches that efficiently model cell-cell interactions with rules governed by differential equations spanning larger physical scales to achieve a systems-level understanding of the biology (Figure 2B). For example, combining CA models with PDEs has facilitated investigation of tumor-immune interactions during tumor growth (Mallet and De Pillis, 2006). One hybrid model revealed how spatial and phenotypic heterogeneity can lead to immunosuppression (Wells et al., 2015). These hybrid approaches will enable researchers to bridge existing multivariate datasets and observations to generate more holistic models of tumor immune interactions capable of predicting emergent phenomena that may be difficult to discern with existing experimental approaches.
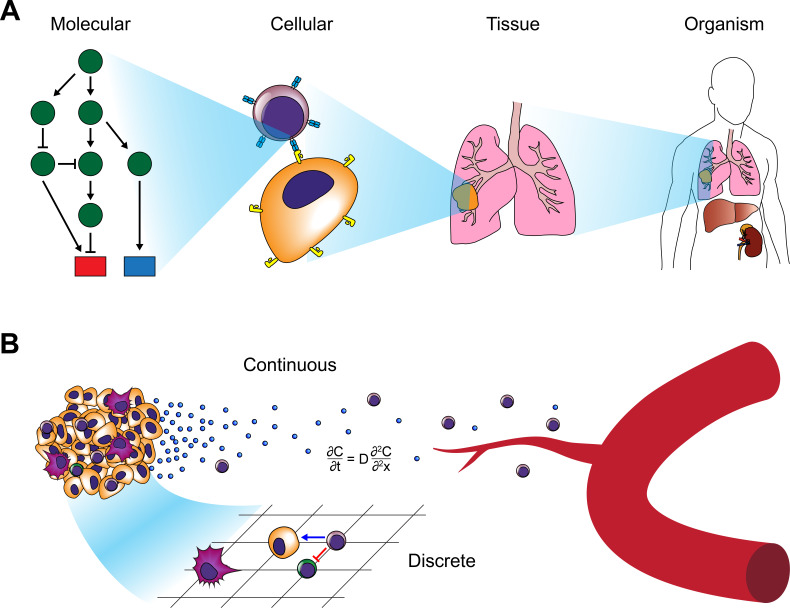
Figure 2. Modeling approaches for cancer systems immunology.
(A) Modeling approaches used in cancer systems immunology operate within or across multiple scales in order to describe how tumors interact with the immune system. (B) Hybrid models, in particular, combine continuous models (e.g. differential equations) with discrete models (e.g. CA or ABM).
Bioinformatics tools for analyzing systems-level data
In addition to the use of computational approaches to develop models of tumor-immune interactions, bioinformaticians have developed a large array of tools for analyzing the data that are produced by the technologies described earlier. While the full extent of these tools is beyond the scope of this review, we highlight a few topics that are particularly relevant to cancer systems immunology.
The reduced cost and increased accessibility of DNA microarrays and NGS have resulted in vast amounts of publicly available human datasets. The Immunological Genome Project (ImmGen) provides transcriptional profiles from all the major immune subsets in mouse and human (Heng et al., 2008; Shay and Kang, 2013). Resources such as InnateDB, ImmuneXpresso, and the Human Immunology Project Consortium also provide large databases of immune expression profiles and interaction networks (Breuer et al., 2013; Kveler et al., 2018; Brusic et al., 2014). Projects such as the Cancer Genome Atlas (TCGA) have collected –omics data from tens of thousands of patients (The Cancer Genome Atlas, 2006). A recent herculean effort by multiple investigators used TCGA data across 33 cancer types and 10,000 tumors to identify six immune subtypes conserved across cancers that can inform outcome predictions and identify regulatory networks independent of tumor type (Thorsson et al., 2018). By performing a pan-cancer meta-analysis of transcriptional profiles in 18,000 patients using a tool called PRECOG, researchers identified specific signatures of distinct leukocyte infiltration that correlates with outcome (Gentles et al., 2015), while another study correlated the antigenicity of tumors with immune responses to characterize the ‘antigenome’ of tumors (Angelova et al., 2015). One of the challenges that arises when analyzing these data is the heterogeneous nature of the tissue collected for these bulk analyses. The sequenced tumors typically contain not only malignant cells but also a variety of stromal and immune populations making it difficult to determine whether observed differences in for example, gene expression or allele frequency, reflect changes within a tumor, changes in other cells, or simply differences in cellular makeup. To address this challenge, bioinformaticians have developed deconvolution algorithms that can approximate cellular content from bulk transcriptomic data (Racle et al., 2017; Newman et al., 2015; Newman et al., 2019; Ahn et al., 2013; Yoshihara et al., 2013; Gong and Szustakowski, 2013; Li et al., 2016b; Becht et al., 2016; Shen-Orr et al., 2010; Zhong et al., 2013; Aran et al., 2017; Quon et al., 2013; Shen-Orr and Gaujoux, 2013; Vallania et al., 2018; Du et al., 2019). CIBERSORTx is capable of not only deconvolving cellular mixtures, but also quantifying cell-type-specific gene expression profiles (Newman et al., 2019). It performs this analysis by developing signature matrices derived from scRNA-seq and FACS sorted bulk RNA-seq datasets. These types of approaches will allow systems biologists to leverage the prodigious amounts of publicly available bulk RNA-seq data to discover how specific immune subsets interact with tumors and influence clinical outcome.
Even with advances in deconvolution, however, scRNA-seq remains one of the most effective methods for evaluating immune profiles within tumors (Shalek et al., 2013; Zilionis et al., 2019; Puram et al., 2017; Azizi et al., 2018; Tirosh et al., 2016; Schelker et al., 2017; Qiu et al., 2019; Zheng et al., 2017). This approach enables interrogation of the immune composition of tumors in an unbiased fashion. The amount of data and diversity of cell types can render interpretation of the datasets challenging, however. Computational biologists have developed a number of tools to analyze these datasets and present the findings in an interpretable fashion (Stegle et al., 2015; Chen et al., 2019a). Typically, expression profiles are clustered in an unsupervised manner and visualized using dimensional reduction techniques (Macosko et al., 2015; Jaitin et al., 2014; Maaten and Hinton, 2008; Žurauskienė and Yau, 2016; Xu and Su, 2015; Guo et al., 2015; Grün et al., 2015; Kiselev et al., 2017; Becht et al., 2019; Levine et al., 2015). Dimensional reduction and visualization approaches such as principal component analysis (PCA) (Pearson, 1901), diffusion maps (Coifman et al., 2005), t-distributed stochastic neighbor embedding (t-SNE) (Maaten and Hinton, 2008), and uniform approximation and projection (UMAP) (McInnes et al., 2018) enable researchers to quickly visualize changes in cell populations. Many ‘all-in-one’ pipelines, such as Seurat, incorporate the normalization, clustering, and visualization algorithms in a single R package to assist in efficient interpretation (Butler et al., 2018; Stuart et al., 2019). A major challenge in scRNA-seq analysis is the occurrence of batch effects. For example, the effects may result in the T cells of one patient clustering more closely with macrophages from the same patient than with T cells from another patient. Such effects are especially prominent when attempting to compare data acquired on different platforms (e.g. 10X Chromium vs. inDrop vs. Fluidigm C1). Furthermore, scRNA-seq captures only a small percentage of the transcripts in any given cell due to undersampling and technical limitationss, resulting in dropout. A variety of techniques have been developed to deal with batch effects and dropout by imputing expression of zero-read genes and defining anchors onto which clusters can be mapped (Stuart et al., 2019; van Dijk et al., 2018). Using a method termed ‘Biscuit’, one approach normalizes and clusters cells simultaneously using co-expression of genes to identify cell types, which are then normalized independently and dropout gene expression is imputed (Azizi et al., 2018; Prabhakaran, 2016). This approach enabled robust comparisons of scRNA-seq datasets across platforms, and was used to demonstrate the diversity of T cell states in human breast tumors.
Single-cell cytometry and imaging technologies also produce large high-parameter datasets whose interpretation has been aided by the development of computational tools. For example, spanning-tree progression analysis of density-normalized events (SPADE) overcomes the limitations of biaxial plots frequently employed in the analysis of conventional flow cytometry to facilitate analysis of mass cytometry data (Bendall et al., 2011; Qiu et al., 2011). This approach uses agglomerative clustering on down-sampled data along with a minimum spanning tree approach to generate a visual representation upon which the original data is displayed. Like many of the mass cytometry analysis tools, SPADE does not rely upon preexisting knowledge and enables an agnostic interpretation of the data. Other methods include viSNE (an adapted version of t-SNE) (Amir et al., 2013), FlowSOM (Van Gassen, 2015), Citrus (Bruggner et al., 2014), PhenoGraph (Levine et al., 2015), X-shift (Samusik et al., 2016). Another tool, Wanderlust, is capable of inferring a trajectory continuum of cell states from mass cytometry data and has been applied to understanding the transitions during B cell development (Bendall et al., 2014). In collaboration with the lab of Garry Nolan, our group developed a tool known as Scaffold Maps that uses manual gating of mass cytometry data to define landmark nodes in combination with force-directed layouts to generate a graphical reference map of the immune system that can be used to compare tissues, species, or other parameters (Spitzer et al., 2015). By augmenting this approach with statistical inference adopted from the significance analysis of microarrays (SAM) (Bair and Tibshirani, 2004), we applied this approach to interrogating cancer immunotherapy in genetically-engineered mouse models of breast cancer and melanoma (Spitzer et al., 2017). These analyses revealed the importance of secondary lymphoid tissues in orchestrating anti-tumor immune responses as well as identified an emergent CD4+ T cell population that is a key element of effective immunotherapy. As with mass cytometry, high-parameter imaging modalities require the advent of new analysis tools. A recent study exploring immune infiltration in triple-negative breast cancer patients by MIBI used a deep-learning approach to aid in image segmentation and revealed populational co-occurrence patterns that correlate with prognosis (Keren et al., 2018). Deconvolution approaches have also been applied in combination with multiplexed fluorescence confocal imaging to interrogate immune interactions in entire organs, such as lymph nodes (Gerner et al., 2012). This approach was recently used to demonstrate how autoreactive T cells are regulated by clustering with Tregs and migratory dendritic cells in lymph nodes (Liu et al., 2015). To visualize interactions between immune cells such as T cell interactions with antigen-presenting DCs, researchers have traditionally used two-photon excitation microscopy (Stoll et al., 2002; Mempel et al., 2004; Miller et al., 2002). In a recent study, researchers developed a deep convolution neural network to identify and characterize DC-T cell interactions in fixed tissue samples, thus enabling quantification of these interactions tissues from mice and humans without requiring the use of transgenic animals expressing fluorescent reporters (Liarski et al., 2019). These analyses could have important implications for understanding how tumors interact with the immune system during malignant progression.
Systems approaches to understanding the roles of specific immune subsets in the tumor immune microenvironment
Studies investigating the immune status of tumors have revealed that the prognosis of patients often strongly correlates with the degree of T cell infiltration into the local tumor microenvironment (TME) (Galon et al., 2006; Binnewies et al., 2018; Gajewski et al., 2013). Immune infiltrates have also served as effective prognostic biomarkers of response to immune checkpoint blockade (ICB) (Herbst et al., 2014; Tumeh et al., 2014). Tumor immunologists frequently refer to tumors as ‘hot’ or ‘cold’ (or ‘deserts’, in extreme cases) to describe the degree of infiltration of immune cells beyond the tumor margin, but these crude epithets fail to capture the breadth of nuance, and presumably prognostic value, that can be extracted using systems biology approaches to interrogate the TME. Furthermore, the TME does not exist in isolation, but rather is the product of constant communication with the entire organism (Egeblad et al., 2010) rendering its analysis particularly amenable to systems approaches. While most systems analyses have focused on immune responses within the primary tumor or peripheral blood, some recent studies have extended their analyses to the TME of metastatic sites such as LNs (Puram et al., 2017; Tirosh et al., 2016; Kim et al., 2020). Further studies are needed to comprehensively interrogate systemic immune responses to metastases, as lymph node metastases can render the systemic immune response permissive to tumor progression (Reticker-Flynn et al., 2020), and the invocation of systemic immunity is required for the efficacy of immunotherapy (Spitzer et al., 2017).
Macrophages
In the mid-nineteenth century, Rudolf Virchow first recognized that tumors frequently contain leukocytes. He posited that chronic inflammation lies at the origins of tumors (Virchow, 1863; Virchow, 1858), leading Dvorak, 1986, to describe tumors as ‘wounds that do not heal’, a century later. Many of the initial investigations into the TME focused on the role of myeloid cells in promoting tumorigenesis and metastasis (Joyce and Pollard, 2009; Qian and Pollard, 2010; Coussens and Werb, 2002; Engblom et al., 2016). In particular, researchers have focused on macrophages as key instigators of both tumor-promoting inflammation as well as immunosuppression. While macrophages represent essential components of innate immunity due to their capacity to scavenge for microbial pathogens, drive new blood vessel formation, and process and present antigens to lymphocytes, they have long been associated with a range of pathologies including atherosclerosis, cirrhosis, neurodegeneration, and malignancy (Murray and Wynn, 2011). Nearly all solid tumors exhibit evidence of macrophage involvement regardless of the presence of other infiltrating immune types. While tumor-associated macrophages (TAMs) have been reviewed extensively elsewhere (Lewis and Pollard, 2006), we focus here on recent systems biology approaches employed to elucidate their roles in the TME.
Macrophages within tissues are derived from one of two sources: either, they differentiate from circulating bone-marrow-derived monocytes that have extravasated from blood vessels or, in the case of some specialized tissues, they were seeded during development by macrophages derived from the yolk sac or monocytes from the fetal liver (Ginhoux and Guilliams, 2016; Epelman et al., 2014). Langerhans cells of the epidermis (Merad et al., 2002), microglia of the brain (Ginhoux et al., 2010; Ajami et al., 2007; Yona et al., 2013), Kupffer cells of the liver (Yona et al., 2013), and alveolar macrophages of the lungs (Yona et al., 2013; Hashimoto et al., 2013; Guilliams et al., 2013) are all examples of the latter ontogeny and are known as tissue-resident macrophages. Circulating bone-marrow-derived monocytes serve as the other major macrophage source and the primary source after birth. The majority of these monocytes are known as inflammatory or classical monocytes. They express high levels of Ly6C (in mice) and are recruited to sites of inflammation, often through CCR2, where they differentiate into macrophages (although such differentiation typically does not occur during steady state conditions) (Jakubzick et al., 2013; Tsou et al., 2007; Serbina and Pamer, 2006). Ly6Clo nonclassical or patrolling monocytes typically remain within vessels and crawl along the endothelial walls to clean up debris from dying endothelium (Auffray et al., 2007; Carlin et al., 2013).
The majority of TAM research has focused on macrophages derived from inflammatory monocytes and recruited to the TME (Kitamura et al., 2015; Richards et al., 2013). Classically, macrophages have been divided into M1 and M2 macrophages in an attempt to mimic the nomenclature of T helper (Th) cells (Mills et al., 2000). M1 macrophages (or classically activated macrophages) were thought to represent a state of polarization induced by Th1-derived cytokines such as IFN-γ or microbial stimuli (e.g. LPS). They exhibit elevated MHC-II expression and produce nitrous oxide (NO), reactive oxygen species (ROS), TNF-α, and a milieu of inflammatory cytokines (e.g. IL-12, IL-23, IL-6, IL-1, etc.) (Dalton et al., 1993; Mantovani et al., 2004; Atri et al., 1801). In contrast, M2 macrophages (alternatively-activated macrophages) were initially reported to be polarized by Th2 cytokines such as IL-4 and IL-13 that drive alternative macrophage activation (Stein et al., 1992; Doyle et al., 1994; Gordon, 2003). These macrophages express arginase 1, IL-10, CCL17, CCL22, and CCL24 and are generally considered anti-inflammatory and immunosuppressive (Biswas and Mantovani, 2010). Consequently, M1 macrophages have traditionally been associated with anti-tumor effects while M2 macrophages were considered pro-tumor (though M1 ROS production has been suggested to play a role in tumorigenesis) (Kratochvill et al., 2015; Zhang et al., 2013). Over time, it became apparent that the initial nomenclature, derived primarily from in vitro activation experiments, was likely not sufficient to classify the M2 macrophage states, in particular. Distinctions between Th2 cytokine production, IL-10-mediated immunosuppression, and immune complex and TLR stimulation have led to an effort to subset M2 macrophages into three groups (Mantovani et al., 2004).
Recently, a number of studies that have employed systems approaches have revealed that this binary interpretation of macrophage polarization is vastly oversimplified and fails to capture the diversity of phenotypes exhibited by macrophages (Mosser and Edwards, 2008; Xue et al., 2014; Murray et al., 2014; Martinez and Gordon, 2014; Lavin et al., 2014; Ginhoux et al., 2016; Reinartz et al., 2014; Zhang et al., 2019). One group of investigators interrogated the regulatory landscape of tissue-resident macrophages across a variety of organs by combining RNA-seq, ChIP-seq, and ATAC-seq (Lavin et al., 2014). In this study, the corresponding tissue-resident macrophages from the brain, lungs, liver, spleen, peritoneum, ileum, and colon were purified by FACS, and subjected to these analyses to reveal distinct gene expression, enhancer methylation, and chromatin states depending on their tissue of residence. In accordance with reports describing the considerable plasticity of macrophages, this study also showed that transplanted macrophages could be reprogrammed by their new tissue of residence in a manner that mimics the regulatory landscape of macrophages in those tissues. Seeking to characterize the diversity of macrophage activation states, other investigators performed transcriptional profiling of human macrophages following in vitro activation with a variety of stimuli and found that the cells exhibit a range of distinct states that extend well beyond the conventional M1/M2 polarization axis (Xue et al., 2014). Similarly, TAMs frequently fail to conform to the traditional M1/M2 polarization model (Aras and Zaidi, 2017). In one study that used mass cytometry to profile macrophages and T cells within the tumors of 73 clear cell renal cell carcinoma patients, the authors characterized 17 distinct TAM phenotypes (Chevrier et al., 2017). In lung adenocarcinoma, researchers used both MARS-seq and mass cytometry to reveal that tumor-specific macrophages exhibit distinct profiles from other mononuclear phagocytes, including lung-specific macrophages, conventional macrophages, DCs, and monocytes (Lavin et al., 2017). Characterization of myeloid cell diversity in lung adenocarcinoma using scRNA-seq identified multiple populations of neutrophils, DCs, monocytes, and macrophages that were conserved across patients and species (Zilionis et al., 2019). Of note, while some of the macrophage populations exhibited enrichment for M2 gene signatures, they did not cluster to a singular state and the myeloid cells, in general, exhibit a spectrum of states within tumors. Similarly, another study performed scRNA-seq on 45,000 leukocytes from human breast tumors along with corresponding blood, lymph nodes, and normal breast tissue (Azizi et al., 2018). Using diffusion maps (Coifman et al., 2005), they characterized the heterogeneity in both the T cell and myeloid compartments. In particular, they noted considerable diversity in the myeloid compartments, and surprisingly, found a strong positive correlation between M1 and M2 gene signatures (Figure 3). The three TAM populations that they identified often expressed both gene signatures in the same cells, again suggesting that the original M1/M2 polarization concept does not recapitulate what occurs in vivo, particularly in human tumors. Taken together, these studies suggest that macrophages within tumors do not comport to the theorized discrete M1/M2 polarization states, but rather exist within a spectrum of activation states that even permits concomitant expression of the two profiles. While mixtures of the transcriptional and epigenetic repression by the opposing cytokines IL-4 and IFN-γ may be partially responsible for the mixed phenotypes exhibited in vivo where complex mixtures of cytokines are present (Piccolo et al., 2017), it still remains unclear how such inhibitory programs could result in coexpression of both gene sets within the same cells. Adding to the complexity of TAM characterization, a number of recent studies investigating macrophage ontogeny in the context of tumors have revealed that TAMs are not only derived from bone marrow-derived monocyte precursors, but also tissue-resident macrophages seeded during embryogenesis. A recent study that used parabiosis and congenic mice revealed that TAMs in pancreatic ductal adenocarcinoma are derived from both blood monocytes and tissue-resident macrophages and that these populations serve distinct functions during tumor progression (Zhu et al., 2017). Similarly, lineage-tracing approaches, scRNA-seq, and ATAC-seq have all been used to reveal that both microglia and bone marrow-derived macrophages are involved in gliomas and exhibit distinct profiles and contributions to tumor progression (Bowman et al., 2016; Müller et al., 2017). Through an appreciation of the diversity, ontogeny, and complexity of TAM phenotypes cancer systems biologists are beginning to reveal how the interplay of these populations can facilitate tumor progression.
Figure 3. Systems approaches to the TME.
Many systems approaches have been applied to analyzing the TME to reveal the cellular makeup and relationships between immune cells at the single-cell level. Studies have revealed heterogeneity among myeloid and lymphocyte populations, trafficking of cDC subsets to the draining LNs, and epigenetic exhaustion profiles of T cells infiltrating tumors following ICB.
Dendritic Cells
Compared to macrophages, dendritic cells (DCs) represent a relative minority of the myeloid cells present within most tumors. Nonetheless, DCs are critical orchestrators of immune responses due to their exquisite capacity to present antigens and license, stimulate, or suppress T cells (Banchereau and Steinman, 1998). They can present antigens in the TME to T cells in situ or in lymph nodes (LNs) by accruing tumor debris that has drained there through the lymphatics or by themselves trafficking from the TME into LNs. Their essential role in choreographing the adaptive immune response across an organism makes them an ideal subject for integrated multi-level systems biology investigations.
Many cells of the DC lineage, including classical DCs (cDCs) and plasmacytoid DCs (pDCs), are derived from a common bone-marrow progenitor, the common DC precursor (CDP), which gives rise to a lineage distinct from other leukocytes (Onai et al., 2007; Naik et al., 2007; Liu et al., 2009; Merad et al., 2013). Traditionally, DCs have been described as belonging to four subsets: cDCs, pDCs, monocyte-derived DCs (MoDCs), and Langerhan cells (LCs), which closely resemble other tissue-resident macrophages in gene expression and phenotype (Satpathy et al., 2012; Eisenbarth, 2019). cDCs, so called as they were the first DCs described (Steinman and Cohn, 1973), are potent antigen presenting cells that efficiently phagocytose antigens and license T cells. Consequently, they play a critical role in facilitating anti- or pro-tumor T cell responses both in the local TME and LNs. cDCs can be further divided into two subsets: cDC1 and cDC2. cDC1 express the chemokine receptor XCR1 (Bachem et al., 2012; Dorner et al., 2009), require the AP-1 transcription factor BATF3 for development (Hildner et al., 2008; Murphy et al., 2013), and are characterized by their ability to cross-present antigens to CD8+ T cells. In lymphoid tissues, cDC1s typically express CD8α, while peripheral cDC1s express integrin αE (CD103) in mice (both express BDCA3 in humans) and exhibit the ability to efficiently migrate to LNs to present antigen. Both typically lack CD11b. cDC2 have been primarily associated with activation of CD4+ T cells, though both cDC subsets can activate both T cell subsets (Eisenbarth, 2019; Bedoui et al., 2009). While a number of markers have proven useful in delineating DCs subsets, recent unbiased mass cytometry and scRNA-seq studies have added clarity and revealed correlations between DC subsets across anatomical locations and species (Guilliams et al., 2016; Villani et al., 2017; See et al., 2017; Alcántara-Hernández et al., 2017).
It is likely that all of the DC subsets play important roles in tumor progression. pDCs, in particular, have been suggested to play an immunosuppressive role (Demoulin et al., 2013; Sisirak et al., 2012; Mitchell et al., 2018; Zhang et al., 2017c; Labidi-Galy et al., 2011), yet the cDC1 subset is perhaps the best-studied due to its relevance in priming antitumor CD8+ T cells, while MoDCs are frequently the most numerous in the TME. cDCs are critical for antitumor T-cell-mediated immunity (Hildner et al., 2008; Fuertes et al., 2011). They exist at the borders of tumors and exhibit prolonged interactions with tumor-specific CD8+ T cells, yet they frequently fail to stimulate the T cells to mediate an antitumor effect (Engelhardt et al., 2012; Boissonnas et al., 2013; Broz et al., 2014). Furthermore, migratory CD103+ peripheral cDC1s traffic to tumor-draining LNs in a CCR7-dependent fashion, where they interact with tumor-specific T cells and transfer tumor antigens to other LN resident DC subsets, at much higher levels than migratory CD11b+ DCs (Roberts et al., 2016; Salmon et al., 2016). FcγR engagement of immune complexes (ICs) by DCs induces their CCR-7 dependent migration to LNs (Clatworthy et al., 2014), and we have shown that the combination of DC adjuvants with tumor-bound allogeneic antibody ICs induce robust anti-tumor T cell responses (Carmi et al., 2015). Vaccines expressing Flt3 ligand (Flt3L), which induces CDP lineage commitment, have been shown to improve ICB efficacy (Curran and Allison, 2009).
Systems-level analyses have helped extend our understanding of cDC functions in tumors. One study, in which mass cytometry was used to evaluate the TME during early- and late-stage tumor development, showed that Flt3L injections vastly increases the numbers of CD103+ DCs in the TME (Salmon et al., 2016). Furthermore, these DCs transport antigen to the draining LNs where they augment PD-L1 ICB to elicit anti-tumor T cell responses. Using mass cytometry to profile the evolution of the immune repertoire at an organism-wide level in response to immunotherapy, we found that in the initiation of anti-tumor immune responses, CD103+ DCs in the tumor microenvironment exhibit a strong increase in proliferation (Spitzer et al., 2017). Furthermore, the cDC frequencies decrease in the local TME during the therapy initiation phase while increasing in the draining LNs during the rejection phase, suggesting that they may become activated at the tumors and subsequently traffic to LNs to facilitate the generation of anti-tumor T cell responses. Using scRNA-seq, one study found that migration of cDC2s from tumors to the draining LNs is required for priming of CD4+ Tconv and eliciting a potent antitumor response, but Tregs inhibit their efficacy likely by preventing their migration to the LNs (Figure 3; Binnewies et al., 2019). A recent study combined 10X Chromium with SMART-seq scRNA-seq platforms to investigate the cellular diversity of hepatocellular carcinoma (HCC) and found that the droplet and plate-based approaches identify distinct populations (Zhang et al., 2019). Additionally, they identified a LAMP3+ subset of DCs that appeared to migrate from the tumor to draining LNs from their RNA velocity analysis, but these DCs share transcriptional features with both cDC subsets as well as pDCs. This population appears to be inducible from multiple lineages upon stimulation with various DC adjuvants, although it remains unclear whether this subset represents an activation state or a different state of differentiation. These agnostic systems approaches have been particularly useful in understanding these types of complex interactions where DCs are interacting with many different cell types in multiple organs in manners that change considerably over the timecourse of the immune response.
T cells
While investigations into adaptive immune responses to tumors have long been a major focus of tumor immunologists, the recent successes of immune checkpoint blockade (ICB) and engineered chimeric antigen receptor T cells (CAR-T cells) have stimulated a renewed interest in understanding T cell interactions with tumors. Consequently, many novel systems approaches have been applied to T cells. Due to their ability to recognize tumor-specific antigens and kill tumor cells in an antigen-specific manner, T cells represent one of the most critical components of anti-tumor immune responses. Nonetheless, T cells frequently fail to eliminate tumors likely due to immune evasion (DuPage et al., 2012; Matsushita et al., 2012; Pereira et al., 2017; Sade-Feldman et al., 2017) or suppression (Joshi et al., 2015; Willimsky and Blankenstein, 2005; Pauken and Wherry, 2015) mechanisms of the tumors. Furthermore, while a variety of cells and conditions can promote broad immunosuppression of a host, T cells can become subject to tolerance or exhaustion in a manner that impairs tumor-specific immunity without affecting the ability of the immune system to defend against unrelated pathogens (Schietinger and Greenberg, 2014). It is this latter feature that renders tumors such pernicious adversaries in efforts at both detecting and combatting malignancies.
Systems approaches have aided our understanding of T cell responses to tumors in a variety of contexts. One of the mechanisms by which tumor-specific T cells are thought to become dysfunctional is through processes related to exhaustion. T cell exhaustion, first identified in chronic viral infections (Zajac et al., 1998; Gallimore et al., 1998), is induced by persistent exposure to antigen accompanied by failure to eliminate it (Wherry, 2011). It can take multiple forms and likely evolved as a mechanism to avoid inflammation-induced pathologies in which pathogens cannot be resolved (Blank et al., 2019). It is worth noting that TEx are not entirely ineffective and exhibit some anti-pathogen efficacy even if unable to eliminate the pathogen. Features of exhaustion include elevated expression of inhibitory receptors (e.g. PD1, Lag3, Tim-3, etc.), reduced cytokine expression, reduced proliferation, altered metabolism, and reduced effector function (McLane et al., 2019). In tumors, T cells often acquire a phenotype that is analogous to exhaustion, although it is unclear whether this dysfunctional state exhibits a similar capacity to be reprogrammed, as is often evidenced in the context of viral infections (Philip and Schietinger, 2019).
Through the use of systems approaches to evaluate the regulatory landscape of tumor-specific T cells, what has become abundantly clear is that, like most T cell states (Zhang et al., 2012; Shih et al., 2016; Yu et al., 2017), the exhausted phenotype of these cells is regulated at an epigenetic level, suggesting that TEx represent a distinct cell type (Figure 3; Sen et al., 2016). Evaluation of chromatin accessibility landscapes by ATAC-seq has revealed that naïve, effector, memory, and exhausted T cells exhibit profound differences in open chromatin regions (OCR) (Sen et al., 2016). While PD-1 signaling blockade can reinvigorate exhausted T cells, it induces only moderate changes in the OCRs that do not revert the cells to a Tmem epigenetic profile, perhaps explaining why the reinvigoration is transient (Pauken et al., 2016). Yet while these epigenetic states persist during PD-1 blockade, as also evidenced through bisulfite sequencing, combining DNA demethylating agents with PD-1 blockade has shown the potential to reverse the exhaustion state and improve antitumor response (Ghoneim et al., 2017). Recent studies combining differential gene expression and OCR analyses between Tmem and TEx. suggest that epigenetic control of the exhaustion state is mediated by the transcription factor TOX, which is induced by chronic TCR stimulation, and in particular, calcineurin and NFAT signaling and is responsible for upregulation of inhibitory receptors such as PD1 (Khan et al., 2019; Alfei et al., 2019; Scott et al., 2019). Notably, inhibition of calcineurin signaling following TOX induction by treatment with FK506 or cyclosporin A did not ablate TOX expression or the TEx phenotype, suggesting that once induced, TOX expression remains stable (Khan et al., 2019). Augmenting these studies, scRNAs-eq has revealed similar TOX-driven phenotypes in the context of chronic viral infection (Yao et al., 2019). Similarly, by combining transcriptional analysis with genome-wide H3K4me3 and H3K27me3 analyses, researchers identified a critical role for the NR4A1 transcription factor in instilling the TEx state by inhibiting binding of the AP-1 transcription factor to binding sites of effector-related genes (Liu et al., 2019). These effects extend to cell therapies, where genetic ablation of NR4A genes can prevent exhaustion in CAR-T cells and improve tumor clearance (Chen et al., 2019b). Another CAR-T study found similar effects with ablation of TOX and TOX2 and suggested that TOX and NR4A positively regulate each other (Seo et al., 2019).
Studies interrogating the stability of T cell dysfunction and exhaustion in the context of viral infection, autoimmunity, and malignancy have identified a number of states, some of which are defined by their inherent reversibility and others by their proliferative or progenitor capacity, although they are frequently referred to as reprogrammable-non-reprogrammable, partial-complete, or progenitor-terminal exhaustion (Philip et al., 2017; McLane et al., 2019; Schietinger et al., 2016; Long et al., 2016; Blackburn et al., 2008; Paley et al., 2012; Im et al., 2016). Progenitor TEx, or less-differentiated TEx, express high levels of TCF-1 or T-bet and exhibit the capacity to replenish the terminally differentiated TEx (Paley et al., 2012; Im et al., 2016; Wu et al., 2016; Utzschneider et al., 2016). Of note, these subsets typically respond quite differently upon treatment with PD-1 blockade with the progenitors expanding during therapy. As these states appear to be largely regulated at an epigenetic level, ATAC-seq has been used to reveal distinct chromatin states that distinguish dysfunctional tumor-specific CD8+ T cells that can be recovered from those that are resistant to reprogramming (Philip et al., 2017). Additional technologies have facilitated systems analyses of T cell exhaustion. A recent droplet-based ATAC-seq protocol that enables massively parallel scATAC-seq profiling was used to interrogate T cell exhaustion in tumors in the context of ICB (Satpathy et al., 2019). The results revealed a massive expansion of TEx in patients post treatment with PD1 blockade. The results further revealed differences in OCRs corresponding to intermediate and terminally-exhausted TEx as well as a shared regulatory program between TEx and Tfh. A recent study that combined MARS-seq with single-cell TCR sequencing in melanoma patients to evaluate clonality of dysfunctional T cells revealed that the transcriptional profile of T cells forms a gradient of states exhibiting a range of profiles from highly cytotoxic to highly dysfunctional with shared clones occupying multiple transitional states of dysfunction (Li et al., 2019) Surprisingly, although TEx are thought to have a reduced proliferative capacity, the most proliferative T cells (by Ki67 and clonal expansion) were the dysfunctional T cells.
In addition to exhaustion, systems analyses of the TME have revealed a variety of other T cell features. By combining scRNA-seq with TCR sequencing, one group of investigators identified specific subsets of CD8+ T cells and Tregs that are enriched in hepatocellular carcinoma compared to other tissues and identified a gene, LAYN, that inhibits IFN-γ production (Zheng et al., 2017). The same investigators used this approach to characterize fate decisions in tumors, revealing a variety of relationships between various T cell subsets in the TME (Zhang et al., 2018; Guo et al., 2018). Studies interrogating the tumor specificity of tumor-infiltrating T cells by TCR sequencing (Scheper et al., 2019) and mass cytometry (Simoni et al., 2018) have revealed that many T cells within the TME do not actually recognize tumor antigens. In breast cancer, researchers used scRNA-seq to reveal that T cells with transcriptional profiles analogous to resident memory T cells (TRM) can be found within tumors and correlate with improved prognosis (Savas et al., 2018). Using scRNA-seq, ATAC-seq, and TCR-seq to query ICB responsiveness in melanoma patients revealed a variety of T cell-related factors that correspond to ICB efficacy including TCF7 expression on tumors and CD39 blockade (Sade-Feldman et al., 2018). An additional study also suggests that T cells exist along a continuum of differentiation states evidenced by scRNA-seq analyses and that tumor-involved Tregs may exhibit more heterogeneity than previously appreciated (Azizi et al., 2018). In agreement with scRNA-seq reports, a mass cytometry approach found that the lung cancer TME is enriched for T cells that are dysfunctional or regulatory (Lavin et al., 2017).
B cells
While far fewer studies have interrogated the roles of B cells in tumor progression and antitumor immunity than T cells, tumor-infiltrating B cells can be found in most solid malignancies and are readily amenable to the same types of systems analyses. The primary function of B cells is to orchestrate the humoral immune response through the production of antibodies, but there exist a number of different subsets of B cells that have additional functions (Allman and Pillai, 2008). Some B cells can elicit immunosuppressive activity, including in the context of malignancy, through a variety of mechanisms including expression of PD-L1 and production of IL-10, IL-35, and TGF-β leading some to refer to this subset as regulatory B cells (Bregs) in analogy to Tregs (Schioppa et al., 2011; Yanaba et al., 2008; Rosser and Mauri, 2015; DiLillo et al., 2010; Khan et al., 2015; Olkhanud et al., 2011; Huang et al., 2017). Production of cytokines from B cell subsets such as Bregs, B10 cells, or B1 cells can induce a myriad of effects, including repolarization of macrophage populations, induction of Tregs, or activation of survival signals within tumor cells themselves (Wong et al., 2010; Mielle et al., 2018; Ammirante et al., 2010). Furthermore, B cells can promote lymphangiogenesis (Angeli et al., 2006), which, in turn, can enable trafficking of DCs or tumor cells themselves from primary tumors to draining LNs (Roberts et al., 2016; Binnewies et al., 2019; Stacker et al., 2014; Tammela and Alitalo, 2010). Consequently, B cells have frequently been associated with promoting tumorigenesis and metastasis (de Visser et al., 2005; Shah et al., 2005; Qin et al., 1998; Affara et al., 2014; Brodt and Gordon, 1982). These immunosuppressive mechanisms typically occur in a manner that does not require antigen recognition by the BCR (Shah et al., 2005). Yet other studies have suggested that the production of antibodies themselves may promote tumor progression and metastasis by activation of Fc Receptors (FcRs) on myeloid cells, induction of tumor-intrinsic signaling through activation of surface receptors, promotion of pro-inflammatory granulocyte responses, promotion of angiogenesis, and suppression of cellular immunity (Qin et al., 1998; Pucci et al., 2016; Gu et al., 2019; Andreu et al., 2010; Barbera-Guillem et al., 1999; Nyhus et al., 2001; Tan and Coussens, 2007).
Although B cells have the potential to elicit these tumor-promoting effects, they have also been implicated as key regulators of antitumor immunity in the context of immunotherapy. Tumors often acquire tertiary lymphoid structures (TLSs), which closely resemble lymphoid follicles, containing T cells, B cells, FDCs, Tfh-like cells, fibroblast reticular cells (FRCs), and even high endothial venules (HEVs) (Jones et al., 2016; Goc et al., 2013; Dieu-Nosjean et al., 2014; Colbeck et al., 2017). Although TLSs can serve as a source of protumoral Tregs (Joshi et al., 2015), their presence in tumors has classically correlated with improved survival in a variety of cancers (Colbeck et al., 2017; Ladányi et al., 2007; Dieu-Nosjean et al., 2008; Pitzalis et al., 2014). Like SLOs, these TLSs contain germinal centers (GCs) where B cells can proliferate and undergo class switching and somatic hypermutation (GeurtsvanKessel et al., 2009; Perros et al., 2012; Neyt et al., 2012), and the presence and differentiation of B cells, in particular, within GCs correlates with improved prognosis (Germain et al., 2014). Thus, activation of humoral responses within TLS can improve responses, particularly in the context of immunotherapy (Germain et al., 2014; Helmink et al., 2020; Montfort et al., 2017; Sautès-Fridman et al., 2016; Cottrell et al., 2018; Sautès-Fridman et al., 2019). Furthermore, while FcR binding of tumor-immune complexes has been suggested to promote tumor progression (Andreu et al., 2010), we have found that combining tumor-binding antibodies with DC adjuvants and CD40 agonists results in extremely potent anti-tumor responses in a manner that is FcR-dependent (Spitzer et al., 2017; Carmi et al., 2015; Carmi et al., 2016).
Given the importance of B cells in both tumor progression and antitumor responses, researchers have begun to apply a variety of systems-level approaches to elucidate their roles in malignancy. While the use of TCR repertoire profiling is discussed in detail in the following section, analogous approaches have been developed for BCRs and employed in the context of malignancy to identify the sequences of tumor-reactive antibodies and identify their cognate antigens (Katoh et al., 2017). Building upon their TRUST algorithm developed to identify TCR CDR3 sequences from bulk RNA-seq data (Li et al., 2016a), researchers adapted their approach to BCRs to evaluate humoral responses in TCGA data across 32 cancer types (Hu et al., 2019). They characterized broad clonal expansion across malignancies and suggested roles for subclass switching in conjunction with defective ADCC. The roles that these alterations play in tumor progression or anti-tumor immune responses, however, remain to be clarified. Similarly, as part of their characterization of six distinct conserved immune subtypes using TCGA data, researchers employed immunoglobulin heavy chain repertoire profiling using V’DJer (Mose et al., 2016) on bulk RNA-seq data. This analysis revealed high variance of IgH diversity depending on immune subtype with the IFN-γ and TGFβ dominated subtypes exhibiting high variance and the ‘lymphocyte depleted’ and ‘immunologically quiet’ subtypes exhibiting considerably lower diversity.
To understand the role of TLS B cells during immunotherapy, one group combined bulk RNA-seq of melanoma and renal cell carcinoma (RCC) responders and non-responders with deconvolution algorithms to identify a strong enrichment in B cell signatures in responders. They further analyzed the BCRs of these patients from the bulk RNA-seq and found increases in both clonal diversity and expansion of individual clones in responders, suggesting antigen-specific humoral responses. Finally, using mass cytometry, they demonstrated an enrichment for memory B cells, plasma cells, and GC B cells specifically within the tumors of responders, suggesting a role for B cell activation and humoral responses in patients benefiting from ICB (Helmink et al., 2020). Other researchers combined novel GEMMs with BCR sequencing and scRNA-seq to identify a role for Tfh-induced B cells in promoting ICB responses (Hollern et al., 2019). While not as common as existing T cell analyses, these types of multimodal systems approaches to understanding the involvement of B cells in malignancy and associated therapeutic responses will be critical for uncovering the role that these lymphocytes play in tumor progression and treatment.
NK cells, non-canonical APCs, and other myeloid cells
Although T cells, B cells, TAMS, and DCs have been the subject of the majority of systems analyses of the TME, other lymphocytes, myeloid cells, and non-hematopoietic APCs play important roles in tumor progression. Similarly, the importance of NK cells and other innate lymphoid cells (ILCs) (Cerwenka and Lanier, 2001; Wu and Lanier, 2003; Marcus et al., 2014; Malmberg et al., 2017; Hsu et al., 2018; Bruchard and Ghiringhelli, 2019) and PMNs (Szczerba et al., 2019; Fridlender et al., 2009; Nozawa et al., 2006; Piccard et al., 2012; Eruslanov et al., 2014; Singhal et al., 2016) in tumor progression have been well documented but are only beginning to be explored at a systems level (Zilionis et al., 2019; Horowitz et al., 2013). In addition to DCs, macrophages, and B cells, there exist a number of non-professional APCs capable of inducing tolerance (Turley et al., 2010). Many of these cells, such as lymphatic endothelial cells and fibroblastic reticular cells, exist in or near SLOs and can induce tolerance or immunosuppression, including in the context of tumors (Cohen et al., 2010; Nichols et al., 2007; Lund et al., 2012; Lund et al., 2016; Swartz, 2014; Fletcher et al., 2010; Fletcher et al., 2015; Lee et al., 2007). The functional relevance of these cells in the induction of tumor-specific immune tolerance is in its nascent days of exploration, and systems approaches will certainly aid in advancing our understanding of their role in tumor progression.
For over a decade there has been interest in a group of cells frequently associated with tumors referred to as ‘myeloid derived suppressor cells’ (MDSCs). This nomenclature was based upon expression of the integrin subunit αM (CD11b) and staining (in mice) for a marker known as ‘Gr-1’ with an antibody that exhibits a high affinity for Ly-6G and weak affinity for Ly-6C (Gabrilovich et al., 2007). It should be noted that CD11b, Ly-6G, and Ly-6C are expressed on a wide range of immune cells at various levels including monocytes, neutrophils (PMNs), macrophages, DCs, NK cells, and even T cells. Numerous studies, including our own, have revealed that cells bearing these markers can promote tumor progression, metastasis, or suppression of T cell immunity (Reticker-Flynn and Bhatia, 2015; Gabrilovich, 2017). Nonetheless, expression of these markers, on their own, does not confer immune suppressive function. Indeed, anti-Gr-1 antibodies are frequently used to deplete neutrophils, although such antibodies also deplete some monocytes and T cells due to their Ly-6C expression (Daley et al., 2008; Faget et al., 2018). Furthermore, many studies have demonstrated the immune-suppressive and tumor-promoting capacities of PMNs (Fridlender et al., 2009; Piccard et al., 2012), monocytes (Qian et al., 2011; Jung et al., 2017), macrophages (Noy and Pollard, 2014), and DCs (Kenkel et al., 2017; Shurin et al., 2013) in tumors, making it unclear whether MDSCs represent a distinct activation state or lineage from those cell types. In an attempt to clarify the nature of this population, researchers have further divided MDSCs into granulocytic and monocytic lineages, but acknowledge that the differentiating markers have no impact on the suppressive function of these cells (Youn et al., 2008). Analogous subsets have been described in humans and their identification also relies upon markers shared by many known hematopoietic subsets (Bronte et al., 2016; Mandruzzato et al., 2016). Reliance upon these nonspecific markers has resulted in a concerning number of publications claiming MDSC classification without functionally validating the suppressive capacity of the cells. Functional analysis of sorted populations does not prove that the cells of interest are distinct from other myeloid populations (e.g. monocytes, macrophages, DCs, or neutrophils), but is capable of determining whether some cells in the sorted population have suppressive capacity (Bronte et al., 2016).
Systems biology approaches provide a more comprehensive agnostic approach toward evaluating the myeloid repertoire, as has been discussed above. One study purports to identify a specific marker, LOX-1, of PMN-MDSCs by bulk RNA-seq, though this marker is only upregulated in human PMN-MDSCs (Condamine et al., 2016). Separation of the PMN-MDSC population from PMNs, however, was performed by density centrifugation, which results in mixed populations, highlighting the inadequacy of bulk analysis approaches for populations without unique markers. While LOX-1+ cells exhibit an increased capacity to suppress T cell responses, it is unclear whether this is a feature of all LOX-1+ cells or a subset and whether the marker represents a distinct lineage rather than an activation or maturation state of PMNs. Furthermore, genetic ablation of the gene (Olr1) in mice had no impact on the suppressive function of the cells in vitro nor growth of tumors in wild-type mice reconstituted with Olr1-/- bone marrow following lethal irradiation. In contrast to bulk approaches, single-cell analyses such as scRNA-seq are particularly useful for identifying distinguishing features of individual cells, evaluating heterogeneity of populations, and reconstructing lineage relationships between populations. Indeed, when unbiased clustering of systems level single-cell data has been performed under a myriad of conditions, none of the studies report the existence of distinct MDSC clusters (Spitzer et al., 2017; Puram et al., 2017; Azizi et al., 2018; Tirosh et al., 2016; Binnewies et al., 2019; Sade-Feldman et al., 2018). In one recent exception, researchers labeled a myeloid cluster ‘MDSC-like macrophages’ due to their expression of S100A family genes, but the suppressive capacity of these cells was not evaluated. Furthermore, these cells fell along a continuum with other macrophages on diffusion maps and did not distinctly segregate from other myeloid populations in the UMAP projections. Current transcriptional, epigenetic, and proteomic analyses do not support the notion that MDSCs represent a singular or dual lineage(s) or distinct differentiation states. Most likely, they represent a heterogeneous plastic phenotypic state of neutrophils, monocytes, macrophages, and their precursors, some of which exhibit immune suppressive capacity. Thus, it is best to use caution when employing a nomenclature that ascribes function based solely upon unrelated marker expression.
T cell receptor repertoires and neoantigens: understanding and harnessing tumor-immune specificity
While the generation of effective naturally occurring or therapeutically induced immune responses typically requires the involvement of many immune cell types, T cells are frequently the most critical element of these responses. Patient prognosis often correlates with the degree of T cell infiltration into tumors (Galon et al., 2006; Zhang et al., 2003), and adoptive transfer of ex vivo stimulated autologous tumor infiltrating lymphocytes remains one of the most effective tumor immunotherapies (Rosenberg et al., 2008; Rosenberg et al., 1988). Similarly, CAR-T therapy involves engineering T cells to directly recognize tumors, and ICB works by stimulating or reinvigorating tumor-reactive T cells. A critical factor explaining the efficacy of T cell responses to tumors is the ability of the TCR to distinguish malignant from healthy tissue by its overexpression of normal antigens, re-expression of embryonic antigens, or expression of neoantigens (mutated proteins expressed only by the malignant cells). Neoantigens are frequently genomic in origin, resulting from point mutations, indels, or translocations, but can also arise from post-translational modifications such as phosphorylation or glycosylation (Bräunlein and Krackhardt, 1702; Cobbold et al., 2013; Zarling et al., 2006). Some cancers may escape T cell surveillance by reducing or eliminating expression of these neoantigens in a process known as ‘immunoediting’ (DuPage et al., 2012; Matsushita et al., 2012; Schreiber et al., 2011), while others escape by downregulating MHC-I presentation (Pereira et al., 2017; Sade-Feldman et al., 2017; Gettinger et al., 2017; Zaretsky et al., 2016; Sahin et al., 2017). Furthermore, efficacy of ICB is correlated with neoantigen burden for a variety of cancers (Yarchoan et al., 2017; Snyder et al., 2014; Carbone et al., 2017; Rizvi et al., 2015a; Galsky et al., 2017; McGranahan et al., 2016), and tumor mutational burden (TMB) may be a better predictor of ICB response than PD-L1-positive staining in some instances (Hellmann et al., 2018). Furthermore, the degree to which neoantigens are shared across tumor clones also correlates with ICB efficacy (McGranahan et al., 2016). Consequently, bioinformaticians and systems biologists have developed an array of tools and approaches for predicting and quantifying neoantigens as well as mapping T cell clonality and neoantigen recognition (Liu and Mardis, 2017).
As effective anti-tumor immune responses typically involve T cell recognition of neoantigens, approaches have been developed to vaccinate patients against the neoantigens of their tumors. Early attempts at cancer vaccines and cell therapies did not rely upon a priori knowledge of the specific neoantigens of the tumors (Fong and Engleman, 2000; Rosenberg and Restifo, 2015). Building off initial proof-of-principal studies in mice (Mandelboim et al., 1994; Mandelboim et al., 1995; Castle et al., 2012), a variety of strategies have recently been designed to specifically target the neoantigens of patient tumors (Schumacher and Schreiber, 2015; Hacohen et al., 2013). The existence of mutations within tumors does not mean that those mutations will serve as neoantigens. In order for a given mutation to result in T cell recognition, it must be in a protein coding region, be non-synonymous, be in a gene that is expressed by the tumor cells, be maintained following proteasomal degradation, result in a peptide that can be loaded onto the specific MHC-I molecules of that patient, and, of course, be recognized by the TCR of a T cell that can enter the tumor. A variety of tools have been developed to use WES data (often combined with RNA-seq data) to predict potential neoantigens in a tumor (Bjerregaard et al., 2017; Lundegaard et al., 2008; Gowthaman et al., 2010; Rajasagi et al., 2014; Duan et al., 2014; Hundal et al., 2016; Łuksza et al., 2017). Additionally, MS has been used to characterize the peptidome of tumors to identify neoantigens directly, frequently by affinity purification of HLA molecules and MS analysis of eluted HLA-bound peptides (Creech, 2018; Cox et al., 1994; Pritchard et al., 2015; Bassani-Sternberg et al., 2010). Using these types of tools, researchers have begun testing pipelines for developing personalized neoantigen vaccines in humans (Sahin et al., 2017; Ott et al., 2017; Carreno et al., 2015; Hu et al., 2017b). These approaches typically begin with excision of tumors and WES to identify candidate neoantigen peptides. In one case, long peptides (15 to 30 amino acids) were synthesized containing the top candidate peptides and administered to patients in conjunction with immune adjuvants in multiple priming and boosting injections. Use of the long peptides was chosen to enable elicitation of both CD4+ and CD8+ T cell responses. To improve HLA-binding prediction, the same researchers used an LC-MS/MS approach wherein peptides were eluted from single HLA allele-expressing cell lines and subjected to MS resulting in the identification of 24,000 peptides and their cognate HLA class I molecules (Abelin et al., 2017). Furthermore, using this database of peptide-MHC interactions, they trained a neural network algorithm to predict peptide loading with a higher degree of accuracy than preexisting binding affinity-based approaches. The initial neoantigen vaccine trials have now been extended to glioblastoma, a notoriously immune cold tumor, and appear to generate antigen-specific T cells and increases in tumor infiltration by the T cells (Keskin et al., 2019).
In addition to vaccine design, systems approaches to neoantigen identification have been employed for TIL therapy. Following analogous approaches as used for vaccines, researchers identified candidate peptides based on WES of 75 patients with GI cancers. They then screened TIL cultures by ELISPOT for reactivity in response to autologous DCs pulsed with the peptides or transfected with minigenes harboring the mutations. By subsequently sequencing TCRs of T cells responding to particular neoantigens they were then able to transduce new T cells with these TCRs to enable greater T cell expansion and the ability to generate personalized T cell therapies (Parkhurst et al., 2019). These types of approaches highlight the potential benefits that systems biology can bring to patient care and personalized medicine. Only by taking advantage of the synergies of high-throughput technologies, novel bioinformatics approaches, mathematical models, and the ability to monitor and predict immune responses across an entire patient can such an approach be rendered feasible and uncover new avenues for patient care and tumor immunotherapy.
While antitumor T cell-mediated immunity is typically predicated upon recognition of tumor-specific antigens, it also requires a TCR repertoire capable of recognizing those epitopes. The profound diversity of the TCR is not germline encoded but rather is the consequence of V(D)J rearrangement that results in the generation of more possible sequences than there are T cells within an individual (Arstila et al., 1999). While conventional T cells recognize only specific antigens, the TCR is relatively cross-reactive compared to antibodies. One elegant systems-level study used yeast display of peptide-MHC constructs to reveal that a given TCR is capable of recognizing hundreds of distinct peptides provided that they contain specific ‘hot spots’ where the TCR contacts the complex (Birnbaum et al., 2014). The peptides shared among a cross-reactive TCR bear many similarities, permitting researchers to predict potential naturally-occurring ligands given a TCR sequence. The group that developed this approach subsequently applied it to CRC by sequencing patient tumors and TCRs to determine peptide-MHC ligands for expanded TILs (Gee et al., 2018). Epitope prediction revealed TCR-specificity including multiple TCRs that recognized non-mutated self-antigens. Nonetheless, prediction of TCR ligands from their sequences remains one of the holy grails of immunology. To address this need, researchers developed GLIPH, an algorithm capable of grouping TCRs based on predicted shared epitopes using similarity of the CDR3 regions and demonstrated that they could predict shared binding partners across individuals (Glanville et al., 2017). Another study combined MHC tetramer selection with single-cell TCR sequencing to develop an algorithm, TCRdist, that also groups TCRs of related specificities (Dash et al., 2017). Such approaches might help researchers understand whether distinct clones in a patient recognize similar antigens, perhaps present at different tumor sites or even across different patients with similar malignancies. Cancer systems immunologists could exploit existing TCR sequencing data to determine the antigens most frequently recognized by T cells in patients responding to ICB, for example.
In addition to identifying epitopes, understanding the clonal evolution of the T cell response as well as the phenotypes of T cells bearing TCRs of a particular specificity can be highly informative when considering the immune responses to tumors. To investigate the relationships between functions of T cells and their TCR specificity, researchers developed a single-cell sequencing approach for T cells that combines targeted RNA-seq with TCRα and TCRβ gene sequencing (Han et al., 2014). Applying their approach to CRC, they found expanded clones within the tumors that shared specificity and were absent from adjacent normal tissue. Similarly, other researchers combined scATAC-seq with TCR-seq to evaluate clonal relationships with epigenetic profiles and applied it to patients with cutaneous T cell lymphoma to identify differences in the regulatory pathways between normal and leukemic T cells (Satpathy et al., 2018). In a recent study, this same group combined single-cell TCR and RNA sequencing on nearly 80,000 cells from patients with basal and squamous cell carcinomas before and after ICB treatment with anti-PD1 (Yost et al., 2019). By comparing clonotypes with phenotypes, they showed that exhausted and effector CD8+ T cells in tumors shared clonotypes with memory T cells but not with each other, suggesting that these distinct states are not shared between the same T cell clones. Even more notably, they found considerable expansion of TEx post-treatment that did not exist before therapy, with only minor contributions from pre-existing TCF7+ clones. These clones can be found within the blood at much lower percentages. In contrast with conventional wisdom suggesting that ICB works by reinvigorating existing T cells within the TME, these results suggest that anti-PD1 stimulates recruitment of new tumor-specific T cells to the TME and are in agreement with our own findings that trafficking of lymphocytes from SLOs is required for effective immunotherapy (Spitzer et al., 2017). While this approach cannot rule out the existence of rare T cell clones in the TME prior to treatment that exist below the sequencing depth and expand following treatment, the data suggest this is unlikely and future complementary systems-level studies interrogating clonal trafficking could help shed light on this question.
Nonetheless, while these TEx clones that are tumor-specific can be found within blood of treated patients and recruitment of T cells from SLOs is important for treatment efficacy, some of these effects may be transient, and it remains unclear which T cell populations are the most critical for effective immunotherapy. It is possible that Tmem and cells from both TEx states within the TME and extratumoral tissues play important roles during immunotherapy. It is important to note that autologous transplant of ex vivo expanded tumor-infiltrating lymphocytes (TIL therapy) has proven effective, and even curative, in a number of malignancies including melanoma, breast cancer, and multiple GI cancers (Rosenberg et al., 1988; Rosenberg and Restifo, 2015; Dudley et al., 2002; Restifo et al., 2012; Tran et al., 2014; Tran et al., 2016; Zacharakis et al., 2018). Moreover, in the case of melanoma, some patients who have failed ICB still benefit from TIL therapy (Sarnaik et al., 2020; Rohaan et al., 2018). These successes highlight the fact that there exist tumor-specific T cells within tumors that can be expanded and used to eliminate tumors. Thus, it is unclear whether these T cells reflect exhausted or dysfunctional T cells whose state is reversible in the context of ex vivo expansion with or without engineering or whether there exist a minority subset of tumor-specific T cells that are not in any state of exhaustion and can be expanded and act as TEff and Tmem once reinfused. In either case, it is quite reasonable to expect that ICB and TIL therapy have highly distinct effects on the nature of the T cells eliciting the anti-tumor immunity. A systems-level characterization of TIL approaches might help shed light on the states of the expanded T cells, whether they were derived from progenitor TEx or other T cell subsets, and to what extent the ex vivo processing is capable of reprogramming them.
The microbiome and its effects on tumor immunity
Microbiota consist of the microorganisms that inhabit a host and include bacteria, fungi, viruses, and archaea. Sites such as the skin, respiratory tract, gastrointestinal tract, and vagina are colonized by these organisms, which play critical roles in health and disease. Systems approaches and –omics, in particular, have been utilized extensively to analyze the microbiota, resulting in the adoption of the term ‘microbiome’ to represent the collection of these organisms in a host. The microbiome affects everything from obesity, to resistance to colonization by pathogenic bacteria, to cancer (Ridaura et al., 2013; Petersen et al., 2019; Jacobson et al., 2018; Iida et al., 2013; Buffie et al., 2015; van Nood et al., 2013; Viaud et al., 2013; Sivan et al., 2015; Vetizou et al., 2015; Gopalakrishnan et al., 2018; Routy et al., 2018; Tanoue et al., 2019; Matson et al., 2018; Garrett, 2015; Reticker-Flynn and Engleman, 2019). While the microbiome mediates some of these effects directly (e.g. production of short-chain fatty acids), many are the indirect result of effects upon the immune system (Tanoue et al., 2019; Honda and Littman, 2016; Mazmanian et al., 2005; Atarashi et al., 2017; Atarashi et al., 2013; Ivanov et al., 2009; Gaboriau-Routhiau et al., 2009). In an effort to identify specific microbial components that are capable of influencing the adaptive immune response, researchers have colonized germ-free mice with feces from healthy human donors. Through 16S rRNA sequencing of the caecal contents of mice exhibiting enrichment of particular T cell subsets in their colons following fecal transplant or in comparison to mice under different housing conditions, they were able to define commensal consortia capable of inducing expansion of Tregs (Atarashi et al., 2013), TH1 (Atarashi et al., 2017), TH17 (Ivanov et al., 2009; Gaboriau-Routhiau et al., 2009), or IFN-γ+ CD8+ T cells (Tanoue et al., 2019). The last of these resulted in expansion of the T cells not only within the intestines, but also throughout the host in a manner that was protective against Listeria monocytogenes and augmented the efficacy of ICB. Similarly, among tumor-bearing mice, a comparison of germ-free to specific pathogen-free mice or mice from different vendors demonstrated differences in tumor growth depending on the microbiota. Moreover, specific commensals affected responses to ICB, with Bifidobacterium aiding anti-PD-1 (Sivan et al., 2015) and Bacteroidales fragilis aiding anti-CTLA-4 (Vetizou et al., 2015). To investigate whether the microbiome influences responses to ICB in humans, researchers characterized the diversity and composition of the microbiomes of patients receiving these therapies. These results showed that increased diversity and enrichment for Ruminococcaceae family bacteria correlate with improved response to anti-PD-1 and increases in CD8+ T cell activity (Gopalakrishnan et al., 2018) and that Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium may also augment responses by expanding tumor-specific CD8+ T cells without affecting Treg numbers (Matson et al., 2018). Similarly, fecal transplant into mice from human ICB responders resulted in increased efficacy of PD-1 blockade compared to that from non-responders, possibly due to the effects on recruitment of a CD4+ T cell subset (Routy et al., 2018). The ability to identify defined microbiota capable of improving responses to tumor immunotherapy is extremely exciting and would have been highly challenging without the –omics-level profiling technologies. It remains unclear, however, precisely how changes in the intestinal microbiota elicit systemic changes in adaptive immunity that extend beyond the gastrointestinal tract and against antigens that are not being presented there. Systems approaches will be critical to reveal the mechanisms by which this antigen-specific systemic immunity is established in response to alterations in the microbiota. Ultimately, these investigations are likely to lead to new ways of improving patient responses to ICB and possibly to altogether novel immunotherapies.
Systems approaches to designing, monitoring, and evaluating clinical responses to tumor immunotherapy
Cancer immunotherapy, and ICB in particular, represents one of the most significant advances in oncology in decades and has the ability to elicit durable responses and cures in some patients with advanced stage malignancies (Rosenberg and Restifo, 2015; Brahmer et al., 2012; Hamid et al., 2013; Hodi et al., 2010; Nghiem et al., 2016; Pardoll, 2012; Robert et al., 2015; Sharma and Allison, 2015; Rizvi et al., 2015b; Grupp et al., 2013; Brentjens et al., 2013; Porter et al., 2011; June et al., 2018). Nonetheless, while subsets of patients with melanoma, RCC, Hodgkin’s lymphoma, NSCLC, urothelial cancer, and MSI high GI cancers have experienced durable responses and even complete tumor regression (Zappasodi et al., 2018), the majority of patients who receive the therapy do not exhibit such responses. Thus, there is an urgent need to distinguish in advance of treatment which patients will respond to which therapies as well as learn why certain patients exhibit durable responses while others either fail to respond at all or relapse after varying periods of response. The tools and approaches used by systems biologists to investigate tumor immune biology have enabled a variety of new approaches for designing and evaluating clinical responses. In many ways, most clinical practice could be construed as systems biology. Clinicians integrate cellular and molecular blood biomarkers (e.g. blood chemistry, complete blood counts, hematocrit, serum immunoglobulin levels, etc.), imaging, and biophysical measurements (e.g. temperature, blood pressure, etc.) in a longitudinal manner to gain an understanding of disease progression. Thus, the practice of medicine epitomizes the multiscale integrated analyses that lie at the core of systems biology. Indeed, pharmaceutical companies have used systems biology approaches in drug discovery and clinical trial design (Butcher et al., 2004). This holistic integration of multi-level datasets and the quantitation tools that accompany them render medicine amenable to the recent advances in cancer systems immunology and their application to evaluating tumor immunotherapy (Figure 4).
Figure 4. Clinical applications of cancer systems immunology.
Tumor and peripheral blood samples are frequently harvested from patients in a longitudinal fashion. Model organisms such as mice enable researchers to query as many tissues as desired. These samples can be subjected to high-dimensional analysis platforms such as various NGS modalities or mass cytometry. Results from these analyses have been used to monitor responses to ICB (including immune repertoire analysis), mapping changes in the immune system across an organism, and for the design of personalized neoantigen vaccines.
Many of the systems approaches discussed in this review are now being applied to advancing our understanding of the effects of existing immunotherapies and designing new approaches. As discussed in the previous section, researchers and clinicians have combined WES of patient tumors with HLA-binding and TCR prediction algorithms to design personalized neoantigen peptide vaccines (Figure 4; Ott et al., 2017; Hu et al., 2017b; Keskin et al., 2019). Staining for targeted costimulatory molecules (e.g. PD-L1) on tumors has exhibited variable predictive power in ICB, showing positive correlations in many studies but failing to predict responders whose tumors appear negative for the markers (Topalian et al., 2012; Nishino et al., 2017). In addition to the utility of WES in vaccine design, evaluation of neoantigen burden by WES has revealed that the response to anti-CTLA-4 (Snyder et al., 2014) and anti-PD1 (Rizvi et al., 2015a) therapies correlates with mutational burden. Recent studies have sought to exploit mass cytometry to develop high-dimensional cellular biomarkers of treatment response to ICB (Krieg et al., 2018; Wistuba-Hamprecht et al., 2017). One study identified not only differences in lymphocyte populations but also evidence of classical monocyte activation in PBMCs of patients who responded to anti-PD1 (Krieg et al., 2018). Additionally, standardized mass cytometry-based immunophenotyping approaches have been developed for monitoring clinical responses to immunotherapies (Hartmann et al., 2019), and this approach is currently being applied to monitoring responses to DC vaccination trials (Nowicki et al., 2019). Similarly, scRNA-seq approaches are being considered for monitoring immune responses to therapies and evaluating tumor heterogeneity, and will become increasingly feasible with the continual reduction in cost of these technologies (Shalek and Benson, 2017; Kim et al., 2016).
In addition to monitoring therapy in patients, systems approaches can reveal underlying biology about responses to treatment. A recent study revealing the clonal replacement of exhausted T cells following ICB is one such example demonstrating the power of integrative systems analyses to improve our understanding of the mechanisms of ICB (Figure 4; Yost et al., 2019). We believe that it is important to gain a holistic understanding of how immunity is orchestrated across an organism in order to understand the basis of immune responses in cancer. By combining our visualization method known as Statistical Scaffold with mass cytometry, we were able to generate an organism-wide map of differences in immune responses that distinguish effective from ineffective immunotherapy (Spitzer et al., 2017). This approach allowed us to compare immune repertoires in distinct tissues to each other and across treatment conditions, facilitating a systems-level understanding of immune responses across an organism. In addition to identifying differences in key immune populations such as a subset activated memory CD4+ T cells, we found that the key differentiator of effective immune responses is its orchestration from extratumoral sites such as LNs rather than a simple reinvigoration of existing TILs. Information gleaned from these types of systems-level analyses should facilitate the design of approaches that augment our current arsenal of immunotherapies (Figure 4).
Expanding the future potential of cancer systems immunology
Technological and computational advances in the life sciences and the profound clinical successes of tumor immunotherapy have ushered the fields of systems biology and tumor immunology to the forefront of biomedical research. Understandably, the application of systems biology to tumor immunology is intuitive and has generated considerable excitement. Once lauded as representing the future of optimal cancer treatment and potential cures, personalized medicine fell out of fashion with the failure of targeted therapies to realize their promise (Nature Biotechnology, 2012). While –omics technologies bolstered the original enthusiasm for the field, they were ultimately applied in a reductionist fashion. Personalized medicine in cancer used to be construed as identifying oncogenic mutations and designing and treating with targeted small molecule or biological inhibitors of these mutations. These approaches demonstrated the innate shortcoming of reductionist analyses; that is, they fail to account for the inherent complexity of the tumor and its context within the patient. Escape mechanisms can involve a myriad of molecules and cell types requiring systems-level analyses to understand the emergent behavior of the tumor and its interactions with other cell types. In contrast to targeted therapies, immunotherapy exhibits the capacity, in many instances, to evolve with the tumor. Furthermore, it is capable of addressing the multifaceted components of the tumor that confer its malignant potential. The inherent systems-level nature of antitumor immunity thus requires a complementary set of approaches to interrogate the responsible immune cells and molecules, and to inform the design and implementation of new and existing therapies. Indeed, personalized medicine is experiencing a resurgence due to the potential of immunotherapies such as CAR-T cells and neoantigen vaccines to enable tumor targeting with a greater precision than previously possible. With an increased understanding of the dynamics underlying the coevolution of tumors and their immune responses during additional immunotherapies, such as ICB, systems biology will enhance our capacity to design and deliver personalized care to patients with advanced malignancies.
Cancer systems immunology is at an important transition point in its maturation. To date, preclinical and clinical studies exploiting systems approaches have generated profound amounts of data enabling characterization of immune responses and the generation of data rich atlases and accompanying tools for analysis. These important advances have laid the groundwork for discovery in the field of tumor immunology. The challenge moving forward will be to expand these approaches to uncover new biology that can be functionally validated. Many of the studies in the field have been largely descriptive and devoid of functional validation. In order to ensure that the field does not fall short of its promise, cancer systems immunologists will need to meet the higher bar of not only characterizing differences in immune responses to tumors but also performing the requisite experiments to determine the significance of the findings. Compared to other fields in cancer, this task is considerably more challenging. By definition, systems immunology typically uncovers interactions that involve many cellular components operating across multiple organ systems and timescales making perturbation of the components in a physiologically relevant manner difficult. Investigations in immunocompetent animal models, despite their challenges, represent some of the best approaches for testing the hypotheses generated from systems analyses. These models have served as the backbone for many discoveries in the field of immunology and will be necessary for advancing our understanding of tumor immunology. Future studies in the field should utilize advances in modeling approaches, such as ABM, to inform preclinical studies and focus the parameter space to one that is experimentally feasible. In some instances, where animal models are incapable of predicting patient responses, the use of systems approaches to integrate data sets and model human biology can inform the design of new therapies. Despite the challenges that confront the field, cancer systems immunology will continue to provide discoveries that lead to the next generation of life-saving immunotherapies.
Acknowledgements
The authors thank members of the Engleman Lab for insightful discussions. This work was supported by NIH grant U54 CA209971 (Engleman).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Nathan E Reticker-Flynn, Email: retickerflynn@stanford.edu.
Edgar G Engleman, Email: edgareng@stanford.edu.
Jeffrey Settleman, Pfizer, United States.
Yutaka Kawakami, Keio University School of Medicine, Japan.
Funding Information
This paper was supported by the following grant:
National Cancer Institute U54 CA209971 to Edgar G Engleman.
Additional information
Competing interests
No competing interests declared.
References
- Abdelsamed HA, Moustaki A, Fan Y, Dogra P, Ghoneim HE, Zebley CC, Triplett BM, Sekaly RP, Youngblood B. Human memory CD8 T cell effector potential is epigenetically preserved during in vivo homeostasis. Journal of Experimental Medicine. 2017;214:1593–1606. doi: 10.1084/jem.20161760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelin JG, Keskin DB, Sarkizova S, Hartigan CR, Zhang W, Sidney J, Stevens J, Lane W, Zhang GL, Eisenhaure TM, Clauser KR, Hacohen N, Rooney MS, Carr SA, Wu CJ. Mass spectrometry profiling of HLA-Associated peptidomes in Mono-allelic cells enables more accurate epitope prediction. Immunity. 2017;46:315–326. doi: 10.1016/j.immuni.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q, Ma Y, Wiesen JF, Wong MH, Kulesz-Martin M, Irving B, Coussens LM. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–821. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Yuan Y, Parmigiani G, Suraokar MB, Diao L, Wistuba II, Wang W. DeMix: deconvolution for mixed Cancer transcriptomes using raw measured data. Bioinformatics. 2013;29:1865–1871. doi: 10.1093/bioinformatics/btt301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nature Neuroscience. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Akhtar-Zaidi B, Cowper-Sal-lari R, Corradin O, Saiakhova A, Bartels CF, Balasubramanian D, Myeroff L, Lutterbaugh J, Jarrar A, Kalady MF, Willis J, Moore JH, Tesar PJ, Laframboise T, Markowitz S, Lupien M, Scacheri PC. Epigenomic enhancer profiling defines a signature of Colon cancer. Science. 2012;336:736–739. doi: 10.1126/science.1217277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcántara-Hernández M, Leylek R, Wagar LE, Engleman EG, Keler T, Marinkovich MP, Davis MM, Nolan GP, Idoyaga J. High-Dimensional phenotypic mapping of human dendritic cells reveals interindividual variation and tissue specialization. Immunity. 2017;47:1037–1050. doi: 10.1016/j.immuni.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, Utzschneider DT, von Hoesslin M, Cullen JG, Fan Y, Eisenberg V, Wohlleber D, Steiger K, Merkler D, Delorenzi M, Knolle PA, Cohen CJ, Thimme R, Youngblood B, Zehn D. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature. 2019;571:265–269. doi: 10.1038/s41586-019-1326-9. [DOI] [PubMed] [Google Scholar]
- Alfonso JC, Schaadt NS, Schönmeyer R, Brieu N, Forestier G, Wemmert C, Feuerhake F, Hatzikirou H. In-silico insights on the prognostic potential of immune cell infiltration patterns in the breast lobular epithelium. Scientific Reports. 2016;6:33322. doi: 10.1038/srep33322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman D, Pillai S. Peripheral B cell subsets. Current Opinion in Immunology. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altrock PM, Liu LL, Michor F. The mathematics of Cancer: integrating quantitative models. Nature Reviews Cancer. 2015;15:730–745. doi: 10.1038/nrc4029. [DOI] [PubMed] [Google Scholar]
- Amir AD, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC, Shenfeld DK, Krishnaswamy S, Nolan GP, Pe'er D. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nature Biotechnology. 2013;31:545–552. doi: 10.1038/nbt.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate Cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G. Introduction of an agent-based multi-scale modular architecture for dynamic knowledge representation of acute inflammation. Theoretical Biology and Medical Modelling. 2008;5:11. doi: 10.1186/1742-4682-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, Naldini L, de Visser KE, De Palma M, Coussens LM. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli V, Ginhoux F, Llodrà J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Angelo M, Bendall SC, Finck R, Hale MB, Hitzman C, Borowsky AD, Levenson RM, Lowe JB, Liu SD, Zhao S, Natkunam Y, Nolan GP. Multiplexed ion beam imaging of human breast tumors. Nature Medicine. 2014;20:436–442. doi: 10.1038/nm.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova M, Charoentong P, Hackl H, Fischer ML, Snajder R, Krogsdam AM, Waldner MJ, Bindea G, Mlecnik B, Galon J, Trajanoski Z. Characterization of the immunophenotypes and antigenomes of colorectal cancers reveals distinct tumor escape mechanisms and novel targets for immunotherapy. Genome Biology. 2015;16:64. doi: 10.1186/s13059-015-0620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angermueller C, Clark SJ, Lee HJ, Macaulay IC, Teng MJ, Hu TX, Krueger F, Smallwood S, Ponting CP, Voet T, Kelsey G, Stegle O, Reik W. Parallel single-cell sequencing links transcriptional and epigenetic heterogeneity. Nature Methods. 2016;13:229–232. doi: 10.1038/nmeth.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anna SL, Bontoux N, Stone HA. Formation of dispersions using “flow focusing” in microchannels. Applied Physics Letters. 2003;82:364–366. doi: 10.1063/1.1537519. [DOI] [Google Scholar]
- Araki Y, Wang Z, Zang C, Wood WH, Schones D, Cui K, Roh TY, Lhotsky B, Wersto RP, Peng W, Becker KG, Zhao K, Weng NP. Genome-wide analysis of histone methylation reveals chromatin state-based regulation of gene transcription and function of memory CD8+ T cells. Immunity. 2009;30:912–925. doi: 10.1016/j.immuni.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biology. 2017;18:220. doi: 10.1186/s13059-017-1349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aras S, Zaidi MR. TAMeless traitors: macrophages in Cancer progression and metastasis. British Journal of Cancer. 2017;117:1583–1591. doi: 10.1038/bjc.2017.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archetti M. Evolutionary game theory of growth factor production: implications for tumour heterogeneity and resistance to therapies. British Journal of Cancer. 2013;109:1056–1062. doi: 10.1038/bjc.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of clostridia strains from the human Microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Suda W, Luo C, Kawaguchi T, Motoo I, Narushima S, Kiguchi Y, Yasuma K, Watanabe E, Tanoue T, Thaiss CA, Sato M, Toyooka K, Said HS, Yamagami H, Rice SA, Gevers D, Johnson RC, Segre JA, Chen K, Kolls JK, Elinav E, Morita H, Xavier RJ, Hattori M, Honda K. Ectopic colonization of oral Bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017;358:359–365. doi: 10.1126/science.aan4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atri C, Guerfali F, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. International Journal of Molecular Sciences. 1801;19:1801. doi: 10.3390/ijms19061801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M, Choi K, Fromme RM, Dao P, McKenney PT, Wasti RC, Kadaveru K, Mazutis L, Rudensky AY, Pe'er D. Single-Cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174:1293–1308. doi: 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem A, Hartung E, Güttler S, Mora A, Zhou X, Hegemann A, Plantinga M, Mazzini E, Stoitzner P, Gurka S, Henn V, Mages HW, Kroczek RA. Expression of XCR1 characterizes the Batf3-Dependent lineage of dendritic cells capable of antigen Cross-Presentation. Frontiers in Immunology. 2012;3:214. doi: 10.3389/fimmu.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair E, Tibshirani R. Semi-Supervised methods to predict patient survival from gene expression data. PLOS Biology. 2004;2:e108. doi: 10.1371/journal.pbio.0020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bandura DR, Baranov VI, Ornatsky OI, Antonov A, Kinach R, Lou X, Pavlov S, Vorobiev S, Dick JE, Tanner SD. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma Time-of-Flight mass spectrometry. Analytical Chemistry. 2009;81:6813–6822. doi: 10.1021/ac901049w. [DOI] [PubMed] [Google Scholar]
- Bangasser BL, Odde DJ. Master equation-based analysis of a motor-clutch model for cell traction force. Cellular and Molecular Bioengineering. 2013;6:449–459. doi: 10.1007/s12195-013-0296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbera-Guillem E, May KF, Nyhus JK, Nelson MB. Promotion of tumor invasion by cooperation of granulocytes and macrophages activated by anti-tumor antibodies. Neoplasia. 1999;1:453–460. doi: 10.1038/sj.neo.7900054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Basanta D, Scott JG, Fishman MN, Ayala G, Hayward SW, Anderson AR. Investigating prostate cancer tumour-stroma interactions: clinical and biological insights from an evolutionary game. British Journal of Cancer. 2012;106:174–181. doi: 10.1038/bjc.2011.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslan T, Kendall J, Rodgers L, Cox H, Riggs M, Stepansky A, Troge J, Ravi K, Esposito D, Lakshmi B, Wigler M, Navin N, Hicks J. Genome-wide copy number analysis of single cells. Nature Protocols. 2012;7:1024–1041. doi: 10.1038/nprot.2012.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani-Sternberg M, Barnea E, Beer I, Avivi I, Katz T, Admon A. Soluble plasma HLA peptidome as a potential source for Cancer biomarkers. PNAS. 2010;107:18769–18776. doi: 10.1073/pnas.1008501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, Selves J, Laurent-Puig P, Sautès-Fridman C, Fridman WH, de Reyniès A. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biology. 2016;17:218. doi: 10.1186/s13059-016-1070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becht E, McInnes L, Healy J, Dutertre C-A, Kwok IWH, Ng LG, Ginhoux F, Newell EW. Dimensionality reduction for visualizing single-cell data using UMAP. Nature Biotechnology. 2019;37:38–44. doi: 10.1038/nbt.4314. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nature Immunology. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- Bellomo N, Firmani B, Guerri L. Bifurcation analysis for a nonlinear system of integro-differential equations modelling tumor-immune cells competition. Applied Mathematics Letters. 1999;12:39–44. doi: 10.1016/S0893-9659(98)00146-3. [DOI] [Google Scholar]
- Bellomo N, Delitala M. From the mathematical kinetic, and stochastic game theory to modelling mutations, onset, progression and immune competition of cancer cells. Physics of Life Reviews. 2008;5:183–206. doi: 10.1016/j.plrev.2008.07.001. [DOI] [Google Scholar]
- Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, Gangadhar TC, Amaravadi RK, Schuchter LM, Feldman MD, Ishwaran H, Vonderheide RH, Maity A, Wherry EJ, Minn AJ. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167:1540–1554. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall SC, Simonds EF, Qiu P, Amir AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, Plevritis SK, Sachs K, Pe'er D, Tanner SD, Nolan GP. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall SC, Nolan GP, Roederer M, Chattopadhyay PK. A deep profiler's guide to cytometry. Trends in Immunology. 2012;33:323–332. doi: 10.1016/j.it.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall SC, Davis KL, Amir AD, Tadmor MD, Simonds EF, Chen TJ, Shenfeld DK, Nolan GP, Pe'er D. Single-cell trajectory detection uncovers progression and regulatory coordination in human B cell development. Cell. 2014;157:714–725. doi: 10.1016/j.cell.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennun SV, Hizal DB, Heffner K, Can O, Zhang H, Betenbaugh MJ. Systems glycobiology: integrating glycogenomics, glycoproteomics, glycomics, and other 'Omics Data Sets to Characterize Cellular Glycosylation Processes. Journal of Molecular Biology. 2016;428:3337–3352. doi: 10.1016/j.jmb.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Berdasco M, Esteller M. Aberrant epigenetic landscape in Cancer: how cellular identity Goes awry. Developmental Cell. 2010;19:698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Berger AH, Brooks AN, Wu X, Shrestha Y, Chouinard C, Piccioni F, Bagul M, Kamburov A, Imielinski M, Hogstrom L, Zhu C, Yang X, Pantel S, Sakai R, Watson J, Kaplan N, Campbell JD, Singh S, Root DE, Narayan R, Natoli T, Lahr DL, Tirosh I, Tamayo P, Getz G, Wong B, Doench J, Subramanian A, Golub TR, Meyerson M, Boehm JS. High-throughput Phenotyping of Lung Cancer Somatic Mutations. Cancer Cell. 2016;30:214–228. doi: 10.1016/j.ccell.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertozzi CR, Sasisekharan R. Glycomics. In: Varki A, Cummings RD, Esko JD, editors. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor Laboratory Press; 2009. pp. 2015–2017. [PubMed] [Google Scholar]
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnewies M, Mujal AM, Pollack JL, Combes AJ, Hardison EA, Barry KC, Tsui J, Ruhland MK, Kersten K, Abushawish MA, Spasic M, Giurintano JP, Chan V, Daud AI, Ha P, Ye CJ, Roberts EW, Krummel MF. Unleashing Type-2 dendritic cells to drive protective antitumor CD4+ T Cell Immunity. Cell. 2019;177:556–571. doi: 10.1016/j.cell.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum ME, Mendoza JL, Sethi DK, Dong S, Glanville J, Dobbins J, Ozkan E, Davis MM, Wucherpfennig KW, Garcia KC. Deconstructing the peptide-MHC specificity of T cell recognition. Cell. 2014;157:1073–1087. doi: 10.1016/j.cell.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature Immunology. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- Bjerregaard A-M, Nielsen M, Hadrup SR, Szallasi Z, Eklund AC. MuPeXI: prediction of neo-epitopes from tumor sequencing data. Cancer Immunology, Immunotherapy. 2017;66:1123–1130. doi: 10.1007/s00262-017-2001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. PNAS. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, Schietinger A, Schumacher TN, Schwartzberg PL, Sharpe AH, Speiser DE, Wherry EJ, Youngblood BA, Zehn D. Defining 'T cell exhaustion'. Nature Reviews Immunology. 2019;19:665–674. doi: 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissonnas A, Licata F, Poupel L, Jacquelin S, Fetler L, Krumeich S, Théry C, Amigorena S, Combadière C. CD8+ tumor-infiltrating T cells are trapped in the tumor-dendritic cell network. Neoplasia. 2013;15:85–IN26. doi: 10.1593/neo.121572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth MJ, Branco MR, Ficz G, Oxley D, Krueger F, Reik W, Balasubramanian S. Quantitative sequencing of 5-methylcytosine and 5-hydroxymethylcytosine at single-base resolution. Science. 2012;336:934–937. doi: 10.1126/science.1220671. [DOI] [PubMed] [Google Scholar]
- Booth MJ, Marsico G, Bachman M, Beraldi D, Balasubramanian S. Quantitative sequencing of 5-formylcytosine in DNA at single-base resolution. Nature Chemistry. 2014;6:435–440. doi: 10.1038/nchem.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, Dhara S, Simpson K, Gardner EE, Iacobuzio-Donahue CA, Brennan CW, Tabar V, Gutin PH, Joyce JA. Macrophage ontogeny underlies differences in Tumor-Specific education in brain malignancies. Cell Reports. 2016;17:2445–2459. doi: 10.1016/j.celrep.2016.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle AP, Davis S, Shulha HP, Meltzer P, Margulies EH, Weng Z, Furey TS, Crawford GE. High-resolution mapping and characterization of open chromatin across the genome. Cell. 2008;132:311–322. doi: 10.1016/j.cell.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced Cancer. New England Journal of Medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräunlein E, Krackhardt AM. Identification and characterization of neoantigens as well as respective immune responses in Cancer patients. Frontiers in Immunology. 1702;8:1702. doi: 10.3389/fimmu.2017.01702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, Wasielewska T, He Q, Bernal Y, Rijo IV, Hedvat C, Kobos R, Curran K, Steinherz P, Jurcic J, Rosenblat T, Maslak P, Frattini M, Sadelain M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Science Translational Medicine. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer K, Foroushani AK, Laird MR, Chen C, Sribnaia A, Lo R, Winsor GL, Hancock RE, Brinkman FS, Lynn DJ. InnateDB: systems biology of innate immunity and beyond--recent updates and continuing curation. Nucleic Acids Research. 2013;41:D1228–D1233. doi: 10.1093/nar/gks1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodt P, Gordon J. Natural resistance mechanisms may play a role in protection against chemical carcinogenesis. Cancer Immunology Immunotherapy. 1982;13:125–127. doi: 10.1007/BF00205312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nature Communications. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, Amigorena S, Van't Veer LJ, Sperling AI, Wolf DM, Krummel MF. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker DK, Proctor EA, Haigis KM, Lauffenburger DA. Computational translation of genomic responses from experimental model systems to humans. PLOS Computational Biology. 2019;15:e1006286. doi: 10.1371/journal.pcbi.1006286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker DK, Lauffenburger DA. Translating preclinical models to humans. Science. 2020;367:742–743. doi: 10.1126/science.aay8086. [DOI] [PubMed] [Google Scholar]
- Bruchard M, Ghiringhelli F. Deciphering the roles of innate lymphoid cells in Cancer. Frontiers in Immunology. 2019;10:656. doi: 10.3389/fimmu.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggner RV, Bodenmiller B, Dill DL, Tibshirani RJ, Nolan GP. Automated identification of stratifying signatures in cellular subpopulations. PNAS. 2014;111:E2770–E2777. doi: 10.1073/pnas.1408792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusic V, Gottardo R, Kleinstein SH, Davis MM, HIPC steering committee Computational resources for high-dimensional immune analysis from the human immunology project consortium. Nature Biotechnology. 2014;32:146–148. doi: 10.1038/nbt.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nature Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Wu B, Litzenburger UM, Ruff D, Gonzales ML, Snyder MP, Chang HY, Greenleaf WJ. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature. 2015;523:486–490. doi: 10.1038/nature14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Corces MR, Lareau CA, Wu B, Schep AN, Aryee MJ, Majeti R, Chang HY, Greenleaf WJ. Integrated Single-Cell analysis maps the continuous regulatory landscape of human hematopoietic differentiation. Cell. 2018;173:1535–1548. doi: 10.1016/j.cell.2018.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MR, Jenq RR, Taur Y, Sander C, Cross JR, Toussaint NC, Xavier JB, Pamer EG. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger M, Groudine M. Functional and mechanistic diversity of distal transcription enhancers. Cell. 2011;144:327–339. doi: 10.1016/j.cell.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M, Tarakhovsky A. Epigenetic control of immunity. Cold Spring Harbor Perspectives in Biology. 2014;6:a024174. doi: 10.1101/cshperspect.a024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC, Berg EL, Kunkel EJ. Systems biology in drug discovery. Nature Biotechnology. 2004;22:1253–1259. doi: 10.1038/nbt1017. [DOI] [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nature Biotechnology. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Cusanovich DA, Ramani V, Aghamirzaie D, Pliner HA, Hill AJ, Daza RM, McFaline-Figueroa JL, Packer JS, Christiansen L, Steemers FJ, Adey AC, Trapnell C, Shendure J. Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science. 2018;361:1380–1385. doi: 10.1126/science.aau0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli RM. Imaging mass spectrometry: molecular microscopy for the new age of biology and medicine. Proteomics. 2016;16:1607–1612. doi: 10.1002/pmic.201600133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, Ready N, Hiltermann TJN, Nair S, Juergens R, Peters S, Minenza E, Wrangle JM, Rodriguez-Abreu D, Borghaei H, Blumenschein GR, Villaruz LC, Havel L, Krejci J, Corral Jaime J, Chang H, Geese WJ, Bhagavatheeswaran P, Chen AC, Socinski MA, CheckMate 026 Investigators First-Line nivolumab in stage IV or recurrent Non-Small-Cell lung Cancer. New England Journal of Medicine. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmi Y, Spitzer MH, Linde IL, Burt BM, Prestwood TR, Perlman N, Davidson MG, Kenkel JA, Segal E, Pusapati GV, Bhattacharya N, Engleman EG. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature. 2015;521:99–104. doi: 10.1038/nature14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmi Y, Prestwood TR, Spitzer MH, Linde IL, Chabon J, Reticker-Flynn NE, Bhattacharya N, Zhang H, Zhang X, Basto PA, Burt BM, Alonso MN, Engleman EG. Akt and SHP-1 are DC-intrinsic checkpoints for tumor immunity. JCI Insight. 2016;1:89020. doi: 10.1172/jci.insight.89020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER, Linette GP. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle JC, Kreiter S, Diekmann J, Löwer M, van de Roemer N, de Graaf J, Selmi A, Diken M, Boegel S, Paret C, Koslowski M, Kuhn AN, Britten CM, Huber C, Türeci O, Sahin U. Exploiting the mutanome for tumor Vaccination. Cancer Research. 2012;72:1081–1091. doi: 10.1158/0008-5472.CAN-11-3722. [DOI] [PubMed] [Google Scholar]
- Cerwenka A, Lanier LL. Natural killer cells, viruses and Cancer. Nature Reviews Immunology. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- Chamseddine IM, Rejniak KA. Hybrid modeling frameworks of tumor development and treatment. Wiley Interdisciplinary Reviews: Systems Biology and Medicine. 2019;14:1461. doi: 10.1002/wsbm.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N, Wesemann DR. Analyzing immunoglobulin repertoires. Frontiers in Immunology. 2018;9:462. doi: 10.3389/fimmu.2018.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Boettiger AN, Moffitt JR, Wang S, Zhuang X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science. 2015a;348:aaa6090. doi: 10.1126/science.aaa6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Chen Z, Wu D, Zhang L, Lin X, Su J, Rodriguez B, Xi Y, Xia Z, Chen X, Shi X, Wang Q, Li W. Broad H3K4me3 is associated with increased transcription elongation and enhancer activity at tumor-suppressor genes. Nature Genetics. 2015b;47:1149–1157. doi: 10.1038/ng.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Li C, Peng X, Zhou Z, Weinstein JN, Liang H, Cancer Genome Atlas Research Network A Pan-Cancer analysis of enhancer expression in nearly 9000 patient samples. Cell. 2018;173:386–399. doi: 10.1016/j.cell.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Ning B, Shi T. Single-Cell RNA-Seq technologies and related computational data analysis. Frontiers in Genetics. 2019a;10:317. doi: 10.3389/fgene.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, López-Moyado IF, Seo H, Lio CJ, Hempleman LJ, Sekiya T, Yoshimura A, Scott-Browne JP, Rao A. NR4A transcription factors limit CAR T cell function in solid tumours. Nature. 2019b;567:530–534. doi: 10.1038/s41586-019-0985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, Ries CH, Ailles L, Jewett MAS, Moch H, van den Broek M, Beisel C, Stadler MB, Gedye C, Reis B, Pe'er D, Bodenmiller B. An immune atlas of clear cell renal cell carcinoma. Cell. 2017;169:736–749. doi: 10.1016/j.cell.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiacchio F, Pennisi M, Russo G, Motta S, Pappalardo F. Agent-Based modeling of the immune system: netlogo, a promising framework. BioMed Research International. 2014;2014:1–6. doi: 10.1155/2014/907171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikina MD, Troyanskaya OG. Accurate quantification of functional analogy among close homologs. PLOS Computational Biology. 2011;7:e1001074. doi: 10.1371/journal.pcbi.1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HY, Hofree M, Ideker T. A decade of systems biology. Annual Review of Cell and Developmental Biology. 2010;26:721–744. doi: 10.1146/annurev-cellbio-100109-104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, Davidson NE, Martino S, Livingston R, Ingle JN, Perez EA, Carpenter J, Hurd D, Holland JF, Smith BL, Sartor CI, Leung EH, Abrams J, Schilsky RL, Muss HB, Norton L. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast Cancer: first report of intergroup trial C9741/Cancer and leukemia group B trial 9741. Journal of Clinical Oncology. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- Clark SJ, Smallwood SA, Lee HJ, Krueger F, Reik W, Kelsey G. Genome-wide base-resolution mapping of DNA methylation in single cells using single-cell bisulfite sequencing (scBS-seq) Nature Protocols. 2017;12:534–547. doi: 10.1038/nprot.2016.187. [DOI] [PubMed] [Google Scholar]
- Clatworthy MR, Aronin CE, Mathews RJ, Morgan NY, Smith KG, Germain RN. Immune complexes stimulate CCR7-dependent dendritic cell migration to lymph nodes. Nature Medicine. 2014;20:1458–1463. doi: 10.1038/nm.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold M, De La Peña H, Norris A, Polefrone JM, Qian J, English AM, Cummings KL, Penny S, Turner JE, Cottine J, Abelin JG, Malaker SA, Zarling AL, Huang HW, Goodyear O, Freeman SD, Shabanowitz J, Pratt G, Craddock C, Williams ME, Hunt DF, Engelhard VH. MHC class I-associated phosphopeptides are the targets of memory-like immunity in leukemia. Science Translational Medicine. 2013;5:203ra125. doi: 10.1126/scitranslmed.3006061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, Farr AG, Tung KS, Engelhard VH. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. The Journal of Experimental Medicine. 2010;207:681–688. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coifman RR, Lafon S, Lee AB, Maggioni M, Nadler B, Warner F, Zucker SW. Geometric diffusions as a tool for harmonic analysis and structure definition of data: diffusion maps. PNAS. 2005;102:7426–7431. doi: 10.1073/pnas.0500334102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbeck EJ, Ager A, Gallimore A, Jones GW. Tertiary lymphoid structures in Cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Frontiers in Immunology. 2017;8:1830. doi: 10.3389/fimmu.2017.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, Partlova S, Garfall A, Vogl DT, Xu X, Knight SC, Malietzis G, Lee GH, Eruslanov E, Albelda SM, Wang X, Mehta JL, Bewtra M, Rustgi A, Hockstein N, Witt R, Masters G, Nam B, Smirnov D, Sepulveda MA, Gabrilovich DI. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in Cancer patients. Science Immunology. 2016;1:aaf8943. doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces MR, Buenrostro JD, Wu B, Greenside PG, Chan SM, Koenig JL, Snyder MP, Pritchard JK, Kundaje A, Greenleaf WJ, Majeti R, Chang HY. Lineage-specific and single-cell chromatin accessibility charts human hematopoiesis and leukemia evolution. Nature Genetics. 2016;48:1193–1203. doi: 10.1038/ng.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces MR, Granja JM, Shams S, Louie BH, Seoane JA, Zhou W, Silva TC, Groeneveld C, Wong CK, Cho SW, Satpathy AT, Mumbach MR, Hoadley KA, Robertson AG, Sheffield NC, Felau I, Castro MAA, Berman BP, Staudt LM, Zenklusen JC, Laird PW, Curtis C, Greenleaf WJ, Chang HY. The chromatin accessibility landscape of primary human cancers. Science. 2018;362:eaav1898. doi: 10.1126/science.aav1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun AF, Cai L. Dense transcript profiling in single cells by image correlation decoding. Nature Methods. 2016;13:657–660. doi: 10.1038/nmeth.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, Rekhtman N, Anders RA, Cuda JD, Illei PB, Gabrielson E, Askin FB, Niknafs N, Smith KN, Velez MJ, Sauter JL, Isbell JM, Jones DR, Battafarano RJ, Yang SC, Danilova L, Wolchok JD, Topalian SL, Velculescu VE, Pardoll DM, Brahmer JR, Hellmann MD, Chaft JE, Cimino-Mathews A, Taube JM. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC) Annals of Oncology. 2018;29:1853–1860. doi: 10.1093/annonc/mdy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- Creech AL. The role of mass spectrometry and proteogenomics in the advancement of HLA epitope prediction. Proteomics. 2018;18:1700259. doi: 10.1002/pmic.201700259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creixell P, Reimand J, Haider S, Wu G, Shibata T, Vazquez M, Mustonen V, Gonzalez-Perez A, Pearson J, Sander C, Raphael BJ, Marks DS, Ouellette BFF, Valencia A, Bader GD, Boutros PC, Stuart JM, Linding R, Lopez-Bigas N, Stein LD, Mutation Consequences and Pathway Analysis Working Group of the International Cancer Genome Consortium Pathway and network analysis of Cancer genomes. Nature Methods. 2015;12:615–621. doi: 10.1038/nmeth.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings RD, Pierce JM. The challenge and promise of glycomics. Chemistry & Biology. 2014;21:1–15. doi: 10.1016/j.chembiol.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran MA, Allison JP. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Research. 2009;69:7747–7755. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusanovich DA, Daza R, Adey A, Pliner HA, Christiansen L, Gunderson KL, Steemers FJ, Trapnell C, Shendure J. Multiplex single cell profiling of chromatin accessibility by combinatorial cellular indexing. Science. 2015;348:910–914. doi: 10.1126/science.aab1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusanovich DA, Hill AJ, Aghamirzaie D, Daza RM, Pliner HA, Berletch JB, Filippova GN, Huang X, Christiansen L, DeWitt WS, Lee C, Regalado SG, Read DF, Steemers FJ, Disteche CM, Trapnell C, Shendure J. A Single-Cell atlas of in Vivo Mammalian Chromatin Accessibility. Cell. 2018;174:1309–1324. doi: 10.1016/j.cell.2018.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. Journal of Leukocyte Biology. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Dash P, Fiore-Gartland AJ, Hertz T, Wang GC, Sharma S, Souquette A, Crawford JC, Clemens EB, Nguyen THO, Kedzierska K, La Gruta NL, Bradley P, Thomas PG. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature. 2017;547:89–93. doi: 10.1038/nature22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datlinger P, Rendeiro AF, Schmidl C, Krausgruber T, Traxler P, Klughammer J, Schuster LC, Kuchler A, Alpar D, Bock C. Pooled CRISPR screening with single-cell transcriptome readout. Nature Methods. 2017;14:297–301. doi: 10.1038/nmeth.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JO, Telenius JM, McGowan SJ, Roberts NA, Taylor S, Higgs DR, Hughes JR. Multiplexed analysis of chromosome conformation at vastly improved sensitivity. Nature Methods. 2016;13:74–80. doi: 10.1038/nmeth.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JO, Oudelaar AM, Higgs DR, Hughes JR. How best to identify chromosomal interactions: a comparison of approaches. Nature Methods. 2017;14:125–134. doi: 10.1038/nmeth.4146. [DOI] [PubMed] [Google Scholar]
- Davis MM, Tato CM, Furman D. Systems immunology: just getting started. Nature Immunology. 2017;18:725–732. doi: 10.1038/ni.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Deisboeck TS, Wang Z, Macklin P, Cristini V. Multiscale cancer modeling. Annual Review of Biomedical Engineering. 2011;13:127–155. doi: 10.1146/annurev-bioeng-071910-124729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- Dekker J. Gene regulation in the third dimension. Science. 2008;319:1793–1794. doi: 10.1126/science.1152850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoulin S, Herfs M, Delvenne P, Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. Journal of Leukocyte Biology. 2013;93:343–352. doi: 10.1189/jlb.0812397. [DOI] [PubMed] [Google Scholar]
- Denayer T, Stöhr T, Van Roy M. Animal models in translational medicine: validation and prediction. New Horizons in Translational Medicine. 2014;2:5–11. doi: 10.1016/j.nhtm.2014.08.001. [DOI] [Google Scholar]
- Denny SK, Yang D, Chuang CH, Brady JJ, Lim JS, Grüner BM, Chiou SH, Schep AN, Baral J, Hamard C, Antoine M, Wislez M, Kong CS, Connolly AJ, Park KS, Sage J, Greenleaf WJ, Winslow MM. Nfib promotes metastasis through a widespread increase in chromatin accessibility. Cell. 2016;166:328–342. doi: 10.1016/j.cell.2016.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, Lebecque S, Fridman WH, Cadranel J. Long-term survival for patients with non-small-cell lung Cancer with intratumoral lymphoid structures. Journal of Clinical Oncology. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- Dieu-Nosjean MC, Goc J, Giraldo NA, Sautès-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends in Immunology. 2014;35:571–580. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Annals of the New York Academy of Sciences. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- Ding J, Adiconis X, Simmons SK, Kowalczyk MS, Hession CC, Marjanovic ND, Hughes TK, Wadsworth MH, Burks T, Nguyen LT, Kwon JYH, Barak B, Ge W, Kedaigle AJ, Carroll S, Li S, Hacohen N, Rozenblatt-Rosen O, Shalek AK, Villani AC, Regev A, Levin JZ. Systematic comparison of single-cell and single-nucleus RNA-sequencing methods. Nature Biotechnology. 2020;38:737–746. doi: 10.1038/s41587-020-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit A, Parnas O, Li B, Chen J, Fulco CP, Jerby-Arnon L, Marjanovic ND, Dionne D, Burks T, Raychowdhury R, Adamson B, Norman TM, Lander ES, Weissman JS, Friedman N, Regev A. Perturb-Seq: dissecting molecular circuits with scalable Single-Cell RNA profiling of pooled genetic screens. Cell. 2016;167:1853–1866. doi: 10.1016/j.cell.2016.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, Smith I, Tothova Z, Wilen C, Orchard R, Virgin HW, Listgarten J, Root DE. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nature Biotechnology. 2016;34:184–191. doi: 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong MB, Wang G, Chow RD, Ye L, Zhu L, Dai X, Park JJ, Kim HR, Errami Y, Guzman CD, Zhou X, Chen KY, Renauer PA, Du Y, Shen J, Lam SZ, Zhou JJ, Lannin DR, Herbst RS, Chen S. Systematic immunotherapy target discovery using Genome-Scale In Vivo CRISPR Screens in CD8 T Cells. Cell. 2019;178:1189–1204. doi: 10.1016/j.cell.2019.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner BG, Dorner MB, Zhou X, Opitz C, Mora A, Güttler S, Hutloff A, Mages HW, Ranke K, Schaefer M, Jack RS, Henn V, Kroczek RA. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity. 2009;31:823–833. doi: 10.1016/j.immuni.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Dostie J, Richmond TA, Arnaout RA, Selzer RR, Lee WL, Honan TA, Rubio ED, Krumm A, Lamb J, Nusbaum C, Green RD, Dekker J. Chromosome conformation capture carbon copy (5C): a massively parallel solution for mapping interactions between genomic elements. Genome Research. 2006;16:1299–1309. doi: 10.1101/gr.5571506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AG, Herbein G, Montaner LJ, Minty AJ, Caput D, Ferrara P, Gordon S. Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-γ. European Journal of Immunology. 1994;24:1441–1445. doi: 10.1002/eji.1830240630. [DOI] [PubMed] [Google Scholar]
- Drasdo D, Höhme S. A single-cell-based model of tumor growth in vitro: monolayers and spheroids. Physical Biology. 2005;2:133–147. doi: 10.1088/1478-3975/2/3/001. [DOI] [PubMed] [Google Scholar]
- Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nature Immunology. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- Dréau D. An Agent-Based Model of Solid Tumor Progression. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. [Google Scholar]
- Du R, Carey V, Weiss ST. deconvSeq: deconvolution of cell mixture distribution in sequencing data. Bioinformatics. 2019;35:5095–5102. doi: 10.1093/bioinformatics/btz444. [DOI] [PubMed] [Google Scholar]
- Duan F, Duitama J, Al Seesi S, Ayres CM, Corcelli SA, Pawashe AP, Blanchard T, McMahon D, Sidney J, Sette A, Baker BM, Mandoiu II, Srivastava PK. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. Journal of Experimental Medicine. 2014;211:2231–2248. doi: 10.1084/jem.20141308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies Cancer immunoediting. Nature. 2012;482:405–409. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. New England Journal of Medicine. 1986;315:1650–1659. doi: 10.1158/2326-6066.CIR-14-0209. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Developmental Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nature Reviews Immunology. 2019;19:89–103. doi: 10.1038/s41577-018-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS, Sivajothi S, Armstrong TD, Engle DD, Yu KH, Hao Y, Wolfgang CL, Park Y, Preall J, Jaffee EM, Califano A, Robson P, Tuveson DA. Cross-Species Single-Cell analysis of pancreatic ductal adenocarcinoma reveals Antigen-Presenting Cancer-Associated fibroblasts. Cancer Discovery. 2019;9:1102–1123. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enderling H, Hlatky L, Hahnfeldt P. Immunoediting: evidence of the multifaceted role of the immune system in self-metastatic tumor growth. Theoretical Biology and Medical Modelling. 2012;9:31. doi: 10.1186/1742-4682-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom C, Pfirschke C, Pittet MJ. The role of myeloid cells in Cancer therapies. Nature Reviews Cancer. 2016;16:447–462. doi: 10.1038/nrc.2016.54. [DOI] [PubMed] [Google Scholar]
- Engelhardt JJ, Boldajipour B, Beemiller P, Pandurangi P, Sorensen C, Werb Z, Egeblad M, Krummel MF. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell. 2012;21:402–417. doi: 10.1016/j.ccr.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A, Litzky L, Hancock WW, Conejo-Garcia JR, Feldman M, Albelda SM, Singhal S. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. Journal of Clinical Investigation. 2014;124:5466–5480. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. Epigenetics in Cancer. New England Journal of Medicine. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- Esumi S, Wu SX, Yanagawa Y, Obata K, Sugimoto Y, Tamamaki N. Method for single-cell microarray analysis and application to gene-expression profiling of GABAergic neuron progenitors. Neuroscience Research. 2008;60:439–451. doi: 10.1016/j.neures.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Faget J, Boivin G, Ancey P-B, Gkasti A, Mussard J, Engblom C, Pfirschke C, Vazquez J, Bendriss-Vermare N, Caux C, Vozenin M-C, Pittet MJ, Gunzer M, Meylan E. Efficient and specific Ly6G+ cell depletion: A change in the current practices toward more relevant functional analyses of neutrophils. bioRxiv. 2018 doi: 10.1101/498881. [DOI]
- Faratian D. Cancer Systems Biology Systems Biology in Drug Discovery and Development. In: Yan Q, editor. Methods and Protocols. Totowa, NJ: Humana Press; 2010. pp. 245–263. [Google Scholar]
- Farlik M, Sheffield NC, Nuzzo A, Datlinger P, Schönegger A, Klughammer J, Bock C. Single-cell DNA methylome sequencing and bioinformatic inference of epigenomic cell-state dynamics. Cell Reports. 2015;10:1386–1397. doi: 10.1016/j.celrep.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of Cancer. Science. 2017;357:eaal2380. doi: 10.1126/science.aal2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, Collier AR, Boyd RL, Turley SJ. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. The Journal of Experimental Medicine. 2010;207:689–697. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher AL, Acton SE, Knoblich K. Lymph node fibroblastic reticular cells in health and disease. Nature Reviews Immunology. 2015;15:350–361. doi: 10.1038/nri3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong L, Engleman EG. Dendritic cells in Cancer immunotherapy. Annual Review of Immunology. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. PNAS. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. The Journal of Experimental Medicine. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, Orlov YL, Velkov S, Ho A, Mei PH, Chew EG, Huang PY, Welboren WJ, Han Y, Ooi HS, Ariyaratne PN, Vega VB, Luo Y, Tan PY, Choy PY, Wansa KD, Zhao B, Lim KS, Leow SC, Yow JS, Joseph R, Li H, Desai KV, Thomsen JS, Lee YK, Karuturi RK, Herve T, Bourque G, Stunnenberg HG, Ruan X, Cacheux-Rataboul V, Sung WK, Liu ET, Wei CL, Cheung E, Ruan Y. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature. 2009;462:58–64. doi: 10.1038/nature08497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulwyler MJ. Electronic separation of biological cells by volume. Science. 1965;150:910–911. doi: 10.1126/science.150.3698.910. [DOI] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. The key role of segmented filamentous Bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Research. 2007;67:425–426. doi: 10.1158/0008-5472.CAN-06-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI. Myeloid-Derived suppressor cells. Cancer Immunology Research. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nature Immunology. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore A, Glithero A, Godkin A, Tissot AC, Plückthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. Journal of Experimental Medicine. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Galsky MD, Saci A, Szabo PM, Azrilevich A, Horak C, Lambert A, Siefker-Radtke A, Necchi A, Sharma P. Impact of zumor mutation burden on nivolumab efficacy in second-line urothelial carcinoma patients: exploratory analysis of the phase ii checkmate 275 study. Annals of Oncology. 2017;28:v296–v297. doi: 10.1093/annonc/mdx371.003. [DOI] [Google Scholar]
- Garcia-Garijo A, Fajardo CA, Gros A. Determinants for neoantigen identification. Frontiers in Immunology. 2019;10:1392. doi: 10.3389/fimmu.2019.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway LA, Lander ES. Lessons from the Cancer genome. Cell. 2013;153:17–37. doi: 10.1016/j.cell.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Garrett WS. Cancer and the microbiota. Science. 2015;348:80–86. doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenbee C. Macrophage-mediated immunoediting drives ductal carcinoma evolution: space is the game changer. bioRxiv. 2019 doi: 10.1101/594598. [DOI]
- Gawad C, Koh W, Quake SR. Single-cell genome sequencing: current state of the science. Nature Reviews Genetics. 2016;17:175–188. doi: 10.1038/nrg.2015.16. [DOI] [PubMed] [Google Scholar]
- Gee MH, Han A, Lofgren SM, Beausang JF, Mendoza JL, Birnbaum ME, Bethune MT, Fischer S, Yang X, Gomez-Eerland R, Bingham DB, Sibener LV, Fernandes RA, Velasco A, Baltimore D, Schumacher TN, Khatri P, Quake SR, Davis MM, Garcia KC. Antigen identification for orphan T cell receptors expressed on Tumor-Infiltrating lymphocytes. Cell. 2018;172:549–563. doi: 10.1016/j.cell.2017.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M, West RB, Plevritis SK, Alizadeh AA. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nature Medicine. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes MJ, Sevinsky CJ, Sood A, Adak S, Bello MO, Bordwell A, Can A, Corwin A, Dinn S, Filkins RJ, Hollman D, Kamath V, Kaanumalle S, Kenny K, Larsen M, Lazare M, Li Q, Lowes C, McCulloch CC, McDonough E, Montalto MC, Pang Z, Rittscher J, Santamaria-Pang A, Sarachan BD, Seel ML, Seppo A, Shaikh K, Sui Y, Zhang J, Ginty F. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. PNAS. 2013;110:11982–11987. doi: 10.1073/pnas.1300136110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlee P, Anderson AR. An evolutionary hybrid cellular automaton model of solid tumour growth. Journal of Theoretical Biology. 2007;246:583–603. doi: 10.1016/j.jtbi.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain RN, Meier-Schellersheim M, Nita-Lazar A, Fraser ID. Systems biology in immunology: a computational modeling perspective. Annual Review of Immunology. 2011;29:527–585. doi: 10.1146/annurev-immunol-030409-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, Validire P, Damotte D, Alifano M, Magdeleinat P, Cremer I, Teillaud JL, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung Cancer. American Journal of Respiratory and Critical Care Medicine. 2014;189:832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- Germain RN. Will systems biology deliver its promise and contribute to the development of new or improved vaccines? what really constitutes the study of "Systems Biology" and How Might Such an Approach Facilitate Vaccine Design. Cold Spring Harbor Perspectives in Biology. 2018;10:a033308. doi: 10.1101/cshperspect.a033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity. 2012;37:364–376. doi: 10.1016/j.immuni.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, Wurtz A, Dong W, Cai G, Melnick MA, Du VY, Schlessinger J, Goldberg SB, Chiang A, Sanmamed MF, Melero I, Agorreta J, Montuenga LM, Lifton R, Ferrone S, Kavathas P, Rimm DL, Kaech SM, Schalper K, Herbst RS, Politi K. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung Cancer. Cancer Discovery. 2017;7:1420–1435. doi: 10.1158/2159-8290.CD-17-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, Lambrecht BN. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. The Journal of Experimental Medicine. 2009;206:2339–2349. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffarizadeh A, Heiland R, Friedman SH, Mumenthaler SM, Macklin P. PhysiCell: an open source physics-based cell simulator for 3-D multicellular systems. PLOS Computational Biology. 2018;14:e1005991. doi: 10.1371/journal.pcbi.1005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharib WH, Robinson-Rechavi M. When orthologs diverge between human and mouse. Briefings in Bioinformatics. 2011;12:436–441. doi: 10.1093/bib/bbr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoneim HE, Fan Y, Moustaki A, Abdelsamed HA, Dash P, Dogra P, Carter R, Awad W, Neale G, Thomas PG, Youngblood B. De novo epigenetic programs inhibit PD-1 Blockade-Mediated T cell rejuvenation. Cell. 2017;170:142–157. doi: 10.1016/j.cell.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierahn TM, Wadsworth MH, Hughes TK, Bryson BD, Butler A, Satija R, Fortune S, Love JC, Shalek AK. Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nature Methods. 2017;14:395–398. doi: 10.1038/nmeth.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesen C, Wang HA, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, Schüffler PJ, Grolimund D, Buhmann JM, Brandt S, Varga Z, Wild PJ, Günther D, Bodenmiller B. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nature Methods. 2014;11:417–422. doi: 10.1038/nmeth.2869. [DOI] [PubMed] [Google Scholar]
- Gilmour DS, Lis JT. In vivo interactions of RNA polymerase II with genes of Drosophila melanogaster. Molecular and Cellular Biology. 1985;5:2009–2018. doi: 10.1128/MCB.5.8.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nature Immunology. 2016;17:34–40. doi: 10.1038/ni.3324. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Guilliams M. Tissue-Resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Glanville J, Huang H, Nau A, Hatton O, Wagar LE, Rubelt F, Ji X, Han A, Krams SM, Pettus C, Haas N, Arlehamn CSL, Sette A, Boyd SD, Scriba TJ, Martinez OM, Davis MM. Identifying specificity groups in the T cell receptor repertoire. Nature. 2017;547:94–98. doi: 10.1038/nature22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass G, Papin JA, Mandell JW. SIMPLE: a sequential immunoperoxidase labeling and erasing method. Journal of Histochemistry & Cytochemistry. 2009;57:899–905. doi: 10.1369/jhc.2009.953612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goc J, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Characteristics of tertiary lymphoid structures in primary cancers. OncoImmunology. 2013;2:e26836. doi: 10.4161/onci.26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltsev Y, Samusik N, Kennedy-Darling J, Bhate S, Hale M, Vazquez G, Black S, Nolan GP. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell. 2018;174:968–981. doi: 10.1016/j.cell.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C, Milberg O, Wang B, Vicini P, Narwal R, Roskos L, Popel AS. A computational multiscale agent-based model for simulating spatio-temporal tumour immune response to PD1 and PDL1 inhibition. Journal of the Royal Society Interface. 2017;14:20170320. doi: 10.1098/rsif.2017.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T, Szustakowski JD. DeconRNASeq: a statistical framework for deconvolution of heterogeneous tissue samples based on mRNA-Seq data. Bioinformatics. 2013;29:1083–1085. doi: 10.1093/bioinformatics/btt090. [DOI] [PubMed] [Google Scholar]
- Good Z, Sarno J, Jager A, Samusik N, Aghaeepour N, Simonds EF, White L, Lacayo NJ, Fantl WJ, Fazio G, Gaipa G, Biondi A, Tibshirani R, Bendall SC, Nolan GP, Davis KL. Single-cell developmental classification of B cell precursor acute lymphoblastic leukemia at diagnosis reveals predictors of relapse. Nature Medicine. 2018;24:474–483. doi: 10.1038/nm.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good Z, Borges L, Vivanco Gonzalez N, Sahaf B, Samusik N, Tibshirani R, Nolan GP, Bendall SC. Proliferation tracing with single-cell mass cytometry optimizes generation of stem cell memory-like T cells. Nature Biotechnology. 2019;37:259–266. doi: 10.1038/s41587-019-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nature Reviews Immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gowthaman U, Chodisetti SB, Parihar P, Agrewala JN. Evaluation of different generic in silico methods for predicting HLA class I binding peptide vaccine candidates using a reverse approach. Amino Acids. 2010;39:1333–1342. doi: 10.1007/s00726-010-0579-2. [DOI] [PubMed] [Google Scholar]
- Grosselin K, Durand A, Marsolier J, Poitou A, Marangoni E, Nemati F, Dahmani A, Lameiras S, Reyal F, Frenoy O, Pousse Y, Reichen M, Woolfe A, Brenan C, Griffiths AD, Vallot C, Gérard A. High-throughput single-cell ChIP-seq identifies heterogeneity of chromatin states in breast Cancer. Nature Genetics. 2019;51:1060–1066. doi: 10.1038/s41588-019-0424-9. [DOI] [PubMed] [Google Scholar]
- Grün D, Lyubimova A, Kester L, Wiebrands K, Basak O, Sasaki N, Clevers H, van Oudenaarden A. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature. 2015;525:251–255. doi: 10.1038/nature14966. [DOI] [PubMed] [Google Scholar]
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. New England Journal of Medicine. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Liu Y, Fu L, Zhai L, Zhu J, Han Y, Jiang Y, Zhang Y, Zhang P, Jiang Z, Zhang X, Cao X. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nature Medicine. 2019;25:312–322. doi: 10.1038/s41591-018-0309-y. [DOI] [PubMed] [Google Scholar]
- Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. The Journal of Experimental Medicine. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, Van Gassen S, Chen J, Poidinger M, De Prijck S, Tavernier SJ, Low I, Irac SE, Mattar CN, Sumatoh HR, Low GHL, Chung TJK, Chan DKH, Tan KK, Hon TLK, Fossum E, Bogen B, Choolani M, Chan JKY, Larbi A, Luche H, Henri S, Saeys Y, Newell EW, Lambrecht BN, Malissen B, Ginhoux F. Unsupervised High-Dimensional analysis aligns dendritic cells across tissues and species. Immunity. 2016;45:669–684. doi: 10.1016/j.immuni.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Zhu P, Wu X, Li X, Wen L, Tang F. Single-cell methylome landscapes of mouse embryonic stem cells and early embryos analyzed using reduced representation bisulfite sequencing. Genome Research. 2013;23:2126–2135. doi: 10.1101/gr.161679.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Wang H, Potter SS, Whitsett JA, Xu Y. SINCERA: a pipeline for Single-Cell RNA-Seq profiling analysis. PLOS Computational Biology. 2015;11:e1004575. doi: 10.1371/journal.pcbi.1004575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zhang Y, Zheng L, Zheng C, Song J, Zhang Q, Kang B, Liu Z, Jin L, Xing R, Gao R, Zhang L, Dong M, Hu X, Ren X, Kirchhoff D, Roider HG, Yan T, Zhang Z. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nature Medicine. 2018;24:978–985. doi: 10.1038/s41591-018-0045-3. [DOI] [PubMed] [Google Scholar]
- Habib N, Li Y, Heidenreich M, Swiech L, Avraham-Davidi I, Trombetta JJ, Hession C, Zhang F, Regev A. Div-Seq: single-nucleus RNA-Seq reveals dynamics of rare adult newborn neurons. Science. 2016;353:925–928. doi: 10.1126/science.aad7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib N, Avraham-Davidi I, Basu A, Burks T, Shekhar K, Hofree M, Choudhury SR, Aguet F, Gelfand E, Ardlie K, Weitz DA, Rozenblatt-Rosen O, Zhang F, Regev A. Massively parallel single-nucleus RNA-seq with DroNc-seq. Nature Methods. 2017;14:955–958. doi: 10.1038/nmeth.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ. Getting personal with neoantigen-based therapeutic Cancer vaccines. Cancer Immunology Research. 2013;1:11–15. doi: 10.1158/2326-6066.CIR-13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. New England Journal of Medicine. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Bagheri N, Bradshaw EM, Hafler DA, Lauffenburger DA, Love JC. Polyfunctional responses by human T cells result from sequential release of cytokines. PNAS. 2012;109:1607–1612. doi: 10.1073/pnas.1117194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han A, Glanville J, Hansmann L, Davis MM. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nature Biotechnology. 2014;32:684–692. doi: 10.1038/nbt.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Li H, Guan Y, Huang J. Immune repertoire: a potential biomarker and therapeutic for hepatocellular carcinoma. Cancer Letters. 2016;379:206–212. doi: 10.1016/j.canlet.2015.06.022. [DOI] [PubMed] [Google Scholar]
- Haque A, Engel J, Teichmann SA, Lönnberg T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Medicine. 2017;9:75. doi: 10.1186/s13073-017-0467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann FJ, Babdor J, Gherardini PF, Amir ED, Jones K, Sahaf B, Marquez DM, Krutzik P, O'Donnell E, Sigal N, Maecker HT, Meyer E, Spitzer MH, Bendall SC. Comprehensive immune monitoring of clinical trials to advance human immunotherapy. Cell Reports. 2019;28:819–831. doi: 10.1016/j.celrep.2019.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, García-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimshony T, Wagner F, Sher N, Yanai I. CEL-Seq: single-cell RNA-Seq by multiplexed linear amplification. Cell Reports. 2012;2:666–673. doi: 10.1016/j.celrep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- He B, Xing S, Chen C, Gao P, Teng L, Shan Q, Gullicksrud JA, Martin MD, Yu S, Harty JT, Badovinac VP, Tan K, Xue HH. CD8+ T cells utilize highly dynamic enhancer repertoires and regulatory circuitry in response to infections. Immunity. 2016;45:1341–1354. doi: 10.1016/j.immuni.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, Ni A, Novik JB, Mangarin LMB, Abu-Akeel M, Liu C, Sauter JL, Rekhtman N, Chang E, Callahan MK, Chaft JE, Voss MH, Tenet M, Li XM, Covello K, Renninger A, Vitazka P, Geese WJ, Borghaei H, Rudin CM, Antonia SJ, Swanton C, Hammerbacher J, Merghoub T, McGranahan N, Snyder A, Wolchok JD. Genomic features of response to combination immunotherapy in patients with advanced Non-Small-Cell lung Cancer. Cancer Cell. 2018;33:843–852. doi: 10.1016/j.ccell.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, Gopalakrishnan V, Xi Y, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu W, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau PO, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong S, Bailey M, Warren S, Rao D, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautès-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang L, Wargo JA. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng TS, Painter MW, Immunological Genome Project Consortium The immunological genome project: networks of gene expression in immune cells. Nature Immunology. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- Henning AN, Roychoudhuri R, Restifo NP. Epigenetic control of CD8+ T cell differentiation. Nature Reviews Immunology. 2018;18:340–356. doi: 10.1038/nri.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SL, Farmer D, Marszalek K, Cadera E, Liang HE, Xu Y, Schlissel MS, Skok JA. Association between the Igk and Igh immunoglobulin loci mediated by the 3' Igk enhancer induces 'decontraction' of the Igh locus in pre-B cells. Nature Immunology. 2008;9:396–404. doi: 10.1038/ni1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcombe M, Adra S, Bicak M, Chin S, Coakley S, Graham AI, Green J, Greenough C, Jackson D, Kiran M, MacNeil S, Maleki-Dizaji A, McMinn P, Pogson M, Poole R, Qwarnstrom E, Ratnieks F, Rolfe MD, Smallwood R, Sun T, Worth D. Modelling complex biological systems using an agent-based approach. Integr. Biol. 2012;4:53–64. doi: 10.1039/C1IB00042J. [DOI] [PubMed] [Google Scholar]
- Hollern DP, Xu N, Thennavan A, Glodowski C, Garcia-Recio S, Mott KR, He X, Garay JP, Carey-Ewend K, Marron D, Ford J, Liu S, Vick SC, Martin M, Parker JS, Vincent BG, Serody JS, Perou CM. B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast Cancer. Cell. 2019;179:1191–1206. doi: 10.1016/j.cell.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134:714–717. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Honda K, Littman DR. The Microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- Hood L, Heath JR, Phelps ME, Lin B. Systems biology and new technologies enable predictive and preventative medicine. Science. 2004;306:640–643. doi: 10.1126/science.1104635. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, Dekker CL, Mackey S, Maecker H, Swan GE, Davis MM, Norman PJ, Guethlein LA, Desai M, Parham P, Blish CA. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Science Translational Medicine. 2013;5:208ra145. doi: 10.1126/scitranslmed.3006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi N, Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. PNAS. 1976;73:3628–3632. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, Azimi CS, Scheer AK, Randolph HE, Thompson TW, Zhang L, Iannello A, Mathur N, Jardine KE, Kirn GA, Bell JC, McBurney MW, Raulet DH, Ardolino M. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. Journal of Clinical Investigation. 2018;128:4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Fabyanic E, Kwon DY, Tang S, Zhou Z, Wu H. Dissecting Cell-Type composition and Activity-Dependent transcriptional state in mammalian brains by massively parallel Single-Nucleus RNA-Seq. Molecular Cell. 2017a;68:1006–1015. doi: 10.1016/j.molcel.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for Cancer. Nature Reviews Immunology. 2017b;18:168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Zhang J, Wang J, Fu J, Li T, Zheng X, Wang B, Gu S, Jiang P, Fan J, Ying X, Zhang J, Carroll MC, Wucherpfennig KW, Hacohen N, Zhang F, Zhang P, Liu JS, Li B, Liu XS. Landscape of B cell immunity and related immune evasion in human cancers. Nature Genetics. 2019;51:560–567. doi: 10.1038/s41588-018-0339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A, Cheng L, He M, Nie J, Wang J, Jiang K. Interleukin-35 on B cell and T cell induction and regulation. Journal of Inflammation. 2017;14:16. doi: 10.1186/s12950-017-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Roberts N, McGowan S, Hay D, Giannoulatou E, Lynch M, De Gobbi M, Taylor S, Gibbons R, Higgs DR. Analysis of hundreds of cis-regulatory landscapes at high resolution in a single, high-throughput experiment. Nature Genetics. 2014;46:205–212. doi: 10.1038/ng.2871. [DOI] [PubMed] [Google Scholar]
- Hulett HR, Bonner WA, Barrett J, Herzenberg LA. Cell sorting: automated separation of mammalian cells as a function of intracellular fluorescence. Science. 1969;166:747–749. doi: 10.1126/science.166.3906.747. [DOI] [PubMed] [Google Scholar]
- Hundal J, Carreno BM, Petti AA, Linette GP, Griffith OL, Mardis ER, Griffith M. pVAC-Seq: A genome-guided in silico approach to identifying tumor neoantigens. Genome Medicine. 2016;8:11. doi: 10.1186/s13073-016-0264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideker T, Galitski T, Hood L. A new approach to decoding life: systems biology. Annual Review of Genomics and Human Genetics. 2001;2:343–372. doi: 10.1146/annurev.genom.2.1.343. [DOI] [PubMed] [Google Scholar]
- Ideker T. Integrated Genomic and Proteomic Analyses of a Systematically Perturbed Metabolic Network. Science. 2001;292:929–934. doi: 10.1126/science.292.5518.929. [DOI] [PubMed] [Google Scholar]
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS. Commensal Bacteria control Cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, Sharpe AH, Freeman GJ, Germain RN, Nakaya HI, Xue HH, Ahmed R. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Human Genome Sequencing Consortium Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- Irish JM, Doxie DB. High-dimensional single-cell cancer biology. Current Topics in Microbiology and Immunology. 2014;377:1–21. doi: 10.1007/82_2014_367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S, Kjällquist U, Moliner A, Zajac P, Fan JB, Lönnerberg P, Linnarsson S. Characterization of the single-cell transcriptional landscape by highly multiplex RNA-seq. Genome Research. 2011;21:1160–1167. doi: 10.1101/gr.110882.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous Bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A, Lam L, Rajendram M, Tamburini F, Honeycutt J, Pham T, Van Treuren W, Pruss K, Stabler SR, Lugo K, Bouley DM, Vilches-Moure JG, Smith M, Sonnenburg JL, Bhatt AS, Huang KC, Monack D. A gut Commensal-Produced metabolite mediates colonization resistance to Salmonella infection. Cell Host & Microbe. 2018;24:296–307. doi: 10.1016/j.chom.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby MA, Duncavage EJ, Walter MJ. Implications of tumor clonal heterogeneity in the Era of Next-Generation sequencing. Trends in Cancer. 2015;1:231–241. doi: 10.1016/j.trecan.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Jaitin DA, Kenigsberg E, Keren-Shaul H, Elefant N, Paul F, Zaretsky I, Mildner A, Cohen N, Jung S, Tanay A, Amit I. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science. 2014;343:776–779. doi: 10.1126/science.1247651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaitin DA, Weiner A, Yofe I, Lara-Astiaso D, Keren-Shaul H, David E, Salame TM, Tanay A, van Oudenaarden A, Amit I. Dissecting immune circuits by linking CRISPR-Pooled screens with Single-Cell RNA-Seq. Cell. 2016;167:1883–1896. doi: 10.1016/j.cell.2016.11.039. [DOI] [PubMed] [Google Scholar]
- Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma'ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J, Quaranta V, Cummings PT. An off-lattice hybrid discrete-continuum model of tumor growth and invasion. Biophysical Journal. 2010;98:37–47. doi: 10.1016/j.bpj.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Schonnesen AA, Ma KY. Ushering in integrated T cell repertoire profiling in Cancer. Trends in Cancer. 2019;5:85–94. doi: 10.1016/j.trecan.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Torquato S. Emergent behaviors from a cellular automaton model for invasive tumor growth in heterogeneous microenvironments. PLOS Computational Biology. 2011;7:e1002314. doi: 10.1371/journal.pcbi.1002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson TM, Coughlan HD, Lun ATL, Bediaga NG, Naselli G, Garnham AL, Harrison LC, Smyth GK, Allan RS. Genome-wide analysis reveals no evidence of trans chromosomal regulation of mammalian immune development. PLOS Genetics. 2018;14:e1007431. doi: 10.1371/journal.pgen.1007431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- Jones GW, Hill DG, Jones SA. Understanding immune cells in tertiary lymphoid organ development: it is all starting to come together. Frontiers in Immunology. 2016;7:401. doi: 10.3389/fimmu.2016.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, DuPage M, Tammela T, Kerper NR, Farago AF, Robbins R, Crowley DM, Bronson RT, Jacks T. Regulatory T cells in Tumor-Associated tertiary lymphoid structures suppress Anti-tumor T cell responses. Immunity. 2015;43:579–590. doi: 10.1016/j.immuni.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature Reviews Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- Jung K, Heishi T, Khan OF, Kowalski PS, Incio J, Rahbari NN, Chung E, Clark JW, Willett CG, Luster AD, Yun SH, Langer R, Anderson DG, Padera TP, Jain RK, Fukumura D. Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. Journal of Clinical Investigation. 2017;127:3039–3051. doi: 10.1172/JCI93182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- Katoh H, Komura D, Konishi H, Suzuki R, Yamamoto A, Kakiuchi M, Sato R, Ushiku T, Yamamoto S, Tatsuno K, Oshima T, Nomura S, Seto Y, Fukayama M, Aburatani H, Ishikawa S. Immunogenetic profiling for gastric cancers identifies sulfated glycosaminoglycans as major and functional B cell antigens in human malignancies. Cell Reports. 2017;20:1073–1087. doi: 10.1016/j.celrep.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Kaufman M, Urbain J, Thomas R. Towards a logical analysis of the immune response. Journal of Theoretical Biology. 1985;114:527–561. doi: 10.1016/S0022-5193(85)80042-4. [DOI] [PubMed] [Google Scholar]
- Ke R, Mignardi M, Pacureanu A, Svedlund J, Botling J, Wählby C, Nilsson M. In situ sequencing for RNA analysis in preserved tissue and cells. Nature Methods. 2013;10:857–860. doi: 10.1038/nmeth.2563. [DOI] [PubMed] [Google Scholar]
- Kenkel JA, Tseng WW, Davidson MG, Tolentino LL, Choi O, Bhattacharya N, Seeley ES, Winer DA, Reticker-Flynn NE, Engleman EG. An immunosuppressive dendritic cell subset accumulates at secondary sites and promotes metastasis in pancreatic Cancer. Cancer Research. 2017;77:4158–4170. doi: 10.1158/0008-5472.CAN-16-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren L, Bosse M, Marquez D, Angoshtari R, Jain S, Varma S, Yang SR, Kurian A, Van Valen D, West R, Bendall SC, Angelo M. A structured Tumor-Immune microenvironment in triple negative breast Cancer revealed by multiplexed ion beam imaging. Cell. 2018;174:1373–1387. doi: 10.1016/j.cell.2018.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, Oliveira G, Giobbie-Hurder A, Felt K, Gjini E, Shukla SA, Hu Z, Li L, Le PM, Allesøe RL, Richman AR, Kowalczyk MS, Abdelrahman S, Geduldig JE, Charbonneau S, Pelton K, Iorgulescu JB, Elagina L, Zhang W, Olive O, McCluskey C, Olsen LR, Stevens J, Lane WJ, Salazar AM, Daley H, Wen PY, Chiocca EA, Harden M, Lennon NJ, Gabriel S, Getz G, Lander ES, Regev A, Ritz J, Neuberg D, Rodig SJ, Ligon KL, Suvà ML, Wucherpfennig KW, Hacohen N, Fritsch EF, Livak KJ, Ott PA, Wu CJ, Reardon DA. Neoantigen vaccine generates intratumoral T cell responses in phase ib glioblastoma trial. Nature. 2019;565:234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khain E, Sander LM. Dynamics and pattern formation in invasive tumor growth. Physical Review Letters. 2006;96:188103. doi: 10.1103/PhysRevLett.96.188103. [DOI] [PubMed] [Google Scholar]
- Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B cells are critical regulators of humoral immunity. Nature Communications. 2015;6:5997. doi: 10.1038/ncomms6997. [DOI] [PubMed] [Google Scholar]
- Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, Attanasio J, Yan P, George SM, Bengsch B, Staupe RP, Donahue G, Xu W, Amaravadi RK, Xu X, Karakousis GC, Mitchell TC, Schuchter LM, Kaye J, Berger SL, Wherry EJ. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature. 2019;571:211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KT, Lee HW, Lee HO, Song HJ, Jeong daE, Shin S, Kim H, Shin Y, Nam DH, Jeong BC, Kirsch DG, Joo KM, Park WY. Application of single-cell RNA sequencing in optimizing a combinatorial therapeutic strategy in metastatic renal cell carcinoma. Genome Biology. 2016;17:80. doi: 10.1186/s13059-016-0945-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Kim HK, Lee K, Hong Y, Cho JH, Choi JW, Lee JI, Suh YL, Ku BM, Eum HH, Choi S, Choi YL, Joung JG, Park WY, Jung HA, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ, Lee HO. Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nature Communications. 2020;11:2285. doi: 10.1038/s41467-020-16164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselev VY, Kirschner K, Schaub MT, Andrews T, Yiu A, Chandra T, Natarajan KN, Reik W, Barahona M, Green AR, Hemberg M. SC3: consensus clustering of single-cell RNA-seq data. Nature Methods. 2017;14:483–486. doi: 10.1038/nmeth.4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nature Reviews Immunology. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano H. Systems biology: a brief overview. Science. 2002;295:1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, Peshkin L, Weitz DA, Kirschner MW. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell. 2015;161:1187–1201. doi: 10.1016/j.cell.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch I. Petri nets in systems biology. Software & Systems Modeling. 2015;14:703–710. doi: 10.1007/s10270-014-0421-5. [DOI] [Google Scholar]
- Kompauer M, Heiles S, Spengler B. Atmospheric pressure MALDI mass spectrometry imaging of tissues and cells at 1.4-μm lateral resolution. Nature Methods. 2017;14:90–96. doi: 10.1038/nmeth.4071. [DOI] [PubMed] [Google Scholar]
- Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, Nishimasu H, Nureki O, Zhang F. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin EV. Comparative genomics, minimal gene-sets and the last universal common ancestor. Nature Reviews Microbiology. 2003;1:127–136. doi: 10.1038/nrmicro751. [DOI] [PubMed] [Google Scholar]
- Koonin EV. Orthologs, paralogs, and evolutionary genomics. Annual Review of Genetics. 2005;39:309–338. doi: 10.1146/annurev.genet.39.073003.114725. [DOI] [PubMed] [Google Scholar]
- Korsunsky I, McGovern K, LaGatta T, Olde Loohuis L, Grosso-Applewhite T, Griffeth N, Mishra B. Systems biology of Cancer: a challenging expedition for clinical and quantitative biologists. Frontiers in Bioengineering and Biotechnology. 2014;2:27. doi: 10.3389/fbioe.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochvill F, Neale G, Haverkamp JM, Van de Velde LA, Smith AM, Kawauchi D, McEvoy J, Roussel MF, Dyer MA, Qualls JE, Murray PJ. TNF counterbalances the emergence of M2 tumor macrophages. Cell Reports. 2015;12:1902–1914. doi: 10.1016/j.celrep.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreeger PK, Lauffenburger DA. Cancer systems biology: a network modeling perspective. Carcinogenesis. 2010;31:2–8. doi: 10.1093/carcin/bgp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM, Dummer R, Robinson MD, Levesque MP, Becher B. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nature Medicine. 2018;24:144–153. doi: 10.1038/nm.4466. [DOI] [PubMed] [Google Scholar]
- Kshitiz, Ellison DD, Suhail Y, Afzal J, Woo L, Kilic O, Spees J, Levchenko A. Dynamic secretome of bone marrow-derived stromal cells reveals a cardioprotective biochemical cocktail. PNAS. 2019;116:14374–14383. doi: 10.1073/pnas.1902598116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kveler K, Starosvetsky E, Ziv-Kenet A, Kalugny Y, Gorelik Y, Shalev-Malul G, Aizenbud-Reshef N, Dubovik T, Briller M, Campbell J, Rieckmann JC, Asbeh N, Rimar D, Meissner F, Wiser J, Shen-Orr SS. Immune-centric network of cytokines and cells in disease context identified by computational mining of PubMed. Nature Biotechnology. 2018;36:651–659. doi: 10.1038/nbt.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labidi-Galy SI, Sisirak V, Meeus P, Gobert M, Treilleux I, Bajard A, Combes JD, Faget J, Mithieux F, Cassignol A, Tredan O, Durand I, Ménétrier-Caux C, Caux C, Blay JY, Ray-Coquard I, Bendriss-Vermare N. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian Cancer. Cancer Research. 2011;71:5423–5434. doi: 10.1158/0008-5472.CAN-11-0367. [DOI] [PubMed] [Google Scholar]
- Lacar B, Linker SB, Jaeger BN, Krishnaswami SR, Barron JJ, Kelder MJE, Parylak SL, Paquola ACM, Venepally P, Novotny M, O'Connor C, Fitzpatrick C, Erwin JA, Hsu JY, Husband D, McConnell MJ, Lasken R, Gage FH. Nuclear RNA-seq of single neurons reveals molecular signatures of activation. Nature Communications. 2016;7:11022. doi: 10.1038/ncomms11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladányi A, Kiss J, Somlai B, Gilde K, Fejős Z, Mohos A, Gaudi I, Tímár J. Density of DC-LAMP+ mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunology, Immunotherapy. 2007;56:1459–1469. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann Y, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Raymond C, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blöcker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowki J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ, Szustakowki J, International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annual Review of Immunology. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Latonen L, Afyounian E, Jylhä A, Nättinen J, Aapola U, Annala M, Kivinummi KK, Tammela TTL, Beuerman RW, Uusitalo H, Nykter M, Visakorpi T. Integrative proteomics in prostate Cancer uncovers robustness against genomic and transcriptomic aberrations during disease progression. Nature Communications. 2018;9:1176. doi: 10.1038/s41467-018-03573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, Meinhof K, Chow A, Kim-Shulze S, Wolf A, Medaglia C, Li H, Rytlewski JA, Emerson RO, Solovyov A, Greenbaum BD, Sanders C, Vignali M, Beasley MB, Flores R, Gnjatic S, Pe'er D, Rahman A, Amit I, Merad M. Innate immune landscape in early lung adenocarcinoma by paired Single-Cell analyses. Cell. 2017;169:750–765. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, Meyerson M, Gabriel SB, Lander ES, Getz G. Discovery and saturation analysis of Cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nature Immunology. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- Lee JH, Daugharthy ER, Scheiman J, Kalhor R, Yang JL, Ferrante TC, Terry R, Jeanty SS, Li C, Amamoto R, Peters DT, Turczyk BM, Marblestone AH, Inverso SA, Bernard A, Mali P, Rios X, Aach J, Church GM. Highly multiplexed subcellular RNA sequencing in situ. Science. 2014;343:1360–1363. doi: 10.1126/science.1250212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letort G, Montagud A, Stoll G, Heiland R, Barillot E, Macklin P, Zinovyev A, Calzone L. PhysiBoSS: a multi-scale agent-based modelling framework integrating physical dimension and cell signalling. Bioinformatics. 2019;35:1188–1196. doi: 10.1093/bioinformatics/bty766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettice LA, Heaney SJ, Purdie LA, Li L, de Beer P, Oostra BA, Goode D, Elgar G, Hill RE, de Graaff E. A long-range shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Human Molecular Genetics. 2003;12:1725–1735. doi: 10.1093/hmg/ddg180. [DOI] [PubMed] [Google Scholar]
- Levesque MJ, Raj A. Single-chromosome transcriptional profiling reveals chromosomal gene expression regulation. Nature Methods. 2013;10:246–248. doi: 10.1038/nmeth.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JH, Simonds EF, Bendall SC, Davis KL, Amir AD, Tadmor MD, Litvin O, Fienberg HG, Jager A, Zunder ER, Finck R, Gedman AL, Radtke I, Downing JR, Pe'er D, Nolan GP. Data-Driven phenotypic dissection of AML reveals Progenitor-like cells that correlate with prognosis. Cell. 2015;162:184–197. doi: 10.1016/j.cell.2015.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Research. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Li G, Zan H, Xu Z, Casali P. Epigenetics of the antibody response. Trends in Immunology. 2013;34:460–470. doi: 10.1016/j.it.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Li T, Pignon JC, Wang B, Wang J, Shukla SA, Dou R, Chen Q, Hodi FS, Choueiri TK, Wu C, Hacohen N, Signoretti S, Liu JS, Liu XS. Landscape of tumor-infiltrating T cell repertoire of human cancers. Nature Genetics. 2016a;48:725–732. doi: 10.1038/ng.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J, Jiang P, Shen H, Aster JC, Rodig S, Signoretti S, Liu JS, Liu XS. Comprehensive analyses of tumor immunity: implications for Cancer immunotherapy. Genome Biology. 2016b;17:174. doi: 10.1186/s13059-016-1028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, van der Leun AM, Yofe I, Lubling Y, Gelbard-Solodkin D, van Akkooi ACJ, van den Braber M, Rozeman EA, Haanen J, Blank CU, Horlings HM, David E, Baran Y, Bercovich A, Lifshitz A, Schumacher TN, Tanay A, Amit I. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell. 2019;176:775–789. doi: 10.1016/j.cell.2018.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liarski VM, Sibley A, van Panhuys N, Ai J, Chang A, Kennedy D, Merolle M, Germain RN, Giger ML, Clark MR. Quantifying in situ adaptive immune cell cognate interactions in humans. Nature Immunology. 2019;20:503–513. doi: 10.1038/s41590-019-0315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau B, Tan B, Teo G, Zhang P, Choo A, Rudd PM. Shotgun glycomics identifies Tumor-Associated glycan ligands bound by an ovarian Carcinoma-Specific monoclonal antibody. Scientific Reports. 2017;7:14489. doi: 10.1038/s41598-017-15123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, Sandstrom R, Bernstein B, Bender MA, Groudine M, Gnirke A, Stamatoyannopoulos J, Mirny LA, Lander ES, Dekker J. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson JD, Engleman EG. Role of CD4 in normal immunity and HIV infection. Immunological Reviews. 1989;109:93–117. doi: 10.1111/j.1600-065X.1989.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Lin CY, Lovén J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JR, Fallahi-Sichani M, Sorger PK. Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method. Nature Communications. 2015;6:8390. doi: 10.1038/ncomms9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling JQ, Li T, Hu JF, Vu TH, Chen HL, Qiu XW, Cherry AM, Hoffman AR. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science. 2006;312:269–272. doi: 10.1126/science.1123191. [DOI] [PubMed] [Google Scholar]
- Linnemann C, Heemskerk B, Kvistborg P, Kluin RJ, Bolotin DA, Chen X, Bresser K, Nieuwland M, Schotte R, Michels S, Gomez-Eerland R, Jahn L, Hombrink P, Legrand N, Shu CJ, Mamedov IZ, Velds A, Blank CU, Haanen JB, Turchaninova MA, Kerkhoven RM, Spits H, Hadrup SR, Heemskerk MH, Blankenstein T, Chudakov DM, Bendle GM, Schumacher TN. High-throughput identification of antigen-specific TCRs by TCR gene capture. Nature Medicine. 2013;19:1534–1541. doi: 10.1038/nm.3359. [DOI] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, Edsall L, Antosiewicz-Bourget J, Stewart R, Ruotti V, Millar AH, Thomson JA, Ren B, Ecker JR. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Gerner MY, Van Panhuys N, Levine AG, Rudensky AY, Germain RN. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature. 2015;528:225–230. doi: 10.1038/nature16169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhu Y, Lin LW, Ding SK, Lin XC, Zhong KL, Pan K, Dai Y. The composition and variation of the BCR CDR3s in gastric cancer. Oncology Letters. 2018;16:239–246. doi: 10.3892/ol.2018.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang Y, Lu H, Li J, Yan X, Xiao M, Hao J, Alekseev A, Khong H, Chen T, Huang R, Wu J, Zhao Q, Wu Q, Xu S, Wang X, Jin W, Yu S, Wang Y, Wei L, Wang A, Zhong B, Ni L, Liu X, Nurieva R, Ye L, Tian Q, Bian XW, Dong C. Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature. 2019;567:525–529. doi: 10.1038/s41586-019-0979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Mardis ER. Applications of immunogenomics to Cancer. Cell. 2017;168:600–612. doi: 10.1016/j.cell.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SA, Thorpe J, DeBerg HA, Gersuk V, Eddy J, Harris KM, Ehlers M, Herold KC, Nepom GT, Linsley PS. Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes. Science Immunology. 2016;1:eaai7793. doi: 10.1126/sciimmunol.aai7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubeck E, Coskun AF, Zhiyentayev T, Ahmad M, Cai L. Single-cell in situ RNA profiling by sequential hybridization. Nature Methods. 2014;11:360–361. doi: 10.1038/nmeth.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubeck E, Cai L. Single-cell systems biology by super-resolution imaging and combinatorial labeling. Nature Methods. 2012;9:743–748. doi: 10.1038/nmeth.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łuksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A, Rizvi NA, Merghoub T, Levine AJ, Chan TA, Wolchok JD, Greenbaum BD. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature. 2017;551:517–520. doi: 10.1038/nature24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Reports. 2012;1:191–199. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Lund AW, Wagner M, Fankhauser M, Steinskog ES, Broggi MA, Spranger S, Gajewski TF, Alitalo K, Eikesdal HP, Wiig H, Swartz MA. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. Journal of Clinical Investigation. 2016;126:3389–3402. doi: 10.1172/JCI79434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundegaard C, Lund O, Nielsen M. Accurate approximation method for prediction of class I MHC affinities for peptides of length 8, 10 and 11 using prediction tools trained on 9mers. Bioinformatics. 2008;24:1397–1398. doi: 10.1093/bioinformatics/btn128. [DOI] [PubMed] [Google Scholar]
- Ma C, Fan R, Ahmad H, Shi Q, Comin-Anduix B, Chodon T, Koya RC, Liu CC, Kwong GA, Radu CG, Ribas A, Heath JR. A clinical microchip for evaluation of single immune cells reveals high functional heterogeneity in phenotypically similar T cells. Nature Medicine. 2011;17:738–743. doi: 10.1038/nm.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaten L, Hinton G. Visualizing data using t-SNE. Journal of Machine Learning Research. 2008;9:2579–2605. [Google Scholar]
- Machado D, Costa RS, Rocha M, Ferreira EC, Tidor B, Rocha I. Modeling formalisms in systems biology. AMB Express. 2011;1:45. doi: 10.1186/2191-0855-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA. Highly parallel Genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai Z, Liu H. Boolean network-based analysis of the apoptosis network: irreversible apoptosis and stable surviving. Journal of Theoretical Biology. 2009;259:760–769. doi: 10.1016/j.jtbi.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in Cancer treatment. American Journal of Translational Research. 2014;6:114–118. [PMC free article] [PubMed] [Google Scholar]
- Makridakis M, Vlahou A. Secretome proteomics for discovery of cancer biomarkers. Journal of Proteomics. 2010;73:2291–2305. doi: 10.1016/j.jprot.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Mallet DG, De Pillis LG. A cellular automata model of tumor-immune system interactions. Journal of Theoretical Biology. 2006;239:334–350. doi: 10.1016/j.jtbi.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Malmberg KJ, Carlsten M, Björklund A, Sohlberg E, Bryceson YT, Ljunggren HG. Natural killer cell-mediated immunosurveillance of human Cancer. Seminars in Immunology. 2017;31:20–29. doi: 10.1016/j.smim.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Mandelboim O, Berke G, Fridkin M, Feldman M, Eisenstein M, Eisenbach L. CTL induction by a tumour-associated antigen octapeptide derived from a murine lung carcinoma. Nature. 1994;369:67–71. doi: 10.1038/369067a0. [DOI] [PubMed] [Google Scholar]
- Mandelboim O, Vadai E, Fridkin M, Katz-Hillel A, Feldman M, Berke G, Eisenbach L. Regression of established murine carcinoma metastases following vaccination with tumour-associated antigen peptides. Nature Medicine. 1995;1:1179–1183. doi: 10.1038/nm1195-1179. [DOI] [PubMed] [Google Scholar]
- Mandruzzato S, Brandau S, Britten CM, Bronte V, Damuzzo V, Gouttefangeas C, Maurer D, Ottensmeier C, van der Burg SH, Welters MJP, Walter S. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunology, Immunotherapy. 2016;65:161–169. doi: 10.1007/s00262-015-1782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Marcus A, Gowen BG, Thompson TW, Iannello A, Ardolino M, Deng W, Wang L, Shifrin N, Raulet DH. Recognition of tumors by the innate immune system and natural killer cells. Advances in Immunology. 2014;122:91–128. doi: 10.1016/B978-0-12-800267-4.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Reports. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materi W, Wishart DS. Computational systems biology in Cancer: modeling methods and applications. Gene Regulation and Systems Biology. 2007;1:117762500700100. doi: 10.1177/117762500700100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen YS, Shea LK, Hundal J, Wendl MC, Demeter R, Wylie T, Allison JP, Smyth MJ, Old LJ, Mardis ER, Schreiber RD. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzavinos A, Chaplain MA, Kuznetsov VA. Mathematical modelling of the spatio-temporal response of cytotoxic T-lymphocytes to a solid tumour. Mathematical Medicine and Biology. 2004;21:1–34. doi: 10.1093/imammb/21.1.1. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic Bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TB, Shafi S, Murugaesu N, Mitter R, Akarca AU, Linares J, Marafioti T, Henry JY, Van Allen EM, Miao D, Schilling B, Schadendorf D, Garraway LA, Makarov V, Rizvi NA, Snyder A, Hellmann MD, Merghoub T, Wolchok JD, Shukla SA, Wu CJ, Peggs KS, Chan TA, Hadrup SR, Quezada SA, Swanton C. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes L, Healy J, Melville J. Umap: uniform manifold approximation and projection for dimension reduction. arXiv. 2018 https://arxiv.org/abs/1802.03426
- McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and Cancer. Annual Review of Immunology. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- Meissner A, Gnirke A, Bell GW, Ramsahoye B, Lander ES, Jaenisch R. Reduced representation bisulfite sequencing for comparative high-resolution DNA methylation analysis. Nucleic Acids Research. 2005;33:5868–5877. doi: 10.1093/nar/gki901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner F, Scheltema RA, Mollenkopf HJ, Mann M. Direct proteomic quantification of the secretome of activated immune cells. Science. 2013;340:475–478. doi: 10.1126/science.1232578. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nature Immunology. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annual Review of Immunology. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertins P, Mani DR, Ruggles KV, Gillette MA, Clauser KR, Wang P, Wang X, Qiao JW, Cao S, Petralia F, Kawaler E, Mundt F, Krug K, Tu Z, Lei JT, Gatza ML, Wilkerson M, Perou CM, Yellapantula V, Huang KL, Lin C, McLellan MD, Yan P, Davies SR, Townsend RR, Skates SJ, Wang J, Zhang B, Kinsinger CR, Mesri M, Rodriguez H, Ding L, Paulovich AG, Fenyö D, Ellis MJ, Carr SA, NCI CPTAC Proteogenomics connects somatic mutations to signalling in breast Cancer. Nature. 2016;534:55–62. doi: 10.1038/nature18003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. The Journal of Immunology. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Meyer AS, Heiser LM. Systems biology approaches to measure and model phenotypic heterogeneity in cancer. Current Opinion in Systems Biology. 2019;17:35–40. doi: 10.1016/j.coisb.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, Nowak MA. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–1270. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- Mielle J, Audo R, Hahne M, Macia L, Combe B, Morel J, Daien C. IL-10 producing B cells ability to induce regulatory T cells is maintained in rheumatoid arthritis. Frontiers in Immunology. 2018;9:961. doi: 10.3389/fimmu.2018.00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. The Journal of Immunology. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- Mistry AM, Greenplate AR, Ihrie RA, Irish JM. Beyond the message: advantages of snapshot proteomics with single-cell mass cytometry in solid tumors. The FEBS Journal. 2019;286:1523–1539. doi: 10.1111/febs.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D, Chintala S, Dey M. Plasmacytoid dendritic cell in immunity and cancer. Journal of Neuroimmunology. 2018;322:63–73. doi: 10.1016/j.jneuroim.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Montfort A, Pearce O, Maniati E, Vincent BG, Bixby L, Böhm S, Dowe T, Wilkes EH, Chakravarty P, Thompson R, Topping J, Cutillas PR, Lockley M, Serody JS, Capasso M, Balkwill FR. A strong B-cell response is part of the immune landscape in human High-Grade serous ovarian metastases. Clinical Cancer Research. 2017;23:250–262. doi: 10.1158/1078-0432.CCR-16-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A, Bottino C, Pende D, Tripodi G, Tambussi G, Viale O, Orengo A, Barbaresi M, Merli A, Ciccone E. Identification of four subsets of human CD3-CD16+ natural killer (NK) cells by the expression of clonally distributed functional surface molecules: correlation between subset assignment of NK clones and ability to mediate specific alloantigen recognition. The Journal of Experimental Medicine. 1990;172:1589–1598. doi: 10.1084/jem.172.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mose LE, Selitsky SR, Bixby LM, Marron DL, Iglesia MD, Serody JS, Perou CM, Vincent BG, Parker JS. Assembly-based inference of B-cell receptor repertoires from short read RNA sequencing data with V'DJer. Bioinformatics. 2016;32:3729–3734. doi: 10.1093/bioinformatics/btw526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature Reviews Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S, Kohanbash G, Liu SJ, Alvarado B, Carrera D, Bhaduri A, Watchmaker PB, Yagnik G, Di Lullo E, Malatesta M, Amankulor NM, Kriegstein AR, Lim DA, Aghi M, Okada H, Diaz A. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biology. 2017;18:234. doi: 10.1186/s13059-017-1362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumbach MR, Rubin AJ, Flynn RA, Dai C, Khavari PA, Greenleaf WJ, Chang HY. HiChIP: efficient and sensitive analysis of protein-directed genome architecture. Nature Methods. 2016;13:919–922. doi: 10.1038/nmeth.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumbach MR, Satpathy AT, Boyle EA, Dai C, Gowen BG, Cho SW, Nguyen ML, Rubin AJ, Granja JM, Kazane KR, Wei Y, Nguyen T, Greenside PG, Corces MR, Tycko J, Simeonov DR, Suliman N, Li R, Xu J, Flynn RA, Kundaje A, Khavari PA, Marson A, Corn JE, Quertermous T, Greenleaf WJ, Chang HY. Enhancer connectome in primary human cells identifies target genes of disease-associated DNA elements. Nature Genetics. 2017;49:1602–1612. doi: 10.1038/ng.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TL, Tussiwand R, Murphy KM. Specificity through cooperation: batf-irf interactions control immune-regulatory networks. Nature Reviews Immunology. 2013;13:499–509. doi: 10.1038/nri3470. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature Reviews Immunology. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa SA, Hoheisel JD, Alhamdani MS. Secretome profiling with antibody microarrays. Molecular BioSystems. 2011;7:1795–1801. doi: 10.1039/c1mb05071k. [DOI] [PubMed] [Google Scholar]
- Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Molecular & Cellular Proteomics : MCP. 2012;11:M111.014647. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Lubling Y, Stevens TJ, Schoenfelder S, Yaffe E, Dean W, Laue ED, Tanay A, Fraser P. Single-cell Hi-C reveals cell-to-cell variability in chromosome structure. Nature. 2013;502:59–64. doi: 10.1038/nature12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O'Keeffe M, Bahlo M, Papenfuss A, Kwak JY, Wu L, Shortman K. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nature Immunology. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- Nature Biotechnology What happened to personalized medicine? Nature Biotechnology. 2012;30:1. doi: 10.1038/nbt.2096. [DOI] [PubMed] [Google Scholar]
- Navin N, Kendall J, Troge J, Andrews P, Rodgers L, McIndoo J, Cook K, Stepansky A, Levy D, Esposito D, Muthuswamy L, Krasnitz A, McCombie WR, Hicks J, Wigler M. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EW, Sigal N, Nair N, Kidd BA, Greenberg HB, Davis MM. Combinatorial tetramer staining and mass cytometry analysis facilitate T-cell epitope mapping and characterization. Nature Biotechnology. 2013;31:623–629. doi: 10.1038/nbt.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EW, Davis MM. Beyond model antigens: high-dimensional methods for the analysis of antigen-specific T cells. Nature Biotechnology. 2014;32:149–157. doi: 10.1038/nbt.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nature Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, Khodadoust MS, Esfahani MS, Luca BA, Steiner D, Diehn M, Alizadeh AA. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nature Biotechnology. 2019;37:773–782. doi: 10.1038/s41587-019-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends in Immunology. 2012;33:297–305. doi: 10.1016/j.it.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA. PD-1 blockade with pembrolizumab in advanced Merkel-Cell carcinoma. New England Journal of Medicine. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. The Journal of Immunology. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nature Reviews Drug Discovery. 2002;1:153–161. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Lindon JC, Lindon, Metabonomics JC. Metabonomics. Nature. 2008;455:1054–1056. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, Zhao K, Levens D. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 2012;151:68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nature Reviews Clinical Oncology. 2017;14:655–668. doi: 10.1038/nrclinonc.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrup DL, Zhao K. Application of ChIP-Seq and related techniques to the study of immune function. Immunity. 2011;34:830–842. doi: 10.1016/j.immuni.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton KA, Gong C, Jamalian S, Popel AS. Multiscale Agent-Based and hybrid modeling of the tumor immune microenvironment. Processes. 2019;7:37. doi: 10.3390/pr7010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton L, Simon R. Growth curve of an experimental solid tumor following radiotherapy. JNCI: Journal of the National Cancer Institute. 1977;58:1735–1741. doi: 10.1093/jnci/58.6.1735. [DOI] [PubMed] [Google Scholar]
- Nowak MA, Sigmund K. Evolutionary dynamics of biological games. Science. 2004;303:793–799. doi: 10.1126/science.1093411. [DOI] [PubMed] [Google Scholar]
- Nowicki TS, Berent-Maoz B, Cheung-Lau G, Huang RR, Wang X, Tsoi J, Kaplan-Lefko P, Cabrera P, Tran J, Pang J, Macabali M, Garcilazo IP, Carretero IB, Kalbasi A, Cochran AJ, Grasso CS, Hu-Lieskovan S, Chmielowski B, Comin-Anduix B, Singh A, Ribas A. A pilot trial of the combination of transgenic NY-ESO-1-reactive adoptive cellular therapy with dendritic cell vaccination with or without ipilimumab. Clinical Cancer Research. 2019;25:2096–2108. doi: 10.1158/1078-0432.CCR-18-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. PNAS. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhus JK, Wolford CC, Friece CR, Nelson MB, Sampsel JW, Barbera-Guillem E. IgG-recognizing shed tumor-associated antigens can promote tumor invasion and metastasis. Cancer Immunology, Immunotherapy. 2001;50:361–372. doi: 10.1007/s002620100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell GM, Foe VE. An agent-based model contrasts opposite effects of dynamic and stable microtubules on cleavage furrow positioning. Journal of Cell Biology. 2008;183:471–483. doi: 10.1083/jcb.200807129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, DeNicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic Cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast Cancer metastasis by converting resting CD4⁺ T cells to T-regulatory cells. Cancer Research. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson B, Li Y, Lin Y, Liu ET, Patnaik A. Mouse models for Cancer immunotherapy research. Cancer Discovery. 2018;8:1358–1365. doi: 10.1158/2159-8290.CD-18-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nature Immunology. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MR, Sherratt JA. Modelling the macrophage invasion of tumours: effects on growth and composition. Mathematical Medicine and Biology. 1998;15:165–185. doi: 10.1093/imammb/15.2.165. [DOI] [PubMed] [Google Scholar]
- Ozik J, Collier N, Wozniak JM, Macal C, Cockrell C, Friedman SH, Ghaffarizadeh A, Heiland R, An G, Macklin P. High-throughput cancer hypothesis testing with an integrated PhysiCell-EMEWS workflow. BMC Bioinformatics. 2018;19:483. doi: 10.1186/s12859-018-2510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco JM, Santos FC, Dingli D. The ecology of cancer from an evolutionary game theory perspective. Interface Focus. 2014;4:20140019. doi: 10.1098/rsfs.2014.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page DB, Yuan J, Redmond D, Wen YH, Durack JC, Emerson R, Solomon S, Dong Z, Wong P, Comstock C, Diab A, Sung J, Maybody M, Morris E, Brogi E, Morrow M, Sacchini V, Elemento O, Robins H, Patil S, Allison JP, Wolchok JD, Hudis C, Norton L, McArthur HL. Deep sequencing of T-cell receptor DNA as a biomarker of clonally expanded TILs in breast Cancer after immunotherapy. Cancer Immunology Research. 2016;4:835–844. doi: 10.1158/2326-6066.CIR-16-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, Wherry EJ. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappalardo F, Martinez Forero I, Pennisi M, Palazon A, Melero I, Motta S. SimB16: modeling induced immune system response against B16-melanoma. PLOS ONE. 2011;6:e26523. doi: 10.1371/journal.pone.0026523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DS, Robertson-Tessi M, Luddy KA, Maini PK, Bonsall MB, Gatenby RA, Anderson ARA. The goldilocks window of personalized chemotherapy: getting the immune response just right. Cancer Research. 2019;79:5302–5315. doi: 10.1158/0008-5472.CAN-18-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst MR, Robbins PF, Tran E, Prickett TD, Gartner JJ, Jia L, Ivey G, Li YF, El-Gamil M, Lalani A, Crystal JS, Sachs A, Groh E, Ray S, Ngo LT, Kivitz S, Pasetto A, Yossef R, Lowery FJ, Goff SL, Lo W, Cafri G, Deniger DC, Malekzadeh P, Ahmadzadeh M, Wunderlich JR, Somerville RPT, Rosenberg SA. Unique neoantigens arise from somatic mutations in patients with gastrointestinal cancers. Cancer Discovery. 2019;9:1022–1035. doi: 10.1158/2159-8290.CD-18-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas O, Jovanovic M, Eisenhaure TM, Herbst RH, Dixit A, Ye CJ, Przybylski D, Platt RJ, Tirosh I, Sanjana NE, Shalem O, Satija R, Raychowdhury R, Mertins P, Carr SA, Zhang F, Hacohen N, Regev A. A Genome-wide CRISPR screen in primary immune cells to dissect regulatory networks. Cell. 2015;162:675–686. doi: 10.1016/j.cell.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, Barnitz RA, Bartman C, Bengsch B, Huang AC, Schenkel JM, Vahedi G, Haining WN, Berger SL, Wherry EJ. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends in Immunology. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson K. LIII. On lines and planes of closest fit to systems of points in space. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 1901;2:559–572. doi: 10.1080/14786440109462720. [DOI] [Google Scholar]
- Pech MF, Fong LE, Villalta JE, Chan LJ, Kharbanda S, O'Brien JJ, McAllister FE, Firestone AJ, Jan CH, Settleman J. Systematic identification of cancer cell vulnerabilities to natural killer cell-mediated immune surveillance. eLife. 2019;8:e47362. doi: 10.7554/eLife.47362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W, Kotarski J, Tarkowski R, Dou Y, Cho K, Hensley-Alford S, Munkarah A, Liu R, Zou W. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C, Gimenez-Xavier P, Pros E, Pajares MJ, Moro M, Gomez A, Navarro A, Condom E, Moran S, Gomez-Lopez G, Graña O, Rubio-Camarillo M, Martinez-Martí A, Yokota J, Carretero J, Galbis JM, Nadal E, Pisano D, Sozzi G, Felip E, Montuenga LM, Roz L, Villanueva A, Sanchez-Cespedes M. Genomic profiling of Patient-Derived xenografts for lung Cancer identifies B2M Inactivation Impairing Immunorecognition. Clinical Cancer Research. 2017;23:3203–3213. doi: 10.1158/1078-0432.CCR-16-1946-T. [DOI] [PubMed] [Google Scholar]
- Perros F, Dorfmüller P, Montani D, Hammad H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S, Cohen-Kaminsky S, Humbert M, Lambrecht BN. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2012;185:311–321. doi: 10.1164/rccm.201105-0927OC. [DOI] [PubMed] [Google Scholar]
- Petersen C, Bell R, Klag KA, Lee SH, Soto R, Ghazaryan A, Buhrke K, Ekiz HA, Ost KS, Boudina S, O'Connell RM, Cox JE, Villanueva CJ, Stephens WZ, Round JL. T cell-mediated regulation of the Microbiota protects against obesity. Science. 2019;365:eaat9351. doi: 10.1126/science.aat9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri CA. Kommunikation Mit Automaten. Mathematisches Institut der Universität Bonn; 1962. [Google Scholar]
- Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, Scott AC, Viale A, Lauer P, Merghoub T, Hellmann MD, Wolchok JD, Leslie CS, Schietinger A. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545:452–456. doi: 10.1038/nature22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip M, Schietinger A. Heterogeneity and fate choice: t cell exhaustion in Cancer and chronic infections. Current Opinion in Immunology. 2019;58:98–103. doi: 10.1016/j.coi.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Critical Reviews in Oncology/Hematology. 2012;82:296–309. doi: 10.1016/j.critrevonc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Piccolo V, Curina A, Genua M, Ghisletti S, Simonatto M, Sabò A, Amati B, Ostuni R, Natoli G. Opposing macrophage polarization programs show extensive epigenomic and transcriptional cross-talk. Nature Immunology. 2017;18:530–540. doi: 10.1038/ni.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nature Reviews Immunology. 2014;14:447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- Pogson M, Holcombe M, Smallwood R, Qwarnstrom E. Introducing spatial information into predictive NF-kappaB modelling--an agent-based approach. PLOS ONE. 2008;3:e2367. doi: 10.1371/journal.pone.0002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleszczuk J, Macklin P, Enderling H. Agent-Based modeling of Cancer stem cell driven solid tumor growth. Methods in Molecular Biology. 2016;1516:335–346. doi: 10.1007/7651_2016_346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. New England Journal of Medicine. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourhasanzade F, Sabzpoushan SH, Alizadeh AM, Esmati E. An agent-based model of avascular tumor growth: immune response tendency to prevent Cancer development. Simulation. 2017;93:641–657. doi: 10.1177/0037549717699072. [DOI] [Google Scholar]
- Poussin C, Mathis C, Alexopoulos LG, Messinis DE, Dulize RH, Belcastro V, Melas IN, Sakellaropoulos T, Rhrissorrakrai K, Bilal E, Meyer P, Talikka M, Boué S, Norel R, Rice JJ, Stolovitzky G, Ivanov NV, Peitsch MC, Hoeng J. The species translation challenge-a systems biology perspective on human and rat bronchial epithelial cells. Scientific Data. 2014;1:140009. doi: 10.1038/sdata.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran S. Dirichlet process mixture model for correcting technical variation insingle-cell gene expression data. International Conference on Machine Learning; 2016. [PMC free article] [PubMed] [Google Scholar]
- Pritchard AL, Hastie ML, Neller M, Gorman JJ, Schmidt CW, Hayward NK. Exploration of peptides bound to MHC class I molecules in melanoma. Pigment Cell & Melanoma Research. 2015;28:281–294. doi: 10.1111/pcmr.12357. [DOI] [PubMed] [Google Scholar]
- Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, Alvarez D, Sprachman M, Evavold C, Magnuson A, von Andrian UH, Glatz K, Breakefield XO, Mempel TR, Weissleder R, Pittet MJ. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science. 2016;352:242–246. doi: 10.1126/science.aaf1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S, Rodman C, Luo CL, Mroz EA, Emerick KS, Deschler DG, Varvares MA, Mylvaganam R, Rozenblatt-Rosen O, Rocco JW, Faquin WC, Lin DT, Regev A, Bernstein BE. Single-Cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck Cancer. Cell. 2017;171:1611–1624. doi: 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Richter G, Schüler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nature Medicine. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- Qiu P, Simonds EF, Bendall SC, Gibbs KD, Bruggner RV, Linderman MD, Sachs K, Nolan GP, Plevritis SK. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nature Biotechnology. 2011;29:886–891. doi: 10.1038/nbt.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Hong R, Zhuang Z, Li Y, Zhu L, Lin X, Zheng Q, Liu S, Zhang K, Huang M, Lee K, Lu Q, Xia W, Xu F, Wang X, Tang J, Xiao X, Wei W, Yuan Z, Shi Y, Hou Y, Zhang X, Wang J, Yang H, Zhan Q, Li B, Wang S. A Single-Cell immune atlas of triple negative breast Cancer reveals novel immune cell subsets. bioRxiv. 2019 doi: 10.1101/566968. [DOI]
- Quon G, Haider S, Deshwar AG, Cui A, Boutros PC, Morris Q. Computational purification of individual tumor gene expression profiles leads to significant improvements in prognostic prediction. Genome Medicine. 2013;5:29. doi: 10.1186/gm433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racle J, de Jonge K, Baumgaertner P, Speiser DE, Gfeller D. Simultaneous enumeration of cancer and immune cell types from bulk tumor gene expression data. eLife. 2017;6:e26476. doi: 10.7554/eLife.26476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasagi M, Shukla SA, Fritsch EF, Keskin DB, DeLuca D, Carmona E, Zhang W, Sougnez C, Cibulskis K, Sidney J, Stevenson K, Ritz J, Neuberg D, Brusic V, Gabriel S, Lander ES, Getz G, Hacohen N, Wu CJ. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014;124:453–462. doi: 10.1182/blood-2014-04-567933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani V, Deng X, Qiu R, Gunderson KL, Steemers FJ, Disteche CM, Noble WS, Duan Z, Shendure J. Massively multiplex single-cell Hi-C. Nature Methods. 2017;14:263–266. doi: 10.1038/nmeth.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsköld D, Luo S, Wang YC, Li R, Deng Q, Faridani OR, Daniels GA, Khrebtukova I, Loring JF, Laurent LC, Schroth GP, Sandberg R. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nature Biotechnology. 2012;30:777–782. doi: 10.1038/nbt.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES, Aiden EL. A 3D map of the human genome at Kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, Krause M, Schwörer AM, Wagner U, Müller-Brüsselbach S, Müller R. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. International Journal of Cancer. 2014;134:32–42. doi: 10.1002/ijc.28335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner K, Singer K, Koehl GE, Geissler EK, Peter K, Siska PJ, Kreutz M. Metabolic hallmarks of tumor and immune cells in the tumor microenvironment. Frontiers in Immunology. 2017;8:248. doi: 10.3389/fimmu.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature Reviews Immunology. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reticker-Flynn NE, Zhang W, Basto PA, Bejnood A, Martins MM, Kenkel JA, Linde IL, Bagchi S, Yuan R, Cheng J, Tolentino LL, Choi O, Wu N, Chang S, Kong C, Gentles AJ, Sunwoo JB, Plevritis SK, Engleman EG. Lymph node colonization alters the systemic immune response to enable metastasis to distant tissues. SSRN Electronic Journal. 2020 doi: 10.2139/ssrn.3624427. [DOI]
- Reticker-Flynn NE, Bhatia SN. Aberrant glycosylation promotes lung Cancer metastasis through adhesion to galectins in the metastatic niche. Cancer Discovery. 2015;5:168–181. doi: 10.1158/2159-8290.CD-13-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reticker-Flynn NE, Engleman EG. A gut punch fights cancer and infection. Nature. 2019;565:573–574. doi: 10.1038/d41586-019-00133-w. [DOI] [PubMed] [Google Scholar]
- Rhrissorrakrai K, Belcastro V, Bilal E, Norel R, Poussin C, Mathis C, Dulize RH, Ivanov NV, Alexopoulos L, Rice JJ, Peitsch MC, Stolovitzky G, Meyer P, Hoeng J. Understanding the limits of animal models as predictors of human biology: lessons learned from the sbv IMPROVER species translation challenge. Bioinformatics. 2015;31:471–483. doi: 10.1093/bioinformatics/btu611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DM, Hettinger J, Feuerer M. Monocytes and macrophages in Cancer: development and functions. Cancer Microenvironment. 2013;6:179–191. doi: 10.1007/s12307-012-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut Microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015a;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, Campos LT, Gandara DR, Levy BP, Nair SG, Zalcman G, Wolf J, Souquet PJ, Baldini E, Cappuzzo F, Chouaid C, Dowlati A, Sanborn R, Lopez-Chavez A, Grohe C, Huber RM, Harbison CT, Baudelet C, Lestini BJ, Ramalingam SS. Activity and safety of Nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. The Lancet Oncology. 2015b;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. The New England Journal of Medicine. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, Kaisho T, Bogunovic D, Bhardwaj N, Krummel MF. Critical role for CD103(+)/CD141(+) Dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell. 2016;30:324–336. doi: 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez RM, Suarez-Alvarez B, Lavín JL, Mosén-Ansorena D, Baragaño Raneros A, Márquez-Kisinousky L, Aransay AM, Lopez-Larrea C. Epigenetic networks regulate the transcriptional program in memory and terminally differentiated CD8+ T cells. The Journal of Immunology. 2017;198:937–949. doi: 10.4049/jimmunol.1601102. [DOI] [PubMed] [Google Scholar]
- Rohaan MW, van den Berg JH, Kvistborg P, Haanen J. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. Journal for ImmunoTherapy of Cancer. 2018;6:102. doi: 10.1186/s40425-018-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. New England Journal of Medicine. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nature Reviews Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Rotem A, Ram O, Shoresh N, Sperling RA, Goren A, Weitz DA, Bernstein BE. Single-cell ChIP-seq reveals cell subpopulations defined by chromatin state. Nature Biotechnology. 2015;33:1165–1172. doi: 10.1038/nbt.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- Russ BE, Olshanksy M, Smallwood HS, Li J, Denton AE, Prier JE, Stock AT, Croom HA, Cullen JG, Nguyen ML, Rowe S, Olson MR, Finkelstein DB, Kelso A, Thomas PG, Speed TP, Rao S, Turner SJ. Distinct epigenetic signatures delineate transcriptional programs during virus-specific CD8(+) T cell differentiation. Immunity. 2014;41:853–865. doi: 10.1016/j.immuni.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabò A, Kress TR, Pelizzola M, de Pretis S, Gorski MM, Tesi A, Morelli MJ, Bora P, Doni M, Verrecchia A, Tonelli C, Fagà G, Bianchi V, Ronchi A, Low D, Müller H, Guccione E, Campaner S, Amati B. Selective transcriptional regulation by myc in cellular growth control and lymphomagenesis. Nature. 2014;511:488–492. doi: 10.1038/nature13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann A, Heiner M, Koch I. Application of Petri net based analysis techniques to signal transduction pathways. BMC Bioinformatics. 2006;7:482. doi: 10.1186/1471-2105-7-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane J-P, Bjorgaard SL, Hammond MR, Vitzthum H, Blackmon SM, Frederick DT, Hazar-Rethinam M, Nadres BA, Van Seventer EE, Shukla SA, Yizhak K, Ray JP, Rosebrock D, Livitz D, Adalsteinsson V, Getz G, Duncan LM, Li B, Corcoran RB, Lawrence DP, Stemmer-Rachamimov A, Boland GM, Landau DA, Flaherty KT, Sullivan RJ, Hacohen N. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nature Communications. 2017;8:1136. doi: 10.1038/s41467-017-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, Lieb DJ, Chen JH, Frederick DT, Barzily-Rokni M, Freeman SS, Reuben A, Hoover PJ, Villani A-C, Ivanova E, Portell A, Lizotte PH, Aref AR, Eliane J-P, Hammond MR, Vitzthum H, Blackmon SM, Li B, Gopalakrishnan V, Reddy SM, Cooper ZA, Paweletz CP, Barbie DA, Stemmer-Rachamimov A, Flaherty KT, Wargo JA, Boland GM, Sullivan RJ, Getz G, Hacohen N. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell. 2018;175:998–1013. doi: 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Rodriguez J, Simeoni L, Lindquist JA, Hemenway R, Bommhardt U, Arndt B, Haus UU, Weismantel R, Gilles ED, Klamt S, Schraven B. A logical model provides insights into T cell receptor signaling. PLOS Computational Biology. 2007;3:e163. doi: 10.1371/journal.pcbi.0030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Müller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Brück AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Höller C, Utikal J, Huber C, Loquai C, Türeci Ö. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against Cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, Chakarov S, Rivera C, Hogstad B, Bosenberg M, Hashimoto D, Gnjatic S, Bhardwaj N, Palucka AK, Brown BD, Brody J, Ginhoux F, Merad M. Expansion and activation of CD103(+) Dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samusik N, Good Z, Spitzer MH, Davis KL, Nolan GP. Automated mapping of phenotype space with single-cell data. Nature Methods. 2016;13:493–496. doi: 10.1038/nmeth.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia S, Chakravarty D, Daian F, Gao Q, Bailey MH, Liang WW, Foltz SM, Shmulevich I, Ding L, Heins Z, Ochoa A, Gross B, Gao J, Zhang H, Kundra R, Kandoth C, Bahceci I, Dervishi L, Dogrusoz U, Zhou W, Shen H, Laird PW, Way GP, Greene CS, Liang H, Xiao Y, Wang C, Iavarone A, Berger AH, Bivona TG, Lazar AJ, Hammer GD, Giordano T, Kwong LN, McArthur G, Huang C, Tward AD, Frederick MJ, McCormick F, Meyerson M, Van Allen EM, Cherniack AD, Ciriello G, Sander C, Schultz N, Cancer Genome Atlas Research Network Oncogenic signaling pathways in the Cancer genome atlas. Cell. 2018;173:321–337. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnaik A, Khushalani NI, Chesney JA, Lewis KD, Medina TM, Kluger HM, Thomas SS, Domingo Musibay E, Pavlick AC, Whitman ED, Martin-Algarra S, Corrie PG, Lutzky J, Hamid O, Wu R, Shi W, Fardis M, Weber JS, Larkin JMG, Kirkwood JM. Long-term follow up of lifileucel (LN-144) cryopreserved autologous tumor infiltrating lymphocyte therapy in patients with advanced melanoma progressed on multiple prior therapies. Journal of Clinical Oncology. 2020;38:10006. doi: 10.1200/JCO.2020.38.15_suppl.10006. [DOI] [Google Scholar]
- Satomaa T, Heiskanen A, Leonardsson I, Angström J, Olonen A, Blomqvist M, Salovuori N, Haglund C, Teneberg S, Natunen J, Carpén O, Saarinen J. Analysis of the human Cancer glycome identifies a novel group of tumor-associated N-acetylglucosamine glycan antigens. Cancer Research. 2009;69:5811–5819. doi: 10.1158/0008-5472.CAN-08-0289. [DOI] [PubMed] [Google Scholar]
- Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nature Immunology. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy AT, Saligrama N, Buenrostro JD, Wei Y, Wu B, Rubin AJ, Granja JM, Lareau CA, Li R, Qi Y, Parker KR, Mumbach MR, Serratelli WS, Gennert DG, Schep AN, Corces MR, Khodadoust MS, Kim YH, Khavari PA, Greenleaf WJ, Davis MM, Chang HY. Transcript-indexed ATAC-seq for precision immune profiling. Nature Medicine. 2018;24:580–590. doi: 10.1038/s41591-018-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy AT, Granja JM, Yost KE, Qi Y, Meschi F, McDermott GP, Olsen BN, Mumbach MR, Pierce SE, Corces MR, Shah P, Bell JC, Jhutty D, Nemec CM, Wang J, Wang L, Yin Y, Giresi PG, Chang ALS, Zheng GXY, Greenleaf WJ, Chang HY. Massively parallel single-cell chromatin landscapes of human immune cell development and intratumoral T cell exhaustion. Nature Biotechnology. 2019;37:925–936. doi: 10.1038/s41587-019-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautès-Fridman C, Lawand M, Giraldo NA, Kaplon H, Germain C, Fridman WH, Dieu-Nosjean MC. Tertiary lymphoid structures in cancers: prognostic value, regulation, and manipulation for therapeutic intervention. Frontiers in Immunology. 2016;7:407. doi: 10.3389/fimmu.2016.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of Cancer immunotherapy. Nature Reviews Cancer. 2019;19:307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, Salgado R, Byrne DJ, Teo ZL, Dushyanthen S, Byrne A, Wein L, Luen SJ, Poliness C, Nightingale SS, Skandarajah AS, Gyorki DE, Thornton CM, Beavis PA, Fox SB, Darcy PK, Speed TP, Mackay LK, Neeson PJ, Loi S. Single-cell profiling of breast Cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nature Medicine. 2018;24:986–993. doi: 10.1038/s41591-018-0078-7. [DOI] [PubMed] [Google Scholar]
- Scharer CD, Bally AP, Gandham B, Boss JM. Cutting edge: chromatin accessibility programs CD8 T cell memory. The Journal of Immunology. 2017;198:2238–2243. doi: 10.4049/jimmunol.1602086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelker M, Feau S, Du J, Ranu N, Klipp E, MacBeath G, Schoeberl B, Raue A. Estimation of immune cell content in tumour tissue using single-cell RNA-seq data. Nature Communications. 2017;8:2032. doi: 10.1038/s41467-017-02289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper W, Kelderman S, Fanchi LF, Linnemann C, Bendle G, de Rooij MAJ, Hirt C, Mezzadra R, Slagter M, Dijkstra K, Kluin RJC, Snaebjornsson P, Milne K, Nelson BH, Zijlmans H, Kenter G, Voest EE, Haanen J, Schumacher TN. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nature Medicine. 2019;25:89–94. doi: 10.1038/s41591-018-0266-5. [DOI] [PubMed] [Google Scholar]
- Schietinger A, Philip M, Krisnawan VE, Chiu EY, Delrow JJ, Basom RS, Lauer P, Brockstedt DG, Knoblaugh SE, Hämmerling GJ, Schell TD, Garbi N, Greenberg PD. Tumor-Specific T cell dysfunction is a dynamic Antigen-Driven differentiation program initiated early during tumorigenesis. Immunity. 2016;45:389–401. doi: 10.1016/j.immuni.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends in Immunology. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM, Balkwill FR. B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. PNAS. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidl C, Delacher M, Huehn J, Feuerer M. Epigenetic mechanisms regulating T-cell responses. Journal of Allergy and Clinical Immunology. 2018;142:728–743. doi: 10.1016/j.jaci.2018.07.014. [DOI] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Schubert W, Bonnekoh B, Pommer AJ, Philipsen L, Böckelmann R, Malykh Y, Gollnick H, Friedenberger M, Bode M, Dress AW. Analyzing proteome topology and function by automated multidimensional fluorescence microscopy. Nature Biotechnology. 2006;24:1270–1278. doi: 10.1038/nbt1250. [DOI] [PubMed] [Google Scholar]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- Scott AC, Dündar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, Trivedi P, Menocal L, Appleby H, Camara S, Zamarin D, Walther T, Snyder A, Femia MR, Comen EA, Wen HY, Hellmann MD, Anandasabapathy N, Liu Y, Altorki NK, Lauer P, Levy O, Glickman MS, Kaye J, Betel D, Philip M, Schietinger A. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019;571:270–274. doi: 10.1038/s41586-019-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See P, Dutertre CA, Chen J, Günther P, McGovern N, Irac SE, Gunawan M, Beyer M, Händler K, Duan K, Sumatoh HRB, Ruffin N, Jouve M, Gea-Mallorquí E, Hennekam RCM, Lim T, Yip CC, Wen M, Malleret B, Low I, Shadan NB, Fen CFS, Tay A, Lum J, Zolezzi F, Larbi A, Poidinger M, Chan JKY, Chen Q, Rénia L, Haniffa M, Benaroch P, Schlitzer A, Schultze JL, Newell EW, Ginhoux F. Mapping the human DC lineage through the integration of high-dimensional techniques. Science. 2017;356:eaag3009. doi: 10.1126/science.aag3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiden PE, Celada F. A model for simulating cognate recognition and response in the immune system. Journal of Theoretical Biology. 1992;158:329–357. doi: 10.1016/S0022-5193(05)80737-4. [DOI] [PubMed] [Google Scholar]
- Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao HW, Godec J, LaFleur MW, Brown FD, Tonnerre P, Chung RT, Tully DC, Allen TM, Frahm N, Lauer GM, Wherry EJ, Yosef N, Haining WN. The epigenetic landscape of T cell exhaustion. Science. 2016;354:1165–1169. doi: 10.1126/science.aae0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H, Chen J, González-Avalos E, Samaniego-Castruita D, Das A, Wang YH, López-Moyado IF, Georges RO, Zhang W, Onodera A, Wu CJ, Lu LF, Hogan PG, Bhandoola A, Rao A. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. PNAS. 2019;116:12410–12415. doi: 10.1073/pnas.1905675116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nature Immunology. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Shafer MER. Cross-Species analysis of Single-Cell transcriptomic data. Frontiers in Cell and Developmental Biology. 2019;7:175. doi: 10.3389/fcell.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, Shin SU, Nechustan H, Challita-Eid PM, Segal BM, Yi KH, Rosenblatt JD. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. International Journal of Cancer. 2005;117:574–586. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- Shalek AK, Satija R, Adiconis X, Gertner RS, Gaublomme JT, Raychowdhury R, Schwartz S, Yosef N, Malboeuf C, Lu D, Trombetta JJ, Gennert D, Gnirke A, Goren A, Hacohen N, Levin JZ, Park H, Regev A. Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature. 2013;498:236–240. doi: 10.1038/nature12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalek AK, Benson M. Single-cell analyses to tailor treatments. Science Translational Medicine. 2017;9:eaan4730. doi: 10.1126/scitranslmed.aan4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F. Genome-Scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- Shay T, Kang J. Immunological Genome Project and systems immunology. Trends in Immunology. 2013;34:602–609. doi: 10.1016/j.it.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Orr SS, Tibshirani R, Khatri P, Bodian DL, Staedtler F, Perry NM, Hastie T, Sarwal MM, Davis MM, Butte AJ. Cell type-specific gene expression differences in complex tissues. Nature Methods. 2010;7:287–289. doi: 10.1038/nmeth.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Orr SS, Gaujoux R. Computational deconvolution: extracting cell type-specific information from heterogeneous samples. Current Opinion in Immunology. 2013;25:571–578. doi: 10.1016/j.coi.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrut E, Carnevale J, Tobin V, Roth TL, Woo JM, Bui CT, Li PJ, Diolaiti ME, Ashworth A, Marson A. Genome-wide CRISPR screens in primary human T cells reveal key regulators of immune function. Cell. 2018;175:1958–1971. doi: 10.1016/j.cell.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HY, Sciumè G, Mikami Y, Guo L, Sun HW, Brooks SR, Urban JF, Davis FP, Kanno Y, O'Shea JJ. Developmental acquisition of regulomes underlies innate lymphoid cell functionality. Cell. 2016;165:1120–1133. doi: 10.1016/j.cell.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin GV, Ma Y, Shurin MR. Immunosuppressive mechanisms of regulatory dendritic cells in Cancer. Cancer Microenvironment. 2013;6:159–167. doi: 10.1007/s12307-013-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, Duan K, Ang N, Poidinger M, Lee YY, Larbi A, Khng AJ, Tan E, Fu C, Mathew R, Teo M, Lim WT, Toh CK, Ong BH, Koh T, Hillmer AM, Takano A, Lim TKH, Tan EH, Zhai W, Tan DSW, Tan IB, Newell EW. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557:575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- Simonis M, Klous P, Splinter E, Moshkin Y, Willemsen R, de Wit E, van Steensel B, de Laat W. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C) Nature Genetics. 2006;38:1348–1354. doi: 10.1038/ng1896. [DOI] [PubMed] [Google Scholar]
- Sims JS, Grinshpun B, Feng Y, Ung TH, Neira JA, Samanamud JL, Canoll P, Shen Y, Sims PA, Bruce JN. Diversity and divergence of the glioma-infiltrating T-cell receptor repertoire. PNAS. 2016;113:E3529–E3537. doi: 10.1073/pnas.1601012113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal S, Bhojnagarwala PS, O'Brien S, Moon EK, Garfall AL, Rao AS, Quatromoni JG, Stephen TL, Litzky L, Deshpande C, Feldman MD, Hancock WW, Conejo-Garcia JR, Albelda SM, Eruslanov EB. Origin and role of a subset of Tumor-Associated neutrophils with Antigen-Presenting cell features in Early-Stage human lung Cancer. Cancer Cell. 2016;30:120–135. doi: 10.1016/j.ccell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisirak V, Faget J, Gobert M, Goutagny N, Vey N, Treilleux I, Renaudineau S, Poyet G, Labidi-Galy SI, Goddard-Leon S, Durand I, Le Mercier I, Bajard A, Bachelot T, Puisieux A, Puisieux I, Blay JY, Ménétrier-Caux C, Caux C, Bendriss-Vermare N. Impaired IFN-α production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast Cancer progression. Cancer Research. 2012;72:5188–5197. doi: 10.1158/0008-5472.CAN-11-3468. [DOI] [PubMed] [Google Scholar]
- Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene PJ, Henikoff S. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife. 2017;6:e21856. doi: 10.7554/eLife.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood SA, Lee HJ, Angermueller C, Krueger F, Saadeh H, Peat J, Andrews SR, Stegle O, Reik W, Kelsey G. Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nature Methods. 2014;11:817–820. doi: 10.1038/nmeth.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. New England Journal of Medicine. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soheilypour M, Mofrad MRK. Agent‐based modeling in molecular systems biology. BioEssays. 2018;40:1800020. doi: 10.1002/bies.201800020. [DOI] [PubMed] [Google Scholar]
- Song X, Lasanajak Y, Xia B, Heimburg-Molinaro J, Rhea JM, Ju H, Zhao C, Molinaro RJ, Cummings RD, Smith DF. Shotgun glycomics: a microarray strategy for functional glycomics. Nature Methods. 2011;8:85–90. doi: 10.1038/nmeth.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spektor R, Tippens ND, Mimoso CA, Soloway PD. methyl-ATAC-seq measures DNA methylation at accessible chromatin. Genome Research. 2019;29:969–977. doi: 10.1101/gr.245399.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer MH, Gherardini PF, Fragiadakis GK, Bhattacharya N, Yuan RT, Hotson AN, Finck R, Carmi Y, Zunder ER, Fantl WJ, Bendall SC, Engleman EG, Nolan GP. IMMUNOLOGY. An interactive reference framework for modeling a dynamic immune system. Science. 2015;349:1259425. doi: 10.1126/science.1259425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, Gherardini PF, Prestwood TR, Chabon J, Bendall SC, Fong L, Nolan GP, Engleman EG. Systemic immunity is required for effective Cancer immunotherapy. Cell. 2017;168:487–502. doi: 10.1016/j.cell.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in Cancer. Nature Reviews Cancer. 2014;14:159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- Stanková K, Brown JS, Dalton WS, Gatenby RA. Optimizing Cancer treatment using game theory: a review. JAMA Oncology. 2019;5:96–103. doi: 10.1001/jamaoncol.2018.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnes CO. Coley's toxins in perspective. Nature. 1992;357:11–12. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- Stegle O, Teichmann SA, Marioni JC. Computational and analytical challenges in single-cell transcriptomics. Nature Reviews Genetics. 2015;16:133–145. doi: 10.1038/nrg3833. [DOI] [PubMed] [Google Scholar]
- Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. The Journal of Experimental Medicine. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. morphology, quantitation, tissue distribution. The Journal of Experimental Medicine. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens TJ, Lando D, Basu S, Atkinson LP, Cao Y, Lee SF, Leeb M, Wohlfahrt KJ, Boucher W, O'Shaughnessy-Kirwan A, Cramard J, Faure AJ, Ralser M, Blanco E, Morey L, Sansó M, Palayret MGS, Lehner B, Di Croce L, Wutz A, Hendrich B, Klenerman D, Laue ED. 3d structures of individual mammalian genomes studied by single-cell Hi-C. Nature. 2017;544:59–64. doi: 10.1038/nature21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- Stoll G, Viara E, Barillot E, Calzone L. Continuous time boolean modeling for biological signaling: application of Gillespie algorithm. BMC Systems Biology. 2012;6:116. doi: 10.1186/1752-0509-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, Hao Y, Stoeckius M, Smibert P, Satija R. Comprehensive Integration of Single-Cell Data. Cell. 2019;177:1888–1902. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart T, Satija R. Integrative single-cell analysis. Nature Reviews Genetics. 2019;20:257–272. doi: 10.1038/s41576-019-0093-7. [DOI] [PubMed] [Google Scholar]
- Suhail Y, Cain MP, Vanaja K, Kurywchak PA, Levchenko A, Kalluri R, Kshitiz Systems Biology of Cancer Metastasis. Cell Systems. 2019;9:109–127. doi: 10.1016/j.cels.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Li T, Song X, Huang L, Zang Q, Xu J, Bi N, Jiao G, Hao Y, Chen Y, Zhang R, Luo Z, Li X, Wang L, Wang Z, Song Y, He J, Abliz Z. Spatially resolved metabolomics to discover tumor-associated metabolic alterations. PNAS. 2019;116:52–57. doi: 10.1073/pnas.1808950116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvà ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339:1567–1570. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadling L, Capone S, Antrobus RD, Brown A, Richardson R, Newell EW, Halliday J, Kelly C, Bowen D, Fergusson J, Kurioka A, Ammendola V, Del Sorbo M, Grazioli F, Esposito ML, Siani L, Traboni C, Hill A, Colloca S, Davis M, Nicosia A, Cortese R, Folgori A, Klenerman P, Barnes E. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Science Translational Medicine. 2014;6:261ra153. doi: 10.1126/scitranslmed.3009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan J, Boulgakov AA, Hernandez ET, Bardo AM, Bachman JL, Marotta J, Johnson AM, Anslyn EV, Marcotte EM. Highly parallel single-molecule identification of proteins in zeptomole-scale mixtures. Nature Biotechnology. 2018;36:1076–1082. doi: 10.1038/nbt.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MA. Immunomodulatory roles of lymphatic vessels in Cancer progression. Cancer Immunology Research. 2014;2:701–707. doi: 10.1158/2326-6066.CIR-14-0115. [DOI] [PubMed] [Google Scholar]
- Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, Beisel C, Kurzeder C, Heinzelmann-Schwarz V, Rochlitz C, Weber WP, Beerenwinkel N, Aceto N. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–557. doi: 10.1038/s41586-019-0915-y. [DOI] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Tan TT, Coussens LM. Humoral immunity, inflammation and Cancer. Current Opinion in Immunology. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, Lao K, Surani MA. mRNA-Seq whole-transcriptome analysis of a single cell. Nature Methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- Tang F, Barbacioru C, Nordman E, Li B, Xu N, Bashkirov VI, Lao K, Surani MA. RNA-Seq analysis to capture the transcriptome landscape of a single cell. Nature Protocols. 2010;5:516–535. doi: 10.1038/nprot.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, Shiota A, Takeshita K, Yasuma-Mitobe K, Riethmacher D, Kaisho T, Norman JM, Mucida D, Suematsu M, Yaguchi T, Bucci V, Inoue T, Kawakami Y, Olle B, Roberts B, Hattori M, Xavier RJ, Atarashi K, Honda K. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565:600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- Tariq MA, Kim HJ, Jejelowo O, Pourmand N. Whole-transcriptome RNAseq analysis from minute amount of total RNA. Nucleic Acids Research. 2011;39:e120. doi: 10.1093/nar/gkr547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas The Cancer genome atlas program. [November 15, 2019];2006 https://www.cancer.gov/tcga
- Thomas R. Boolean formalization of genetic control circuits. Journal of Theoretical Biology. 1973;42:563–585. doi: 10.1016/0022-5193(73)90247-6. [DOI] [PubMed] [Google Scholar]
- Thorsen T, Roberts RW, Arnold FH, Quake SR. Dynamic pattern formation in a vesicle-generating microfluidic device. Physical Review Letters. 2001;86:4163–4166. doi: 10.1103/PhysRevLett.86.4163. [DOI] [PubMed] [Google Scholar]
- Thorsen T, Maerkl SJ, Quake SR. Microfluidic large-scale integration. Science. 2002;298:580–584. doi: 10.1126/science.1076996. [DOI] [PubMed] [Google Scholar]
- Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, Ziv E, Culhane AC, Paull EO, Sivakumar IKA, Gentles AJ, Malhotra R, Farshidfar F, Colaprico A, Parker JS, Mose LE, Vo NS, Liu J, Liu Y, Rader J, Dhankani V, Reynolds SM, Bowlby R, Califano A, Cherniack AD, Anastassiou D, Bedognetti D, Mokrab Y, Newman AM, Rao A, Chen K, Krasnitz A, Hu H, Malta TM, Noushmehr H, Pedamallu CS, Bullman S, Ojesina AI, Lamb A, Zhou W, Shen H, Choueiri TK, Weinstein JN, Guinney J, Saltz J, Holt RA, Rabkin CS, Lazar AJ, Serody JS, Demicco EG, Disis ML, Vincent BG, Shmulevich I, Cancer Genome Atlas Research Network The immune landscape of Cancer. Immunity. 2018;48:812–830. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thul PJ, Åkesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, Alm T, Asplund A, Björk L, Breckels LM, Bäckström A, Danielsson F, Fagerberg L, Fall J, Gatto L, Gnann C, Hober S, Hjelmare M, Johansson F, Lee S, Lindskog C, Mulder J, Mulvey CM, Nilsson P, Oksvold P, Rockberg J, Schutten R, Schwenk JM, Sivertsson Å, Sjöstedt E, Skogs M, Stadler C, Sullivan DP, Tegel H, Winsnes C, Zhang C, Zwahlen M, Mardinoglu A, Pontén F, von Feilitzen K, Lilley KS, Uhlén M, Lundberg E. A subcellular map of the human proteome. Science. 2017;356:eaal3321. doi: 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, Sheffield NC, Stergachis AB, Wang H, Vernot B, Garg K, John S, Sandstrom R, Bates D, Boatman L, Canfield TK, Diegel M, Dunn D, Ebersol AK, Frum T, Giste E, Johnson AK, Johnson EM, Kutyavin T, Lajoie B, Lee BK, Lee K, London D, Lotakis D, Neph S, Neri F, Nguyen ED, Qu H, Reynolds AP, Roach V, Safi A, Sanchez ME, Sanyal A, Shafer A, Simon JM, Song L, Vong S, Weaver M, Yan Y, Zhang Z, Zhang Z, Lenhard B, Tewari M, Dorschner MO, Hansen RS, Navas PA, Stamatoyannopoulos G, Iyer VR, Lieb JD, Sunyaev SR, Akey JM, Sabo PJ, Kaul R, Furey TS, Dekker J, Crawford GE, Stamatoyannopoulos JA. The accessible chromatin landscape of the human genome. Nature. 2012;489:75–82. doi: 10.1038/nature11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice JD, Song H, Lyon AD, Ismagilov RF. Formation of droplets and mixing in multiphase microfluidics at low values of the Reynolds and the capillary numbers. Langmuir. 2003;19:9127–9133. doi: 10.1021/la030090w. [DOI] [Google Scholar]
- Tirosh I, Izar B, Prakadan SM, Wadsworth MH, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, Fallahi-Sichani M, Dutton-Regester K, Lin JR, Cohen O, Shah P, Lu D, Genshaft AS, Hughes TK, Ziegler CG, Kazer SW, Gaillard A, Kolb KE, Villani AC, Johannessen CM, Andreev AY, Van Allen EM, Bertagnolli M, Sorger PK, Sullivan RJ, Flaherty KT, Frederick DT, Jané-Valbuena J, Yoon CH, Rozenblatt-Rosen O, Shalek AK, Regev A, Garraway LA. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjalsma H, Bolhuis A, Jongbloed JD, Bron S, van Dijl JM. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiology and Molecular Biology Reviews. 2000;64:515–547. doi: 10.1128/MMBR.64.3.515-547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in Cancer. New England Journal of Medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosches MA, Yamawaki TM, Naumann RK, Jacobi AA, Tushev G, Laurent G. Evolution of pallium, Hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science. 2018;360:881–888. doi: 10.1126/science.aar4237. [DOI] [PubMed] [Google Scholar]
- Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, Parkhurst MR, Yang JC, Rosenberg SA. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial Cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, Kriley IR, Rosenberg SA. T-Cell transfer therapy targeting mutant KRAS in Cancer. New England Journal of Medicine. 2016;375:2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. Journal of Clinical Investigation. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa T, Kumar S, Borkar RN, Azimi V, Thibault G, Chang YH, Balter A, Kawashima R, Choe G, Sauer D, El Rassi E, Clayburgh DR, Kulesz-Martin MF, Lutz ER, Zheng L, Jaffee EM, Leyshock P, Margolin AA, Mori M, Gray JW, Flint PW, Coussens LM. Quantitative multiplex immunohistochemistry reveals Myeloid-Inflamed Tumor-Immune complexity associated with poor prognosis. Cell Reports. 2017;19:203–217. doi: 10.1016/j.celrep.2017.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turing AM. The chemical basis of morphogenesis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1952;237:37–72. doi: 10.1098/rstb.1952.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley SJ, Fletcher AL, Elpek KG. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nature Reviews Immunology. 2010;10:813–825. doi: 10.1038/nri2886. [DOI] [PubMed] [Google Scholar]
- Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science. 2000;288:113–116. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, Danilo M, Alfei F, Hofmann M, Wieland D, Pradervand S, Thimme R, Zehn D, Held W. T cell factor 1-Expressing Memory-like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity. 2016;45:415–427. doi: 10.1016/j.immuni.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Vahedi G, Kanno Y, Furumoto Y, Jiang K, Parker SC, Erdos MR, Davis SR, Roychoudhuri R, Restifo NP, Gadina M, Tang Z, Ruan Y, Collins FS, Sartorelli V, O'Shea JJ. Super-enhancers delineate disease-associated regulatory nodes in T cells. Nature. 2015;520:558–562. doi: 10.1038/nature14154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallania F, Tam A, Lofgren S, Schaffert S, Azad TD, Bongen E, Haynes W, Alsup M, Alonso M, Davis M, Engleman E, Khatri P. Leveraging heterogeneity across multiple datasets increases cell-mixture deconvolution accuracy and reduces biological and technical biases. Nature Communications. 2018;9:4735. doi: 10.1038/s41467-018-07242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink SC, Sage F, Vértesy Á, Spanjaard B, Peterson-Maduro J, Baron CS, Robin C, van Oudenaarden A. Single-cell sequencing reveals dissociation-induced gene expression in tissue subpopulations. Nature Methods. 2017;14:935–936. doi: 10.1038/nmeth.4437. [DOI] [PubMed] [Google Scholar]
- van Dijk D, Sharma R, Nainys J, Yim K, Kathail P, Carr AJ, Burdziak C, Moon KR, Chaffer CL, Pattabiraman D, Bierie B, Mazutis L, Wolf G, Krishnaswamy S, Pe'er D. Recovering gene interactions from Single-Cell data using data diffusion. Cell. 2018;174:716–729. doi: 10.1016/j.cell.2018.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gassen S. FlowSOM: using self-organizing maps for visualization and interpretation of cytometry data. Cytometry Part A. 2015;87:636–645. doi: 10.1002/cyto.a.22625. [DOI] [PubMed] [Google Scholar]
- van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. New England Journal of Medicine. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng ML, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers YH, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint NN, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn-Deen E, Wolfe K, Zaveri J, Zaveri K, Abril JF, Guigó R, Campbell MJ, Sjolander KV, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes-Stine J, Caulk P, Chiang YH, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A, Zhu X. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Vera J. A Translational Medicine Context Envisioning the Application of Systems Biology in Cancer Immunology. In: Rezaei N, editor. Cancer Immunology. Springer Berlin Heidelberg; 2015. pp. 429–449. [DOI] [Google Scholar]
- Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CPM, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Berard M, Nigou J, Opolon P, Eggermont A, Woerther P-L, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut Microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Bérard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Doré J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L. The intestinal Microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira LS, Vera-Licona P. Computing signal transduction in signaling networks modeled as boolean networks, petri nets and Hypergraphs. bioRxiv. 2019 doi: 10.1101/272344. [DOI]
- Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, Jardine L, Dixon D, Stephenson E, Nilsson E, Grundberg I, McDonald D, Filby A, Li W, De Jager PL, Rozenblatt-Rosen O, Lane AA, Haniffa M, Regev A, Hacohen N. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356:eaah4573. doi: 10.1126/science.aah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow R. Die cellularpathologie in ihrer begrundung auf physiologische und pathologische gewebelehre. Zwanzig Vorlesungen Gehalten Wahrend Der Monate Februar, Marz Und 1858 [Google Scholar]
- Virchow R. Cellular pathology as based upon physiological and pathological histology. JB Lippincott. 1863;383:32770. doi: 10.5962/bhl.title.32770. [DOI] [PubMed] [Google Scholar]
- Wählby C, Erlandsson F, Bengtsson E, Zetterberg A. Sequential immunofluorescence staining and image analysis for detection of large numbers of antigens in individual cell nuclei. Cytometry. 2002;47:32–41. doi: 10.1002/cyto.10026. [DOI] [PubMed] [Google Scholar]
- Wang Y, Waters J, Leung ML, Unruh A, Roh W, Shi X, Chen K, Scheet P, Vattathil S, Liang H, Multani A, Zhang H, Zhao R, Michor F, Meric-Bernstam F, Navin NE. Clonal evolution in breast Cancer revealed by single nucleus genome sequencing. Nature. 2014a;512:155–160. doi: 10.1038/nature13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014b;343:80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Chow RD, Bai Z, Zhu L, Errami Y, Dai X, Dong MB, Ye L, Zhang X, Renauer PA, Park JJ, Shen L, Ye H, Fuchs CS, Chen S. Multiplexed activation of endogenous genes by CRISPRa elicits potent antitumor immunity. Nature Immunology. 2019;20:1494–1505. doi: 10.1038/s41590-019-0500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. The Journal of General Physiology. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Thompson CB. Metabolic reprogramming: a Cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman TH, Theory S. Biology—View of a Biologist. Berlin, Heidelberg: Springer Berlin Heidelberg; 1968. [Google Scholar]
- Wells DK, Chuang Y, Knapp LM, Brockmann D, Kath WL, Leonard JN. Spatial and functional heterogeneities shape collective behavior of tumor-immune networks. PLOS Computational Biology. 2015;11:e1004181. doi: 10.1371/journal.pcbi.1004181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner HM, Mills GB, Ram PT. Cancer systems biology: a peek into the future of patient care? Nature Reviews Clinical Oncology. 2014;11:167–176. doi: 10.1038/nrclinonc.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nature Immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, Cheng D, Jewell K, Arndt D, Sawhney S, Fung C, Nikolai L, Lewis M, Coutouly M-A, Forsythe I, Tang P, Shrivastava S, Jeroncic K, Stothard P, Amegbey G, Block D, Hau DD, Wagner J, Miniaci J, Clements M, Gebremedhin M, Guo N, Zhang Y, Duggan GE, MacInnis GD, Weljie AM, Dowlatabadi R, Bamforth F, Clive D, Greiner R, Li L, Marrie T, Sykes BD, Vogel HJ, Querengesser L. HMDB: the human metabolome database. Nucleic Acids Research. 2007;35:D521–D526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistuba-Hamprecht K, Martens A, Weide B, Teng KW, Zelba H, Guffart E, Chen J, Garbe C, Newell EW, Larbi A, Pawelec G. Establishing high dimensional immune signatures from peripheral blood via mass cytometry in a discovery cohort of stage IV melanoma patients. The Journal of Immunology. 2017;198:927–936. doi: 10.4049/jimmunol.1600875. [DOI] [PubMed] [Google Scholar]
- Woelke AL, Murgueitio MS, Preissner R. Theoretical modeling techniques and their impact on tumor immunology. Clinical and Developmental Immunology. 2010;2010:1–11. doi: 10.1155/2010/271794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X, Abastado JP, Lam KP, Biswas SK. Macrophage polarization to a unique phenotype driven by B cells. European Journal of Immunology. 2010;40:2296–2307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- Woodsworth DJ, Castellarin M, Holt RA. Sequence analysis of T-cell repertoires in health and disease. Genome Medicine. 2013;5:98. doi: 10.1186/gm502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AR, Neff NF, Kalisky T, Dalerba P, Treutlein B, Rothenberg ME, Mburu FM, Mantalas GL, Sim S, Clarke MF, Quake SR. Quantitative assessment of single-cell RNA-sequencing methods. Nature Methods. 2014;11:41–46. doi: 10.1038/nmeth.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Ji Y, Moseman EA, Xu HC, Manglani M, Kirby M, Anderson SM, Handon R, Kenyon E, Elkahloun A, Wu W, Lang PA, Gattinoni L, McGavern DB, Schwartzberg PL. The TCF1-Bcl6 Axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Science Immunology. 2016;1:eaai8593. doi: 10.1126/sciimmunol.aai8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lanier LL. Natural killer cells and Cancer. Advances in Cancer Research. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- Xu X, Hou Y, Yin X, Bao L, Tang A, Song L, Li F, Tsang S, Wu K, Wu H, He W, Zeng L, Xing M, Wu R, Jiang H, Liu X, Cao D, Guo G, Hu X, Gui Y, Li Z, Xie W, Sun X, Shi M, Cai Z, Wang B, Zhong M, Li J, Lu Z, Gu N, Zhang X, Goodman L, Bolund L, Wang J, Yang H, Kristiansen K, Dean M, Li Y, Wang J. Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell. 2012;148:886–895. doi: 10.1016/j.cell.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Su Z. Identification of cell types from single-cell transcriptomes using a novel clustering method. Bioinformatics. 2015;31:1974–1980. doi: 10.1093/bioinformatics/btv088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E, Freeman TC, Ulas T, Schultze JL. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Yang K, Han X. Lipidomics: Techniques, Applications, and Outcomes Related to Biomedical Sciences. Trends in Biochemical Sciences. 2016;41:954–969. doi: 10.1016/j.tibs.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Sun HW, Lacey NE, Ji Y, Moseman EA, Shih HY, Heuston EF, Kirby M, Anderson S, Cheng J, Khan O, Handon R, Reilley J, Fioravanti J, Hu J, Gossa S, Wherry EJ, Gattinoni L, McGavern DB, O'Shea JJ, Schwartzberg PL, Wu T. Single-cell RNA-seq reveals TOX as a key regulator of CD8+ T cell persistence in chronic infection. Nature Immunology. 2019;20:890–901. doi: 10.1038/s41590-019-0403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. New England Journal of Medicine. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, Treviño V, Shen H, Laird PW, Levine DA, Carter SL, Getz G, Stemke-Hale K, Mills GB, Verhaak RG. Inferring tumour purity and stromal and immune cell admixture from expression data. Nature Communications. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, McNamara KL, Granja JM, Sarin KY, Brown RA, Gupta RK, Curtis C, Bucktrout SL, Davis MM, Chang ALS, Chang HY. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nature Medicine. 2019;25:1251–1259. doi: 10.1038/s41591-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. The Journal of Immunology. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Hon GC, Szulwach KE, Song CX, Zhang L, Kim A, Li X, Dai Q, Shen Y, Park B, Min JH, Jin P, Ren B, He C. Base-resolution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell. 2012;149:1368–1380. doi: 10.1016/j.cell.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Zhang K, Milner JJ, Toma C, Chen R, Scott-Browne JP, Pereira RM, Crotty S, Chang JT, Pipkin ME, Wang W, Goldrath AW. Epigenetic landscapes reveal transcription factors that regulate CD8+ T cell differentiation. Nature Immunology. 2017;18:573–582. doi: 10.1038/ni.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharakis N, Chinnasamy H, Black M, Xu H, Lu YC, Zheng Z, Pasetto A, Langhan M, Shelton T, Prickett T, Gartner J, Jia L, Trebska-McGowan K, Somerville RP, Robbins PF, Rosenberg SA, Goff SL, Feldman SA. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast Cancer. Nature Medicine. 2018;24:724–730. doi: 10.1038/s41591-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. Journal of Experimental Medicine. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappasodi R, Merghoub T, Wolchok JD. Emerging concepts for immune checkpoint Blockade-Based combination therapies. Cancer Cell. 2018;33:581–598. doi: 10.1016/j.ccell.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A. Mutations associated with acquired resistance to PD-1 blockade in melanoma. New England Journal of Medicine. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling AL, Polefrone JM, Evans AM, Mikesh LM, Shabanowitz J, Lewis ST, Engelhard VH, Hunt DF. Identification of class I MHC-associated phosphopeptides as targets for Cancer immunotherapy. PNAS. 2006;103:14889–14894. doi: 10.1073/pnas.0604045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian Cancer. New England Journal of Medicine. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- Zhang JA, Mortazavi A, Williams BA, Wold BJ, Rothenberg EV. Dynamic transformations of genome-wide epigenetic marking and transcriptional control establish T cell identity. Cell. 2012;149:467–482. doi: 10.1016/j.cell.2012.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu ZG. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Research. 2013;23:898–914. doi: 10.1038/cr.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cunningham JJ, Brown JS, Gatenby RA. Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate Cancer. Nature Communications. 2017a;8:5. doi: 10.1038/s41467-017-01968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Feng Q, Wang C, Zeng X, Du Y, Lin L, Wu J, Fu L, Yang K, Xu X, Xu H, Zhao Y, Li X, Schoenauer UH, Stadlmayr A, Saksena NK, Tilg H, Datz C, Liu X. Characterization of the B cell receptor repertoire in the intestinal mucosa and of Tumor-Infiltrating lymphocytes in colorectal adenoma and carcinoma. The Journal of Immunology. 2017b;198:3719–3728. doi: 10.4049/jimmunol.1602039. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gregorio JD, Iwahori T, Zhang X, Choi O, Tolentino LL, Prestwood T, Carmi Y, Engleman EG. A distinct subset of plasmacytoid dendritic cells induces activation and differentiation of B and T lymphocytes. PNAS. 2017c;114:1988–1993. doi: 10.1073/pnas.1610630114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu X, Zheng L, Zhang Y, Li Y, Fang Q, Gao R, Kang B, Zhang Q, Huang JY, Konno H, Guo X, Ye Y, Gao S, Wang S, Hu X, Ren X, Shen Z, Ouyang W, Zhang Z. Lineage tracking reveals dynamic relationships of T cells in colorectal Cancer. Nature. 2018;564:268–272. doi: 10.1038/s41586-018-0694-x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, Modak M, Carotta S, Haslinger C, Kind D, Peet GW, Zhong G, Lu S, Zhu W, Mao Y, Xiao M, Bergmann M, Hu X, Kerkar SP, Vogt AB, Pflanz S, Liu K, Peng J, Ren X, Zhang Z. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019;179:829–845. doi: 10.1016/j.cell.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Tavoosidana G, Sjölinder M, Göndör A, Mariano P, Wang S, Kanduri C, Lezcano M, Sandhu KS, Singh U, Pant V, Tiwari V, Kurukuti S, Ohlsson R. Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nature Genetics. 2006;38:1341–1347. doi: 10.1038/ng1891. [DOI] [PubMed] [Google Scholar]
- Zheng C, Zheng L, Yoo J-K, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, Liu Z, Dong M, Hu X, Ouyang W, Peng J, Zhang Z. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell. 2017;169:1342–1356. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- Zheng M, Tian SZ, Capurso D, Kim M, Maurya R, Lee B, Piecuch E, Gong L, Zhu JJ, Li Z, Wong CH, Ngan CY, Wang P, Ruan X, Wei C-L, Ruan Y. Multiplex chromatin interactions with single-molecule precision. Nature. 2019;566:558–562. doi: 10.1038/s41586-019-0949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Wan Y-W, Pang K, Chow LML, Liu Z. Digital sorting of complex tissues for cell type-specific gene expression profiles. BMC Bioinformatics. 2013;14:89. doi: 10.1186/1471-2105-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Shaffer DR, Alvarez Arias DA, Nakazaki Y, Pos W, Torres AJ, Cremasco V, Dougan SK, Cowley GS, Elpek K, Brogdon J, Lamb J, Turley SJ, Ploegh HL, Root DE, Love JC, Dranoff G, Hacohen N, Cantor H, Wucherpfennig KW. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature. 2014;506:52–57. doi: 10.1038/nature12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, Cullinan DR, Luo J, Bearden AR, Lavine KJ, Yokoyama WM, Hawkins WG, Fields RC, Randolph GJ, DeNardo DG. Tissue-Resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity. 2017;47:323–338. doi: 10.1016/j.immuni.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenhain C, Vieth B, Parekh S, Reinius B, Guillaumet-Adkins A, Smets M, Leonhardt H, Heyn H, Hellmann I, Enard W. Comparative analysis of Single-Cell RNA sequencing methods. Molecular Cell. 2017;65:631–643. doi: 10.1016/j.molcel.2017.01.023. [DOI] [PubMed] [Google Scholar]
- Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, Krishnan I, Maroni G, Meyerovitz CV, Kerwin CM, Choi S, Richards WG, De Rienzo A, Tenen DG, Bueno R, Levantini E, Pittet MJ, Klein AM. Single-Cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50:1317–1334. doi: 10.1016/j.immuni.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žurauskienė J, Yau C. pcaReduce: hierarchical clustering of single cell transcriptional profiles. BMC Bioinformatics. 2016;17:140. doi: 10.1186/s12859-016-0984-y. [DOI] [PMC free article] [PubMed] [Google Scholar]