Abstract
Objectives
The effectiveness of treatment incorporating relapse prevention medications for OUD is typically examined in research using rigidly pre-defined endpoints of success versus failure, usually over a single episode of care. But this perspective may not adequately portray the non-linear trajectories typical of real-world treatment courses in this chronic, remitting and relapsing disorder.
Methods
This descriptive study examined 12 month treatment trajectories of n=60 patients enrolled at a single site of a larger multi-site RCT examining the comparative effectiveness of buprenorphine versus extended release naltrexone. While the parent study provided medication treatment through the research protocol for 6 months, this study documents treatment up to 12 months, including medications, provided through standard community resources (Treatment As Usual; TAU) outside of the protocol.
Results
Some patients continued medications past the end of the study intervention while others did not. Some patients initiated medications other than the one assigned by the study. Some patients switched from one medication to the other. Many patients returned to treatment following one or more periods of drop-out and/or relapse. Patients utilized multiple episodes of bed-based care, including short-term acute residential and long-term residential treatment, as well as recovery housing supports. Described trajectories are also depicted graphically. At 12 months, while rates of continuous treatment retention were low (8%), rates of cross-sectional treatment engagement including return to treatment after drop out were higher (35%).
Conclusions
This description of non-linear treatment trajectories highlights the potential benefits of flexibility and optimism in the promotion of re-engagement despite interim outcomes that might traditionally be considered “failure” endpoints.
Introduction:
The US opioid epidemic continues to worsen (CDC, 2015) with well-described morbidity and mortality, including overdose deaths (Lewis et al., 2017). Relapse prevention medication, or Medication for Opioid Use Disorder (MOUD), is the standard of care. While longer duration of treatment is associated with better patient outcomes, unfortunately, outcomes are still severely limited by poor treatment engagement and retention (Ruetsch et al., 2017). Despite innovation in treatment approaches4 patient dropout at 6 months often exceeds 50% (Miller & Carroll 2006; Vo et al., 2016). There are myriad reasons for poor engagement and dropout, and the field has struggled to respond (Dalabol et al., 2010). Since no single barrier accounts for all dropout and no single strategy can address retention for every patient, it is important to examine the multiple patient trajectories and individual treatment courses to attempt to match patients to optimal interventions.
Among other treatment matching strategy questions, there is controversy in the field regarding which relapse prevention medication is best for whom and under what circumstances (Potter et al., 2013). For instance, is patient choice of medication superior to professional clinical recommendation? How can continuing care be facilitated beyond crisis-driven, acute episodes of specialty SUD withdrawal management, hospitalization or other residential treatment? What psychosocial interventions and platforms are most synergistic to the effectiveness of MOUD? When are extended residential supports needed? While these and other questions are critical, they are not easily accounted for in typical research studies.
With the notable exception of a small body of naturalistic, longitudinal follow-up studies in OUD (eg: Hser et al., 2001; Hser et al., 2016; Potter et al., 2014), research methodology for clinical effectiveness typically assigns treatment according to a rigid algorithm without the typical fluidity of real world treatment that includes: patient choice, switching of treatments based on clinical response and patient/clinician preference, or stopping and restarting treatment, etc. Furthermore, research outcomes typically formulate success as uni-dimensional measures over the course of linear trajectories through a single persistent episode of care, either cross-sectionally (such as percent relapse-free survival at 6 months) or cumulatively (such as percent opioid positive urine tests over 6 months). One particular limitation of that perspective is that treatment dropout is seen as a terminal poor outcome, while treatment re-initiation after dropout, a very typical feature of real world treatment as usual, is not captured as a positive outcome. This perspective also tends to reflect predominant but unrealistic clinical thinking, that relapse and dropout represent an ending in failure, and that effective treatment is anticipated to be a “horserace” that ends with the success of persistent abstinence.
The XBOT study conducted by the National Drug Abuse Clinical Trials Network was a multi-site, two-arm, 24-week, parallel-group, open-label, randomized trial comparing the effectiveness and safety of 6 months of treatment with Extended Release Naltrexone (XR-NTX) versus buprenorphine (BUP-NX) for relapse prevention in patients with OUD (Lee et al., 2016). The present study examines qualitative features of patient trajectories for subjects enrolled at one site for that study, both during and following the specified study period, including treatments both within and outside of the study, both before and after dropout from the index treatment episode.
Methods:
Brief Characteristics of Parent Study
The methods and design (Lee et al., 2016; Nunes et al., 2016), and results (Lee et al., 2018) of the parent multi-site trial are presented elsewhere. Avery Road treatment Center (ARTC) in Rockville MD was one of eight sites in the larger study (clinicaltrials.gov: NCT02032433). Subjects were those enrolled into the parent study at the Avery Road Treatment Center (ARTC) site. ARTC is a public sector (State- and County-funded), safety net, short-term residential addiction treatment program that includes withdrawal management (ASAM Level 3.7 and 3.7 WM). For the parent study, participants seeking acute care for opioid use disorders (OUD) were recruited during an index residential treatment episode from the routine patient flow at the site. Subjects were randomized to treatment with either BUP-NX or XR-NTX, having agreed that they would accept either as a randomized assignment. Patients were inducted onto the assigned medication through the study, and then continued through the study in outpatient medication treatment for 6 months. During the study intervention, assigned mediations were provided through the study for up to 6 months. For the purposes of the parent study, those subjects that did not start assigned medication, discontinued assigned medication, or met relapse criteria (defined as 7 consecutive days or 4 consecutive weeks of non-study opioid use in a 28-day period as assessed by self-report and by weekly urine testing with missing samples imputed as positive) were considered to have discontinued study treatment. Patients were followed weekly during study treatment for 6 months, and then again at 1 and 3 months post end of treatment.
Present Study
Routine, non-standardized community treatment (Treatment As Usual; TAU) was offered (but not required) for all study participants in addition to study treatment as part of the parent study protocol. At the ARTC program site, all subjects were also offered non-study TAU at the termination of study treatment, which included recommended relapse prevention medication treatment (MAT) through ARTC’s affiliated outpatient programs or other local providers. (Medications were not available outside of the study at most of the other sites.) For the purposes of the present study, we describe naturalistic treatment trajectories both in and out of the treatment provided by the parent study. This includes treatment after the period of study intervention (6 months) out to a total of 12 months after randomization. It includes treatment with medications other than those assigned through the study, treatment after discontinuation of study medication, treatment after relapse and/or dropout. Non-study medications and other non-study treatment (e.g. treatment with non-assigned medications, treatment after relapse/dropout up to 6 months, and all treatment after 6 months) were provided through usual clinical care using patients’ standard benefits. For the purposes of the present study, all known sources of treatment were considered, whether study-assigned or not. Both BUP-NX and XR-NTX were available to patients through Medicaid or commercial insurance coverage, supplemented by supplies of both medications made available from the local County Health Department.
There were N=63 participants consented at the ARTC program site (total parent study N=570). Of these, 3 subjects dropped out without receiving study medication, were lost to follow-up for the duration of the study period, and were excluded from consideration in the present descriptive study.
In addition to the parent study data set, additional data was abstracted from site clinical charts and communication with outside providers regarding patients’ naturalistic treatment course during and following study participation up to 12 months.
Retention in any particular treatment episode was measured as the number of months in treatment until dropout or discharge, (typically after 4 weeks without any attendance) usually coincident with the date patient met relapse criteria, mirroring the definition in the parent clinical trial12.
Results:
Patient Characteristics
A total of 60 participants are included in the present study. Subjects were average age 31 years old (range 20-55); 70% male; 67% Caucasian American (CA), 18% African American (AA), 15% mixed or other. Compared to the parent study, our sample was slightly younger, more male, more AA, less Hispanic.
Medication treatment
Forty-seven (78% compared to 83% for the parent study) patients were inducted onto study medications as assigned through randomization, and of these, 27 (57%) were also treated with medications through treatment as usual outside of the study protocol at some time over the 12-month course. An additional 13 (28%) patients were treated with non-assigned medications through treatment as usual outside of the study protocol without ever having begun study medication (11 choosing an alternative medication post randomization and 2 inducting their originally assigned medication later within the 12 month period). In sum, 60 participants initiated medication treatment with either buprenorphine (24), XR-NTX (35) or methadone (1). Eighty-three percent (49) of the patients linked to continuing care in some capacity following discharge from acute residential treatment (mostly intensive outpatient with a small number (10) continuing at the outpatient level care which may consist of weekly individual psychosocial sessions). Sixty-two percent (37) were discharged on medications for other co-occurring psychiatric conditions.
Patient Courses:
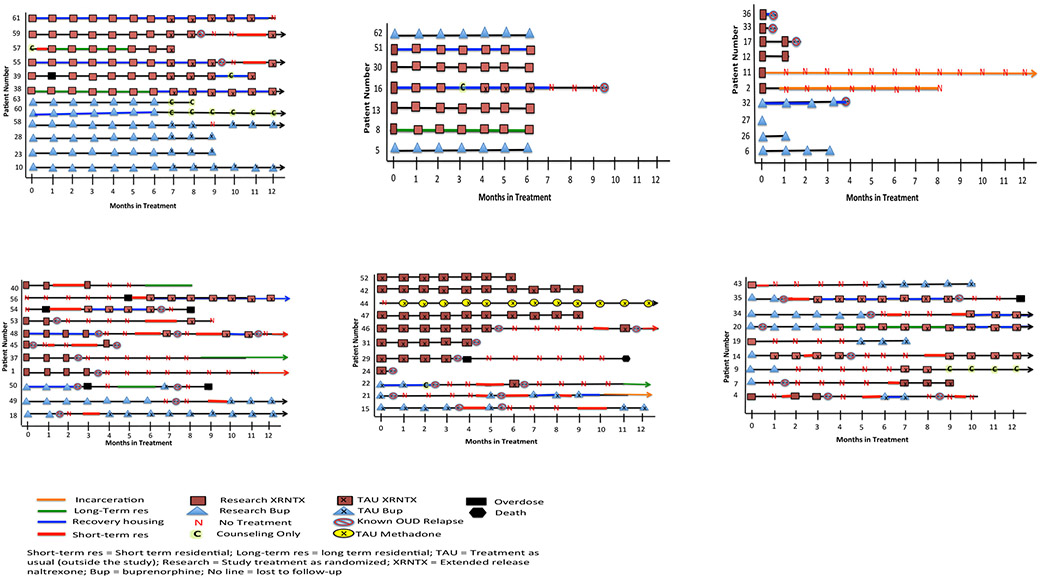
Individual patient courses of treatment are depicted graphically in Figs 1-6, one line per patient, over 12 months. The legend(s) show retention in various treatment conditions, including type of medication, whether the medication was prescribed through study treatment or TAU, residential treatments, counseling only, recovery housing, periods of dropout with no treatment, as well as milestones such as relapse, incarceration and overdose. Patients are grouped in mutually exclusive figures according to several themes.
Fig 1.
a: Patients Who Took Study Medication and Continued TAU After Study Participation
b: Patients Who Completed the Study and Elected to Stop Medication
c: Patients With Early OUD Relapse
d: Patients Who Took Study Medication, Dropped-Out of Treatment, then Re-Engaged in TAU
e: Patients Who Initiated Non-Study Medications in TAU
f: Patients Who Switched Medication
Fig 1 shows a group of 12 patients who took assigned study medication as randomized through 6 months of study treatment, then continued onto that same medication through TAU for at least one month after discontinuation of study treatment. Two patients persisted in treatment continuously to 12 months and beyond (#38, 10). Three others with non-continuous courses (#59, 55, 58) also remained engaged in ongoing treatment beyond 12 months, 2 of whom (#59, 55) had medications reinitiated after relapse and return to residential detox. One patient (#57) did not initiate medications at all until after a relapse and second residential treatment episode within the several weeks following the index residential episode. One patient (#39) had medications reinitiated as an outpatient after an overdose event. Two patients (#63, 60) discontinued medication but remained in counseling treatment.
Fig 2 shows a group of 7 patients who completed the full 6 months of prescribed study medication treatment but did not transition to further TAU treatment. (# 5, 8, 13, 16, 30, 51, and 62). One of these (#16) interrupted treatment with XR-NTX due to pregnancy but resumed after miscarriage.
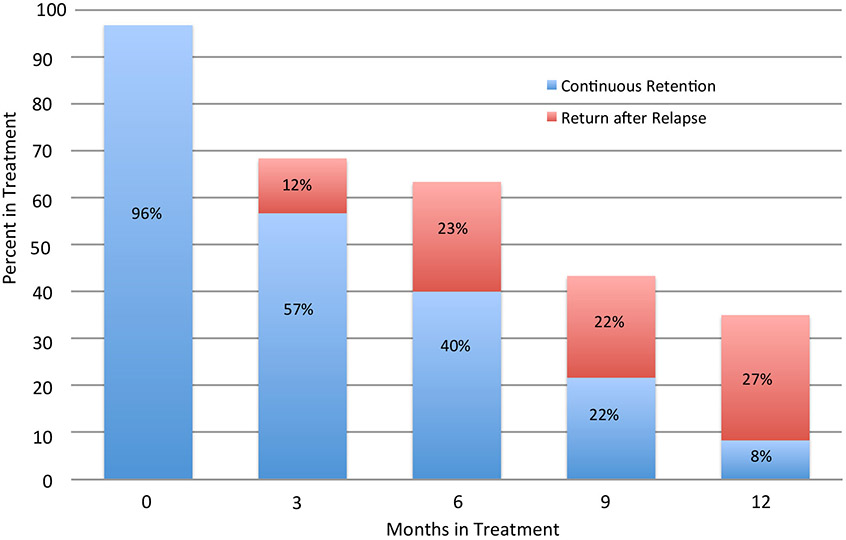
Fig 2:
Percent of Patients In Treatment: Continuous Retention and Return After Relapse
Fig 3 shows a group of 10 patients who discontinued treatment altogether prior to the full prescribed 6 months of study treatment (# 6, 26, 27, 32, 2, 11, 12, 17, 33, 36)
Fig 4 shows a group of 11 patients who discontinued study treatment prior to the full prescribed 6 month duration (or in some cases never even started it, as in cases #56 and #54), but then later reinitiated treatment in TAU, most of them in outpatient treatment involving medication (#56, 53, 48, 45, 49, 18, 56, 54), some in residential treatment (#40, 53, 48, 50, 49, 56, 53, 37, 1), and some in more then one cycle of drop-out and subsequent “re-surfacing.“ (#48, 50, 54).
Fig 5 depicts a group of 11 patients who enrolled in the study, but declined to take their assigned medication after randomization, electing to take a different medication through TAU instead. Eight were randomly assigned to Buprenorphine (bup) but by strong preference from the outset elected treatment with TAU XR-NTX (#52, 42, 47, 46, 31, 29, 24). One was assigned to bup, initially elected XR-NTX but did not get inducted, and instead initiated treatment with methadone within a month (and continued persistently for 12+ months). Three (#22, 21, 15) were randomly assigned to XR-NTX, but by strong preference from the outset elected treatment with TAU bup instead.
Fig 6 depicts a group of 9 patients who started with study medication as randomly assigned but later elected to switch to a different medication, 6 from bup to XR-NTX, and 3 from XR-NTX to bup.
Several additional features of the treatment courses are depicted across all groups. Nineteen patients utilized recovery housing supports (#4, 16, 20, 21, 22, 32, 34, 35, 36, 38, 39, 48, 50, 51, 54, 55, 56, 60, 61). Twenty-five (42%) patients had subsequent episodes of residential treatment following the index episode (#1, 4, 7, 14, 15, 18, 20, 21, 22, 34, 35, 37, 40, 43, 45, 46, 48, 49, 50, 53, 54, 55, 56, 57, 59). Eight patients returned to treatment within a month of relapse (#4, 14, 15, 20, 35, 40, 43, 57), and 17 returned longer than a month after relapse (#1, 7, 18, 21, 22, 34, 48, 49, 50, 53, 54, 55, 56, 59). Of those who returned to care, 17 returned to short term residential, 6 to long term residential and 3 to a combination of both STR and LTR. Nine (36%) had more than one subsequent residential treatment (#4, 14, 15, 21, 22, 34, 40, 46, 48). Two patients were known to have at least one episode of incarceration (#2 and 10).
Mortality
Through the course of the 12 months, 6 (10%) patients had 8 overdoses, 3 of which were fatal. All 8 overdoses occurred while patients were not on any relapse prevention medication and 7 overdoses occurred with patients who were not engaged in any TAU services. One patient was able to re-induct on study XRNTX following the overdose episode while two overdoses prompted return admission to short-term residential care. Two of the three patients with fatal overdoses had a previous overdose in the 12 month period. One patient (#40) died in the 12 month period due to non opioid related causes.
Retention
Fig 7 shows cross-sectional rates of engagement in treatment at months 3, 6, 9, and 12, depicting both the component rates of re-engagement after dropout, and of continuous retention. Over time the total rate of cross-sectional engagement declines, from 69% at month 3 to 35% at month 12. Additionally, among those that were engaged, the proportion that have re-enrolled after dropout increased substantially, in contrast to those that had been continuously retained without dropout, from approximately 15% at month 3 to 75% at month 12. Patients were retained in treatment for an average of 3.9 months (out of 12).
Discussion:
In this descriptive study of medication treatment for OUD, we examined treatment trajectories of 60 patients at a single site of a larger multi-site randomized trial, explicitly broadening the scope of examination to include TAU both during and following the specified study period, treatments both within and outside of the study, and treatment both before and after dropout from the index protocol-specified treatment episode. In contrast to the more homogeneous picture of a hypothetical average subject portrayed by the quantitative outcomes for the aggregate sample means in the parent study, this qualitative view portrays a much more heterogeneous picture of distinct individuals with widely differing treatment courses. This view may help give a broader perspective, more typical of what is seen in real world practice.
One particularly notable feature of the great majority of the treatment courses, is their non-linear quality. While a minority of the patients were unswervingly successful from the outset (see Fig 1) and another small minority had early relapse and dropout without ever re-engaging (see Fig 3), most trajectories seemed to take many twists and turns. Repeated rounds of relapse and remission, as well as treatment drop-out and re-engagement, were more the rule than the exception.
In the context of availability of medication in TAU outside of the study, patient preferences seemed to play a large role in medication choices contrary to research assignment (Fig 5), including switching medications (Fig 6). As in the quantitative results of the as-treated analysis in the parent study, there were no obvious trends indicating that any particular medication choice or sequence was preferable or more likely to be successful (Sullivan et al., 2017). Although it remains unclear whether patient choice was overall better than random assignment (neither was the study sample powered to detect this), the availability and implementation of these choices illustrates an important pathway to treatment engagement when the alternatives were presumably not as palatable or successful.
“Research as usual“ has generally shown 6 month retention rates in the approximate range of 50% for all 3 OUD relapse prevention medications – methadone, buprenorphine and XR-NTX (Mattick et al., 2014; Hser et al., 2016). This has led to mixed interpretations, with both “glass half full“ and “glass half empty“ perspectives. Further, it has generally been thought that real world community treatment as usual would not do as well as highly resourced “boutique“ research results. In this study, conducted at community treatment programs rather than academic research centers, the 6 month continuous relapse-free retention numbers were somewhat lower – approximately 40–45% for the parent study as a whole, and for the single site as well. But in contrast to 40% continuous retention, the cross-sectional treatment engagement rate at 6 months was 62% (at the single site) with the inclusion of return to treatment after dropout. And while <10% were retained continuously at 12 months, a more heartening 35% were engaged in treatment including return after dropout.
It should be noted that several of the groups overlap, with patients that demonstrate more than one of the themes. For example, all of the medication switchers in Fig 5 also exhibited resurfacing in after drop out from study treatment. One patient in Fig 4 who started on non-assigned TAU medication (#22) also later switched medications. Many patients across all of the depicted groups utilized residential treatment and recovery housing supports at different times during their courses, sometimes in multiple episodes. This illustrates the important role of a full continuum of care to support treatment engagement and re-engagement after dropout.
Completion of 6 months of study treatment generally indicated a good prognosis, especially with subsequent TAU medication treatment continuation, consistent with the known relationship between retention and outcome in OUD treatment (Warden et al., 2012; McHugh et al., 2013). But that was not always the case, and several of the 6 month persisters in Fig 1 later dropped out, some re-surfacing later after relapse (#59 and 55). On the other hand, several patients who relapsed and dropped out of treatment before 6 months, later re-engaged with extended periods of subsequent retention and remission (#9, 18, 35, 44, 56).
Over a third of patients (25) had repeated episodes of short-term and/or long-term residential treatment, and several of those had 2 or 3 admissions. This illustrates the importance of bed-based care within the full continuum, both as a re-entry point for crisis stabilization, and most importantly as a portal to further ongoing outpatient care. It is also worth noting the prominent role of recovery housing, with 19 patients having 1 or more stays at a recovery residence program. This illustrates the importance of recovery support services in augmenting engagement in outpatient treatment.
Limitations include: small sample size, lack of a comparison group, and that data from only a single site was used as TAU utilization outside the study and past the duration of the parent study research treatment were not collected at other sites.
Conclusions:
In conclusion, non-linear trajectories are typical of treatment as usual, including multiple episodes of treatment and utilization of various support services across a continuum of care and across different providers. While rates of continuous retention at 12 months in TAU were low in the absence of extra research supports, when returns to treatment after dropout are included, 35% of patients were cross-sectionally engaged in treatment. The expectation of uniformly linear courses of continuous treatment retention and remission is unrealistic, and by defining binary success verses failure may discourage patients and treatment providers from pursuing the desirable and more common trajectory of return to treatment despite expected struggles (Vo et al., 2017). This underscores the role of, and need for, therapeutic optimism as a fundamental approach to patient care in addiction treatment. It also highlights the need to follow patient treated for OUD over longer periods of time, both in research and in standard clinical care. These trajectories of re-engagement after dropout and remission after relapse highlight that the more stereotypical predictors of poorer prognosis can be, and often are, overcome. This is in part because of the heterogeneity and unpredictability of non-linear trajectories. But it is also, and perhaps more importantly, because of the presumed active effect of treatment re-engagement on actually improving prognosis and outcome, despite the conventional odds. Research as usual requires rigid, pre-defined treatment outcomes, but treatment as usual should aspire to the flexibility of always seeking the successes of another round of treatment, and future beginning points, rather than endpoints.
Acknowledgments
Conflicts of interest and sources of funding:
Funded by: NIDA Clinical Trial Network and Maryland Treatment Centers. Dr Fishman has been a consultant for Alkermes, US World Meds, ASAM, Danya/ Mid-Atlantic ATTC.
References:
- Center for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, Mortality File [Database online]. Atlanta, GA: Center for Disease Control and Prevention; Available at: http://www.cdc.gov/nchs/data/health_policy/AADR_drug_poisoning_involving_OA_Heroin_US_2000- 2014.pdf. Updated 2015. [Google Scholar]
- Dalsbol TK, Hammerstrom KT, Vist GE, et al. Psychosocial interventions for retention in drug abuse treatment. Cochrane Database Syst Rev. 2010. doi: 10.1002/14651858.CD00822010 [DOI] [Google Scholar]
- Hser YI, Evans E, Huang D, et al. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction 2016; 111: 695–705. doi:10-1111/add-13238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Hoffman V, Grella CE, et al. A 33-year follow-up narcotics addicts. Arch Gen Psychiatry 2001; 58:503–508. doi: 10.1001/archpsyc.58.5.503 [DOI] [PubMed] [Google Scholar]
- Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet 2018; 391:309–318. doi: 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Nova P, et al. NIDA Clinical Trials Network CTN-0051, Extended-Release Naltrexone vs. Buprenorphine for Opioid Treatment (X:BOT): study design and rationale. Contemp Clin Trials 2016; 50: 253–624. doi: 10.1016/j.cct.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CR, Vo HT, Fishman M. Intranasal naloxone and related strategies for opioid overdose intervention by nonmedical personnel: a review. Subst Abuse Rehab. 2017;8:79–95. doi: 10.2147/SAR.S101700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014; 2: CD002207. doi: 10.1002/14651858.CD002207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Murray HW, Hearon BA, et al. Predictors of dropout from psychosocial treatment in opioid-dependent outpatients. Am J Addict 2013; 22: 18–22. doi: 10.1111/j.1521-0391.2013.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Carroll KM, editors. Rethinking substance abuse: what the science shows, and what we should do about it. New York: The Guilford Press; 2006. [Google Scholar]
- Nunes EV, Lee JD, Sisti D, et al. Ethical and clinical safety considerations in the design of an effectiveness trial: a comparison of buprenorphine versus naltrexone treatment for opioid dependence. Contemp Clin Trials 2016; 51: 34–43. doi: 10.1016/j.cct.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter JS, Marino EN, Hillhouse MP, et al. Buprenorphine/naloxone and methadone maintenance treatment outcomes for opioid analgesic, heroin, and combined users: findings from starting treatment with agonist replacement therapies (START). J of Stud Alc and Drugs 2013; 74(4):605–613. doi: 10.15288/jsad.2013.74.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter JS, Dreifuss JA, Marino EN, et al. The multi-site prescription opioid addiction treatment study: 18-month outcomes. Journal of Substance Abuse Treatment 2014, doi: 10.1016/j.jsat.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruetsch C, Joseph T, Vijay RN, et al. Heterogeneity of Nonadherent Buprenorphine Patients: Subgroup Characteristics and Outcomes. Am J of Managed Care 2017; 23(6): e172–e179. [PubMed] [Google Scholar]
- Schuman-Olivier Z, Weiss DR, Bettina BH, et al. Emerging adult age status predicts poor buprenorphine treatment retention. Journal of Substance Abuse Treatment 2014; 47:202–212. doi: 10.1016/j.jsat.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry 2017; 174: 459–67. doi: 10.1176/appi.ajp.2016.16050548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Substance Abuse. 2016; 37:392–397. doi: 10.1080/08897077.2016.1143435 [DOI] [PubMed] [Google Scholar]
- Vo HT, Burgower R, Rozenberg I, et al. Home-based delivery of XR-NTX in youth with opioid addiction. J Subst Abuse Treat. 2018; 85:84–89. doi: 10.1016/j.jsat.2017.08.007 [DOI] [PubMed] [Google Scholar]
- Warden D, Subramaniam GA, Carmody T, et al. Predictors of attrition with buprenorphine/naloxone treatment in opioid dependent youth. Addict Behav 2012; 37: 1046–53. doi: 10.1016/j.addbeh.2012.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]