Abstract
Background
Maternal human immunodeficiency virus (HIV) infection is associated with lower placental transfer of antibodies specific to several childhood pathogens. Our objective for this study was to evaluate the effect of maternal HIV infection on the placental transfer of respiratory syncytial virus (RSV)-neutralizing antibodies.
Methods
We conducted a cross-sectional study of mothers and their newborn infants at a tertiary hospital in Gaborone, Botswana, between March 2015 and December 2015. We measured serum RSV antibody levels by using a microneutralization assay. We used multivariable linear regression to evaluate the effect of maternal HIV infection on maternal RSV antibody levels, placental transfer of RSV antibodies, and newborn RSV antibody levels.
Results
Of 316 mothers, 154 (49%) were infected with HIV. The placental transfer ratios for RSV antibodies to HIV-exposed, uninfected (HEU) and HIV-unexposed, uninfected infants were 1.02 and 1.15, respectively. The geometric mean titer (95% confidence interval) of RSV-neutralizing antibodies was 2657 (2251–3136) among HEU newborns and 2911 (2543–3331) among HIV-unexposed, uninfected newborns. In multivariable analyses, maternal HIV infection was associated with lower placental transfer of RSV antibodies (P = .02) and a lower level of RSV antibodies among newborns (P = .002). Among HEU newborns, higher birth weight (P = .004) and an undetectable maternal antenatal viral load (P = .01) were associated with more effective placental transfer of RSV antibodies.
Conclusions
Maternal human immunodeficiency virus (HIV) infection is associated with lower mother-to-fetus transfer of serum RSV-neutralizing antibodies. HEU infants should be prioritized for preventive interventions for RSV. Maternal viral suppression through combination antiretroviral therapy has the potential to improve immunity to RSV among HIV-exposed infants.
Keywords: antibody, HIV-exposed uninfected, respiratory syncytial virus
Maternal HIV infection is associated with a lower rate of placental transfer of respiratory syncytial virus antibodies. Maternal viral suppression and higher newborn birth weight predicted more effective antibody transfer to HIV-exposed but uninfected newborns.
Respiratory syncytial virus (RSV) is the leading cause of acute lower respiratory infection during infancy [1]. RSV accounted for 94 600 to 149 400 child deaths in 2015, more than 99% of which occurred in low- and middle-income countries [2, 3]. Although a threshold protective antibody level has not been established, maternally derived RSV antibodies are important for protecting infants from severe RSV infection [4–6]. Compared to age- and sex-matched controls, RSV-infected infants in Mozambique had a significantly lower prevalence and titer of maternally derived RSV antibodies [4]. The gold standard for assessing RSV immunity is the measurement of neutralizing antibodies, which quantifies the functional capacity of serum to neutralize RSV infectivity [7].
Maternal human immunodeficiency virus (HIV) infection impairs the placental transfer of antibodies to a number of common childhood pathogens [8–12]. However, few previous studies on the effect of maternal HIV infection on the transfer of RSV antibodies to infants have been performed [13]. Moreover, the specific maternal or infant factors that influence RSV antibody transfer to HIV-exposed, uninfected (HEU) infants have not been identified. With several candidate RSV vaccines currently in development, an improved understanding of the effect of maternal HIV infection on infant RSV immunity is needed to inform vaccination strategies in areas with a high prevalence of HIV infection. In particular, assessing RSV antibody transfer to HEU infants is important for RSV vaccination strategies that rely on maternal immunization and subsequent maternal–fetal antibody transfer [14].
In this study, we compared placental transfer of serum RSV-neutralizing antibodies to HEU and HIV-unexposed, uninfected (HUU) newborns in Botswana. As a secondary objective, we sought to identify maternal and infant factors that modify the acquisition of these RSV antibodies by HEU infants.
METHODS
Setting
This study was conducted between March 2015 and December 2015 at Princess Marina Hospital, a tertiary hospital in Gaborone, Botswana. RSV infections occur seasonally in Botswana and typically peak between March and June [15]. The HIV prevalence among pregnant women aged 15 to 49 years in Botswana was 26.3% in 2016 [16]. Women in Botswana are offered opt-out HIV testing as part of routine antenatal care. Combination antiretroviral therapy was recommended for all HIV-infected pregnant women in Botswana during the study period. For HIV-infected women who presented for antenatal care before 14 weeks’ gestational age, tenofovir, emtricitabine, and either nevirapine (if the CD4+ cell count was ≤250 cells/μL) or lopinavir–ritonavir (if the CD4+ cell count was >250 cells/μL) were recommended [17]. For HIV-infected women who initiated antiretroviral therapy at ≥14 weeks’ gestational age in the absence of severe anemia or renal insufficiency, Atripla (emtricitabine, tenofovir, and efavirenz) (Gilead, Foster City, California) was recommended [17]. More than 90% of HIV-infected pregnant women in Botswana received combination antiretroviral therapy in 2015, and the estimated mother-to-child HIV transmission rate is lower than 3% [18].
Study Population
Newborns less than 24 hours of age and their mothers were eligible for inclusion in this study. Exclusion criteria were maternal age less than 18 years, newborn birth weight less than 2000 g, multiple gestation, and acute maternal or newborn medical concerns. Postpartum women were approached for enrollment on weekdays. Although recruitment was generally performed consecutively, to ensure that approximately equal numbers of HEU and HUU infants were recruited each month, the number of enrollments of these groups was discussed during biweekly research calls, and recruitment was adjusted accordingly. This was done to minimize the effect of confounding by enrollment month, given the known seasonal circulation of RSV in Botswana [15]. After obtaining written informed consent from the mother, sociodemographic and HIV clinical data were collected through a caregiver questionnaire and review of the medical record. Maternal HIV status was determined using written or electronic documentation of the results of HIV testing performed during the pregnancy or at the time of delivery. For mothers who had not tested positive for HIV previously and who were not tested at the time of delivery, HIV testing was performed using dual parallel rapid testing with the Determine HIV 1/2 (Abbott Laboratories, North Chicago, Illinois) and Uni-Gold Recombigen HIV (Trinity Biotech, Inc, Wicklow, Ireland) tests. Newborns of mothers who tested negative for HIV at delivery or at enrollment were classified as HUU. Newborns of mothers who tested positive for HIV before or during pregnancy, at delivery, or at enrollment were considered HIV exposed. The medical records of HIV-exposed newborns were reviewed for results of HIV DNA polymerase chain reaction (PCR) testing performed at or after birth using the Roche Amplicor 1.5 HIV DNA PCR assay (Roche, Alameda, California).
Laboratory Methods
Venous blood (~1.5 mL) was obtained from the mother and newborn, transported to the National Health Laboratory in Gaborone, and centrifuged. The subsequent serum was stored at −80°C before shipment on dry ice to the Respiratory and Meningeal Pathogens Research Unit in Johannesburg, South Africa. Measurement of neutralizing antibodies to RSV subtype A was performed using an in-house microneutralization assay in 96-well microtiter plates, as previously described [19, 20]. Each sample was tested in duplicate with 12 twofold dilutions of patient serum; 175 plaque-forming units of the long strain of human RSV A (ATCC VR-26) was added to each well, and the plates were incubated and shaken for 90 minutes at 37°C in 5% carbon dioxide. Thereafter, 25 000 HEp2 cells (ATCC CCL23) were added to each well, and the plates were incubated at 37°C in 5% carbon dioxide for 6 days. The plates were then stained with a crystal violet fixative for 24 hours, washed, and dried. RSV-neutralizing antibody titers were determined on the basis of the last sample dilution with a ≤50% intact HEp2 cell monolayer, and the Reed and Muench method was used to calculate the 50% end-point dilution per milliliter (ED50/mL) [21]. The following controls were included for each sample run: an intravenous immunoglobulin plate, for which intravenous immunoglobulin was used instead of patient serum; a second control plate with 2 rows of negative controls (no virus and no serum); 2 rows of positive controls (virus but no serum); and 4 rows for back-titration of the virus. The serum samples from a mother and her newborn were tested on the same microtiter plate. Laboratory personnel were blinded to maternal HIV status and other enrollment data of the study participants.
Statistical Analysis
Our analyses were limited to mother–newborn pairs from whom sufficient serum was obtained for measurement of RSV-neutralizing antibodies. In addition, we excluded HIV-exposed infants who tested positive for HIV according to a PCR assay or for whom no HIV PCR result was available. Baseline characteristics of the study population according to maternal HIV infection status were compared using χ 2 tests for categorical variables and the Student t tests for continuous variables. Given the approximately log-normal distribution of RSV-neutralizing antibody levels in the study population, these values were log transformed for inclusion in multivariable models. We used multivariable linear regression to compare maternal and newborn RSV-neutralizing antibody levels by maternal HIV infection status. We calculated the placental antibody transfer ratio by dividing the newborn RSV antibody level by the maternal RSV antibody level. We used multivariable linear regression to estimate the effect of maternal HIV infection on the placental transfer of RSV antibodies. Last, in analyses limited to HEU newborns, we used multivariable linear regression to identify infant and maternal factors that were associated with placental transfer of RSV antibodies. HIV-infected mothers who did not receive antiretroviral therapy during pregnancy were presumed to have had a detectable antenatal viral load for this analysis. As specified in the study protocol, all analyses adjusted for enrollment month to account for the seasonality of RSV infection. In addition, although not specified a priori, we adjusted analyses for maternal age because of its association with RSV antibody levels in both mothers and newborns. A 2-sided P value of <.05 was considered significant. Study data were managed using Research Electronic Data Capture (REDCap) tools hosted at the Children’s Hospital of Philadelphia [22]. Statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, North Carolina) and R 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria). This study was approved by the Health Research and Development Committee (Ministry of Health, Botswana) and institutional review boards at Princess Marina Hospital, the University of Pennsylvania, McMaster University, and Duke University.
RESULTS
Patient Characteristics
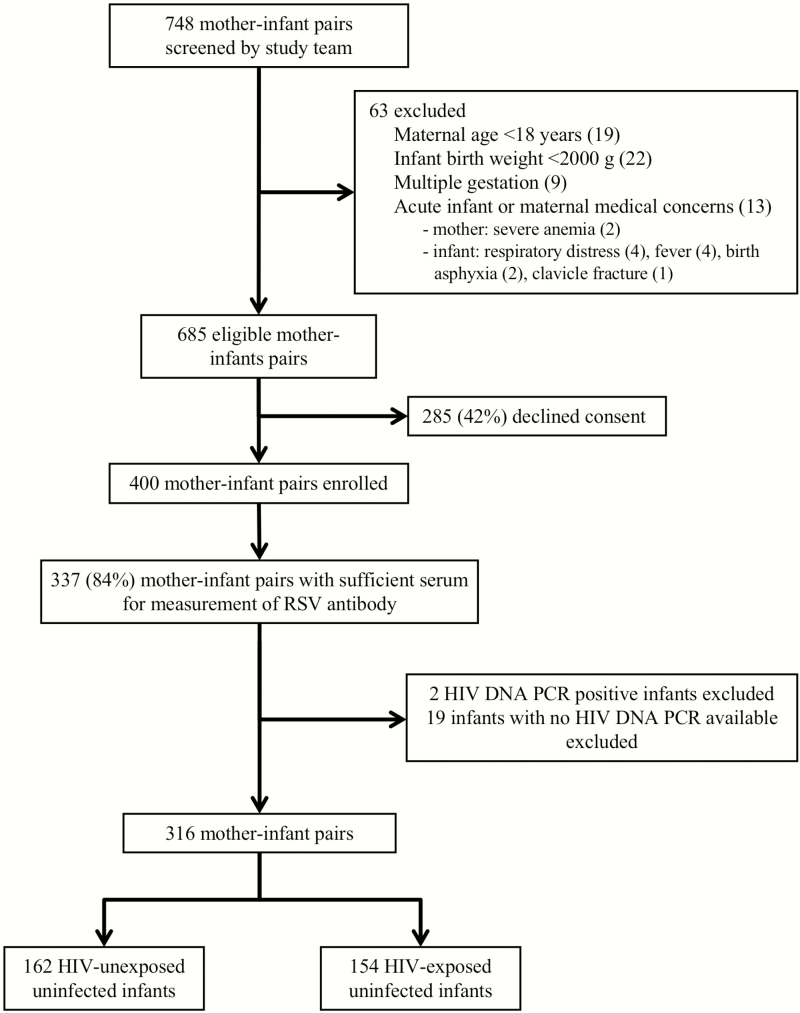
Seven hundred forty-eight mothers and their newborns were screened for eligibility (Figure 1), and 400 mother–newborn pairs were enrolled, including 200 HIV-infected mothers and 200 uninfected mothers. Sufficient serum for the measurement of RSV-neutralizing antibodies was obtained from 337 (84%) mother–newborn pairs. Two HIV-exposed newborns whose HIV PCR test result was positive and 19 newborns with no HIV DNA PCR result available were excluded from these analyses. Characteristics of the final study population of 316 mother–newborn pairs are shown in Table 1. The median birth weight of infants in the study was 3070 g (interquartile range [IQR], 2790–3310 g). Thirty-three (11%) infants had a low birth weight (<2500 g), including 21 HIV-exposed and 12 HIV-unexposed infants. The HIV-infected mothers were older than the HIV-uninfected mothers (median age, 32 vs 24 years, respectively; P < .0001). In addition, household use of wood as a cooking fuel was more frequent among HIV-infected mothers than among HIV-uninfected mothers (73% vs 62%, respectively; P = .04). However, we found no significant differences in newborn birth weights or other measures of socioeconomic status according to maternal HIV status.
Figure 1.
Screening and enrollment of mother–newborn pairs in Gaborone, Botswana, March 2015 to December 2015. Abbreviations: HIV, human immunodeficiency virus; PCR, polymerase chain reaction; RSV, respiratory syncytial virus.
Table 1.
Baseline Characteristics of Mother–Infant Pairs According to Maternal HIV Status
| Characteristic | Maternal HIV Status | P a | ||
|---|---|---|---|---|
| Overall (n = 316) | HIV Infected (n = 154) | HIV Uninfected (n = 162) | ||
| Demographics | ||||
| Maternal age (median [IQR]) (years) | 28 (23–34) | 32 (27–36) | 24 (21–29) | <.0001 |
| Infant sex, female | 168 (53) | 81 (53) | 87 (54) | |
| Birth weight (median [IQR]) (g)b | 3070 (2790–3310) | 3045 (2725–3318) | 3078 (2840–3310) | .39 |
| Socioeconomic factors | ||||
| Maternal education level (n [%]) | .16 | |||
| None or primary school | 23 (7) | 14 (9) | 9 (6) | |
| Secondary school | 221 (70) | 111 (72) | 110 (68) | |
| Tertiary school | 72 (23) | 29 (19) | 43 (27) | |
| Location of primary residence (n [%])c | .50 | |||
| Urban | 98 (31) | 45 (29) | 53 (33) | |
| Semirural or rural | 218 (69) | 109 (71) | 109 (67) | |
| Household electricity (n [%]) | 227 (72) | 104 (68) | 123 (76) | .10 |
| Refrigerator in the home (n [%]) | 199 (63) | 91 (59) | 108 (67) | .16 |
| Piped or private water source (n [%]) | 284 (90) | 141 (92) | 143 (88) | .33 |
| Household use of wood as a cooking fuel (n [%]) | 212 (67) | 112 (73) | 100 (62) | .04 |
| No. of persons in the household (median [IQR]) | 6 (4–8) | 6 (4–8) | 5 (4–7) | .07 |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
a P values were estimated using χ 2 tests for categorical variables and Student t tests for continuous variables.
bData on birth weight were missing for 2 HIV-exposed, uninfected infants.
cUrban areas were those with a population of at least 20 000 people in 2001 according to the Botswana Central Statistics Office.
Most HIV-infected mothers were on combination antiretroviral therapy, had mild or no immunosuppression, and were virally suppressed. Data on antiretroviral therapy during pregnancy were available for 153 (99%) of the 154 HIV-infected mothers. One hundred forty-seven (95%) mothers were on combination antiretroviral therapy for a median of 8 months (IQR, 4–9 months) before they delivered their infant. The most frequent antiretroviral medication regimens were efavirenz, emtricitabine, and tenofovir (Atripla, n = 119), nevirapine, emtricitabine, and tenofovir disoproxil (n = 11), and nevirapine, zidovudine, and lamivudine (n = 11). The median CD4+ cell count during pregnancy was 487 cells/μL (IQR, 337–647 cells/μL), and 92 (94%) of 98 mothers with a viral load during pregnancy had an undetectable level (<400 copies/mL).
RSV Antibody Levels and Placental Transfer
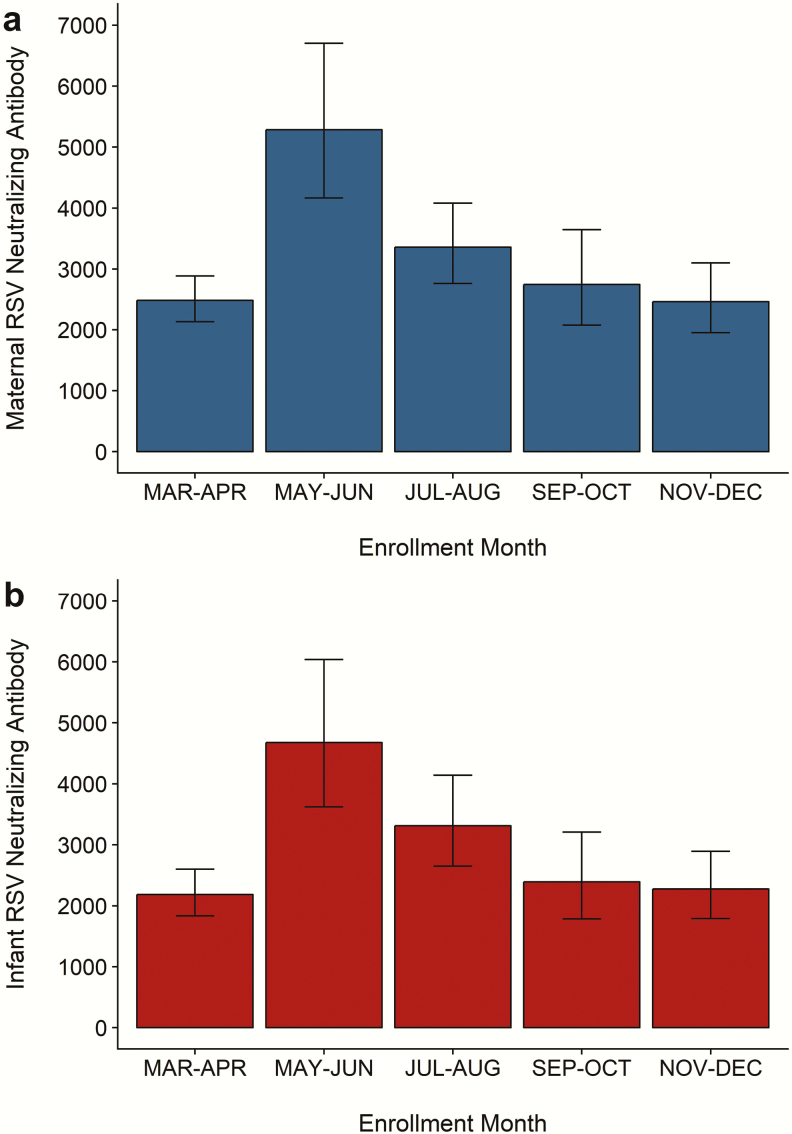
All the mothers and their newborns had a detectable serum RSV-neutralizing antibody level. The geometric mean RSV antibody titer in the mothers was 3039 (95% confidence interval [CI], 2759–3348). The mean placental transfer ratio for RSV antibodies was 1.09 (95% CI, 1.01–1.16). Among infants, the geometric mean RSV antibody titer was 2784 (95% CI, 2504–3095). We observed a marked seasonality to the distribution of RSV antibody levels among the mothers and their infants (Figure 2). The highest geometric mean RSV antibody titers were observed in May and June, which coincides with when the peak in RSV infections typically occurs in Botswana [15]. Age was also strongly associated with the level of maternal serum RSV-neutralizing antibodies. The geometric mean titer of RSV antibodies was 2693 (95% CI, 2427–2989) among mothers younger than 35 years and 4940 (95% CI, 3996–6107) among mothers aged 35 years or older (P < .0001).
Figure 2.
Seasonality of respiratory syncytial virus (RSV)-neutralizing antibodies in mothers and newborns. The bar plots show the geometric mean titers of maternal (a) and newborn (b) RSV-neutralizing antibodies according to enrollment month. The error bars represent 95% confidence limits for the geometric mean titer in each time period. The number of subjects is displayed above each bar.
Serum RSV-neutralizing antibody levels for the mothers and their newborns according to maternal HIV infection status are shown in Table 2. The geometric mean titer of RSV antibodies was 3100 (95% CI, 2675–3591) in HIV-infected mothers and 2983 (95% CI, 2623–3392) in HIV-uninfected mothers. In analyses adjusted for maternal age and enrollment month, maternal HIV infection status was not associated with the level of RSV-neutralizing antibodies among the mothers (P = .09). The mean placental transfer ratios of RSV antibody to HEU and HUU newborns were 1.02 and 1.15, respectively. In adjusted analyses, HIV exposure was associated with lower placental transfer of RSV-neutralizing antibodies (P = .02). The geometric mean titer of RSV antibodies was 2657 (95% CI, 2251–3136) among HEU newborns and 2911 (95% CI, 2543–3331) among HUU newborns. In multivariable analyses, maternal HIV infection was associated with a lower level of RSV-neutralizing antibodies among newborns (P = .002).
Table 2.
Serum RSV-Neutralizing Antibody Levels for Mothers and Newborns According to Maternal HIV Infection
| RSV Antibody Measurement | Maternal HIV Status | P | P adj | |
|---|---|---|---|---|
| HIV Infected (n = 154) | HIV Uninfected (n = 162) | |||
| Maternal GMT (95% CI) | 3100 (2675–3591) | 2983 (2623-3392) | .70 | .09 |
| Placental transfer ratio (95% CI) | 1.02 (0.93–1.12) | 1.15 (1.03-1.27) | .11 | .02 |
| Newborn GMT (95% CI) | 2657 (2251–3136) | 2911 (2543-3331) | .40 | .002 |
Abbreviations: CI, confidence interval; GMT, geometric mean titer; HIV, human immunodeficiency virus; RSV, respiratory syncytial virus.
P values were estimated from Student t tests.
P adj values were estimated from multivariable linear regression models adjusted for enrollment month and maternal age.
Predictors of RSV Antibody Levels and Transfer to HEU Newborns
Table 3 shows predictors of maternal and infant RSV antibody levels and RSV antibody transfer among HIV-infected mothers and their HEU newborns. Higher newborn birth weight and an undetectable maternal antenatal viral load were associated with more effective placental transfer of RSV-neutralizing antibodies from the mothers to their newborns. Among HEU newborns, an undetectable maternal antenatal viral load was associated with higher RSV-neutralizing antibody levels. In analyses in which we compared virally suppressed HIV-infected (n = 92) and HIV-uninfected (n = 162) mothers, we found no significant differences in the geometric mean titers of maternal RSV-neutralizing antibodies (3339 [95% CI, 2759–4041] vs 2983 [95% CI, 2624–3392], respectively; P = .36], mean placental transfer ratios (1.06 [95% CI, 0.94–1.18] vs 1.15 [1.03–1.27], respectively; P = .08), or newborn RSV-neutralizing antibodies (3010 [95% CI, 2411–3759] vs 2911 [95% CI, 2543–3331], respectively; P = .12] according to maternal HIV infection status.
Table 3.
Predictors of RSV-Neutralizing Antibody Levels and Transfer in HIV-Infected Mothers and HIV-Exposed, Uninfected Infants
| Characteristic | N | GMT or Antibody Transfer Ratio (95% CI) | P a |
|---|---|---|---|
| Maternal RSV Antibodies | |||
| Maternal CD4+ count (cells/μL) | .09 | ||
| <350 | 36 | 3172 (2637–3925) | |
| ≥350 | 93 | 2915 (2165–3815) | |
| Maternal viral load (copies/mL) | .69 | ||
| <400 | 92 | 3339 (2759–4041) | |
| ≥400 | 12 | 2216 (1406–3492) | |
| RSV antibody transfer | |||
| Infant birth weight (g) | .004 | ||
| <3000 | 72 | 0.94 (0.80–1.07) | |
| ≥3000 | 82 | 1.10 (0.97–1.23) | |
| Maternal CD4+ count (cells/μL) | .30 | ||
| <350 | 36 | 0.95 (0.75–1.16) | |
| ≥350 | 93 | 1.02 (0.90–1.14) | |
| Maternal viral load (copies/mL) | .01 | ||
| <400 | 92 | 1.06 (0.94–1.18) | |
| ≥400 | 12 | 0.55 (0.33–0.77) | |
| Infant RSV antibodies | |||
| Infant birth weight (g) | .10 | ||
| <3000 | 72 | 2487 (1906–3246) | |
| ≥3000 | 82 | 2815 (2282–3474) | |
| Maternal CD4+ count (cells/μL) | .37 | ||
| <350 | 36 | 2205 (1714–2837) | |
| ≥350 | 93 | 2758 (2200–3458) | |
| Maternal viral load (copies/mL) | .02 | ||
| <400 | 92 | 3010 (2411–3759) | |
| ≥400 | 12 | 1046 (645–1695) |
Abbreviations: CI, confidence interval; GMT, geometric mean titer; HIV, human immunodeficiency virus; RSV, respiratory syncytial virus.
a P values were estimated from multivariable linear regression; all models were adjusted for age and enrollment month.
DISCUSSION
In this study of mother–newborn pairs in Botswana, maternal HIV infection was associated with impaired placental transfer of RSV-neutralizing antibodies to newborns. Older age predicted a higher RSV-neutralizing antibody level among HIV-infected mothers. Among HEU newborns, higher newborn birth weight and an undetectable maternal viral load were associated with more effective transfer of RSV-neutralizing antibodies.
Our results indicate that maternal HIV infection is associated with reduced placental transfer of RSV-neutralizing antibodies. HIV-exposed infants were shown previously to acquire lower levels of antibodies specific to a number of common childhood pathogens, including tetanus, measles, Haemophilus influenzae, Streptococcus pneumoniae, and group B Streptococcus [11, 23–27]. Limited data regarding placental transfer of RSV antibodies to HEU infants exist. In a study of mother–infant pairs in Brazil, RSV antibody levels were actually higher in HEU infants than in HUU infants, although maternal HIV infection was associated with lower placental transfer of RSV-specific antibodies [13]. In that study, enzyme-linked immunosorbent assays were used to quantify total serum RSV immunoglobulin G, and RSV-neutralizing antibodies were not measured specifically. Moreover, the study did not investigate maternal and infant factors that could influence the placental transfer of RSV antibodies to HEU infants. The impaired transfer of RSV antibodies to HEU infants might contribute to the higher risk of RSV-related acute lower respiratory infection in this group [28]. The importance of antibodies for protection from severe RSV disease has been demonstrated by clinical experience with palivizumab, an RSV monoclonal antibody that reduces both the incidence of RSV hospitalization and the duration of moderate or severe RSV illness in infants at high risk [29]. Our results highlight the importance of targeting HEU infants or their mothers for interventions to prevent RSV infection. In particular, several candidate RSV vaccines are currently in development, and HEU infants were shown previously to mount robust antibody responses to other childhood vaccinations [12]. Moreover, a previous study estimated that maternal RSV vaccination has the potential to prevent 29% to 48% of deaths caused by RSV-related acute lower respiratory infection among infants [30].
Several potential mechanisms could account for the lower levels of pathogen-specific antibodies among HEU infants. First, HIV-infected adults might have lower levels of these antibodies than HIV-uninfected adults, as has been found in HIV-infected adults for tetanus, measles, and group B Streptococcus antibodies [8, 31]. Levels of RSV-neutralizing antibodies tended to be higher in HIV-infected mothers than in HIV-uninfected mothers in our cohort, although this result was accounted for, in part, by age differences between these groups. The aforementioned study conducted in Brazil also found that measured RSV antibody levels were higher in HIV-infected women than in HIV-uninfected women [13]. This association might relate to a higher risk of RSV infection among HIV-infected pregnant women, as was suggested in a recent South African study [32]. Second, transfer of pathogen-specific antibodies might be impaired by HIV-related hypergammaglobulinemia, a process that is thought to result from saturation of the neonatal Fc (FcRn) receptors on placental syncytiotrophoblasts that mediate mother-to-fetus antibody transfer [33–35]. Viral suppression was associated previously with resolution of hypergammaglobulinemia in HIV-infected adults, which might partly explain the improved antibody transfer seen in the virally suppressed mothers in our study [36]. Third, HIV might directly impair antibody transfer through an as-yet-unrecognized mechanism. Maternal HIV viral load during pregnancy is an independent predictor of measles antibody transfer, and our findings indicate that the placental transfer of RSV antibodies is influenced similarly by active viral replication [37]. Last, antiretroviral therapy among pregnant women might affect the placental transfer of pathogen-specific antibodies. HIV-infected mothers on combination antiretroviral therapy were previously found to transfer higher levels of measles and pneumococcal antibodies to their infants than did the mothers on zidovudine monotherapy [38].
Other factors associated with serum levels of RSV-neutralizing antibodies in our cohort included maternal age, season, and infant birth weight. Older age was associated with a higher level of RSV-neutralizing antibodies among the mothers. To our knowledge, this association has not been reported previously and could be of importance for future trials of maternal RSV vaccination. It is possible that the higher level of RSV antibodies among older mothers reflects more frequent exposure to RSV as a result of having other young children, although we did not observe an association between the number of household members and maternal RSV antibody level (data not shown). We also found a seasonal distribution of RSV-neutralizing antibody levels; the highest levels were observed in May and June, although enrollment did not span a full calendar year. This association is presumably related to the circulation of RSV within the community, which we previously reported to occur primarily between March and June in Botswana, and the resulting exposure to RSV among pregnant mothers in the cohort [15]. Last, although birth weight did not differ between HEU and HUU infants, it was an independent predictor of placental transfer of RSV-neutralizing antibodies to HEU infants. This result is not surprising, given that birth weight is likely to be strongly correlated with gestational age, and the majority of placental antibody transfer occurs in the third trimester [33].
Our study has several limitations. First, mother–newborn pairs were recruited in the postnatal ward of an urban tertiary hospital. This cohort might not reflect the wider population of pregnant women and infants in sub-Saharan Africa. In addition, maternal age differed substantially between HIV-infected and HIV-uninfected mothers and was associated with the level of maternal RSV-neutralizing antibodies. Studies of several South African cohorts reported similar age differences in the prevalence of HIV infection among pregnant women [8, 10, 39]. We also lacked data in this study on the gestational age of newborns, a factor that was found previously to affect antibody transfer [35]. To account for this lack of data, analyses of newborn RSV antibodies and placental transfer of RSV antibodies were adjusted for newborn birth weight, which was found to correlate with gestational age among HIV-unexposed infants in Botswana [40]. The median CD4+ cell count among HIV-infected mothers in our study was relatively high, and 94% of the HIV-infected mothers in our cohort for whom data were available were virally suppressed, which is unlikely to be representative of pregnant women in many resource-limited settings with a high HIV burden. We did not measure total immunoglobulin G levels and thus are unable to comment on the contribution of HIV-related hypergammaglobulinemia to the reduced rate of placental RSV antibody transfer seen in the HIV-infected women in this study. In addition, the RSV-neutralizing antibody assay that was used in this study was specific to RSV subtype A, which precluded evaluation of antibodies to other subtypes of the virus. However, RSV subtype A accounted for the majority (62%) of RSV strains isolated from patients with severe acute respiratory illnesses in South Africa between 1997 and 2012 [41]. Our approach to measuring RSV antibodies did not include reference sera, which hinders direct comparison of our RSV antibody levels with those reported in other populations. Last, it is unclear if the factors that we identified as influencing the transfer of RSV antibodies to HIV-exposed infants also affect the transfer of antibody specific to other common childhood pathogens.
In conclusion, maternal HIV infection is associated with reduced placental transfer of serum RSV-neutralizing antibodies to infants. Moreover, maternal antenatal viral suppression improved the transfer of RSV antibodies to HEU infants. Several candidate RSV vaccines are currently in development, and these findings indicate that vaccination strategies should prioritize improving RSV immunity among HEU infants.
Notes
Disclaimer. The views expressed in this publication do not necessarily reflect the official policies of the International AIDS Society or ViiV Healthcare.
Financial support. This research was supported by a Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) grant from the International AIDS Society, supported by ViiV Healthcare. This study was also supported in part through core services and support from the Penn Center for AIDS Research, a National Institutes of Health (NIH)-funded program (grant P30-AI045008). M.S.K. and C.K.C. received financial support from the NIH through the Duke Center for AIDS Research (grant P30-AI064518). M.S.K. was supported by an NIH Career Development Award (K23-AI135090). S.M.P. was supported by NIH T32 training grant 5T32-HL007538-33.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis 2018; 217:1356–64. [DOI] [PubMed] [Google Scholar]
- 2. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi T, McAllister DA, O’Brien KL, et al. ; RSV Global Epidemiology Network Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roca A, Abacassamo F, Loscertales MP, et al. Prevalence of respiratory syncytial virus IgG antibodies in infants living in a rural area of Mozambique. J Med Virol 2002; 67:616–23. [DOI] [PubMed] [Google Scholar]
- 5. Stensballe LG, Ravn H, Kristensen K, et al. Seasonal variation of maternally derived respiratory syncytial virus antibodies and association with infant hospitalizations for respiratory syncytial virus. J Pediatr 2009; 154:296–8. [DOI] [PubMed] [Google Scholar]
- 6. Hacimustafaoglu M, Celebi S, Aynaci E, et al. The progression of maternal RSV antibodies in the offspring. Arch Dis Child 2004; 89:52–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Piedra PA, Hause AM, Aideyan L. Respiratory syncytial virus (RSV): neutralizing antibody, a correlate of immune protection. In: Tripp RA, Jorquera PA, eds. Human Respiratory Syncytial Virus: Methods and Protocols. New York, NY: Springer; 2016:77–91. [DOI] [PubMed] [Google Scholar]
- 8. Dangor Z, Kwatra G, Izu A, et al. HIV-1 is associated with lower group B Streptococcus capsular and surface-protein IgG antibody levels and reduced transplacental antibody transfer in pregnant women. J Infect Dis 2015; 212:453–62. [DOI] [PubMed] [Google Scholar]
- 9. Isabel de Moraes-Pinto M, Almeida ACM, Kenj G, et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis 1996; 173:1077–84. [DOI] [PubMed] [Google Scholar]
- 10. Jallow S, Cutland CL, Masbou AK, et al. Maternal HIV infection associated with reduced transplacental transfer of measles antibodies and increased susceptibility to disease. J Clin Virol 2017; 94:50–6. [DOI] [PubMed] [Google Scholar]
- 11. Gaensbauer JT, Rakhola JT, Onyango-Makumbi C, et al. Impaired Haemophilus influenzae type b transplacental antibody transmission and declining antibody avidity through the first year of life represent potential vulnerabilities for HIV-exposed but -uninfected infants. Clin Vaccine Immunol 2014; 21:1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones CE, Naidoo S, De Beer C, et al. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA 2011; 305:576–84. [DOI] [PubMed] [Google Scholar]
- 13. Weinberg A, Mussi-Pinhata MM, Yu Q, et al. ; NISDI Perinatal, LILAC, CIRAI Protocols Excess respiratory viral infections and low antibody responses among HIV-exposed, uninfected infants. AIDS 2017; 31:669–79. [DOI] [PubMed] [Google Scholar]
- 14. Modjarrad K, Giersing B, Kaslow DC, et al. ; WHO RSV Vaccine Consultation Expert Group WHO consultation on respiratory syncytial virus vaccine development report from a World Health Organization Meeting held on 23–24 March 2015. Vaccine 2016; 34:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelly MS, Smieja M, Luinstra K, et al. Association of respiratory viruses with outcomes of severe childhood pneumonia in Botswana. PLoS One 2015; 10:e0126593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joint United Nations Programme on HIV/AIDS. UNAIDS estimates 2017: Botswana Available at: http://www.unaids.org/en/regionscountries/countries/botswana. Accessed May 31, 2018.
- 17. Botswana Ministry of Health. Botswana National HIV & AIDS Treatment Guidelines. Gaborone: Botswana Ministry of Health; 2012. [Google Scholar]
- 18. Joint United Nations Programme on HIV/AIDS. Country fact sheet: Botswana Available at: http://www.unaids.org/sites/default/files/media/documents/UNAIDS_GlobalplanCountryfactsheet_botswana_en.pdf. Accessed May 31, 2018.
- 19. Stensballe LG, Ravn H, Kristensen K, et al. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J Allergy Clin Immunol 2009; 123:398–403. [DOI] [PubMed] [Google Scholar]
- 20. Anderson LJ, Hierholzer JC, Bingham PG, Stone YO. Microneutralization test for respiratory syncytial virus based on an enzyme immunoassay. J Clin Microbiol 1985; 22:1050–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol 1938; 27:493–7. [Google Scholar]
- 22. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cumberland P, Shulman CE, Maple PA, et al. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis 2007; 196:550–7. [DOI] [PubMed] [Google Scholar]
- 24. Gupta A, Mathad JS, Yang WT, et al. Maternal pneumococcal capsular IgG antibodies and transplacental transfer are low in South Asian HIV-infected mother-infant pairs. Vaccine 2014; 32:1466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones C, Pollock L, Barnett SM, Battersby A, Kampmann B. Specific antibodies against vaccine-preventable infections: a mother–infant cohort study. BMJ Open 2013; 3:e002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Le Doare K, Allen L, Kampmann B, et al. Anti-group B Streptococcus antibody in infants born to mothers with human immunodeficiency virus (HIV) infection. Vaccine 2015; 33:621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scott S, Cumberland P, Shulman CE, et al. Neonatal measles immunity in rural Kenya: the influence of HIV and placental malaria infections on placental transfer of antibodies and levels of antibody in maternal and cord serum samples. J Infect Dis 2005; 191:1854–60.15871118 [Google Scholar]
- 28. Cohen C, Moyes J, Tempia S, et al. Epidemiology of acute lower respiratory tract infection in HIV-exposed uninfected infants. Pediatrics 2016; 137:e20153272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102:531–7. [PubMed] [Google Scholar]
- 30. Scheltema NM, Kavelaars XM, Thorburn K, et al. Potential impact of maternal vaccination on life-threatening respiratory syncytial virus infection during infancy. Vaccine 2018; 36:4693–700. [DOI] [PubMed] [Google Scholar]
- 31. De Milito A, Nilsson A, Titanji K, et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 2004; 103:2180–6. [DOI] [PubMed] [Google Scholar]
- 32. Madhi SA, Cutland CL, Downs S, et al. Burden of respiratory syncytial virus infection in South African human immunodeficiency virus (HIV)-infected and HIV-uninfected pregnant and postpartum women: a longitudinal cohort study. Clin Infect Dis 2018; 66:1658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abu-Raya B, Smolen KK, Willems F, et al. Transfer of maternal antimicrobial immunity to HIV-exposed uninfected newborns. Front Immunol 2016; 7:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Okoko BJ, Wesuperuma LH, Ota MO, et al. Influence of placental malaria infection and maternal hypergammaglobulinaemia on materno-foetal transfer of measles and tetanus antibodies in a rural west African population. J Health Popul Nutr 2001; 19:59–65. [PubMed] [Google Scholar]
- 35. Palmeira P, Quinello C, Silveira-Lessa AL, et al. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012; 2012:985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Notermans DW, de Jong JJ, Goudsmit J, et al. Potent antiretroviral therapy initiates normalization of hypergammaglobulinemia and a decline in HIV type 1-specific antibody responses. AIDS Res Hum Retroviruses 2001; 17:1003–8. [DOI] [PubMed] [Google Scholar]
- 37. Farquhar C, Nduati R, Haigwood N, et al. High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. J Acquir Immune Defic Syndr 2005; 40:494–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bosire R, Farquhar C, Nduati R, et al. Higher transplacental pathogen-specific antibody transfer among pregnant women randomized to triple antiretroviral treatment versus short course zidovudine. Pediatr Infect Dis J 2018; 37:246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Slogrove AL, Esser MM, Cotton MF, et al. A prospective cohort study of common childhood infections in South African HIV-exposed uninfected and HIV-unexposed infants. Pediatr Infect Dis J 2017; 36:e38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matthews LT, Ribaudo HJ, Parekh NK, et al. Birth weight for gestational age norms for a large cohort of infants born to HIV-negative women in Botswana compared with norms for U.S.-born black infants. BMC Pediatr 2011; 11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pretorius MA, van Niekerk S, Tempia S, et al. Replacement and positive evolution of subtype A and B respiratory syncytial virus G-protein genotypes from 1997–2012 in South Africa. J Infect Dis 2013; 208(Suppl 3):S227–37. [DOI] [PubMed] [Google Scholar]