Abstract
BENTA (B cell Expansion with NF-κB and T cell Anergy) is a novel lymphoproliferative disorder caused by germline, gain-of-function (GOF) mutations in the lymphocyte-restricted scaffolding protein CARD11. Similar somatic CARD11 mutations are found in lymphoid malignancies such as diffuse large B cell lymphoma (DLBCL). Normally, antigen receptor (AgR) engagement converts CARD11 into an active conformation that nucleates a signalosome required for IκB kinase (IKK) activation and NF-κB nuclear translocation. However, GOF CARD11 mutants drive constitutive NF-κB activity without AgR stimulation. Here we show that unlike wild-type CARD11, GOF CARD11 mutants can form large, peculiar cytosolic protein aggregates we term mCADS (mutant CARD11 dependent shells). MALT1 and phospho-IKK are reliably colocalized with mCADS, indicative of active signaling. Moreover, endogenous mCADS are detectable in ABC-DLBCL lines harboring similar GOF CARD11 mutations. The unique aggregation potential of GOF CARD11 mutants may represent a novel therapeutic target for treating BENTA or DLBCL.
Keywords: CARD11, BENTA, NF-kB, B cell lymphoma, Signalosome, Aggregates, MALT1
1. Introduction
We and others have previously described several patients with a novel human congenital lymphoproliferative syndrome we termed B cell Expansion with Nuclear Factor-κB and T cell Anergy (BENTA) [1,2]. Although BENTA patients present with recurrent infections and other signs of immunodeficiency, the distinguishing disease feature is striking polyclonal B cell accumulation that may increase risk for B cell malignancy later in life [3,4]. BENTA disease is caused by germline, gain-of-function (GOF) missense mutations in CARD11, which encodes a large scaffold protein expressed mainly in lymphocytes. CARD11 is best known for its essential role in antigen receptor (AgR)-mediated activation of NF-κB, a key transcription factor complex that governs the expression of many genes that promote proliferation, survival, and effector function [5,6]. If not regulated properly, however, NF-κB becomes a powerful pro-oncogenic factor for lymphomagenesis. Indeed, constitutive NF-κB activity is a hallmark of certain B cell lymphomas, such as the activated B cell-like subtype of diffuse large B cell lymphoma (ABC-DLBCL) [7–9]. A reliance on chronic NF-κB activation renders ABC-DLBCL particularly refractory to standard chemotherapy. In ~10% of ABC-DLBCL, somatic GOF CARD11 mutations similar to those found in BENTA patients are the primary drivers of constitutive NF-κB activity [10],.
AgR stimulation in normal T and B cells employs CARD11 as an active scaffold for nucleating a large multiprotein signalosome that facilitates activation of the IκB kinase (IKK) complex, which ultimately leads to phosphorylation and degradation of IκB and subsequent release of latent, cytosolic NF-κB into the nucleus. In resting cells, the flexible linker or ‘‘inhibitory domain (ID)” maintains CARD11 in an inactive conformation by interacting with and preventing access to the CARD, LATCH (L), and coiled-coil (CC) domains (Fig. 1A). Proximal AgR signaling triggers phosphorylation of several serines in the ID, inducing an ‘‘open” conformation that enables recruitment of BCL10 and MALT1, forming the CARD11-BCL10-MALT1 (CBM) complex [11,12]. The filamentous CBM complex acts as a nexus for recruitment of several other signaling molecules (e.g. TRAF6, TAK1, caspase 8, LUBAC complex) that cooperate in the activation of IKK [13],. GOF CARD11 mutations found in BENTA and DLBCL are concentrated in the CARD, LATCH and CC domains and can decouple ID phosphorylation from CC-dependent CBM complex assembly, resulting in constitutive NF-κB activation [14,15].
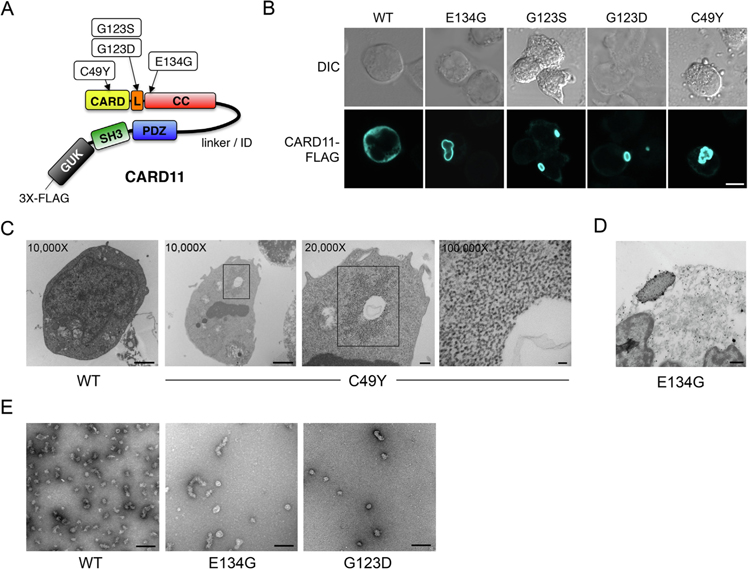
Fig. 1.
Gain-of-function CARD11 mutants form mCADS. (A) Cartoon of CARD11 protein indicating approximate locations of BENTA-associated GOF mutations described. L = LATCH domain, CC = coiled-coil. (B) Confocal immunofluorescent (IF) microscopy analysis of BJAB B cells transfected with WT and GOF CARD11-FLAG mutants, detected using anti-FLAG-AlexaFluor 647 Ab. Merge image includes DAPI staining. Scale bar = 5 μm. (C) JPM50.6 T cells were transfected with WT or C49Y CARD11 and fixed for TEM analysis 24 hrs post-transfection. Numbers indicate magnification, squares denote putative mCADS that are not found in WT cells (n = 30 cells analyzed for each). Data are representative of two independent experiments performed with WT, C49Y, E134G, or G123D CARD11. (D) ImmunoGold staining and TEM analysis of JPM50.6 T cells transfected with E134G CARD11 as prepared in Supp. Fig. 2A. Data are representative of 2 independent experiments. (E) TEM micrographs of recombinant CARD11 (aa 8–302) showing heterogeneous clusters of WT (20–40 nm), E134G (60–120 nm), and G123D (20–40 nm).
Evidence suggests that oligomerization of wild-type (WT) CARD11 at the immunological synapse (IS) is an important feature of normal, AgR-dependent CBM signalosome formation. Both CC-dependent self-association and higher order multimerization through the membrane-associated guanylate kinase (MAGUK) domain is critical for downstream signal propagation [16–18]. We and others have demonstrated that GOF CARD11 mutations, which overwhelmingly reside in and around the CC domain, can induce spontaneous aggregation of CARD11 without AgR stimulation [2,10,19]. Although mutant CARD11 aggregation may correlate with enhanced NF-κB activity, the structure, composition, and signaling capacity of such mutant CARD11 aggregates remains unclear. Biochemical evidence suggests that BCL10/MALT1 interaction with GOF CARD11 mutants is enhanced without affecting association with other critical signaling partners (e.g. TRAF6, TAK1, IKK) [14],. However, imaging studies suggest that the redistribution of CBM signaling components in normal lymphocytes is highly dynamic. Initial microclustering of CARD11 at the IS is followed by the formation of smaller, cytosolic punctate BCL10-MALT1 signaling structures known as POLKADOTS that serve to both stimulate IKK phosphorylation and regulate NF-κB signaling via proteosomal and autophagic degradation of BCL10 [20,21],[22]. Moreover, recent structural studies of the reconstituted CBM complex indicate that CARD11 oligomers nucleate the assembly of BCL10 filaments sheathed in MALT1 [23,24]. These studies collectively suggest that the supramolecular organization of the CBM complex is important for signal amplification and modulation.
To determine how GOF mutations in CARD11 might alter this signalosome, we utilized an imaging approach to carefully interrogate how mutant CARD11 aggregation contributes to persistent NF-κB signaling in T and B cell lines. Here we demonstrate that unlike WT CARD11, GOF CARD11 mutants spontaneously aggregate when ectopically expressed in lymphocytes, and readily assemble into a distinct, cytosolic, often shell-shaped signalosome that facilitates stable IKK phosphorylation and constitutive NF-κB activation. Furthermore, these structures are detectable in ABC-DLBCL cell lines harboring GOF CARD11 mutations. The enhanced, intrinsic aggregation potential and peculiar self-assembly of GOF CARD11 mutant proteins, revealed by mCADS formation, may present a novel target for disrupting chronic CBM signaling without adversely affecting normal AgR signaling to NF-κB.
2. Materials and methods
2.1. Cell culture
All T and B cell lines were cultured in complete RPMI 1640 (Lonza) supplemented with 10–20% heat-inactivated fetal calf serum (Valley Biomed, HyClone), 2 mM glutamine, and 100U/ml each of penicillin and streptomycin (Life Technologies). Jurkat T cells (E6.1) and BJAB B cell lines were purchased from the American Tissue Type Collection. JPM50.6 T cells and MALT1-knockdown (KD) Jurkat T cells were kindly provided by Dr. Lawrence Kane (University of Pittsburgh). NEMO-deficient JM.4.5.2 T cells were a gift from Dr. Brian Schaefer (USUHS). DLBCL lines (OCI-Ly3, WSU-NHL, U2932, HBL-1) were kindly provided by Dr. Louis Staudt (NCI, NIH). In some experiments, T cell lines were stimulated using 1 μg/ml each of anti-CD3e and anti-CD28 agonistic Abs (BD Biosciences).
2.2. DNA cloning and mutagenesis
The human CARD11 expression vector was purchased from InvivoGen. A 3X FLAG tag was inserted using a pair of annealed, overlapping oligonucleotides that encoded the tag and included BamHI (5′) and NheI (3′) overhangs. This fragment was ligated into BamHI/NheI digested pUNO1-huCARD11 plasmid. Site directed mutagenesis for mutant CARD11 constructs was performed using specific primers for linear amplification using 2x Pwo DNA polymerase (Roche), followed by digestion of methylated DNA using DpnI (Thermo Scientific). All plasmids were purified from het-shock transformed competent DH5α E. coli (New England Biolabs) and selected using Blastidicin (InvivoGen).
2.3. Cell transfections
WT, JPM50.6, or JM4.5.2 Jurkat T cells or BJAB B cells (5 × 106/cuvette) were electroporated with 0.5–10 μg DNA in 0.4 mL RPMI plus 10% FBS (no antibiotics) using a BTX Electroporator (BTX Harvard Apparatus) using the following parameters: 260 V, 950 μF, 0Ω for Jurkat cells, and 250 V, 900 μF, 0Ω for BJAB cells. Transfected cells were then incubated in 4 mL RPMI + 10% FBS (no antibiotics) for 4–48 h prior to analysis.
2.4. Confocal immunofluorescence microscopy
Poly-l-lysine coated slides (EMS) were prepared by drawing circular dams using a hydrophobic pen (Super PAP Pen) leaving an opening ~3 mm in diameter (6–8 per slide). Approximately 200,000 cells suspended in 25 μL of PBS were applied into each circle and in a humidified chamber at room temperature (RT) for 30 mins to allow cells to adhere. Cells were then fixed for at least 30 min with an equal volume of 4% paraformaldehyde (Electron Microscopy Services, EMS). Samples were washed 3X with 50 μL PBS/circle using gentle aspiration. Samples were permeabilized with 20 μL 0.1% Triton X100 in PBS for 3–5 mins, washed 3X in PBS, and subsequently blocked with 25 μL 1% FBS in PBS for at least 30 mins. Blocking solution was aspirated, and primary antibodies were applied in 25 μL 1% FBS in PBS. Cell spots were incubated overnight at 4 °C with the following Abs: anti-CARD11 (ab91463, Abcam), anti-BCL10 (clone 331.3), anti-MALT1 (clone B-12), anti-TRAF6 (clone D-10), anti-TAK1 (clone C-9, Santa Cruz Biotech), anti-caspase 8 (clone 3–1-9, BD Biosciences), anti-phospho-IKKα/β (#2694 or #2078, Cell Signaling Technology, CST) and anti-IKKc (GTX101139, GeneTex). Samples were washed again 3X in PBS. Goat anti-rabbit or anti-mouse secondary Abs conjugated to AlexaFluor 488 or 568 (Invitrogen) were then applied in 25 μL 1% FBS in PBS for at 1 hr at RT, followed by 4X washes in PBS. The samples were then stained overnight at 4 °C with an AlexaFluor 647-conjugated anti-FLAG Ab (CST) in 25 μL 1% FBS in PBS. Samples were finally washed 6X with 50 μL PBS and coverslips were applied using Fluoromount M with DAPI (EMS). Fluorescent images were acquired on a Zeiss 710 confocal laser scanning microscope using a 63x oil immersion objective. Images were obtained after first minimizing fluorescence signal saturation for each channel, then maintaining identical laser power and gain settings for all channels throughout the experiment. Image analyses (including scoring for size and frequency of mCADS) and conversion of z-stacks into maximum projection TIFF and MOV files were performed using ZEN software (Zeiss, ver. 2012). Blinded scoring of CARD11 aggregates (defined as punctate spots of any size) and/or mCADS (defined as >1 mM with CARD11 staining enriched around the rim of the aggregate) was performed in at least 4 separate image fields/sample (n = ~50–200 total cells scored) by 3 independent scorers. Average pixel intensity ratios for anti-ph-IKK/anti-FLAG staining in Jurkat vs. JM4.5.2 cells (n = 13–14 cells) were calculated using ImageJ software. For some preliminary analyses, SR-SIM images were collected on identically processed sample slides using an ELYRA PS.1 system (Leica) with a 63x objective.
2.5. Electron microscopy
Transfected cells (~1 × 107) were gently pelleted by centrifugation (10 min at 90×g) and pellets were subsequently fixed at 4 °C in freshly prepared 2% paraformaldehyde/2% glutaraldehyde (EMS) in cacodylate buffer (CB). Following primary fixation, samples were washed 3 × 10 min in CB followed by immersion in 2% osmium tetroxide in CB (OsO4; EMS) for 1 hr. Samples were then washed again in CB, dehydrated in a graduated series of increasing ethanol concentrations, and infiltrated with Spurr’s epoxy resin (EMS). Samples were then polymerized at 70 °C for 11 hrs and thin sections (~70 nm) were cut on a Leica Ultracut UC6 ultramicrotome (Leica). Copper grids containing thin sections were post-stained for 20 min in 2% aqueous uranyl acetate (EMS) and 5 min in Reynold’s lead citrate [25],. Samples were viewed on a JEOL JEM-1011 transmission electron microscope (JEOL USA, Inc.) and images were captured on an AMT XR50S-A digital camera (Advanced Microscopy Techniques). For immunogold labeling, transfected cells were stained as described above with anti-CARD11 (Abcam) followed by anti-rabbit AlexaFluor 488 FluoroNanogold Conjugate (Nanoprobes). Following Ab labeling, cells were incubated in 2% glutaraldehyde (EMS) in CB for 1 h. Samples were then washed 3X 10 min in CB and then incubated in 0.2 M glycine for 10 min to quench any unreacted aldehydes. Following a 2X 10 min wash with ddH2O, samples were immersed in GoldEnhance EM (Nanoprobes) for 5 min. After enhancement of gold nanoparticles, cells were rinsed in ddH2O 3X 10 min, incubated with 2% OsO4 in ddH2O (EMS), and washed again in ddH2O for 3X 10 min. Further processing was identical to cells treated for standard EM as described above. Human CARD11 (8–302) constructs (WT, E134G, and G123S) were expressed in E. coli and purified by Ni-NTA resin (Qiagen) and gel filtration chromatography (Superdex 200 10/300 GL, GE Healthcare) as a void fraction. TEM analysis of purified proteins was performed as previously described [22],. Samples were imaged with a Tecnai G2 Spirit BioTWIN operating at 80 KeV.
2.6. Luminescence assays
Luminescence assays to measure NF-κB activation were performed using a Dual Luciferase kit (Promega). Generally, cells were electroporated as described above with 5 μg CARD11 expression plasmid, 5 μg NF-κB-dependent firefly luciferase reporter plasmid (pNF-κB-Luc), and 0.25 μg of Renilla luciferase constitutive expression plasmid (pRL-TK) for transfection normalization. After 24–48 hrs, samples were processed and read on a Bio-Tek Synergy H1 Mulimode Microplate Reader using Gen5 software. Relative NF-κB activation was determined by normalizing the relative ratio of firefly luciferase signal to Renilla.
2.7. Flow cytometry
CD83 upregulation in transfected BJAB cells was used as a marker of NF-κB activation. Approximately 0.5 × 106 cells were washed in cold FACS buffer (1x PBS, 1% FBS, 0.1% sodium azide) and stained for 30 min on ice with 5 μg of FITC anti-CD83 (BD Biosciences). Cells were subsequently washed in cold FACS buffer and acquired on a C6 Accuri Flow Cytometer (BD Biosciences). GFP reporter expression was used to measure NF-κB activation in transfected JPM50.6 cells. GFP levels were determined following 1 wash in cold FACS buffer and acquired as described above. Data analysis was performed using C6Flow or FlowJo software (BD Biosciences).
2.8. Immunoblotting and in vitro kinase assays
Transfected cells or DLBCL lines (5–10 × 106) were lysed in 1% NP-40 lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 0.5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 1 mM Na3VO4, 1 mM NaF) containing Complete protease inhibitors (Roche) for 30 min on ice. Insoluble material was cleared by 5 min centrifugation at 4 °C (14,000 rpm). Lysates (5–10 μg protein each) were boiled in 2X SDS Lamelli sample buffer + 5% 2-ME for 5 min and resolved on 4–20% Tris glycine SDS-PAGE gels (BioRad). Gels were transferred to nitrocellulose, blocked in 5% milk in TBS/0.1% Tween, and probed with the following Abs: anti-FLAG (clone M2) and anti-b-actin (clone AC15, Sigma), anti-IKKγ/NEMO (#2685) and anti-CARD11 (clone 1D12, Cell Signaling Technology). Bound Abs were detected using HRP-conjugated secondary Abs (Southern Biotech) and enhanced chemiluminescence (Thermo Scientific).
For in vitro kinase assays, 5 × 106 transfected JPM50.6 cells were washed in cold PBS and lysed in 250 μL cold lysis buffer (20 mM Tris-HCl pH 8.0, 150 mM NaCl, 5 mM NaF, 5 mM sodium glycerol phosphate, 1 mM DTT, 0.1% Triton X-100, PhosStop and Compete Protease Inhibitor cocktails (Roche)). Cell lysates were incubated on ice for 1 hr, with 15 sec vortexing every 15 min. Lysates were first centrifuged at 4 °C at low speed (500×g) to remove intact organelles; supernatants were then centrifuged at high speed (14,000 rpm) for 15 min. After removing supernatants (soluble fraction), insoluble pellets were incubated with 27 μL kinase buffer (20 mM HEPES pH 7.3, 2 mM MnCl2, 10 mM MgCl2, 1 mM DTT, 5 mM NaF, 25 mM β-glycerophosphate), 1 μL ATP, and 2 μL GST-IκBα (Rockland) for 30 min at 37 °C. Samples were subsequently boiled upon addition of 30 μL 2x SDS sample buffer and processed for immunoblotting as above. Membranes were probed with the following Abs: anti-MALT1 (clone H300) and anti-IKKβ (clone 24, Santa Cruz Biotechnology), anti-phospho-IκB (clone 14D4) and anti-IκBa (#9242, Cell Signaling Technology), anti-FLAG (M2) and anti-β-actin (AC15, Sigma). Bound Abs were detected using IRDye-conjugated anti-mouse/rabbit secondary Abs on the Odyssey CLx system (LiCOR).
3. Results
3.1. Gain-of-function CARD11 mutants spontaneously form cytosolic protein shells
Previously, we demonstrated that BENTA associated gain-of-function (GOF) mutants of CARD11 drive constitutive NF-κB activity when expressed ectopically as N-terminal Venus fusion proteins. We also showed using confocal microscopy that mutant Venus-CARD11 fusion proteins spontaneously formed intracellular aggregates reminiscent of those seen in BENTA patient lymphocytes, and that BCL10 and MALT1 could be found co-localized with CARD11 in these aggregates [2,19]. However, we noted that mutant Venus-CARD11 fusions did not stimulate NF-κB activation as well FLAG-tagged CARD11 mutants that lacked the fluorescent protein moiety. Venus also dimerizes independently, which could contribute to artifactual aggregation of the CARD11 fusion protein [26],. We therefore utilized BENTA-associated GOF CARD11 mutant constructs for which the N-terminal Venus moiety was replaced with the smaller 3X-FLAG tag on the C-terminus (Fig. 1A). We transiently transfected these CARD11-FLAG constructs into human B (BJAB) and T (JPM50.6) cell lines via electroporation to test their ability to spontaneously aggregate and signal to NF-κB. As expected, WT CARD11 produced a relatively diffuse cytoplasmic expression pattern upon staining with an anti-FLAG antibody (Ab). In striking contrast, mutant CARD11-FLAG proteins aggregated into large shell-like structures in the cytosol (Fig. 1B) that were distinct from globular aggregates we observed with Venus-CARD11 fusions [2],. Herein we refer to these structures as ‘‘mutant CARD11-dependent shells (mCADS)”. We define mCADS as cytosolic aggregates of CARD11 that are (a) >1 μM in diameter, and (b) appear partly ‘‘hollow” based on fluorescence, with an intense fluorescent signal along the ‘‘rim” that is considerably brighter than the diffuse, cytoplasmic signal represented by WT CARD11. Despite considerable variations in size, shape and convolution, several BENTA-associated GOF CARD11 mutants were capable of forming mCADS (Fig. 1B). mCADS increased in size as the amount of ectopic expression was increased, and ectopic CARD11-FLAG expression always outweighed the amount of endogenous CARD11. However, mCADS were still readily detected when GOF CARD11 expression was titrated down to levels closer to with endogenous CARD11 in BJAB cells (Supp. Fig. 1), suggesting this distinct aggregation pattern is enhanced by, but not simply an artifact of, CARD11 overexpression. Moreover, WT CARD11 did not form mCADS-like structures at the highest dose of plasmid transfected (5 μg, Fig. 1B). Both 2- and 3-dimensional projections reconstructed from multiple z-stack images suggested that mCADS were enclosed with anti-FLAG (CARD11) staining primarily concentrated in the outer layer of the shell (Supp Fig. S2A, Supp Video 1). In some cases (e.g. E134G), single mCADS accounted for >30% of the cell volume and physically displaced the nucleus (Supp. Video 1). These results suggest GOF CARD11 mutants can spontaneously assemble into cytosolic protein aggregates with a novel, shell-like superstructure.
We initially hypothesized that mCADS represented membrane-bound vesicles that might be associated with a subcellular structure such as an endosome, lysosome, autophagosome, or aggresome. However, extensive co-stains for relevant markers (e.g. LAMP-1, p62, etc.), cytoskeletal components (e.g. actin, tubulin, septins, vimentin) and organelle-specific dyes (e.g. LysoTracker) suggested mCADS did not colocalize with any known cytosolic or membrane-associated structures. We therefore turned to transmission electron microscopy (TEM) to further characterize the mCADS superstructure. BJAB B cells were transiently transfected with WT or mutant CARD11 expression plasmids and processed for TEM analysis 24 h later. Fig. 1C displays characteristic images from several experiments. In cells expressing GOF CARD11 mutants (e.g. C49Y), we detected unique electron dense structures exclusively in the cytoplasm, ostensibly composed of a filamentous protein matrix with no associated lipid membrane, glycogen or ribosomes (Fig. 1C). These structures were not found in untransfected or WT CARD11 transfected cells. Similar results were obtained in transfected JPM50.6 T cells, although electron dense protein was visible throughout the aggregate (Supp. Fig. S2B). Nevertheless, the edges of these proteinaceous structures were abundantly labeled by immuno-TEM using rabbit anti-FLAG antibody and a NanoGold488-anti-rabbit IgG detection antibody (Fig. 1D), matching mCADS structures visualized in the same cells by immunofluorescent microscopy (Supp. Fig. S2A). These results were congruent with biophysical studies that showed a recombinant, active version of WT CARD11 lacking the ID (amino acids 8–302) could form globular aggregates that nucleate the formation of BCL10 filaments [24],. Similarly, we found that recombinant E134G CARD11 purified from E. coli formed larger, more elongated aggregates in isolation compared to WT or G123D CARD11 (Fig. 1E), suggesting this particular mutation enhanced the intrinsic aggregation potential of CARD11 itself.
3.2. mCADS are sites of active signaling to NF-κB
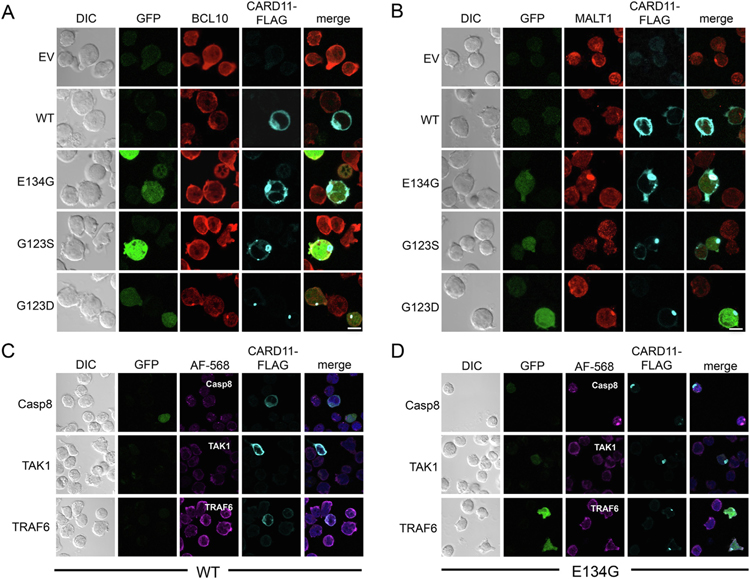
To determine if mCADS represent a higher-order CBM complex, JPM50.6 T cells were transfected with mutant CARD11 expression plasmids and then co-stained for CARD11-FLAG, BCL10 and MALT1. We observed partial colocalization of BCL10 with some mCADS structures (Fig. 2A), particularly at early timepoints after transfection. In contrast, MALT1 was strongly and consistently co-localized with CARD11-FLAG in mCADS formed by all GOF mutants tested (Fig. 2B). These results suggest CBM complex formation occurs at mCADS in the absence of antigen receptor (AgR) ligation. To further characterize the composition of the mCADS signalosome, we co-stained transfected cells for the presence of caspase 8, TRAF6, and TAK1. Although we occasionally observed peripheral association of these molecules with GOF CARD11-FLAG in isolated cells (e.g. caspase 8 in Fig. 2D), we found little evidence of direct, sustained colocalization with mCADS (Fig. 2C–D). Regardless, we often detected NF-κB-driven GFP reporter activity in cells with visible mCADS, although the GFP fluorescence signal was sometimes diminished or lost completely after detergent-based permeabilization for antibody staining. Similar results for component colocalization were noted in BJAB B cells (Supp. Fig. S3). These data suggest that with the exception of MALT1, the association of several signaling partners required for AgR-induced NF-κB activation is partial or transient in mCADS.
Fig. 2.
Differential interaction of CBM signaling proteins with mCADS. (A–D) Confocal IF microscopy analysis of WT and GOF mutants of CARD11-FLAG expressed ectopically in JPM50.6 T cells. Cells were co-stained with Abs against (A) BCL10 and (B) MALT1 as well as anti-FLAG-AlexaFluor 647, 24 hrs post-transfection. JPM50.6 T cells were transfected with (C) WT or (D) E134G CARD11-FLAG then stained with Abs against caspase 8, TAK1, or TRAF6 and FLAG. GFP reporter expression indicates NF-κB activity, although variability of GFP signal was noted after cell permeabilization. Data are representative of >3 independent experiments. Scale bar = 5 μm.
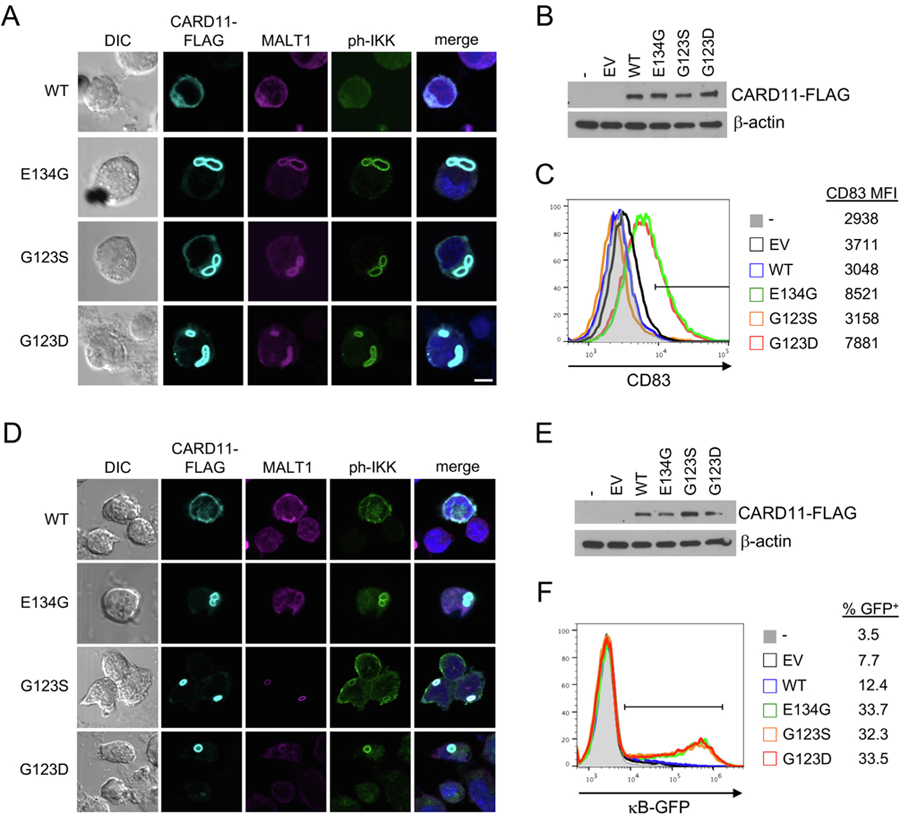
Although we did not detect microtubules or autophagic membranes/proteins surrounding mCADS in transfected cells, it remained possible that mCADS simply represented excess aggregated protein ultimately sequestered for proteolytic degradation rather than active signaling. A critical step in the signaling cascade that activates NF-κB is the phosphorylation of IκB kinase (IKK) α/β, which subsequently triggers phosphorylation and ubiquitin-dependent destruction of IκB to release latent NF-κB for nuclear translocation. To determine if mCADS were directly linked to the ability of GOF CARD11 mutants to mediate constitutive NF-κB activation, transfected samples were co-stained for the presence of phosphorylated IKKα/β (ph-IKK). We observed a robust ph-IKK signal consistently co-localized with GOF CARD11-FLAG mutants and MALT1 in both BJAB (Fig. 3A) and JPM50.6 cells (Fig. 3C), with all CARD11 mutants expressed in roughly equivalent amounts (Fig. 3B, E). This was consistent with an enrichment of total IKK protein within mCADS as well (Supp. Fig. S4). Additional preliminary analyses using super-resolution structured illumination microscopy (SR-SIM) confirmed enrichment of MALT1 and ph-IKK around and within the filamentous superstructure of CARD11-FLAG protein in mCADS (Supp. Fig. S5A–B, Supp Video 2). Importantly, SR-SIM analysis revealed these staining patterns were distinct and not perfectly colocalized, suggesting each signal was specific for the target antigen (Supp. Fig. 5B, Supp. Video 2). Ph-IKK+ mCADS were detected within 4 h post-transfection, becoming more pronounced over 24 h (Supp. Fig. S5C) and persisting for up to 120 h post-transfection. Average size and frequency of mCADS differed between GOF mutants, with E134G CARD11 tending to coalesce into a single, large mCADS per cell by 24 h (Supp. Fig. S5C). The presence of colocalized CARD11-MALT1-ph-IKK in mCADS correlated with constitutive NF-κB activity, as detected by CD83 upregulation (Fig. 3C) or κB-driven GFP reporter expression (Fig. 3F) in BJAB and JPM50.6 cells, respectively. Traditional κB-driven luciferase assays confirmed these readouts (data not shown). Finally, fractionation of JPM50.6 transfectant lysates revealed significant enrichment of CARD11 GOF mutants in the insoluble pellets (Supp. Fig. S6), consistent with aggregated protein. Low levels of MALT1 were also visible; we suspect ubiquitination or other post-translational modifications may alter MALT1 stability or immunoblot antibody detection within these fractions. Remarkably, we also detected enhanced IKK activity in the pellet fractions from GOF mutant transfectants using biochemical in vitro kinase assays with GST-IκB as a substrate, regardless of AgR stimulation (Supp. Fig. S6). By contrast, only small amounts of WT CARD11 were detected in insoluble pellets, consistent with AgR-responsive IKK activity (Supp. Fig. S6). Collectively, our data suggest that mCADS reflect true signalosomes that form spontaneously and direct sustained signaling to NF-κB, rather than inert protein aggregates.
Fig. 3.
mCADS are sites of active IKK signaling. (A) Confocal IF microscopy of BJAB B cells transfected with WT or mutant CARD11-FLAG constructs and stained with anti-FLAG, anti-MALT1, and anti-phospho-IKKα/β Abs. Merge images include DAPI staining. Scale bar = 5 μm. (B) Transfected BJAB cell lysates were separated by SDS-PAGE and immunoblotted for CARD11-FLAG expression. β-actin served as a loading control. (C) Transfected BJAB cells were analyzed by flow cytometry 24 hrs post-transfection using CD83 upregulation as a marker NF-κB activation. (D) Confocal IF microscopy of JPM50.6 T cells transfected with WT or mutant CARD11-FLAG constructs and stained with as in (A). (E) Transfected JPM50.6 cell lysates were separated by SDS-PAGE and immunoblotted for CARD11-FLAG expression. β-actin served as a loading control. (F) NF-κB-driven GFP reporter activity was measured in JPM50.6 transfectants by flow cytometry 24 hrs post-transfection. All data are representative of >3 independent experiments.
3.3. WT CARD11 cannot form mCADS after AgR stimulation
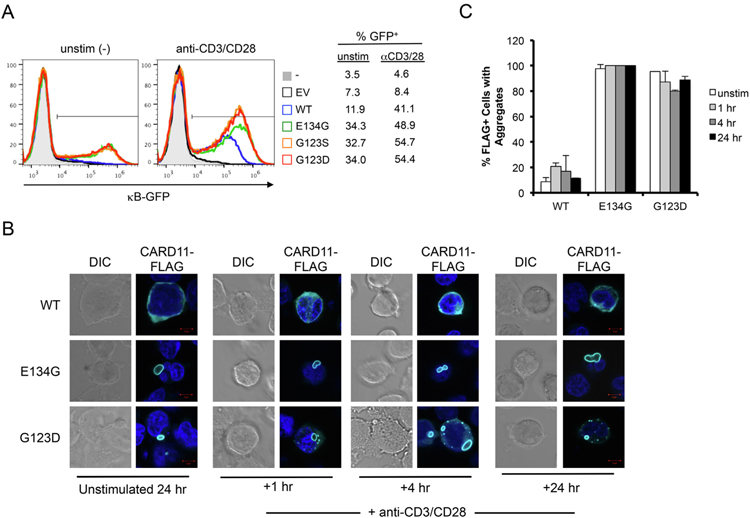
Upon AgR stimulation in normal lymphocytes, WT CARD11 is recruited to lipid rafts localized to the immunological synapse (IS) [12],. Oligomerization of CARD11 through both coiled-coil and MAGUK domain interactions is apparently required for downstream signaling to NF-κB [17,18,27]. We observed neither mCADS formation nor spontaneous NF-κB activation in unstimulated cells ectopically expressing WT CARD11-FLAG (Figs. 1–3). To test whether WT CARD11 could form mCADS-like structures following AgR activation, we activated JPM50.6 cells with agonistic anti-CD3/anti-CD28 antibodies 24 h post-transfection with WT or GOF CARD11 mutants. GFP reporter expression confirmed that transfection of WT CARD11-FLAG rescued robust AgR-induced NF-κB activation as expected (Fig. 4A). Confocal imaging was performed after 1, 4, and 24 h of AgR stimulation. The percentage of FLAG+ cells with any visible CARD11 aggregates, including mCADS, was scored in each set of images. Although we detected an increase in small aggregates of WT CARD11 (<1 mm in diameter) by 1 h, we found no evidence of mCADS formation following AgR stimulation (Fig. 4B–C). In contrast, aggregates of E134G and G123D CARD11 were detected in nearly all FLAG+ cells both before and after AgR stimulation (Fig. 4C), with 80–100% of large aggregates (>1 mm diameter) presenting as ph-IKK+ mCADS. In contrast to large, often single mCADS formed by E134G, G123D mCADS were more frequent per cell, but smaller in average diameter (Fig. 4B). In general, the frequency of small aggregates per cell increased slightly following AgR stimulation (G123D > E134G), correlating with increased κB-GFP reporter expression above the constitutive NF-κB signal detected in unstimulated cells (Fig. 4A–B). Similar results were obtained when cells were strongly stimulated using PMA and ionomycin over the same time course (data not shown). Collectively, these data imply that mCADS are structurally and kinetically distinct from WT CARD11 protein complexes triggered after AgR engagement.
Fig. 4.
WT CARD11 does not form mCADS after AgR stimulation. (A) JPM50.6 T cells were transfected with empty vector (EV), WT, or GOF CARD11-FLAG mutants. After 24 hrs, cells were left unstimulated (left panel) or stimulated with anti-CD3/CD28 agonistic Abs for 4 hrs. NF-κB-driven GFP reporter activity was measured by flow cytometry. (B) Confocal IF microscopy of JPM50.6 T cells transfected and stimulated as in (A). At 1, 4, and 24 hr post-stimulation, cells were stained using anti-FLAG, anti-MALT1, and anti-ph-IKKαAbs. Merge images include DAPI staining. Scale bar = 5 μm. (C) The % of transfected cells (FLAG+) with visible aggregates was scored in multiple fields (>100 cells/construct/experiment). Average ± SD was calculated from 2 independent scorers. Data are representative of 3 independent experiments.
3.4. mCADS formation and signaling to NF-κB requires BCL10 association and SH3-GUK domain interactions
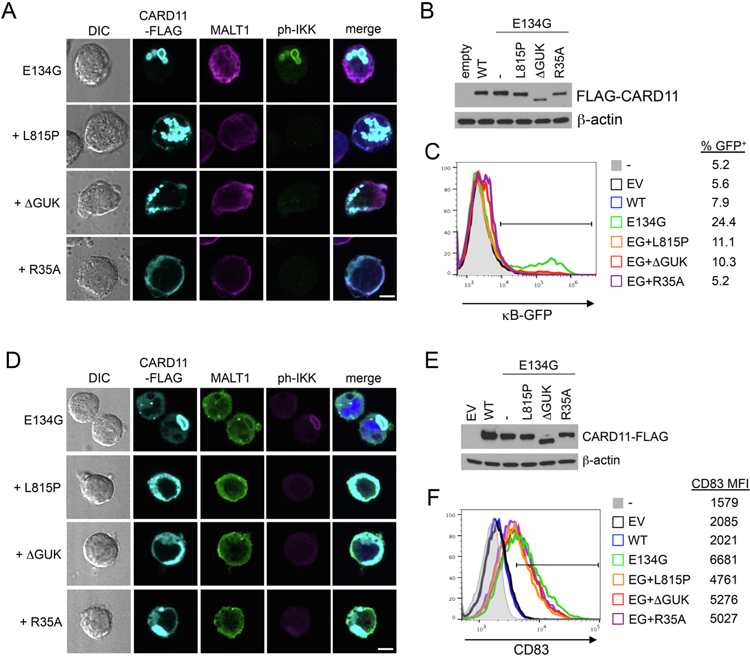
To further delineate structural and functional requirements for mCADS signaling, we introduced known loss-of-function (LOF) mutations that impair AgR-induced WT CARD11 signaling into E134G CARD11. These mutations were shown to disrupt SH3-GUK domain interactions for microcluster formation at the IS (L815P or DGUK) [16,28], or association with BCL10 (R35A) [29],. We then determined the effect of these secondary LOF mutations on E134G CARD11-dependent constitutive NF-κB activation and mCADS formation in transiently transfected cells. Each secondary LOF mutation dramatically disrupted mCADS assembly and enrichment of both MALT1 and ph-IKK in JPM50.6 cells, even when large, non-structured aggregates of CARD11-FLAG protein were clearly present in the cytosol (Fig. 5A). Although E134G CARD11 expression and/or stability remained unaffected (Fig. 5B), LOF mutations neutralized the ability of E134G to stimulate NF-κB-driven GFP reporter expression (Fig. 5C). Similar effects were observed in BJAB cells transfected with LOF mutant E134G constructs (Fig. 5D–E), although only a partial reduction of NF-κB-dependent CD83 expression was observed upon addition of LOF mutations (Fig. 5F). These findings suggest that simple aggregation of CARD11 is not sufficient to drive optimal NF-κB signaling. Instead, both BCL10 recruitment and SH3-GUK domain interactions are critical for higher-order oligomerization, manifesting in the mCADS super-structure, and consequent, constitutive NF-κB activation.
Fig. 5.
Specific LOF mutations disrupt mCADS formation and NF-κB signaling. (A) Confocal IF microscopy of JPM50.6 T cells transfected with E134G CARD11-FLAG constructs ± additional LOF mutations (L815P, ΔGUK, R35A) and stained 24 hrs later using anti-FLAG, anti-MALT1, and anti-ph-IKKα/β Abs. Merge images include DAPI staining. Scale bar = 5 μm. (B) Immunoblotting for CARD11-FLAG expression in lysates made from transfected JPM50.6 cells in (A). β-actin served as a loading control. (C) NF-κB-driven GFP reporter activity was measured in JPM50.6 transfectants in (A) by flow cytometry 24 hrs post-transfection. (D) Confocal IF microscopy of BJAB B cells transfected and stained as in (A). (E) Immunoblotting for CARD11-FLAG expression in lysates made from transfected BJAB cells in (A). β-actin served as a loading control. (F) NF-κB-dependent induction of CD83 expression was measured in BJAB transfectants in (A) by flow cytometry 24 hrs post-transfection. Data are representative of >3 independent experiments.
3.5. mCADS assemble but cannot trigger NF-κB activation in the absence of NEMO
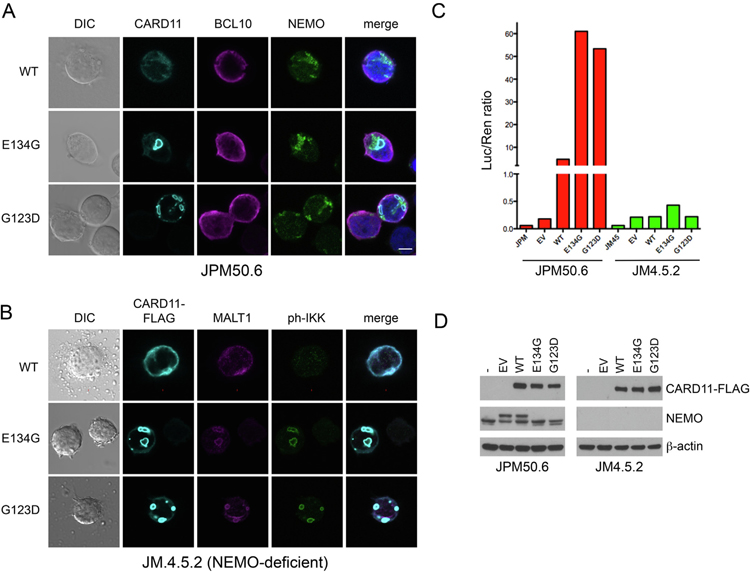
In addition to IKKα/β phosphorylation, CARD11-dependent activation of the IKK complex requires K63-linked ubiquitination of the essential regulatory subunit IKKγ/NEMO [30],. Current models posit that the IKK complex is recruited to the AgR-induced CBM signalosome, where IKKγ is polyubiquitinated by E3 ligases such as TRAF6 [11],. Although GOF mutations enhance BCL10 association with CARD11 and subsequent K63-ubuiqitination of both BCL10 and NEMO [14],, other evidence suggests these events and subsequent BCL10 degradation may occur in spatially distinct protein complexes [21],. In JPM50.6 transfectants, we detected endogenous, punctate NEMO aggregates adjacent to but not closely colocalized with mCADS, with mild enrichment of BCL10 apparent only with CARD11-FLAG (Fig. 6A). To test whether NEMO was required for mCADS assembly and subsequent NF-κB signaling, we transfected NEMO-deficient Jurkat cells (JM4.5.2) with WT, E134G, or G123D CARD11. Surprisingly, we readily observed mCADS formation in NEMO-deficient Jurkat T cells, with colocalization of CARD11-FLAG, MALT1 and ph-IKKα/β (Fig. 6B). Relative to normal Jurkat T cells (ph-IKK/FLAG intensity ratio = 5.9 ± 2.7), the ph-IKK signal appeared less intense in the absence of NEMO (ph-IKK/FLAG intensity ratio = 1.9 ± 1.2). Despite the presence of ph-IKK+ mCADS, luciferase assays indicated that spontaneous NF-κB activity induced by GOF CARD11 mutant expression was severely reduced in the absence of NEMO (Fig. 6C–D). These data suggest that although the mCADS signalosome supports some IKKα/β phosphorylation in NEMO-deficient T cells, NEMO is still required to link mCADS assembly with downstream NF-κB activation, even though NEMO is not closely co-localized with CARD11 or ph-IKKα/β in mCADS.
Fig. 6.
NEMO is dispensable for IKKα/β phosphorylation at mCADS, but required for downstream NF-κB induction. (A) Confocal IF microscopy of JPM50.6 T cells transfected with WT, E134G or G123D CARD11-FLAG constructs stained using anti-FLAG, anti-BCL-10, and anti-NEMO (IKKγ) Abs at 24 hrs post-transfection. (B) Confocal IF microscopy of NEMO-deficient JM4.5.2 T cells transfected with WT, E134G or G123D CARD11-FLAG constructs and stained using anti-FLAG, anti-MALT1, and anti-ph-IKKα/β Abs at 24 hrs post-transfection. Merge images include DAPI staining. Scale bars = 5 μm. (C) NF-κB activity was measured in cell JPM50.6 and JM4.5.2. transfectants from (A) and (B) using a dual luciferase assay. (D) Immunoblotting of lysates prepared from transfected cells for CARD11-FLAG and NEMO expression. β-actin served as a loading control. Data are representative of >3 independent experiments.
3.6. Endogenous mCADS formation in DLBCL harboring GOF CARD11 mutations
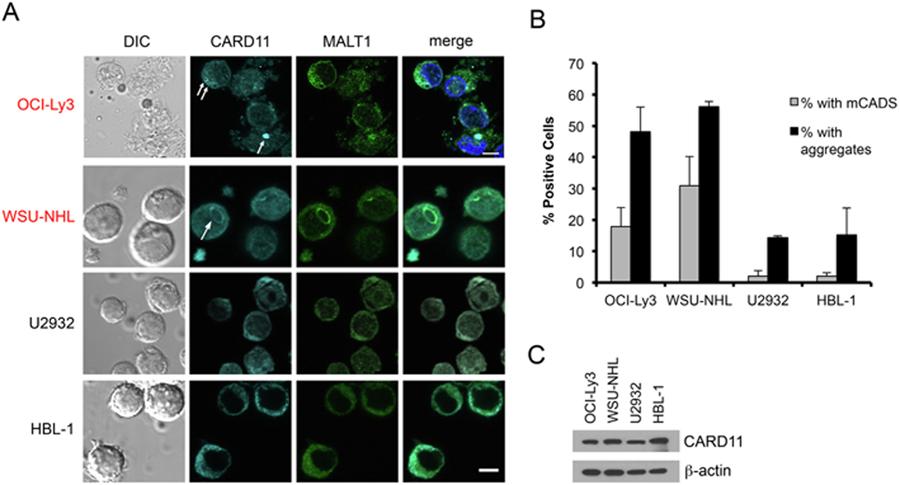
Thus far, our results suggested mCADS may constitute a unique, constitutively active signalosome that is structurally distinct from signalosomes formed by WT CARD11. However, our detection and characterization of mCADS relied on ectopic expression of GOF CARD11 in human cell lines, driven by a relatively strong EF1α-based promoter. Moreover, punctate CARD11 aggregates in BENTA patient lymphocytes do not resemble mCADS using conventional confocal microscopy, although resting primary T and B cells are significantly smaller with far less cytoplasmic volume [2,19]. To establish whether mCADS may represent physiologically relevant structures, we examined four DLBCL cell lines that are dependent on constitutive NF-κB for survival. Importantly, only two of these DLBCL lines carry GOF CARD11 mutations: OCI-Ly3 (activated B cell (ABC) subtype, mutation = L251P [10],) and WSU-NHL (germinal center B cell (GCB) phenotype, mutation = L245P). The other two DLBCL lines (U2932 and HBL-1) contain WT CARD11, yet are dependent on continuous NF-κB activity [31],. Strikingly, confocal microscopy analysis staining for endogenous CARD11 revealed both plentiful small aggregates and readily detectable mCADS (in ~20–30% of cells) in the two CARD11-mutant DLBCL lines (Fig. 7A). The endogenous mCADS found in WSU-NHL cells appeared larger in size (average diameter = ~2–5 mm, versus ~1–2 mm in OCI-Ly3), although ‘‘incomplete” shells and/or fibrils were also present in both lines. In contrast, the frequency of CARD11 aggregates was significantly lower in DLBCL lines lacking CARD11 mutations (U2932, HBL-1), with virtually no mCADS detected (Fig. 7A–B). Immunoblotting revealed that the presence of aggregates and/or mCADS-like structures did not correlate with more relative CARD11 expression (Fig. 7C). These data indicate that GOF mutations in CARD11 contribute to increased aggregation potential and can give rise to endogenous mCADS formation in patient-derived B cell lymphoma lines.
Fig. 7.
Endogenous mCADS formation in DLBCL lines carrying GOF CARD11 mutations. (A) Confocal IF microscopy of DLBCL cell lines stained with anti-CARD11 and anti-MALT1 Abs. Lines marked in red carry GOF CARD11 mutations. Merge images include DAPI staining. Scale bar = 5 μm. (B) The % of cells with mCADS (gray bars) and/or visible aggregates (black bars) was scored in multiple fields (>100 cells/DLBCL line). Average ± SD was calculated from 2 independent scorers. (C) Immunoblot analysis for relative CARD11 protein expression in lysates prepared from DLBCL lines. All data are representative of >3 independent experiments.
4. Discussion
Scaffold proteins are increasingly recognized as critical, evolutionarily designed arbiters of highly regulated intracellular signal transduction [32],. One could therefore predict that even single point mutations capable of altering scaffold function could have profound, potentially pathological effects on cell signaling and function. Here we report that GOF mutations render the CARD11 scaffold capable of spontaneously assembling into a remarkable signalosome structure we refer to as mCADS. Although visible protein aggregation may not be strictly required for GOF CARD11 mutants to activate NF-κB [15],, work from our lab and others suggests the presence of CARD11 aggregates is a readily observed hallmark of elevated NF-κB signaling in BENTA patient lymphocytes and DLBCL tumors harboring GOF CARD11 mutations [2,10,19]. Importantly, the unique protein aggregate pattern of mCADS was not readily apparent in previous studies utilizing CARD11 constructs fused to fluorescent proteins. However, our use of a smaller FLAG tag for ectopic CARD11 expression, coupled with our detection of similar structures in DLBCL lines, unveiled a unique mCADS superstructure capable of efficient but dysregulated activation of NF-κB.
Our microscopy-based approach builds on recent imaging studies indicating that the ‘‘CBM signalosome” cannot simply be regarded as one giant macromolecular machine, but is rather best represented as a dynamic, interconnected network of spatiotemporally separate signaling complexes [11,33]. These studies both complement and amend our understanding of these protein-protein interactions based on more traditional biochemical approaches. Although mCADS are not observed with WT CARD11, our findings are relatively consistent with previous work establishing basic requirements for CARD11-dependent, AgR-induced NF-κB activation, such as BCL10 association, SH3-GUK domain interactions, or NEMO expression. For example, lower levels of BCL10 colocalization we observed in mature mCADS over time likely reflect local turnover of BCL10 via autophagy or proteasomal degradation, as noted with AgR-induced CBM signaling [20,34,35]. MALT1 is also clearly required, as mCADS formation and signaling was severely compromised in MALT1-knockdown Jurkat T cells (Supp. Fig. S7). MALT1 can also directly associate with the CARD CC domain [36],, which may explain our observation of stable colocalization of CARD11 and MALT1 in mCADS even after BCL10 is not reliably detected there. Moreover, our preliminary analyses utilizing pharmacological inhibitors suggest the enzymatic activity of TAK1 and caspase 8 are required for optimal IKK phosphorylation and GOF CARD11-dependent constitutive NF-κB activation in BJAB cells (Supp. Fig. S8), even though both TAK1 and caspase 8 may only be transiently associated with mCADS. It is also possible that WT CARD11 can form highly dynamic, transient mCADS-like structures that are carefully regulated after TCR ligation, only unmasked by inhibiting specific downstream signaling events. Although we did not notice an obvious change in mCADS size or frequency in NEMO-deficient JM4.5.2 cells, future studies should interrogate whether ‘‘WT CARD11 mCADS” might be revealed after blocking key enzymatic steps in the signaling pathway, including MALT1 protease and IKK activity. One major distinguishing dissimilarity between mCADS and WT CARD11 signaling is the apparent uncoupling of mutant CARD11 from upstream signaling. Indeed, our preliminary analyses suggest that GOF mutations still drive mCADS formation and NF-κB activation even when PKCθ is inhibited (Supp. Fig. S9). It will be interesting to further investigate whether other upstream AgR-responsive activators of CARD11 influence mCADS formation or downstream GOF CARD11 signaling.
The filamentous matrix of protein aggregation revealed by our TEM-based analysis of mCADS is reminiscent of filaments of purified BCL10 and MALT1 proteins nucleated by an active, truncated form of CARD11 (aa 8–302) [23,24]. Several lines of evidence suggest CBM complex proteins are indeed prone to form higher order oligomers through several modular domains (e.g. CARD, CC, and tandem Ig-like domains) [16,18],[37–39]. Our findings imply that BENTA and DLBCL-associated GOF mutations can enhance and exploit the intrinsic, CC-dependent oligomerization potential of CARD11, as predicted in silico [24],. Our data also indicate that higher order clustering afforded by SH3-GUK domain interactions is required for mCADS formation [16],. Taken together, we posit that only persistent GOF CARD11 expression can give rise to a stable mCADS conformation for cytosolic CBM signalosomes, which appears distinct from the temporal separation of p62-BCL10-MALT1 POLKADOTS away from WT CARD11 noted in AgR-stimulated effector T cells [21],.
It remains unclear how alterations of intra- and intermolecular interactions forced by GOF mutations can ultimately manifest into the mCADS structures we observed. Although we initially considered that incomplete penetration of antibodies used for immunofluorescent staining of dense CARD11 aggregates might explain this phenomenon, our TEM and initial SR-SIM analyses largely ruled out this possibility. One recent study found that heterodimers and trimers of purified coiled-coil peptides could self-assemble into ‘‘hollow” spheres, making it possible that GOF CARD11 mutants could conform to this type of superstructural organization [40],. On the other hand, our imaging studies also suggest naturally occurring mCADS found in DLBCL lines are infrequent and likely more transient or disordered than those formed upon ectopic expression of GOF CARD11 mutants. This difference could reflect lower expression of CARD11 below a certain threshold required for mCADS formation, or rapid remodeling or breakdown of nascent mCADS by natural feedback mechanisms, such as S608/S637 phosphorylation and/or K48-ubiquitin-directed proteasomal degradation of CARD11, or recruitment of A20, RNF181, and other negative regulators of NF-κB signaling [11],[41–44]. Either phenomenon could also explain why mCADS are not readily detected in BENTA patient lymphocytes, although further super-resolution imaging techniques are required to definitively characterize the small CARD11 aggregates we find in resting T and B cells from BENTA patients [2],. Indeed, the mCADS superstructure was only clearly detected in our experiments once CARD11 aggregates exceeded 1 μm in diameter, even when smaller aggregates were more frequent for ectopically expressed G123D CARD11, or those DLBCL carrying GOF CARD11 mutations. We therefore must conclude that mCADS cannot serve as a reliable diagnostic indicator of the presence of GOF CARD11 mutations in BENTA or DLBCL patients. Indeed, extensive analysis of PBMC samples derived from DLBCL patients suggests that mCADS-like structures are rarely found in primary, circulating lymphoma cells (data not shown).
Even though mCADS are best observed with elevated GOF CARD11 expression, our discovery and characterization of these structures may prove to be clinically relevant. There is significant interest in developing drugs that disrupt precise, stimulus-specific nodes of NF-κB signaling pathways to avoid severe immunosuppression associated with general NF-κB blockade [45],. The CBM signalosome represents an intriguing target for specifically interfering with aberrant lymphocyte activation in inflammatory diseases and malignancies [46,47]. Ideally, the enhanced multimerization potential and distinct assembly of CARD11 aggregates and/or mCADS enforced by GOF mutations in or near the CC could represent a novel therapeutic target for modulating constitutive NF-κB signaling in BENTA or certain DLBCL. If GOF CARD11 oligomerization can occur regardless of linker domain phosphorylation, one could imagine that specific blockade of GOF mutation-induced aggregation may work without compromising AgR-directed WT CARD11 signaling. Recent work delineating the complexity of WT CARD11 regulation through multiple repressive/activating elements in the linker domain lends further credence to this notion [48,49]. Further, detailed structural analyses are required to validate ‘‘druggable” target sites within the dysregulated CBM signalosome induced by GOF mutations. In summation, we hope our findings spur additional validation and characterization of unique biochemical and biophysical characteristics in CBM signalosomes harboring GOF CARD11 mutations.
Supplementary Material
Acknowledgements
We thank Dr. Zhaozhang Li in the USU Genomics core for primer synthesis and sequencing support, Dr. Lawrence Kane and Dr. Louis Staudt for providing cell lines, and Dr. Daisuke Ennishi, Dr. David Scott, and Dr. Randy Gascoyne for providing precious frozen primary cell samples from DLBCL patients. We also thank Dr. Brian Schaefer for critical reading of the manuscript, and Dr. Rachel Cox and Dr. Chou-Zen Giam for helpful discussions. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of Uniformed Services University of the Health Sciences or the United States Department of Defense.
Funding
This work was supported by the National Institutes of Health (1R21AI109187), the Concern Foundation (Conquer Cancer Now Award), and Uniformed Services University.
Footnotes
The authors declare no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cellimm.2020.104129.
References
- [1].Lu HY et al. , The CBM-opathies-a rapidly expanding spectrum of human inborn errors of immunity caused by mutations in the CARD11-BCL10-MALT1 complex, Front. Immunol 9 (2018) 2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Snow AL et al. , Congenital B cell lymphocytosis explained by novel germline CARD11 mutations, J. Exp. Med 209 (12) (2012) 2247–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arjunaraja S, Snow AL, Gain-of-function mutations and immunodeficiency: at a loss for proper tuning of lymphocyte signaling, Curr. Opin. Allergy Clin. Immunol 15 (6) (2015) 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Turvey SE et al. , The CARD11-BCL10-MALT1 (CBM) signalosome complex: stepping into the limelight of human primary immunodeficiency, J. Allergy Clin. Immunol 134 (2) (2014) 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Thome M et al. , Antigen receptor signaling to NF-kappaB via CARMA1, BCL10, and MALT1, Cold Spring Harb. Perspect. Biol 2 (9) (2010) a003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vallabhapurapu S, Karin M, Regulation and function of NF-kappaB transcription factors in the immune system, Annu. Rev. Immunol 27 (2009) 693–733. [DOI] [PubMed] [Google Scholar]
- [7].Shaffer AL 3rd, Young RM, Staudt LM, Pathogenesis of human B cell lymphomas, Annu. Rev. Immunol 30 (2012) 565–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Staudt LM, Oncogenic activation of NF-kappaB, Cold Spring Harb. Perspect. Biol 2 (6) (2010) a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Young RM et al. , B-cell receptor signaling in diffuse large B-cell lymphoma, Semin. Hematol 52 (2) (2015) 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lenz G et al. , Oncogenic CARD11 mutations in human diffuse large B cell lymphoma, Science 319 (5870) (2008) 1676–1679. [DOI] [PubMed] [Google Scholar]
- [11].Paul S, Schaefer BC, A new look at T cell receptor signaling to nuclear factor kappaB, Trends Immunol 34 (6) (2013) 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sommer K et al. , Phosphorylation of the CARMA1 linker controls NF-kappaB activation, Immunity 23 (6) (2005) 561–574. [DOI] [PubMed] [Google Scholar]
- [13].Bedsaul JR et al. , Mechanisms of regulated and dysregulated CARD11 signaling in adaptive immunity and disease, Front. Immunol 9 (2018) 2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chan W, Schaffer TB, Pomerantz JL, A quantitative signaling screen identifies CARD11 mutations in the CARD and LATCH domains that induce Bcl10 ubiquitination and human lymphoma cell survival, Mol. Cell. Biol 33 (2) (2013) 429–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lamason RL et al. , Oncogenic CARD11 mutations induce hyperactive signaling by disrupting autoinhibition by the PKC-responsive inhibitory domain, Biochemistry 49 (38) (2010) 8240–8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hara H et al. , Clustering of CARMA1 through SH3-GUK domain interactions is required for its activation of NF-kappaB signalling, Nat. Commun 6 (2015) 5555. [DOI] [PubMed] [Google Scholar]
- [17].Rawlings DJ, Sommer K, Moreno-Garcia ME, The CARMA1 signalosome links the signalling machinery of adaptive and innate immunity in lymphocytes, Nat. Rev. Immunol 6 (11) (2006) 799–812. [DOI] [PubMed] [Google Scholar]
- [18].Tanner MJ et al. , CARMA1 coiled-coil domain is involved in the oligomerization and subcellular localization of CARMA1 and is required for T cell receptor-induced NF-kappaB activation, J. Biol. Chem 282 (23) (2007) 17141–17147. [DOI] [PubMed] [Google Scholar]
- [19].Brohl AS et al. , Germline CARD11 Mutation in a Patient with Severe Congenital B Cell Lymphocytosis, J. Clin. Immunol 35 (1) (2014) 32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Paul S et al. , Selective autophagy of the adaptor protein Bcl10 modulates T cell receptor activation of NF-kappaB, Immunity 36 (6) (2012) 947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Paul S et al. , T cell receptor signals to NF-kappaB are transmitted by a cytosolic p62-Bcl10-Malt1-IKK signalosome, Sci. Signal 7 (325) (2014) p. ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yokosuka T et al. , Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation, Immunity 29 (4) (2008) 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].David L et al. , Assembly mechanism of the CARMA1-BCL10-MALT1-TRAF6 signalosome, Proc. Natl. Acad. Sci. U.S.A 115 (7) (2018) 1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Qiao Q et al. , Structural architecture of the CARMA1/Bcl10/MALT1 signalosome: nucleation-induced filamentous assembly, Mol. Cell 51 (6) (2013) 766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Reynolds ES, The use of lead citrate at high pH as an electron-opaque stain in electron microscopy, J. Cell Biol 17 (1963) 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shaner NC, Steinbach PA, Tsien RY, A guide to choosing fluorescent proteins, Nat. Methods 2 (12) (2005) 905–909. [DOI] [PubMed] [Google Scholar]
- [27].Schulze-Luehrmann J, Ghosh S, Antigen-receptor signaling to nuclear factor kappa B, Immunity 25 (5) (2006) 701–715. [DOI] [PubMed] [Google Scholar]
- [28].Wang D et al. , CD3/CD28 costimulation-induced NF-kappaB activation is mediated by recruitment of protein kinase C-theta, Bcl10, and IkappaB kinase beta to the immunological synapse through CARMA1, Mol. Cell. Biol 24 (1) (2004) 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li S et al. , Structural insights into the assembly of CARMA1 and BCL10, PLoS ONE 7 (8) (2012) e42775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shambharkar PB et al. , Phosphorylation and ubiquitination of the IkappaB kinase complex by two distinct signaling pathways, EMBO J 26 (7) (2007) 1794–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Davis RE et al. , Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma, Nature 463 (7277) (2010) 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Good MC, Zalatan JG, Lim WA, Scaffold proteins: hubs for controlling the flow of cellular information, Science 332 (6030) (2011) 680–686.21551057 [Google Scholar]
- [33].Rossman JS et al. , POLKADOTS are foci of functional interactions in T-Cell receptor-mediated signaling to NF-kappaB, Mol. Biol. Cell 17 (5) (2006) 2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lobry C et al. , Negative feedback loop in T cell activation through IkappaB kinase-induced phosphorylation and degradation of Bcl10, Proc. Natl. Acad. Sci. U.S.A 104 (3) (2007) 908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Scharschmidt E et al. , Degradation of Bcl10 induced by T-cell activation negatively regulates NF-kappa B signaling, Mol. Cell. Biol 24 (9) (2004) 3860– [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Che T et al. , MALT1/paracaspase is a signaling component downstream of CARMA1 and mediates T cell receptor-induced NF-kappaB activation, J. Biol. Chem 279 (16) (2004) 15870–15876. [DOI] [PubMed] [Google Scholar]
- [37].Guiet C, Vito P, Caspase recruitment domain (CARD)-dependent cytoplasmic filaments mediate bcl10-induced NF-kappaB activation, J. Cell Biol 148 (6) (2000) 1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Holliday MJ et al. , Structures of autoinhibited and polymerized forms of CARD9 reveal mechanisms of CARD9 and CARD11 activation, Nat. Commun 10 (1) (2019) 3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Qiu L, Dhe-Paganon S, Oligomeric structure of the MALT1 tandem Ig-like [DOI] [PMC free article] [PubMed]
- [40].Fletcher JM et al. , Self-assembling cages from coiled-coil peptide modules, Science 340 (6132) (2013) 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Moreno-Garcia ME et al. , Serine 649 phosphorylation within the protein kinase C-regulated domain down-regulates CARMA1 activity in lymphocytes, J. Immunol 183 (11) (2009) 7362–7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Moreno-Garcia ME et al. , MAGUK-controlled ubiquitination of CARMA1 modulates lymphocyte NF-kappaB activity, Mol. Cell. Biol 30 (4) (2010) 922– [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pedersen SM et al. , Negative Regulation of CARD11 Signaling and Lymphoma Cell Survival by the E3 Ubiquitin Ligase RNF181, Mol. Cell. Biol 36 (5) (2016) 794–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bidere N et al. , Casein kinase 1alpha governs antigen-receptor-induced NF-kappaB activation and human lymphoma cell survival, Nature 458 (7234) (2009) 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gilmore TD, Herscovitch M, Inhibitors of NF-kappaB signaling: 785 and counting, Oncogene 25 (51) (2006) 6887–6899. [DOI] [PubMed] [Google Scholar]
- [46].Rosebeck S et al. , From MALT lymphoma to the CBM signalosome: three decades of discovery, Cell Cycle 10 (15) (2011) 2485–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yang C et al. , The CBM signalosome: potential therapeutic target for aggressive lymphoma?, Cytokine Growth Factor Rev 25 (2) (2014) 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jattani RP, Tritapoe JM, Pomerantz JL, Cooperative control of caspase recruitment domain-containing protein 11 (CARD11) signaling by an unusual array of redundant repressive elements, J. Biol. Chem 291 (16) (2016) 8324– [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jattani RP, Tritapoe JM, Pomerantz JL, Intramolecular interactions and regulation of cofactor binding by the four repressive elements in the caspase recruitment domain-containing protein 11 (CARD11) inhibitory domain, J. Biol. Chem 291 (16) (2016) 8338–8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.