Abstract
A direct-from-source rapid musculoskeletal diagnostic panel (MDP) was validated recently. We compared clinical measures to theoretical time points had MDP results been available. The MDP would have significantly decreased the time to pathogen identification (7 hours), time to definitive antimicrobial therapy (22 hours), and hospital length of stay (26.4 hours).
Keywords: diagnostics, infection, osteomyelitis, pediatric, septic arthritis
Acute musculoskeletal infections are common causes of hospitalization of children (incidence, approximately 6 cases per 1000 pediatric hospitalizations) [1, 2].
Pathogen identification for musculoskeletal infections enables targeted antimicrobial therapy but currently relies on traditional time-intensive bacterial culture techniques [3–5]. We recently validated a novel rapid musculoskeletal diagnostic panel (MDP) [6]. The purpose of this study was to quantify the potential clinical effects of this new diagnostic tool.
MATERIALS AND METHODS
MDP Overview and Testing
Details of the study population and the MDP were published previously [6]. In brief, the MDP combines 3 separate rapid tests, including the Xpert MRSA/SA SSTI assay, which identifies the presence of Staphylococcus aureus and genotypic methicillin resistance directly from a specimen, polymerase chain reaction (PCR) assays for the ermA, ermB, and ermC genes, which encode genotypic clindamycin resistance, and a Kingella kingae PCR assay, which targets the rtxA gene [6]. Turnaround times from bone or joint specimen collection until reporting of results were estimated to be 3 hours for the Xpert MRSA/SA SSTI results and 2:00 pm the following calendar day for the ermA/ermB/ermC gene and K kingae PCR assays. These estimates were based on existing Children’s Hospital Colorado microbiology laboratory workflows that allow for specimen extraction once every morning.
Included Patients
Patients were included in the study if they were admitted to the hospital with acute complaints (<2 weeks of symptoms), had a bone or joint specimen sent for microbiological testing, and were started on antimicrobial therapy (not including perioperative antibiotics) after specimen acquisition, indicating suspicion for infection. Patients were excluded if they did not have an infectious indication for biopsy, were aged <6 months or >18 years, or had perforating trauma or decubitus ulcers, severe underlying comorbidity (including cerebral palsy, spina bifida, or immunosuppression), or a head, neck, or central nervous system infection.
Data Analysis
All bone and joint specimens from the included patients were tested using the 3 components of the MDP [6]. A retrospective review of patient charts was managed alongside MDP results using a REDCap electronic data-capture tool hosted at Children’s Hospital Colorado [7]. Initiation of care was defined at the date and time of either the first recorded set of vital signs or the acquisition of a blood or source specimen for culture, whichever occurred first. For patients with a pathogen identified via blood culture, the theoretical rapid multiplex PCR blood culture identification (BCID) turnaround time was recorded as 1 hour after blood culture Gram-stain results were first reported in the electronic medical record.
The time to definitive antimicrobial therapy was defined as the time at which care was initiated until both of the following criteria were met: a patient was being treated with an effective antimicrobial (based on an a priori definition for each identified pathogen) (Supplementary Table 1) and the pathogen was identified and the relevant antimicrobial susceptibility values were available in the electronic medical record. If no pathogen was found in the blood culture, source culture, or MDP, then the time to definitive antimicrobial therapy was defined as 72 hours after source-specimen collection for both the actual and theoretical time points.
The actual discharge date and time were based on the time the discharge order was signed. A theoretical discharge date was calculated for each patient on the basis of the earliest time at which the following criteria were met: MDP results identified a pathogen, 24 hours after the last recorded fever (>38.2°C), 4 hours after the patient’s C-reactive protein level had either decreased to <3 mg/dL was less than 50% of the highest recorded value, and 36 hours after at least 1 negative blood culture result. For patients in whom MDP did not identify a pathogen, the theoretical discharge time was the same as the actual discharge time. This study was approved by the Colorado Multiple Institutional Review Board.
Statistics
Times to outcome for the standard method versus those for theoretical results with the MDP were compared using a Wilcoxon signed rank-sum test to account for departures from normality. For all theoretical calculations, the MDP time was changed to the standard time if it would have been longer, because the MDP would not have affected care in those situations. For comparisons that included the BCID results, the MDP time was changed to the theoretical BCID time if MDP results would have taken longer. Kaplan–Meier curves were plotted for times to pathogen identification, and the results were censored at 48 hours after source-specimen collection if no pathogen was identified. Kaplan–Meier curves were plotted also for times to methicillin resistance and to clindamycin resistance.
RESULTS
Of the 125 unique patients included in the initial validation study, 53 met criteria for inclusion in this retrospective clinical impact study (Supplementary Table 2). When standard culturing techniques were used, a pathogen was identified in 37 (69.8%) of 53 patients, and according to blood culture, a pathogen was identified in 13 (24.5%) of 53 patients. S aureus was identified in 25 (47.2%) of the 53 patients; methicillin-resistant S aureus (MRSA) was identified in 4 (16.0%) of these 25 patients, and clindamycin-resistant S aureus was identified in 2 (8.0%). K kingae was identified in only 1 (1.9%) of the 53 patients with standard microbiologic cultures. Of the 25 patients with S aureus identified on culture, MDP testing identified S aureus in 22 (88%). According to the MDP, 3 (13.6%) of 22 S aureus isolates were identified as MRSA, and all 3 of them were identified as MRSA on culture. Three (13.6%) of 22 S aureus isolates were clindamycin resistant according to the MDP; 1 of these isolates was reported as clindamycin susceptible on culture. We found no patient for whom S aureus was identified with the MDP alone. Only 1 patient would have had a pathogen found on MDP that was missed on standard bacterial culture (positive K kingae PCR result from a 15-month-old patient with monoarticular septic arthritis of the elbow and negative source culture results).
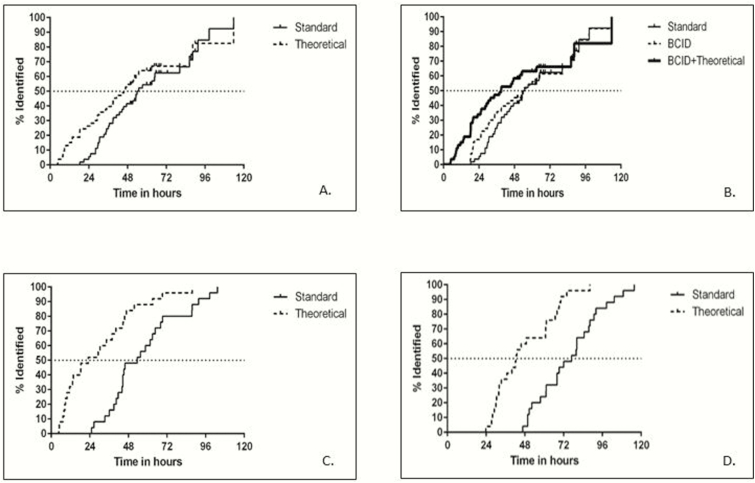
The median time to pathogen identification using the MDP would have been 7 hours faster than standard microbiology culture techniques (45.6 hours [IQR, 23–62] vs 52.5 hours [IQR, 36.1–65.6], respectively; P < .001) (Table 1, Figure 1A). When we incorporated the potential effect of BCID on these results, we found that the median time to pathogen identification would have been slightly decreased in the BCID-available group and decreased more in the BCID-plus-MDP group (52.5 hours [standard] vs 50.5 hours [BCID] vs 39 hours [BCID plus MDP]; P < .001) (Figure 1B). Among patients for whom S aureus was identified in blood or source cultures, the time to methicillin resistance via the MDP would have been 30 hours faster than that via standard microbiology culture techniques (23 vs 53.5 hours, respectively; P < .001) (Figure 1C), and clindamycin resistance would have been reported 34 hours faster (42.5 vs 76.9 hours, respectively; P < .001) (Figure 1D).
Table 1.
Theoretical Effects of the MDP on Clinical Outcomes
| Effect | Actual Results (Median [IQR]) | Theoretical Results (Median [IQR]) | N | P |
|---|---|---|---|---|
| Time to pathogen identification (hours) | 52.5 (36.1–65.6) | 45.6 (23–62) | 53 | <.001 |
| Time to methicillin resistance (hours) | 53.5 (41.7–68) | 23 (9.8–44.6) | 25 | <.001 |
| Time to clindamycin resistance (hours) | 76.9 (61.1–88.3) | 42.5 (31.8–61.2) | 25 | <.001 |
| Vancomycin-hours of therapy per patient | 24.6 (19.4–36.6) | 19.4 (2.7–30.3) | 13 | .13 |
| Total vancomycin-days of therapy | 17.6 | 12.1 | 13 | NA |
| Time to definitive therapy (hours) | 45.1 (28.5–69.8) | 23.5 (3–66.7) | 53 | <.001 |
| Hospital LOS (days) | 4.1 (3.5–5.6) | 3.8 (3–5) | 53 | <.001 |
Abbreviations: IQR, interquartile range; LOS, length of stay; MDP, musculoskeletal diagnostic panel; NA, not applicable.
Figure 1.
Time to pathogen identification and antimicrobial resistance results. (A) Times to pathogen identification for all 53 patients. (B) Times to pathogen identification, including BCID results. The median time according to the standard method was 52.5 hours (interquartile range [IQR], 36.1–65.6 hours), according to BCID was 50.5 hours (IQR, 29.7–65.6 hours), and according to BCID plus the theoretical MDP was 39.0 hours (IQR, 18.4–62.0 hours). (C) Time to methicillin resistance (25 methicillin-sensitive Staphylococcus aureus [MSSA]/methicillin-resistant S aureus [MRSA] samples only). (D) Time to clindamycin resistance (25 MSSA/MRSA samples only).
Thirteen (24.5%) patients received at least 1 dose of vancomycin during their admission, but none of them had a pathogen identified that required vancomycin (ie, clindamycin-resistant MRSA). Among vancomycin-exposed patients, use of the MDP would have decreased the vancomycin length of therapy in 4 (30.8%) of 13 patients. The total cumulative vancomycin-days of therapy would have decreased from 17.6 to 12.1 days of therapy had MDP results been available (Table 1).
The MDP would have decreased the time to definitive therapy by nearly 22 hours (median, 23.5 vs 45.1 hours, respectively; P < .001) (Table 1). On the basis of our a priori MDP discharge criteria, 20 (37.7%) of 53 patients theoretically would have been discharged earlier (median decrease in hospital length of stay [LOS], 26.4 hours; P < .001) (Table 1).
DISCUSSION
No approved rapid diagnostic platforms currently exist for bone and joint specimens from pediatric patients with acute musculoskeletal infection. Our findings suggest that our recently developed rapid MDP could decrease the time to pathogen identification and relevant antimicrobial-susceptibility results, and it could decrease the time to definitive antimicrobial therapy, vancomycin exposure, and hospital LOS.
Nearly one-third of the vancomycin-exposed patients in this cohort would have had vancomycin discontinued earlier or been able to avoid it completely if MDP results had been available during their admission. At institutions with high MRSA rates and frequent usage of empiric vancomycin, identifying MSSA versus MRSA within 3 hours of source-specimen collection would allow providers to choose a narrow initial antimicrobial with less toxicity while awaiting MDP results. In contrast, at an institution such as Children’s Hospital Colorado, where MRSA rates are relatively low (35% among all isolates, 12% among musculoskeletal isolates) and patients are routinely started on empiric cefazolin for acute musculoskeletal infection, the MDP could hasten appropriate MRSA coverage and potentially improve outcomes.
Cost is an important balancing measure when a new potential diagnostic tool is considered. We determined that the MDP would lead, theoretically, to a decrease in LOS and justify the added cost of the MDP. Additional cost benefits are likely to be found from the MDP’s effect on clinical metrics, such as faster time to definitive antimicrobial therapy, although further cost-saving calculations are required to formally determine these potential benefits.
There are important limitations to this study. First, only patients from whom residual source specimens were available were included in the study; therefore, these results might not truly reflect our population of children with musculoskeletal infection. Second, clinical decisions are not always implemented immediately after new microbiological data are reported, and specific patient factors (such as severity of illness) also guide antimicrobial decisions. Therefore, our calculations might not accurately reflect clinical care. Last, our study was performed at a single institution and therefore might not be generalizable to other institutions with different populations, prescribing practices, and antimicrobial resistance rates. It is possible that the MDP would have a greater effect at institutions with higher rates of MRSA, clindamycin resistance, K kingae infection, and/or empiric vancomycin usage.
CONCLUSION
This recently validated MDP has the potential to greatly affect the care of pediatric patients with musculoskeletal infection by decreasing the time to pathogen identification, time to definitive antimicrobial therapy, unnecessary vancomycin exposure, and hospital LOS. Additional prospective studies are needed after effective implementation of the MDP, alongside antimicrobial stewardship partnership, to fully evaluate its effects in a real clinical environment.
Supplementary Material
Notes
Acknowledgments. We thank Chris Robinson, Stacey Hamilton, Kristin Pretty, Ji Yuan, Qi Wei, and Toraj Anarestani with the microbiology laboratory at Children’s Hospital Colorado for their help with the MDP validation study, including banking and documenting convenience bone and joint specimens and erm and Kingella sp PCR development and specimen processing. We also thank Ritu Banerjee at Vanderbilt University School of Medicine for her input and advice with regard to study design and manuscript development.
Financial support. This work was supported by an Innovations Grant from the Children’s Hospital of Colorado, which supported supplies and partial funding of fellowship salary during the project. This publication was supported also by National Institutes of Health/National Center for Research Resources Colorado Clinical and Translational Sciences Institute grant UL1 RR025780.
Disclaimers. The Children’s Hospital of Colorado grant committee played no role in study design, data collection, or interpretation or in the decision to submit the work for publication. Cepheid generously provided the Xpert MRSA/SA SSTI test kits used in the previous MDP validation study but played no role in study design, data analysis, or data interpretation. This article’s contents are the authors’ sole responsibility and do not necessarily represent official National Institutes of Health views.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Arnold SR, Elias D, Buckingham SC, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop 2006; 26:703–8. [DOI] [PubMed] [Google Scholar]
- 2. Dahl LB, Høyland AL, Dramsdahl H, Kaaresen PI. Acute osteomyelitis in children: a population-based retrospective study 1965 to 1994. Scand J Infect Dis 1998; 30:573–7. [DOI] [PubMed] [Google Scholar]
- 3. Funk SS, Copley LA. Acute hematogenous osteomyelitis in children: pathogenesis, diagnosis, and treatment. Orthop Clin North Am 2017; 48:199–208. [DOI] [PubMed] [Google Scholar]
- 4. Gafur OA, Copley LA, Hollmig ST, et al. The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines. J Pediatr Orthop 2008; 28:777–85. [DOI] [PubMed] [Google Scholar]
- 5. Spruiell MD, Searns JB, Heare TC, et al. Clinical care guideline for improving pediatric acute musculoskeletal infection outcomes. J Pediatric Infect Dis Soc 2017; 6:e86–93. [DOI] [PubMed] [Google Scholar]
- 6. Searns JB, Robinson CC, Wei Q, et al. Validation of a novel molecular diagnostic panel for pediatric musculoskeletal infections: integration of the Cepheid Xpert MRSA/SA SSTI and laboratory-developed real-time PCR assays for clindamycin resistance genes and Kingella kingae detection. J Microbiol Methods 2019; 156:60–7. [DOI] [PubMed] [Google Scholar]
- 7. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.