Abstract
Introduction
Before using blood-oxygen-level-dependent magnetic resonance imaging (BOLD MRI) during maternal hyperoxia as a method to detect individual placental dysfunction, it is necessary to understand spatiotemporal variations that represent normal placental function. We investigated the effect of maternal position and Braxton-Hicks contractions on estimates obtained from BOLD MRI of the placenta during maternal hyperoxia.
Methods
For 24 uncomplicated singleton pregnancies (gestational age 27–36 weeks), two separate BOLD MRI datasets were acquired, one in the supine and one in the left lateral maternal position. The maternal oxygenation was adjusted as 5 minutes of room air (21% O2), followed by 5 minutes of 100% FiO2. After datasets were corrected for signal non-uniformities and motion, global and regional BOLD signal changes in R2* and voxel-wise Time-To-Plateau (TTP) in the placenta were measured. The overall placental and uterine volume changes were determined across time to detect contractions.
Results
In mothers without contractions, increases in global placental R2* in the supine position were larger compared to the left lateral position with maternal hyperoxia. Maternal position did not alter global TTP but did result in regional changes in TTP. 57% of the subjects had Braxton-Hicks contractions and 58% of these had global placental R2* decreases during the contraction.
Conclusion
Both maternal position and Braxton-Hicks contractions significantly affect global and regional changes in placental R2* and regional TTP. This suggests that both factors must be taken into account in analyses when comparing placental BOLD signals over time within and between individuals.
Keywords: Placental MRI, BOLD MRI, Maternal position, Maternal hyperoxia, Braxton-Hicks contraction
Introduction
Accurate and clinically meaningful non-invasive assessment of utero-placental function remains an elusive goal in perinatal medicine. Most clinical tests intended to detect utero-placental insufficiency depend on indirect measures such as the non-stress test, fetal biophysical profile and a variety of ultrasound Doppler indices from the maternal and fetal circulation. Blood-oxygen-level-dependent magnetic resonance imaging (BOLD-MRI) during maternal hyperoxia has been proposed as one of several plausible MRI-based methods to characterize placental function by monitoring oxygen transport from mother to fetus utilizing oxygen as a tracer [1–9].
BOLD MRI relies on the observation that the MRI signal intensity in T2* weighted images depends on the relative quantity of deoxyhemoglobin in the blood and therefore reflects blood oxygenation. Manipulation of maternal blood oxygen content by the administration of exogenous oxygen then offers the possibility of characterizing oxygen transport from the maternal to the fetal bloodstream qualitatively and semi-quantitatively with analysis of signal intensity relationships over time. Early studies in animal models and human subjects suggest that such analysis of placental oxygen transport is feasible [1–9]. We have previously demonstrated in a well-controlled experiment (monochorionic diamniotic twins) that numeric parameters such as time to plateau (TTP) obtained with BOLD MRI of the placenta during maternal hyperoxia correlate with placental histologic abnormalities and are longer among fetuses destined to be small for gestational age [6]. Although this new methodology for analysis of placental function is promising, there is significant inter- and intra- subject variation in these BOLD MRI measurements. Therefore, before we can use BOLD MRI with maternal hyperoxia to detect individual placental dysfunction, it is necessary to understand the physiological mechanisms underlying the BOLD MRI changes as well as the spatiotemporal variations that represent normal placental function. Once understood, we can then develop strategies to control for these normal variations and better detect individual placental dysfunction. It is well established that BOLD MRI signals with matern hyperoxia in any organ are affected by hemoglobin concentration, blood volume and blood flow within the tissue [10,11]. Germane to the placenta, intervillous blood flow can vary in space and time [12]. Two factors plausibly affecting intervillous blood flow and therefore BOLD MRI measurements with maternal hyperoxia are maternal position and the presence of pre-labor, or Braxton-Hicks contractions.
One major determinant of intervillous blood flow is maternal mean arterial pressure. Postural hypotension related to aorto-caval compression by the pregnant uterus, resulting in decreased venous return in the inferior vena cava and in some, a resulting vagal reaction, has been recognized clinically for decades [13,14]. Indeed, avoidance of the supine position in late pregnancy is a fundamental principle to prevent the aorto-caval compression syndrome [15,16]. Pregnant women in the third trimester are not uniformly susceptible to aorta-caval compression. Symptoms vary from mother to mother depending on differences in the distribution of cardiac output, circulating blood volume and the effectiveness of the return collateral circulation [16]. Thus, it is plausible that even in the absence of overt aorto-caval compression syndrome, variable aorto-caval compression from variable maternal position and variable maternal physique could lead to variations in placental perfusion pressure, intervillous blood flow and therefore BOLD MRI signal with maternal hyperoxia.
Another major determinant of intervillous blood flow is uterine activity. Classic primate studies by Ramsey and colleagues relying on cineradiography and concurrent manometry of intrauterine and intervillous pressure demonstrated that uterine contractions across all points of gestation significantly reduce intervillous blood flow [12]. Consistent with these findings, more contemporary reports relying on uterine artery Doppler velocimetry in human pregnancy demonstrate changes in resistive indices consistent with decreased uterine artery blood flow during Braxton-Hicks contractions [17,18]. More recently, Sinding and colleagues described spontaneous 4 to 5 minute decreases in placental BOLD MRI signals in 8 of 56 pregnant women (with gestational age 23–40 weeks, placed in left lateral position during the MRI scan) and found that all 8 had concurrent changes in uterine shape consistent with Braxton-Hicks contractions [19]. A more detailed assessment of the effect of Braxton-Hicks contractions on BOLD signal time courses during maternal hyperoxia is needed to determine if and how contractions should be accounted for in future studies.
Before BOLD MRI with maternal hyperoxygenation can be deployed as a reliable research or clinical tool meant to characterize individual placental function, phase 1 studies [20] are needed to characterize the range of results obtained in typical pregnancies and to explore the physiological factors influencing typical spatiotemporal placental function. With this in mind, we conducted a phase 1 human study designed to characterize the relationship between two factors, maternal position and Braxton-Hicks contractions, and numeric parameters obtained with placental BOLD MRI in the third trimester during maternal hyperoxia while monitoring for changes in maternal vital signs and fetal motion.
Methods
Subjects
We screened and recruited pregnant women with uncomplicated singleton pregnancy between 27 and 36 weeks of gestation in the prenatal clinic at Massachusetts General Hospital and Brigham and Women’s Hospital in Boston between April of 2017 and May of 2018. Subjects with multiple gestations, preeclampsia, chronic hypertension, diabetes of any kind, a BMI greater than 30 or fetal malformations were excluded. All subjects were imaged at Boston Children’s Hospital.
The study was reviewed and approved by the institutional review board at all three hospitals. All patients gave informed consent. The study was registered with ClinicalTrials.gov in advance of any recruitment (NCT02297724).
Data Acquisitions
MRI studies were performed on a 3T Skyra scanner (Siemens Healthineers, Erlangen, Germany). For each subject two separate datasets were acquired, one in the supine and one in the left lateral position. The order of the maternal position was randomized, in a manner similar to flipping a coin, into blocks of two subgroups (i.e. 1. first left lateral and second supine; 2. first supine second left lateral). BOLD imaging of the whole uterus was performed using single-shot gradient echo (GE) echo planar imaging (EPI) sequence with TR=5.8–8s, TE= 30– 38ms, in-plane resolution of 3×3mm2 with 3mm thick slices acquired in an interleaved order. The maternal oxygenation was adjusted to provide 5 minutes of room air (21% O2), followed by 5 minutes of 100% FiO2 via a non-rebreathing facial mask. Before each 10-minute BOLD acquisition, half-Fourier acquisition single-shot turbo spin echo (HASTE) images (with 2×2×5 mm resolution) of the whole uterus were acquired for anatomical reference. The first BOLD dataset was acquired at least 15 minutes after maternal positioning. After the first BOLD acquisition, the mother was brought out of the scanner by moving the table and her position changed. Mothers did not stand or sit at any point while changing positions. Maternal repositioning and structural data acquisition for anatomical reference in the second position ensured at least 20 minutes between the BOLD datasets with oxygen exposure after the first position. For each acquisition, maternal blood pressure (diastolic, systolic), arterial oxygen saturation (SaO2), and heart rate (HR) were monitored every 5 minutes.
Data Processing
Before measuring BOLD signal intensity changes in the placenta with maternal hyperoxia, signal inhomogeneities, caused primarily by receive coil positioning, and motion caused primarily by maternal breathing and fetal motion were corrected. The acquired 4 dimensional (4D) images (i.e. the BOLD time series images) were first corrected for signal non-uniformities and then registered to mitigate motion using previous demonstrated method for uterine BOLD MRI [21]. The bias field accounting for signal non-uniformities was estimated using 3D-N4 bias correction implementation in Advanced Normalization Tools (ANTS) registration suite [22,23]. A single bias field was estimated from the average of volumes collected in the first 5 minutes in order to incorporate small variations between the time frames resulting primarily from coil movement. This bias field correction was then applied to each frame. To minimize artifacts from large movements in the average, we compared each volume with the entire series using mean square error (MSE) and excluded the volumes with high MSE from averaging. Note that the volumes collected in the last 5 minutes during maternal hyperoxia were excluded from the average to prevent any effect of the intensity changes due to the maternal oxygenation on the bias correction as the algorithm does not have the prior constraints on this signal change as previously discussed in [21]. All motion correction steps were carried out in Elastix, open source image processing software [24]. For intra-volume motion correction, each volume was separated into two sub-volumes, even and odd slices given the interleaved design, and then registered to the other using the group-wise registration approach [25]. For inter-volume motion correction within the uterus, a reference volume with the least sum of mean square error difference compared to the rest of the volumes in the series was selected as the reference volume. Then, a pairwise non-rigid body transformation was employed between the reference volume and the other volumes following an initial six degrees of freedom rigid transformation. Regions of interest (ROIs) for the placenta and the uterus in the reference frame were manually delineated using ITK-SNAP [26]. Voxel-wise deformation was quantified by computing the determinant of the Jacobian of the transformation generated during inter-volume motion correction within the uterus.
The corrected 4D BOLD data were spatially smoothed with a Gaussian kernel with a width of 5 pixels to improve the signal to noise ratio, and linear interpolation was applied in the temporal domain after outlier rejection. Outlier rejection was performed based on 1) volumes with over-deformation during intra-volume motion correction; and 2) volumes with unexpected average signal intensity change of nearby time frames within the placenta region as explained in [21].
The BOLD signal was converted to normalized R2* (i.e., normalized 1/T2* ) as the indicator of oxygen saturation level changes as follows:
where t is time, S is signal intensity and TE is echo time. For each subject in two different maternal positions, placenta-specific time-intensity curves (i.e., normalized R2* vs. time) were generated after averaging the BOLD signal in the whole placenta at each time point.
Voxel-wise Time-To-Plateau (TTP) in the placenta (i.e., the time required for the signal change due to maternal hyperoxia to reach a plateau) was estimated by fitting normalized R2* time series in placental voxels to a gamma variate function convolved with the oxygen paradigm as described in Luo et al.[6]:
where α and β are gamma function parameters, Δ is the delay time of oxygen arrival, c1 is baseline R2* signal, c2 is the amplitude of normalized R2* or change in R2* from baseline, t is time and Poxy is a step function that describes oxygen paradigm. Summation of Δ and the mode, τ, of the gamma function across voxels give TTP. For better visualization of the TTP maps and BOLD images in two maternal positions we used a placental flattening approach as described in Abulnaga et al. [27] and then for better comparison we performed non-rigid body transformation between flattened images. The average TTP was then calculated by averaging the voxel-wise TTP estimates in the whole placenta.
The overall placental and uterine volume changes were estimated by averaging the log determinant of the Jacobian of the transformation within each region across time. The determinant of the Jacobian of the transformation was generated during inter-volume motion correction, which is a quantitative measure of change in volume such that it provides a ratio of voxel-wise expansion or compression relative to the reference space [28]. Datasets with simultaneous drops in uterine volume (i.e., more than 4% of the overall uterine volume) and placenta volume (i.e., more than 10% of the overall placental volume) were marked as uterine contractions. The threshold values were assigned based on the minimum placental and uterine volume changes estimated in a group of subjects with imaging data having visually detectable local or global signal intensity drop in the placenta. TTP analysis relying on gamma variate function fitting could not be applied to the datasets with uterine contractions due to the unpredictable fluctuation in the BOLD signal during contractions. Datasets with image artifacts were excluded.
Statistical Analysis
Statistical analyses were performed in SAS (version 9.4). Analysis of covariance (ANCOVA) models were used for across subject analysis to examine the relation of maximum change in R2* from baseline to maternal position while checking for influences of placental position, and the experimental order of maternal positions with subject’s ID included as a random effect one at a time. Similarly we examine the relation of maximum change in R2* from baseline to GA after adjusting for maternal position, placental position, and the experimental order of maternal positions. We also checked for the effect of maternal BMI, and maternal vital signs (maternal blood pressure, oxygen saturation, and heart rate) by adding them as fixed variables one at a time while controlling for maternal position, placental position, and the experimental order of maternal positions simultaneously and discarded if non-significant. We performed the analysis for the subjects with and without contraction separately. Similar analyses were performed for average TTP values. The effect of each factor is reported with effect sizes, precision estimates, and associated p-values. Note that group means are expressed as mean ± SD (standard deviation) and mean differences are expressed as mean difference ± SE (standard error).
Results
24 subjects with uncomplicated singleton pregnancy were involved (GA: 31.7w±3d). The order of the maternal position for 16 of 24 subjects was first supine and second left lateral. 11 of 24 subjects (46%) had posterior placenta. 8 of 24 subjects (33%) had no evidence of Braxton-Hicks uterine contractions in either position and were included in the construction of BOLD time- intensity curves and TTP analysis from the placenta in the two maternal positions. Twelve subjects had contraction-related BOLD signal decreases. Three terminated the MRI protocol when they were in the supine position, two due to dizziness and one due to increased contractions and pain. One subject was excluded due to imaging artifacts (i.e. signal loss due to bowel gas susceptibility). Even though we aimed for an equal number of datasets with the two different positioning orders, we collected more data in first supine second left lateral position group as there were more uterine contractions in this cohort, resulting in fewer analyzable BOLD time-intensity curves. Placental position in the uterus was determined using HASTE MR images. Fetal gestational age, maternal position during MRI and placental position in the uterus are listed in Supplementary Table 1.
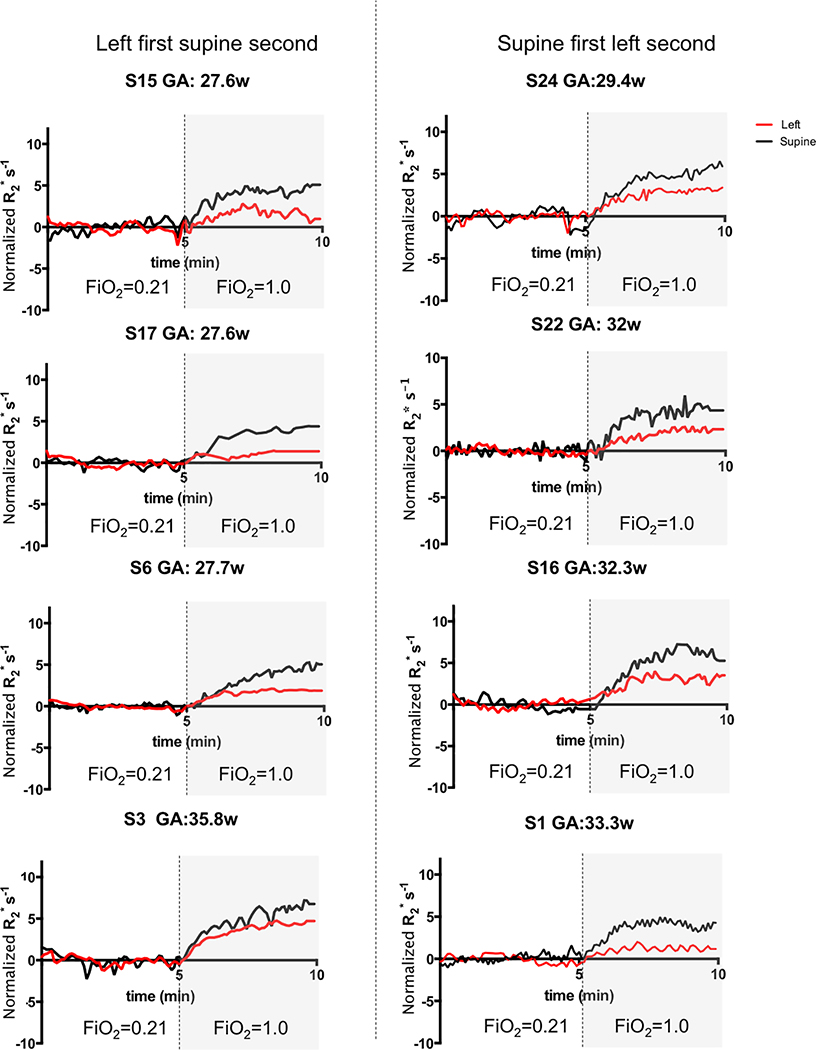
Figure 1 shows the change in R2* from baseline or change in normalized R2* for the entire placenta separately for both positions over time for the eight subjects without evidence of contractions in either supine or left lateral positions. As shown in the figure, the increase in R2* during maternal hyperoxia was higher in the supine position than in the left lateral position for each subject. In fact, the maximum change in R2* (i.e. average change in R2* measured during the last 2 minutes of hyperoxia) estimated in the supine position (4.96 ± 0.91) was significantly higher than in the left lateral position (2.36 ± 1.09) with the difference 2.60 ± 0.36, p<0.0001. There was trend for maximum change in R2* to increase with gestational age but did not reach statistical significance (0.16 units per week of GA with a standard error of 0.11). Other factors including the order of maternal position, placental position in uterus, maternal BMI, and maternal vital signs (i.e. maternal blood pressure, oxygen saturation, and heart rate) had no significant effect on the maximum change in R2*.
Figure 1.
Change of normalized R2* with time in the supine (black) and the left lateral (red) positions for each subject. The vertical dotted line represented the time 100% FiO2 was initiated. Left column: left lateral position first. Right column: supine position first. S = subject, GA = gestational age, w = weeks. Plots were ordered according to the gestational age increasing from top to bottom.
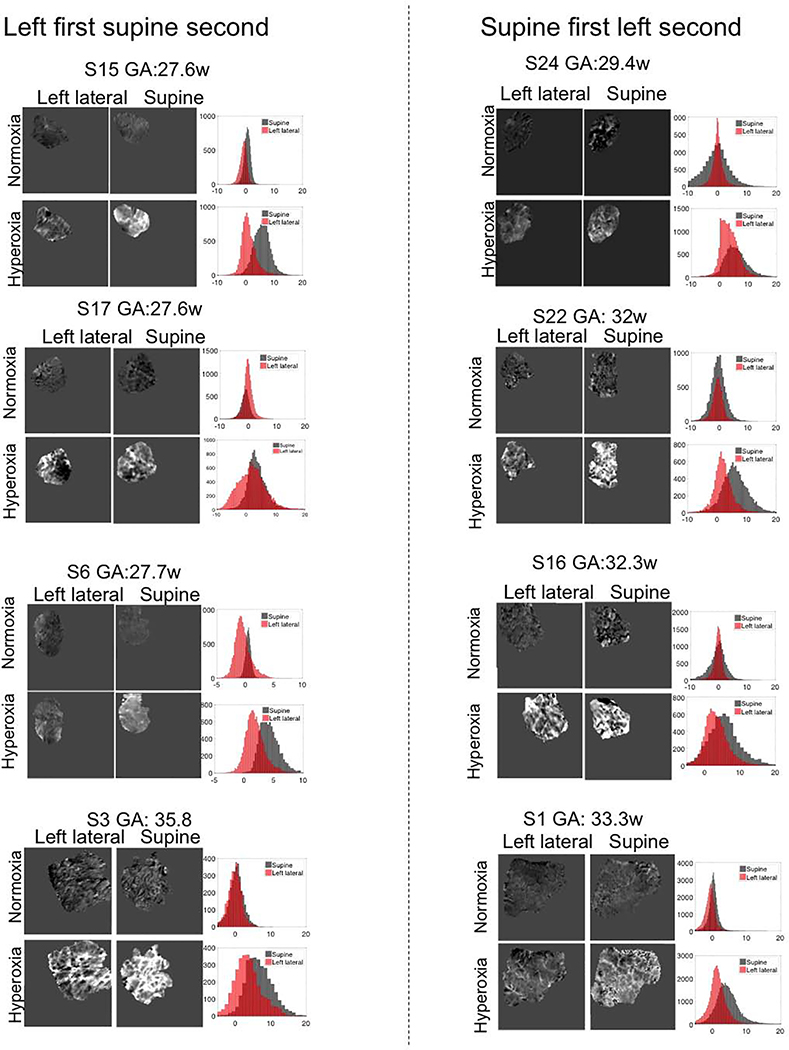
Figure 2 shows the spatial variation in R2* change in the central coronal slice and histograms for whole placenta during baseline and maternal hyperoxia in supine and left lateral positions for the same eight subjects as in Figure 1.
Figure 2.
Coronal views selected as the center slice for placental normalized R2* image after placenta flattening for each subject and histograms in both maternal positions for whole placenta during normoxia and hyperoxia. Left column: left lateral position first. Right column: supine position first. Images were ordered according to the gestational age increasing from top to bottom.
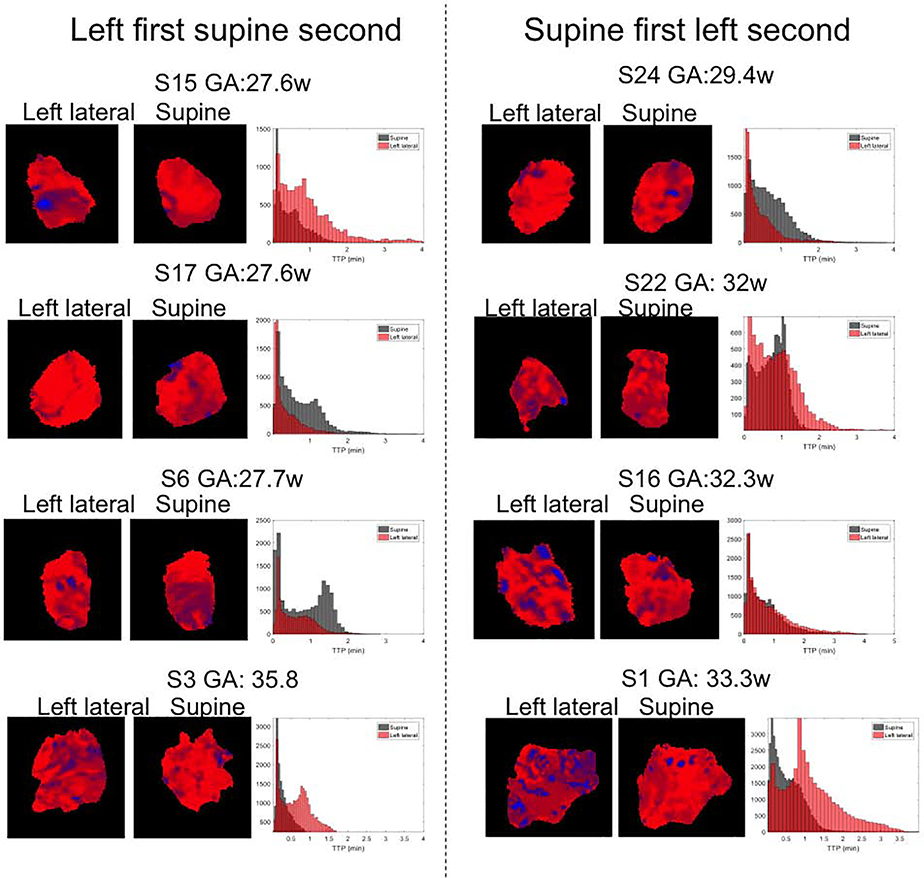
Average TTP values, which can be thought as a dynamic global placental measure, did not show significant differences with maternal position (0.06 ± 0.11, p=0.6). Additionally, in contrast to our previous study of monochorionic-diamniotic twins [6], we did not observe any significant correlation between GA and average TTP (R2=0.05, p=0.43). On the other hand, for each individual, different spatial patterns in TTP maps in the supine versus left lateral position shown in Figure 3 indicate a change in spatiotemporal dynamics of oxygen driven signal change with changes in maternal position. Histogram plots in the same figure show the change in distribution of TTP values over the placenta in two positions, further supporting that position changes spatiotemporal dynamics of oxygen transport.
Figure 3:
Coronal views showing the center slice for TTP maps after placenta flattening for each subject and histograms of TTP values in both maternal positions for whole placenta. Left column: left lateral position first. Right column: supine position first. Images were ordered according to the gestational age increasing from top to bottom.
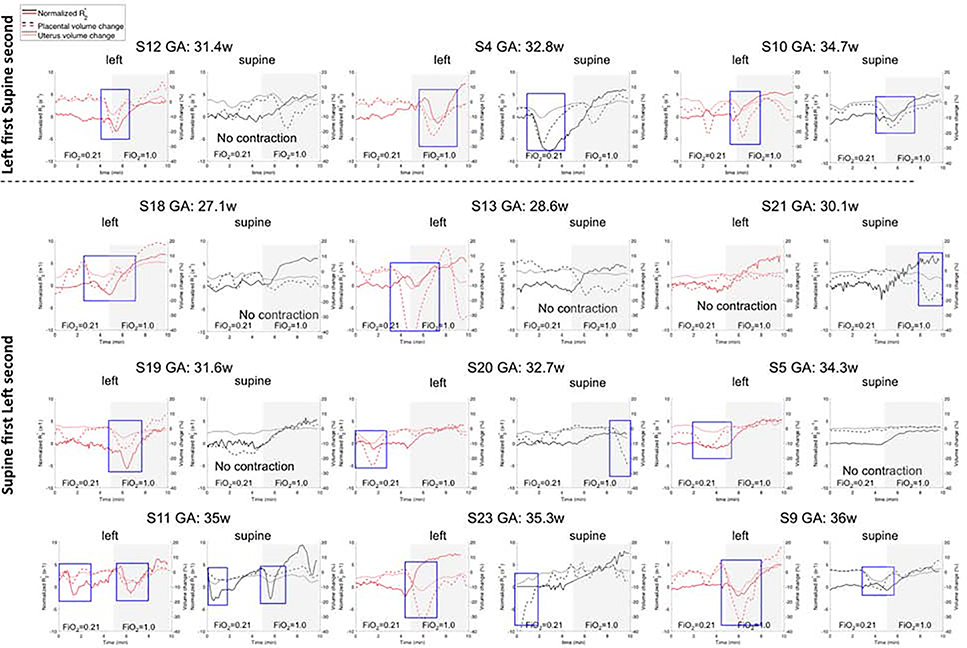
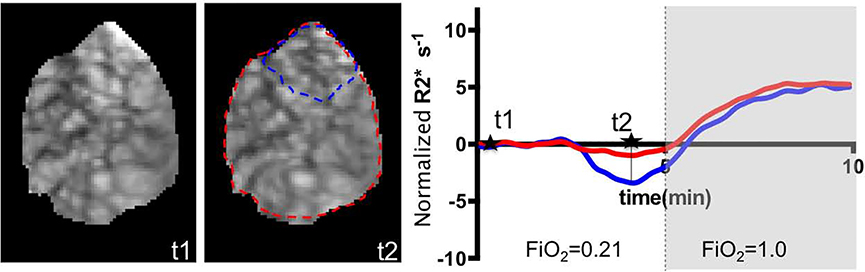
Figure 4 shows the change in R2* relative to baseline and also placental and uterus volume changes over time for the twelve subjects with Braxton-Hicks contractions. The period of contractions were indicated with a blue box. The duration that the effect of a contraction on the volume and signal changes was observed varied between 2 to 4 minutes. Note that even though there is always a random fluctuation in placental and uterus volume curves, at the time of contraction there is a consistent drop in both volumes. The effect of contractions on R2* obscured any effect of maternal positioning. For seven subjects (S4, S9, S11, S12, S18, S19, S23) we observed a global placental BOLD signal drop during the contraction. The maximum drop in normalized R2 during a contraction increased with the amount of placental volume change (0.28 units per % of placental volume change with a standard error of 0.19). For S11, we observed two contractions 5 minutes apart in both positions. As this patient was near term (i.e. she delivered 2 weeks and 6 days later), more frequent contractions were not unexpected. For the remaining five subjects (S5, S10, S13, S20, S21) the contraction was localized and while not large enough to alter the global placental signal, clearly affected the spatial distribution of the signal intensity as seen for Subject 5 (Figure 5).
Figure 4:
Effect of Braxton-Hicks Contractions on change of normalized R2*, placental and uterine volume with time in Supine and Left Lateral Positions. The vertical dotted line represented the time 100% FiO2 was initiated. Top row: left lateral position first. Other three rows: supine position first. S = subject, GA = gestational age, w = weeks. Images were ordered according to the gestational age increasing from left to right. The regions in which simultaneous drops in uterine volume and placenta volume were observed were indicated as a marker of uterine contractions with a blue box.
Figure 5:
BOLD images acquired at t1 and t2 demonstrating the change in the signal during contraction and time activity curves generated for whole placenta (red) compared to the region with a dominant BOLD signal decrease during the contraction (blue).
There was no clinically significant difference in heart rate or arterial oxygen saturation (SaO2) for the different maternal positions (1.9 ± 2.3, p=0.4; 0.2 ± 0.2, p=0.3, respectively) and the minor differences in blood pressure were due to the change in position of the cuff in relation to the heart in the supine position. Supplementary Table 2 shows the average values of maternal blood pressure (diastolic, systolic), SaO2, and heart rate (HR) collected in supine and left lateral positions. Other than the three subjects who withdrew secondary to symptoms consistent with supine hypotensive syndrome, none of the remaining subjects experienced symptoms or showed evidence of the syndrome.
Discussion
In this study we investigated the effect of maternal position and Braxton-Hicks contractions on global and regional placental R2* change and TTP using BOLD MRI time intensity curves with maternal oxygen administration in the third trimester. In mothers without contractions, we demonstrated a consistently greater increase in global placental R2* in the supine position compared to the left lateral position with maternal oxygen administration. Maternal position did not alter global TTP but did result in regional changes in TTP. Finally, Braxton-Hicks contractions can dominate signal R2* change even during maternal hyperoxia. Thus both maternal position and contractions affect the spatiotemporal characteristics of the placental BOLD time course with maternal oxygen administration.
While we have demonstrated a significant effect of maternal positioning on placental BOLD MRI signals obtained with maternal hyper-oxygenation, the exact cause of these changes is unknown. Although changes in maternal arterial and venous hemodynamics may contribute, we believe our results are most consistent with changes in baseline placental oxygenation induced by the supine position. First, maternal administration of oxygen raises the partial pressure of oxygen (pO2) in maternal blood. Relying on the oxygen-hemoglobin saturation curve, changes in oxygen saturation of hemoglobin for a given change in the pO2 are greater at near lower physiologic pO2 levels [29]. Therefore, one would expect greater absolute changes in percent saturation, as we observed in the supine position, when starting at lower hemoglobin oxygen saturation levels. Next, investigators studying the effect of maternal positioning on maternal arterial oxygenation consistently show lower values in the supine position. Relying on arterial blood gas analyses, Awe and colleagues demonstrated that while maternal arterial oxygenation in the third trimester is typically normal in the sitting position, most women will have an abnormal lung alveolar-arterial O2 (A-aO2) gradient in the supine position and more than 50% of these will have an A-aO2 gradient > 20 mmHg [30]. With similar methods, but studying patients in all three trimesters, Spiropoulos and colleagues also reported a significant decrease in maternal arterial oxygen tension in supine position compared to the sitting position in the second and third trimesters [31]. This well recognized change in maternal arterial oxygen saturation is complex, but largely due to a decrease in maternal functional residual capacity of the lungs in the third trimester [32].
Another contributor to the greater change in the placental BOLD MRI signal seen in the supine position may be due to changes in intervillous blood flow induced by changes in maternal position. Radionuclide studies performed with 99mTc and 133Xe demonstrate a decrease in intervillous blood flow associated with the maternal supine position especially in the late gestational ages [33,34]. Studies exploring the effect of maternal position on uterine artery Doppler flow velocity waveforms generally suggest decreased flow with supine compared to sitting or left lateral positions [35]. Finally, if maternal venous return is impaired by extrinsic caval compression, maternal venous effluent from the intervillous space may be impaired causing pooling of a larger volume of lower oxygenated ‘venous’ blood in the placenta when supine.
In addition to a positional effect on the placental BOLD MRI signal, we also noted clear changes in the regional distribution of the TTP of the BOLD signal between the two positions as depicted in Figure 3. This suggests that regional heterogeneities in the spatiotemporal measures of placental oxygen transport are induced by changes in maternal position. This is consistent with the classic studies from Ramsey and colleagues with cineradiography demonstrating that not all of the maternal spiral arteries perfuse the intervillous space at the same time in the hemochorial placenta, emphasizing the changing spatiotemporal nature of placental perfusion [12].
Finally, fifty seven percent of our subjects had Braxton-Hicks contractions and a majority of these (58%) had profound global placental BOLD MRI signal drops during the contraction whether the mother was receiving oxygen or not. We found that the corresponding signal decreases were so great as to obscure any possible changes induced from maternal oxygen administration or change in position. These findings are consistent with the report of Sinding and colleagues [19], who noted similar findings in 8 of 56 women undergoing BOLD MRI in the 3rd trimester. In our study Braxton-Hicks contractions profoundly affected both the baseline BOLD images and dynamic R2* time course signals from the placenta. This is also consistent with the primate cineradiographic studies of Ramsey and Donner demonstrating that even non-labor contractions in the mid trimester (analogous to Braxton-Hicks contractions) can curtail maternal blood flow from the spiral arteries [12]. With such contractions, even within-subject comparisons, such as we attempted while studying the effect of position, were impossible. These findings emphasize the importance of accounting for contractions for accurate analysis of the entire placenta as well as for spatiotemporal analysis.
A recognized limitation of placental BOLD MRI is that it does not directly provide a quantitative measurement of oxygen saturation. To better explain placental hemodynamic changes with maternal positioning, a method to assess baseline oxygenation and blood flow quantitatively would be extremely helpful. Another limitation could be residual effects of the period of oxygen breathing in the first BOLD-MRI study persisting in the placenta into the second study. However, we expect that acquiring the BOLD datasets with oxygen exposure for the two positions 20 minutes apart should allow the BOLD image signal to decrease to baseline after maternal hyperoxygenation in the first position before image acquisition during hyperoxygenation in the second position. We also believe that randomizing the initial position helped to minimize this effect.
A limitation of our study is the inability to distinguish between the effects of changing fetal versus maternal blood oxygenation on the placental BOLD MRI signal. Some of the change in signal is certainly due to the change in oxygen saturation of fetal blood as oxygen is transferred from mother to fetus. Sorensen and colleagues [4] demonstrated visually greater changes from dark to light in areas closer to the fetal surface than the maternal surface with oxygen administration. Because we examined the BOLD MRI time course of the whole placenta since fetal and maternal components are challenging to separate, we are unable to distinguish the relative contributions of changes in normalized R2* imparted by changes in maternal blood oxygen saturation from changes in fetal blood oxygen saturation.
In conclusion, we have demonstrated that for in vivo human placental imaging both maternal position and Braxton-Hicks contractions significantly affect regional changes in R2* and regional TTP obtained with BOLD MRI during maternal oxygen exposure. This suggests that maternal position must be standardized or otherwise addressed in analyses when comparing placental BOLD signals over time both within and between individuals. Similarly, because Braxton-Hicks contractions profoundly affect placental BOLD MRI signals, future investigations must also exclude or account for their presence during any study. Finally, we have demonstrated that in addition to significant changes in the global placental BOLD signals, maternal position also affects the spatial and temporal distribution or regional heterogeneity of BOLD signals obtained from the placenta. This suggests that in addition to uterine contractions, even maternal position may induce regional changes in oxygenation within the placenta that vary over time. The results of our investigation suggest that future placental BOLD MRI investigations relying on baseline R2* or changes in R2* with maternal oxygenation should account for maternal position in the scanner and that changes in R2* with maternal oxygenation will be higher when mothers are supine.
Supplementary Material
Highlights.
Placental R2* increase with maternal hyperoxia is influenced by maternal position.
Maternal position affects the spatiotemporal distribution of placental BOLD signals.
Maternal position does not alter global TTP but results in regional changes in TTP.
Braxton-Hicks contractions can dominate R2* change even during maternal hyperoxia.
Braxton-Hicks contractions significantly affect regional changes in R2*.
Acknowledgments
Grant support
This study was funded by the National Institutes of Health (grant numbers: U01 HD087211, R01 EB017337, P41 EB015902, R01 HD100009).
Footnotes
The authors have no conflict of interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wedegärtner U, Tchirikov M, Schäfer S, Priest AN, Kooijman H, Adam G, Schröder HJ, Functional MR imaging: comparison of BOLD signal intensity changes in fetal organs with fetal and maternal oxyhemoglobin saturation during hypoxia in sheep, Radiology. 238 (2006) 872–880. [DOI] [PubMed] [Google Scholar]
- [2].Sørensen A, Sinding M, Peters DA, Petersen A, Frøkjær JB, Christiansen OB, Uldbjerg N, Placental oxygen transport estimated by the hyperoxic placental BOLD MRI response, Physiol Rep. 3 (2015). 10.14814/phy2.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sørensen A, Pedersen M, Tietze A, Ottosen L, Duus L, Uldbjerg N, BOLD MRI in sheep fetuses: a non-invasive method for measuring changes in tissue oxygenation, Ultrasound Obstet. Gynecol. 34 (2009) 687–692. [DOI] [PubMed] [Google Scholar]
- [4].Sørensen A, Peters D, Fründ E, Lingman G, Christiansen O, Uldbjerg N, Changes in human placental oxygenation during maternal hyperoxia estimated by blood oxygen level- dependent magnetic resonance imaging (BOLD MRI), Ultrasound Obstet. Gynecol 42 (2013) 310–314. [DOI] [PubMed] [Google Scholar]
- [5].Aimot-Macron S, Salomon LJ, Deloison B, Thiam R, Cuenod CA, Clement O, Siauve N, In vivo MRI assessment of placental and foetal oxygenation changes in a rat model of growth restriction using blood oxygen level-dependent (BOLD) magnetic resonance imaging, Eur. Radiol 23 (2013) 1335–1342. [DOI] [PubMed] [Google Scholar]
- [6].Luo J, Abaci Turk E, Bibbo C, Gagoski B, Roberts DJ, Vangel M, Tempany- Afdhal CM, Barnewolt C, Estroff J, Palanisamy A, Barth WH, Zera C, Malpica N, Golland P, Adalsteinsson E, Robinson JN, Grant PE, In Vivo Quantification of Placental Insufficiency by BOLD MRI: A Human Study, Sci. Rep 7 (2017) 3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Huen I, Morris DM, Wright C, Parker GJM, Sibley CP, Johnstone ED, Naish JH, R1 and R2* changes in the human placenta in response to maternal oxygen challenge, Magn. Reson. Med 70 (2013) 1427–1433. [DOI] [PubMed] [Google Scholar]
- [8].Abaci Turk E, Stout JN, Ha C, Luo J, Gagoski B, Yetisir F, Golland P, Wald LL, Adalsteinsson E, Robinson JN, Roberts DJ, Barth WH Jr, Grant PE, Placental MRI: Developing Accurate Quantitative Measures of Oxygenation, Top. Magn. Reson. Imaging 28 (2019) 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sørensen A, Hutter J, Seed M, Grant PE, Gowland P, T2* weighted placental MRI: basic research tool or an emerging clinical test of placental dysfunction?, Ultrasound Obstet. Gynecol (2019). 10.1002/uog.20855. [DOI] [PubMed] [Google Scholar]
- [10].Chalouhi GE, Salomon LJ, BOLD-MRI to explore the oxygenation of fetal organs and of the placenta, BJOG. 121 (2014) 1595. [DOI] [PubMed] [Google Scholar]
- [11].Siauve N, Chalouhi GE, Deloison B, Alison M, Clement O, Ville Y, Salomon LJ, Functional imaging of the human placenta with magnetic resonance, Am. J. Obstet. Gynecol 213 (2015) S103–14. [DOI] [PubMed] [Google Scholar]
- [12].Ramsey EM, Corner GW Jr, Donner MW, Serial and cineradioangiographic visualization of maternal circulation in the primate (hemochorial) placenta, Am. J. Obstet. Gynecol 86 (1963) 213–225. [DOI] [PubMed] [Google Scholar]
- [13].Howard BK, Goodson JH, Mengert WF, Supine Hypotensive Syndrome in Late Pregnancy, Obstetrics & Gynecology. 1 (1953) 371. [PubMed] [Google Scholar]
- [14].Schmitt D, The hypotensive syndrome in the supine position in late pregnancy, N. Engl. J. Med 259 (1958) 1219–1221. [DOI] [PubMed] [Google Scholar]
- [15].Lee AJ, Landau R, Aortocaval Compression Syndrome: Time to Revisit Certain Dogmas, Anesth. Analg 125 (2017) 1975–1985. [DOI] [PubMed] [Google Scholar]
- [16].Kinsella SM, Lohmann G, Supine hypotensive syndrome, Obstet. Gynecol. 83 (1994) 774–788. [PubMed] [Google Scholar]
- [17].Oosterhof H, Dijkstra K, Aarnoudse JG, Uteroplacental Doppler velocimetry during Braxton Hicks’ contractions, Gynecol. Obstet. Invest 34 (1992) 155–158. [DOI] [PubMed] [Google Scholar]
- [18].Bower S, Campbell S, Vyas S, McGirr C, Braxton-Hicks contractions can alter uteroplacental perfusion, Ultrasound Obstet. Gynecol 1 (1991) 46–49. [DOI] [PubMed] [Google Scholar]
- [19].Sinding M, Peters DA, Frøkjær JB, Christiansen OB, Uldbjerg N, Sørensen A, Reduced placental oxygenation during subclinical uterine contractions as assessed by BOLD MRI, Placenta. 39 (2016) 16–20. [DOI] [PubMed] [Google Scholar]
- [20].Gluud C, Gluud LL, Evidence based diagnostics, BMJ. 330 (2005) 724–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Turk EA, Luo J, Gagoski B, Pascau J, Bibbo C, Robinson JN, Grant PE, Adalsteinsson E, Golland P, Malpica N, Spatiotemporal alignment of in utero BOLD-MRI series, J. Magn. Reson. Imaging 46 (2017) 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC, N4ITK: improved N3 bias correction, IEEE Trans. Med. Imaging 29 (2010) 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Avants BB, Tustison N, Song G, Advanced normalization tools (ANTS), Insight J. 2 (2009) 1–35. [Google Scholar]
- [24].Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW, elastix: a toolbox for intensity-based medical image registration, IEEE Trans. Med. Imaging 29 (2010) 196–205. [DOI] [PubMed] [Google Scholar]
- [25].Guyader J-M, Bernardin L, Douglas NHM, Poot DHJ, Niessen WJ, Klein S, Influence of image registration on apparent diffusion coefficient images computed from free-breathing diffusion MR images of the abdomen, Journal of Magnetic Resonance Imaging. 42 (2015) 315–330. 10.1002/jmri.24792. [DOI] [PubMed] [Google Scholar]
- [26].Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G, User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability, Neuroimage. 31 (2006) 1116–1128. [DOI] [PubMed] [Google Scholar]
- [27].Abulnaga SM, Turk EA, Bessmeltsev M, Grant PE, Solomon J, Golland P, Placental Flattening via Volumetric Parameterization, Lecture Notes in Computer Science. (2019) 39–47. 10.1007/978-3-030-32251-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chung MK, Worsley KJ, Paus T, Cherif C, Collins DL, Giedd JN, Rapoport JL, Evans AC, A unified statistical approach to deformation-based morphometry, Neuroimage. 14(2001)595–606. [DOI] [PubMed] [Google Scholar]
- [29].Severinghaus JW, Simple, accurate equations for human blood O2 dissociation computations, J. Appl. Physiol 46 (1979) 599–602. [DOI] [PubMed] [Google Scholar]
- [30].Awe RJ, Nicotra MB, Newsom TD, Viles R, Arterial oxygenation and alveolar-arterial gradients in term pregnancy, Obstet. Gynecol 53 (1979) 182–186. [PubMed] [Google Scholar]
- [31].Spiropoulos K, Prodromaki E, Tsapanos V, Effect of body position on PaO2 and PaCO2 during pregnancy, Gynecol. Obstet. Invest 58 (2004) 22–25. [DOI] [PubMed] [Google Scholar]
- [32].Nørregaard O, Schultz P, Ostergaard A, Dahl R, Lung function and postural changes during pregnancy, Respir. Med 83 (1989) 467–470. [DOI] [PubMed] [Google Scholar]
- [33].Suonio S, Simpanen AL, Olkkonen H, Haring P, Effect of the left lateral recumbent position compared with the supine and upright positions on placental blood flow in normal late pregnancy, Ann. Clin. Res 8 (1976) 22–26. [PubMed] [Google Scholar]
- [34].Kauppila A, Koskinen M, Puolokka J, Tuimala R, Kuikka J, Decreased Intervillous and Unchanged Myometrial Blood Flow in Supine Recumbency, Obstetric Anesthesia Digest. 1 (1981) 9 10.1097/00132582-198103000-00009. [DOI] [PubMed] [Google Scholar]
- [35].Jeffreys RM, Stepanchak W, Lopez B, Hardis J, Clapp JF 3rd, Uterine blood flow during supine rest and exercise after 28 weeks of gestation, BJOG. 113 (2006) 1239–1247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.