Abstract
Background and aims
Overall obesity has recently been established as an independent risk factor for critical illness in patients with coronavirus disease 2019 (COVID-19). The role of fat distribution and especially that of visceral fat, which is often associated with metabolic syndrome, remains unclear. Therefore, this study aims at investigating the association between fat distribution and COVID-19 severity.
Methods
Thirty patients with COVID-19 and a mean age of 65.6 ± 13.1 years from a level-one medical center in Berlin, Germany, were included in the present cross-sectional analysis. COVID-19 was confirmed by polymerase chain reaction (PCR) from nasal and throat swabs. A severe clinical course of COVID-19 was defined by hospitalization in the intensive care unit (ICU) and/or invasive mechanical ventilation. Fat was measured at the level of the first lumbar vertebra on routinely acquired low-dose chest computed tomography (CT).
Results
An increase in visceral fat area (VFA) by ten square centimeters was associated with a 1.37-fold higher likelihood of ICU treatment and a 1.32-fold higher likelihood of mechanical ventilation (adjusted for age and sex). For upper abdominal circumference, each additional centimeter of circumference was associated with a 1.13-fold higher likelihood of ICU treatment and a 1.25-fold higher likelihood of mechanical ventilation.
Conclusions
Our proof-of-concept study suggests that visceral adipose tissue and upper abdominal circumference specifically increase the likelihood of COVID-19 severity. CT-based quantification of visceral adipose tissue and upper abdominal circumference in routine chest CTs may therefore be a simple tool for risk assessment in COVID-19 patients.
Abbreviations: BMI, body mass index; CAP, Canon Aquilion PRIME; COVID 19, coronavirus 19; CT, computed tomography; GEL, GE Lightspeed VCT; ICU, intensive care unit; IL-6, interleukin 6; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome corona virus 2; SFA, subcutaneous fat area; TFA, total fat area; VFA, visceral fat area
Keywords: COVID-19, Obesity, Overweight, Quantification of adipose tissue, Visceral adipose tissue
Highlights
-
•
Previous studies suggested an association between obesity and severe COVID-19.
-
•
So far, research focused exclusively on body mass index as a measure of obesity.
-
•
Routine chest CTs allow quantification of subcutaneous and visceral abdominal fat.
-
•
We found a positive association between visceral fat tissue and COVID-19 severity.
-
•
Chest CTs may be a simple tool for risk assessment in patients with COVID-19.
1. Introduction
In the context of the current unprecedented health crisis due to the pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), evidence has emerged suggesting that obesity might aggravate the course of coronavirus disease 2019 COVID-19 [1,2]. However, previous research has focused exclusively on an increased individual body mass index (BMI) as a risk factor for severe COVID-19 without distinguishing between subcutaneous and visceral adipose tissue. Apart from overall obesity, body fat distribution is an important risk factor for cardiometabolic outcome [1,2]. Visceral adipose tissue is often associated with metabolic syndrome, conferring an increased risk of cardiovascular disease and type 2 diabetes mellitus with subsequent higher morbidity [1,2]. Furthermore, although BMI is currently the most commonly used indicator for assessing overweight/obesity in adults and has high specificity, it is influenced by sex and age and has an inherently low sensitivity for identifying excess fat mass [3]. Furthermore, BMI-based measures cannot distinguish between visceral and subcutaneous fat. Here, computed tomography (CT) with noninvasive postprocessing tools enables the differentiation and quantification of visceral and subcutaneous fat tissue, thus allowing reliable assessment of body fat distribution [4].
Therefore, the aim of this proof-of-concept study was to quantify the subcutaneous and visceral adipose tissue of COVID-19 patients using routinely acquired chest CT scans and to compare the results with the severity of the clinical course in terms of hospitalization with intensive care unit (ICU) treatment and/or invasive mechanical ventilation.
2. Material and methods
Thirty patients with COVID-19 and a mean age of 65.6 ± 13.1 years from a level-one medical center in Berlin, Germany, were included in the present cross-sectional analysis. Patients were treated between March 27 and April 27, 2020 and inclusion criteria were: availability of a (low-dose) chest CT scan with diagnostic image quality and laboratory-confirmed COVID-19. All CT datasets were of diagnostic image quality and none of the patients had to be excluded. COVID-19 was confirmed by polymerase chain reaction (PCR) from nasal and throat swabs on the day of admission. To reduce the false negative rate, at least two repeat PCRs were performed. Additional swabs were obtained to confirm the diagnosis in patients with a positive result and in case of negative results and persisting respiratory symptoms. A severe course of COVID-19 was defined by the requirement of ICU treatment and/or mechanical ventilation. Age and sex were potential confounders, which were corrected for in statistical analysis.
This proof-of-concept study was prior approved by the local ethics committee in accordance with the local laws and regulations, including a waiver of informed consent (EA 4/140/17). It was conducted in accordance with the local laws and regulations and in accordance with the Declaration of Helsinki and Good Clinical Practice.
2.1. CT imaging protocol
CT examinations of study patients were performed on two different types of scanners: three Canon Aquilion PRIME (CAP) scanners and a GE Lightspeed VCT (GEL) scanner. A low-dose chest CT scan was performed for diagnostic purposes or to evaluate the patient's current condition on the day of admission. The CT protocol included the following imaging parameters: highest rotation time available - 0.27 s (CAP) and 0.35 s (GEL), 100 kV, automatic tube modulation between 10 and 100 mA with a noise index of 27 (CAP) and 39 (GEL), collimation of 80 × 0.5 mm (CAP) and 64 × 0.625 mm (GEL), pitch factors of 1.388 (CAP) and 1.375 (GEL). Patients were positioned supine, and nonenhanced scans in caudocranial scan direction were obtained in deep inspiration. We reconstructed 0.5 mm (CAP) and 0.625 mm (GEL) axial slices using soft tissue and lung kernels (Fc01 and Fc85 (CAP), “standard” and “lung” (GEL)) and iterative reconstruction technology (AIDR3D level “moderate” (CAP), ASIR 50% (GEL)).
2.2. Data acquisition
CT fat was measured in a fully automated fashion from chest CTs using Vital's Vitrea™ Advanced Visualization postprocessing applications (Version 7.0, Canon Medical Systems Cooperation, Otawara, Tochigi, Japan), which was designed to isolate and quantify subcutaneous and visceral fat tissue based on a single slice of non-contrast enhanced CT data. Visceral fat area (VFA), subcutaneous fat area (SFA), and total fat area (TFA) were quantified at the level of the mid of the first lumbar vertebra on clinical chest CT scans routinely acquired for COVID-19 [5,6]. Data were then exported as comma-separated values.
As outcome of interest we defined whether the patient had to be transferred to the intensive care unit and if mechanical ventilation was required. The Radiology Information System was searched to determine if the patient had to be been transferred to the ICU and conventional chest radiographs were assessed to assess if patients were intubated.
Other patient information such as age, weight, or height was extracted from the DICOM headers of the CT scans, if available. If this information was missing in the DICOM headers, it was instead extracted from patient reports, resulting in no missing data on age, weight or height. BMI was calculated according to the common formula: weight (kg) / [height (m)]2. Other clinical information, such as comorbidities and laboratory data, were also extracted from patient records. Regarding comorbidities, we assumed a comorbidity was not present if not mentioned in any of the patient records over the last five years. Acquired data were stored in tabular form and then exported as comma-separated values for subsequent statistical analysis.
2.3. Statistical analysis
All statistical analysis was performed using the ‘R’ statistical environment (Version 4.0.0), including the “tidyverse” library [7,8]. Continuous variables were tested for normal distribution using the Shapiro-Wilk test. If normally distributed, they were expressed as mean ± standard deviation. Otherwise, they were presented as median and interquartile range (IQR). Spearman's correlation coefficients were calculated, if correlations were assessed. The three subgroups of patients (normal ward, ICU, and mechanical ventilation) were compared using an analysis of variance with mixed models corrected for age and sex, with the imaging site as random intercept. Afterwards, post-hoc binary tests were applied to compare patients on normal wards with ICU patients and patients on wards with patients requiring mechanical ventilation. Continuous and normally distributed variables were compared with a one-tailed Student's t-test, while the Wilcox rank sum test was used in case of non-normal distribution. Categorical variables were expressed as frequencies and percentages and compared using Fisher's exact test or the Chi-squared test, as appropriate. A multivariate binomial logistic regression model was employed to analyze the relationship between severe clinical courses and quantitative CT features. 95% confidence intervals for odds ratios were calculated using bootstrapping (1000 iterations). A p-value < 0.05 was considered statistically significant.
3. Results
In the present proof-of-concept study, we analyzed chest CT images of 30 patients with confirmed SARS-CoV-2 infection. The study population had a mean age of 65.6 years ± 13.1 years and included 18 men (61.9 years ± 12.8 years) and 12 women (71.2 years ± 11.8 years, p = 0.051). A total of 43% of the patients had to be transferred to the ICU during treatment (n = 13), with men being affected more often than women (9 men vs. 4 women, p = 0.47). Seven of the 13 ICU patients required mechanical ventilation (6 men vs. 1 woman, p = 0.19). Information on height and weight was available in all cases. Table 1, Table 2, Table 3 provide detailed overviews of anthropomorphic and clinical patient characteristics.
Table 1.
Demographic and clinical patient characteristics.
| Characteristic | All patients (n = 30) | BMI groups |
p | Age groups |
p | |||
|---|---|---|---|---|---|---|---|---|
| <25 (n = 11) | ≥25 (n = 19) | ≤50 (n = 4) | 51–64 (n = 7) | ≥65 (n = 19) | ||||
| Male/female sex – No. | 18/12 | 7/4 | 11/8 | 1/1 | 4/0 | 4/3 | 10/9 | 0.27/0.27 |
| Age – years | 65.6 ± 13.1 | 68.8 ± 12.8 | 63.7 ± 12.2 | 0.96 | ||||
| BMI – kg/m2 | 26.4 ± 3 | 25.6 IQR 2.7 | 27.3 IQR 2.4 | 26.3 IQR 2.1 | 0.99 | |||
| Former or current smoker – No. | 4 | 3 | 1 | 0.13 | 0 | 1 | 3 | 1 |
| Coexisting disorders | ||||||||
| Any – No. | 19 | 7 | 12 | 1 | 2 | 5 | 12 | 0.87 |
| Hypertension – No. | 15 | 4 | 11 | 0.45 | 1 | 4 | 10 | 0.75 |
| Diabetes – No. | 5 | 2 | 3 | 1 | 0 | 1 | 4 | 1 |
| Coronary artery disease – No. | 5 | 3 | 2 | 0.33 | 0 | 0 | 5 | 0.27 |
| Heart failure – No. | 2 | 1 | 1 | 1 | 0 | 0 | 2 | 1 |
| COPD – No. | 3 | 1 | 2 | 1 | 0 | 0 | 3 | 0.71 |
| Bronchial asthma – No. | 3 | 0 | 3 | 0.28 | 0 | 3 | 0 | 0.01 |
| Chronic renal disease – No. | 2 | 2 | 0 | 013 | 0 | 0 | 2 | 1 |
| Active malignancy – No. | 3 | 3 | 0 | 0.04 | 1 | 0 | 2 | 0.46 |
This table provides an overview of demographic and clinical patient characteristics. Values for age and BMI are presented as mean ± standard deviation if normally distributed and as median and interquartile range (IQR) if not. p-Values were obtained with Fisher tests for the count variables age and BMI and with ANOVA for all other variables. Abbreviations: No.: number, SD: standard deviation, BMI: body mass index, COPD: chronic obstructive pulmonary disease.
Table 2.
Fat measures and laboratory findings.
| Characteristic | All patients (n = 30) | BMI groups |
p | Age groups |
p | |||
|---|---|---|---|---|---|---|---|---|
| <25 (n = 11) | ≥25 (n = 19) | ≤50 (n = 4) | 51–64 (n = 7) | ≥65 (n = 19) | ||||
| Total fat area (10 cm2) | 15.1 IQR: 7.6 | 12.8 ± 4.9 | 15.7 IQR: 9.7 | 0.05 | 12.6 ± 7.09 | 26.0 ± 13.4 | 15.0 IQR: 4.6 | 0.65 |
| Subcutaneous fat area (10 cm2) | 6.2 IQR: 4.8 | 5.1 ± 12.0 | 8.4 IQR: 7.3 | 0.03 | 5.2 ± 3.6 | 6.2 IQR: 21.2 | 6.3 IQR: 3.6 | 0.64 |
| Visceral fat area (10 cm2) | 8.2 IQR: 5.5 | 7.7 ± 3.6 | 8.8 IQR 5.3 | 0.40 | 7.4 ± 3.8 | 10.8 SD: 1.6 | 6.6 IQR: 4.8 | 0.86 |
| Upper abdominal circumference (cm) | 102.5 ± 8.9 | 97.8 ± 7.5 | 105.3 ± 8.6 | 0.03 | 102.2 ± 3.7 | 110.4 ± 5.3 | 99.7 ± 9.1 | 0.13 |
| C-reactive protein (mg/dL) | 63 IQR: 105.3 | 60 IQR 97 | 96.1 ± 73.3 | 0.30 | 132.1 ± 122 | 103.2 ± 76.9 | 62 IQR: 92.8 | 0.13 |
| White-cell count – per (103/μL) | 6.2 ± 2.7 | 5.4 ± 2.6 | 6.6 ± 2.8 | 0.39 | 5.0 ± 3.5 | 7.1 ± 3.0 | 5.6 IQR: 2.7 | 0.97 |
| Lymphocyte count – per (103/μL) | 0.87 IQR: 0.62 | 0.77 IQR 0.54 | 0.96 IQR 0.63 | 0.53 | 1.1 ± 0.7 | 1.4 IQR: 2.96 | 0.8 IQR: 0.48 | 0.38 |
| Creatinine (mg/dL) | 0.91 IQR: 0.32 | 1.02 IQR: 1.08 | 0.9 IQR 0.26 | 0.78 | 0.78 ± 0.17 | 1 IQR: 0.22 | 0.99 IQR: 0.38 | 0.31 |
This table shows the distribution of fat measures and laboratory findings across different BMI and age groups. Values are presented as mean ± standard deviation if normally distributed and as median and interquartile range (IQR) if not. P-values were obtained with ANOVA for all columns. Abbreviations: IQR: interquartile range, BMI: body mass index.
Table 3.
In-hospital courses of COVID-19 in the 30 study patients.
| Characteristic | All patients (n = 30) | BMI groups |
p | Age groups |
p | |||
|---|---|---|---|---|---|---|---|---|
| <25 (n = 11) | ≥25 (n = 19) | ≤50 (n = 4) | 51–64 (n = 7) | ≥65 (n = 19) | ||||
| Mortality – No. | 2 | 2 | 0 | – | 0 | 1 | 1 | – |
| Normal ward – No. | 17 | 8 | 9 | 0.26 | 2 | 2 | 13 | 0.21 |
| ICU – No. | 13 | 3 | 10 | 0.26 | 2 | 5 | 6 | 0.21 |
| ICU with mechanical ventilation – No. | 7 | 2 | 5 | 1 | 1 | 3 | 3 | 0.39 |
| Number of days in ICU – No. | 13.2 ± 10.2 | 4⁎ | 14.2 ± 10.3 | – | 19; 7⁎⁎ | 4 IQR 7.3 | 15.3 ± 8.3 | – |
| ARDS – No. | 5 | 2 | 3 | 1 | 0 | 2 | 3 | 0.61 |
This table provides an overview of in-hospital courses of COVID-19, including mortality, number of patients in the normal ward, intensive care unit (ICU), and ICU with intubation. Also provided is information on the number of days in ICU and on patients with ARDS. The number of ICU days is given as mean ± standard deviation if the values were normally distributed in the respective subgroup and as median and interquartile range (IQR) if not. We used Fisher tests to calculate p-values for all count variables (mortality, normal ward, ICU, ICU with mechanical ventilation and ARDS) and ANOVA for group comparison regarding the number of days on the ICU. Abbreviations: No.: number, ICU: intensive care unit, ARDS: acute respiratory distress syndrome, BMI: body mass index, SD: standard deviation, IQR: interquartile range.
The number of days in the ICU was missing for two patients, as they were transferred to another site.
Only two patients were in the ICU in this subgroup.
3.1. Differences between patients in the ICU and normal ward
Mean BMI of all patients was 26.4 kg/m2 ± 2.9 kg/m2 (men 26.0 kg/m2 ± 2.24 kg/m2 vs. women 26.9 kg/m2 ± 3.7 kg/m2). Patients who were admitted to the ICU had a BMI of 26.8 kg/m2 ± 2.1 kg/m2 compared to patients who did not require ICU treatment with a BMI of 26.1 kg/m2 ± 3.4 kg/m2 This difference was not significant (p = 0.46).
The median VFA was 82.4 cm2 (IQR 55.2 cm2). Men tended to have more visceral adipose tissue than women (95.4 cm2 vs. 67.3 cm2), and the difference was statistically significant (p = 0.014). Patients admitted to the ICU had a significantly larger TFA compared to patients in the normal ward. When looking at the different fat compartments, it was particularly conspicuous that ICU patients had a significantly larger VFA (96.9 cm2 ± 33.5 cm2) compared to patients in the normal ward (70.0 cm2 ± 28.2 cm2, p = 0.031). Furthermore, patients requiring mechanical ventilation had a significantly larger VFA than patients who could breathe freely (ICU with mechanical ventilation: 124.2 cm2 vs. ICU and free breathing: 96.6 cm2 vs. normal ward and free breathing: 70.0 cm2, p = 0.006) (see Fig. 1 for patient case examples). CT-derived upper abdominal circumference also significantly differed between patients in ICU (107.0 cm ± 8.3 cm) and patients on the normal ward (99.2 cm ± 8.0 cm, p = 0.009) and similarly between patients requiring mechanical ventilation (109.7 cm ± 7.6 cm) and patients without mechanical ventilation (103.8 ± 8.6, p = 0.008).
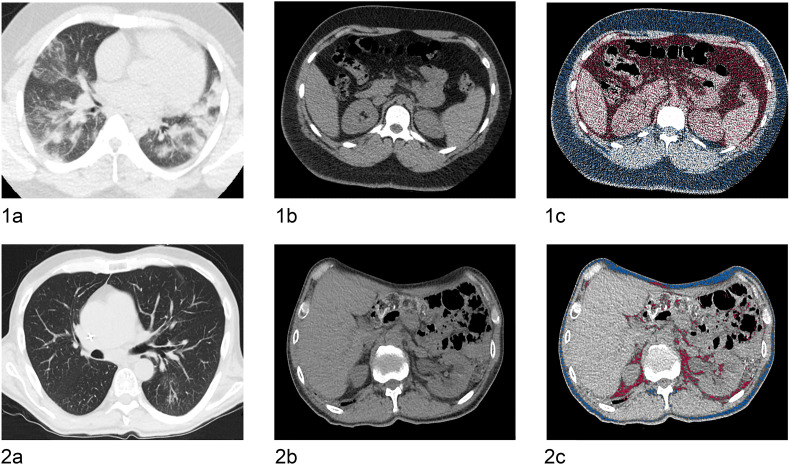
Fig. 1.
Patient examples using the automated post-processing application.
Shows two case examples of a lean and an obese patient with COVID-19. 1a–c show a chest (1a) and abdominal CT scan (1b) of an obese 27-year-old male patient. 1c shows CT-based fat quantification of the subcutaneous (blue color) and visceral adipose tissue (red color) with a visceral fat area of 68.3 cm2, a subcutaneous fat area of 88.5 cm2, and a CT-derived upper abdominal circumference of 109.1 cm. 2a–c show a chest (2a) and abdominal CT scan (2b) of a lean 51-year old male patient. 2c shows CT-based fat quantification of the subcutaneous and visceral adipose tissue with a visceral fat area of 15.4 cm2, subcutaneous fat area of 20.8 cm2, and a CT-derived upper abdominal circumference of 95.9 cm. Of these two patients, the one with the higher fat content had more severe pulmonary COVID-19 infection.
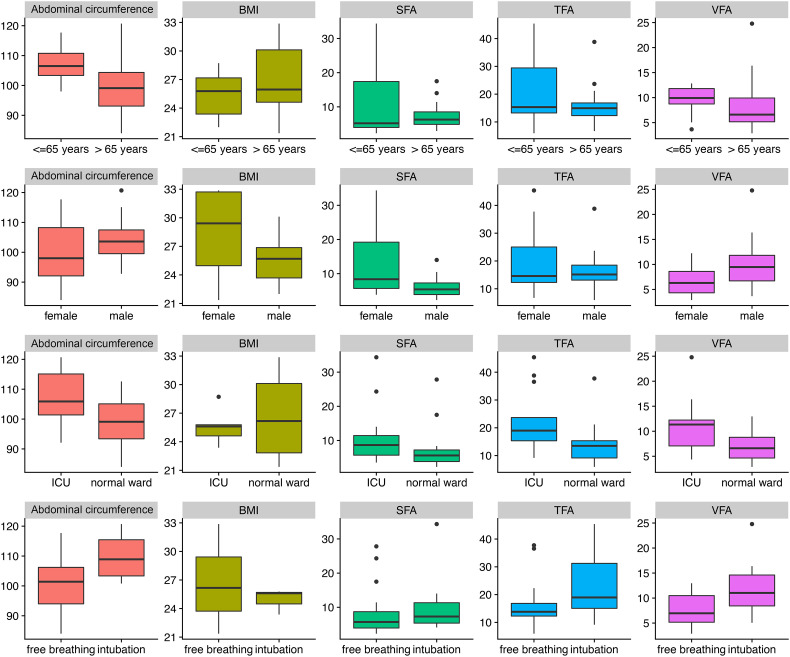
Differences in SFA between patients on the normal ward vs. ICU or patients with vs. without mechanical ventilation were also significant (p = 0.039), but with only low statistical power (0.38). The differences in the various fat tissue measurements and BMI between patient groups are summarized in Table 4 . Fig. 2 provides an overview of the distribution of the different BMI and fat measures.
Table 4.
Differences in fat measures between patient groups.
| Measurement | Normal ward | ICU and free breathing | ICU and mechanical ventilation | p-Values | Power |
|---|---|---|---|---|---|
| Total fat area (cm2) | 135 IQR 61.8 | 204.4 ± 86.9 | 237.3 ± 134.3 | 0.005* | 0.85 |
| Subcutaneous fat area (cm2) | 56.2 IQR 33.8 | 107.57 ± 72.8 | 73 IQR 59.7 | 0.039* | 0.38 |
| Visceral fat area (cm2) | 70.0 ± 28.2 | 96.9 ± 33.5 | 124.2 ± 65.9 | 0.005* | 0.98 |
| Upper abdominal circumference (cm) | 99.2 ± 8.0 | 103.8 ± 8.6 | 109.7 ± 7.6 | 0.011* | 0.76 |
| BMI (kg/m) | 26.1 ± 3.4 | 27.3 ± 2.2 | 26.4 ± 2.2 | 0.579 | 0.09 |
This table gives an overview of differences in fat measures between three patient groups: Group 1 included patients in the normal ward, group 2 included patients treated in the ICU without mechanical ventilation, group 3 included patients treated in the ICU with mechanical ventilation. Values for the groups were expressed as mean ± standard deviation if normally distributed and as median and interquartile range (IQR) if not. All differences were assessed using analysis of variance (ANOVA). Significant p-values are marked with an asterisk. The power analysis was performed post priori. Abbreviations: BMI: body mass index, ICU: intensive care unit.
Fig. 2.
Distribution of different weight and fat measures.
Distribution of abdominal circumference (in cm), body mass index (BMI), subcutaneous fat area (SFA, cm2), total fat area (TFA), and visceral fat area (VFA) across different binary parameters. Line 1 represents the distribution of these fat measures across two age groups (<65 vs. ≥65 years). Line 2 represents the distribution of these fat measures across the two sexes (male, female). Line 3 represents the distribution of these fat measures for intensive care unit (ICU) patients vs. patients in normal wards. Line 4 represents the distribution of these fat measures between freely breathing and mechanically ventilated patients. Abbreviations: BMI: body mass index, SFA: subcutaneous fat area, TFA: total fat area, VFA: visceral fat area.
3.2. Association between BMI and fat area measurements
VFA and BMI showed a moderate correlation with a correlation coefficient of r = 0.53 (95% CI 0.40–0.73, p < 0.001). It is noteworthy that the correlation was stronger for men than for women (r = 0.59 vs. r = 0.52). CT-derived upper abdominal circumference and BMI also showed a moderate correlation with a correlation coefficient of r = 0.59 (95% CI 0.41–0.78, p < 0.001). Again, men showed a slightly stronger correlation between BMI and CT-derived upper abdominal circumference compared to women (r = 0.62 vs. r = 0.58).
3.3. Association between fat area measurements and COVID-19 severity
As VFA and CT-derived upper abdominal circumference differed significantly between patients who required ICU treatment and/or mechanical ventilation and patients who did not require ICU treatment or mechanical ventilation, a logistic regression analysis was conducted. Two models each were constructed for VFA and CT-derived upper abdominal circumference, the first unadjusted for any confounder, the second adjusted for age and sex. Table 5 gives an overview of the odds ratios derived from logistic regression analysis. An increase in VFA by 10 square centimeters was associated with a higher likelihood of ICU treatment and a higher likelihood of mechanical ventilation. The odds ratio, as derived from the model adjusted for age and sex, was 1.37 (95% CI 1.07–1.89) regarding ICU treatment. This means that patients were 1.37 times more likely to require ICU treatment than patients with 10 cm2 less VFA. Similarly, those patients were also 1.32 (95% CI 1.04–1.91) times more likely to require mechanical ventilation compared to patients with 10 cm2 less VFA.
Table 5.
Multivariate logistic regression analysis for ICU treatment and mechanical ventilation.
| Variable | Odds ratio | 95% CI | Outcome |
|---|---|---|---|
| Unadjusted for age and sex | |||
| Visceral fat area (per 10 cm2) | 1.36 | 1.08–1.86 | Patient in ICU |
| Upper abdominal circumference (per 1 cm) | 1.13 | 1.03–1.29 | Patient in ICU |
| Total fat area (per 10 cm2) | 1.11 | 1.02–1.28 | Patient in ICU |
| Visceral fat area (per 10 cm2) | 1.30 | 1.05–1.81 | Mechanical ventilation |
| Upper abdominal circumference (per 1 cm) | 1.17 | 1.04–1.37 | Mechanical ventilation |
| Total fat area (per 10 cm2) | 1.08 | 0.99–1.19 | Mechanical ventilation |
| Adjusted for age and sex | |||
| Visceral fat area (per 10 cm2) | 1.37 | 1.07–1.89 | Patient in ICU |
| Upper abdominal circumference (per 1 cm) | 1.13 | 1.02–1.3 | Patient in ICU |
| Total fat area (per 10 cm2) | 1.13 | 1.03–1.29 | Patient in ICU |
| Visceral fat area (per 10 cm2) | 1.32 | 1.04–1.91 | Mechanical ventilation |
| Upper abdominal circumference (per 1 cm) | 1.25 | 1.05–1.68 | Mechanical ventilation |
| Total fat area (per 10 cm2) | 1.28 | 1.06–1.80 | Mechanical ventilation |
This table provides the odds ratios for ICU treatment and mechanical ventilation, derived from multivariate logistic regression analysis, unadjusted and adjusted for age and sex. Abbreviations: CI: confidence interval, ICU: intensive care unit.
For CT-derived upper abdominal circumference, each additional centimeter of circumference was associated with a 1.13-fold (95% CI 1.02–1.3) higher likelihood of ICU treatment and a 1.25-fold (95% CI 1.05–1.68) higher likelihood of mechanical ventilation.
4. Discussion
In the present proof-of concept study, subcutaneous and visceral adipose tissue was automatically quantified in routinely acquired chest CT scans of COVID-19 patients at the level of the first lumbar vertebra using a post-processing application, which was not previously validated for fat measurements in COVID-19 patients. Apart from the total fat area, we identified visceral adipose tissue and CT-derived upper abdominal circumference as significant indicators of severe courses of COVID-19, while BMI was not an indicator in our analysis. A severe course was assumed when a patient needed ICU treatment and/or mechanical ventilation.
In 2016, the World Health Organization estimated that 1.9 billion adults were overweight and of these, over 650 million were obese [9]. Overall obesity is a state of low-grade systemic inflammation, which contributes to the development of metabolic diseases, such as dyslipidemia or type 2 diabetes mellitus, and may modify immune responses, making the immune system more susceptible to infection [10]. This could also play an important role in the pathogenesis of respiratory disorders [11]. In the context of influenza virus infection, obesity has been suggested to impair memory CD8+ T cell responses to infection, resulting in more severe lung pathology and higher mortality [12]. Particularly central obesity with intra-abdominal deposition of visceral fat tissue has been closely linked to cardiometabolic disorders [13]. Visceral adipose tissue, known to be more metabolically active than subcutaneous adipose tissue, has unique endocrine functions and secretes a variety of adipokines and pro-inflammatory cytokines, including TNF, C-reactive protein, interleukin 6 (IL-6), and leptin [12,14]. Apart from insulin resistance and metabolic syndrome, elevated IL-6 levels are known to be related to chronic inflammatory airways disease, and recent studies report higher levels of IL-6 in COVID-19 non-survivors [15,16]. Early findings from Italy suggest that IL-6 receptor inhibition by administration of the monoclonal antibody tocilizumab might improve respiratory function in COVID-19-related acute respiratory distress syndrome [17]. In addition, leptin was previously found to be related to airway reactivity, and initial findings suggest that leptin levels are elevated in COVID-19 patients with more severe pulmonary inflammation [18,19].
With the shift of COVID-19 epicenters to Europe and now especially North and South America, the impact of obesity on COVID-19 may gain in importance, as these two continents have some of the highest prevalence of obesity worldwide, with obesity taking on epidemic proportions [20]. While previous investigators already identified overall obesity as a risk factor for severe courses of COVID-19, they only used BMI to measure obesity [[21], [22], [23], [24]]. Compared to recent COVID-19 studies from New York and Lille [24,25], which used BMI as a measure of obesity, our ICU patients were of similar age (65.6 ± 13.1 years) as the patients from New York (median 67, ICR 56–77 years), while patients from Lille were younger (median 60, ICR 51–70 years) [26]. Regarding median BMI, the Lille center found that COVID-19 patients with a BMI ≥ 35 kg/m2 required mechanical ventilation significantly more often than COVID-19 patients with a BMI below 25 kg/m2 [24]. In a New York patient population, especially a BMI ≥ 40 kg/m2 was identified to be among the factors most strongly associated with hospitalization of COVID-19 patients [25]. Another study from New York by Palaiodimos et al. examined a minority-predominant population and found that severe obesity was independently associated with a higher in-hospital mortality [27]. Zheng et al. reported patients with metabolic associated fatty liver disease and obesity (BMI > 25 kg/m2) to have a 6-fold higher risk for a severe course of COVID-19 [2]. By comparison, we found a more than 1.37-fold higher likelihood of ICU treatment and a more than 1.32-fold higher likelihood of mechanical ventilation for an increase in VFA by 10 cm2 (adjusted for age and sex).
A current study by Deng et al. found that obesity could also increase severe courses of COVID-19 in younger patients, especially in those with damages of liver and kidneys [28]. Kass et al. also reported, that obesity could shift severe COVID-19 to younger ages after examining the correlation between BMI and age in COVID-19 patients admitted to the ICU at different university hospitals in the United States [29]. Most recently, Stefan et al. underlined the importance of measuring anthropomorphics and metabolic parameters, such as BMI, waist and hip circumferences and levels of glucose and insulin, in addition to the evaluation of standard hospital parameters in order to improve individual risk assessment of COVID-19 patients [1].
It should be noted that the utility of BMI in assessing obesity depends on the assumption of a close correlation of anthropomorphic measures with direct measures of obesity, such as total body fat or visceral and subcutaneous adipose tissue [24]. Here, several studies suggest that, for the same BMI, the amount of body fat is heavily influenced by age and sex, as women tend to have more fat than men with the same BMI, while older persons tend to have more body fat than younger persons with the same BMI [30,31]. This is in line with our correlation coefficient of 0.56 for VFA and BMI and the consistently moderate correlation coefficients reported in the literature, ranging from 0.61 to 0.71 after adjustment for age [26,32,33].
By using CT-based quantification of the visceral and subcutaneous adipose tissue, we chose a more comprehensive approach to measure potential obesity, which allowed us to separately determine different fat compartments such as the subcutaneous and visceral fat area. This is essential as body fat distribution was previously established as an independent risk factor for cardiometabolic outcomes in the general population, next to obesity [2]. Although the post-processing application used in the present study (Vitrea™) was not previously validated for fat measurement in COVID-19 patients, it has been validated for abdominal fat measurement by using cryosection photographs and CT images from the NIH Visible Human Project [34,35]. In addition, it was reported that visceral fat areas obtained from a single slice and waist circumference obtained at the level of the umbilicus (approximately L4 or L5) were highly correlated to the total visceral fat volume [36,37]. Here, it has to be pointed out, that we obtained the fat measurements at the level of the first lumbar vertebra instead of the fourth or fifth vertebra, as the lower levels were not included in the chest CT scans. Kuk et al. previously investigated if the measurement site for visceral and abdominal subcutaneous adipose tissue in CT scans would alter associations with the metabolic syndrome and found no significant difference between Th12-L1, L1-2 and L4-L5 [38]. Therefore, it appears that the first lumbar vertebra (L1) may similarly be used for fat measurement. Still, our deviating measurement method (measurement at the level of L1 instead of L4/5) has to be recognized as a potential shortcoming.
In our COVID-19 population we found that - apart from TFA - VFA and CT-derived upper abdominal circumference were associated with ICU treatment and/or mechanical ventilation. Differences in SFA were also statistically significant, but with only low statistical power, while BMI did not show any significant association with severe courses of COVID-19. However, it has to be acknowledged that the mean BMI was quite low in our study population, with most of the patients being overweight, but not obese. This might explain why BMI was not a significant indicator in our patients. On the other hand, this observation highlights the importance of visceral adipose tissue as a potential indicator of COVID-19 severity in overweight patients with an increased amount of visceral fat tissue who do not meet the criterion for obesity.
To our knowledge, this is the first study that specifically identifies visceral fat and CT-derived upper abdominal circumference as potential risk factors for severe courses of COVID-19. These indicators can be determined without the need for further imaging simply by performing an automated postprocessing analysis of a standard chest CT scan, provided that it was acquired during routine diagnostic workup. However, it should be noted that a CT-based measurement is obviously not less costly than an anthropomorphic measurement of waist circumference. But other than conventional anthropomorphic measurements, CT-based measurements also allow for an assessment of the visceral and subcutaneous adipose tissue. In addition, even simple measurements, such as measurements of the waist circumference, can be more difficult to obtain in critically ill patients. Given that some COVID-19 patients are extremely ill, such easy-to-determine parameters may be of clinical relevance.
The present proof-of-concept study has several limitations: firstly, anthropomorphic measures were derived from DICOM headers or patient records and might have been reported by the patients with possible bias. Secondly, regarding comorbidities, we also extracted this information from the patient records, assuming a comorbidity was not present if not mentioned in any of the patient records over the last five years. We are aware, that this approach might lead to biased results for the presence of comorbidities. Besides there were also missing data on laboratory data. However, as this data was only provided in the sense of additional clinical information and not included in any analysis, we believe, that this does not affect the results of the present study. Thirdly, the post-processing application used for the fat measurements was not previously validated for fat measurements in COVID-19 patients. Besides, CT-based fat measurements were performed at the level of the first lumbar vertebra instead of the fourth or fifth vertebra and we used the upper abdominal circumference instead of the abdominal circumference. This may have affected the reliability of our fat measurements. Finally, a major limitation is the small sample size and the single-center cross-sectional design. Retrospective epidemiological studies in larger populations as well as autopsy studies and clinical trials with the aim to identify individuals most at risk of becoming infected or of developing complications will be needed for a better selection of individual safety measures. Also, it will be important to determine if it is possible to employ prophylactic and therapeutic measures (e.g., treatment modulating the complement system or IL-6 secretion), and if there are long-term consequences of COVID-19 infection on the health of patients with overweight and increased amounts of adipose tissue. Finally, the pathophysiological link between obesity, overweight, visceral adipose tissue and respiratory disease will have to be further investigated.
5. Conclusions
Going beyond the recently established association between BMI-based obesity and severe courses of COVID-19, our results suggest that body fat distribution is also important, with visceral adipose tissue and CT-derived upper abdominal circumference significantly increasing the likelihood of severe courses of COVID-19. Hence, CT-based quantification of visceral adipose tissue and upper abdominal circumference might be a simple tool for risk assessment in COVID-19 patients.
CRediT authorship contribution statement
Antonia Petersen: Validation, Investigation, Data curation, Writing - original draft. Keno Bressem: Conceptualization, Methodology, Software, Formal analysis, Writing - review & editing. Jakob Albrecht: Writing - review & editing. Hans-Martin Thieß: Writing - review & editing. Janis Vahldiek: Writing - review & editing. Bernd Hamm: Resources, Writing - review & editing. Marcus R. Makowski: Writing - review & editing. Alexandra Niehues: Investigation, Writing - review & editing. Stefan M. Niehues: Conceptualization, Methodology, Resources, Writing - review & editing, Supervision, Project administration. Lisa C. Adams: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - original draft, Visualization, Supervision, Project administration.
Declaration of competing interest
BH has received research grants for the Department of Radiology, Charité - Universitätsmedizin Berlin from the following companies and institutions: (1) Abbott, (2) Actelion Pharmaceuticals, (3) Bayer Schering Pharma, (4) Bayer Vital, (5) BRACCO Group, (6) Bristol-Myers Squibb, (7) Charite Research Organisation GmbH, (8) Deutsche Krebshilfe, (9) Dt. Stiftung für Herzforschung, (10) Essex Pharma, (11) EU Programmes, (12) FibrexMedical Inc., (13) Focused Ultrasound Surgery Foundation, (14) Fraunhofer Gesellschaft, (15) Guerbet, (16) INC Research, (17) lnSightec Ud, (18) IPSEN Pharma, (19) Kendlel MorphoSys AG, (20) Lilly GmbH, (21) Lundbeck GmbH, (22) MeVis Medical Solutions AG, (23) Nexus Oncology, (24) Novartis, (25) Parexel Clinical Research Organisation Service, (26) Perceptive, (27) Pfizer GmbH, (28) Philipps, (29) Sanofis-Aventis S.A., (30) Siemens, (31) Spectranetics GmbH, (32) Terumo Medical Corporation, (33) TNS Healthcare GMbH, (34) Toshiba, (35) UCB Pharma, (36) Wyeth Pharma, (37) Zukunftsfond Berlin (TSB), (38) Amgen, (39) AO Foundation, (40) BARD, (41) BBraun, (42) Boehring Ingelheimer, (43) Brainsgate, (44) PPD (Clinical Research Organisation), (45) CELLACT Pharma, (46) Celgene, (47) CeloNova Bio-Sciences, (48) Covance, (49) DC Deviees, Ine. USA, (50) Ganymed, (51) Gilead Sciences, (52) GlaxoSmithKline, (53) ICON (Clinical Research Organisation), (54) Jansen, (55) LUX Bioseienees, (56) MedPass, (57) Merek, (58) Mologen, (59) Nuvisan, (60) Pluristem, (61) Quintiles, (62) Roehe, (63) SehumaeherGmbH (Sponsoring eines Workshops), (64) Seattle Geneties, (65) Symphogen, (66) TauRx Therapeuties Ud, (67) Accovion, (68) AIO: Arbeitsgemeinschaft Internistische Onkologie, (69) ASR Advanced sleep research, (70) Astellas, (71) Theradex, (72) Galena Biopharma, (73) Chiltern, (74) PRAint, (75) lnspiremd, (76) Medronic, (77) Respicardia, (78) Silena Therapeutics, (79) Spectrum Pharmaceuticals, (80) St Jude, (81) TEVA, (82) Theorem, (83) Abbvie, (84) Aesculap, (85) Biotronik, (86) Inventivhealth, (87) ISATherapeutics, (88) LYSARC, (89) MSD, (90) Novocure, (91) Ockham Oncology, (92) Premier-Research, (93) Psi-cro, (94) Tetec-ag, (95) Winicker-Norimed, (96) Achaogen Inc., (97) ADIR, (98) AstraZenaca AB, (99) Demira Inc., (100) Euroscreen S.A., (101) Galmed Research and Development Ltd., (102) GETNE, (103) Guidant Europe NV, (104) Holaira Inc., (105) Immunomedics Inc., (106) Innate Pharma, (107) Isis Pharmaceuticals Inc., (108) Kantar Health GmbH, (109) MedImmune Inc., (110) Medpace Germany GmbH (CRO), (111) Merrimack Pharmaceuticals Inc., (112) Millenium Pharmaceuticals Inc., (113) Orion Corporation Orion Pharma, (114) Pharmacyclics Inc., (115) PIQUR Therapeutics Ltd., (116) Pulmonx International Sárl, (117) Servier (CRO), (118) SGS Life Science Services (CRO), and (119) Treshold Pharmaceuticals Inc. S.M. Niehues has received funding from Bayer Vital, Bracco, Guerbet, Canon Medical Systems and Teleflex medical. The funding had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The remaining authors declare that they have no conflicts of interest and did not receive any funds. There are no patents, products in development, or marketed products to declare.
Acknowledgments
Acknowledgments
MRM is grateful for support from the Deutsche Forschungsgemeinschaft (DFG, SFB 1340/1 2018, 5943/31/41/91). LCA is grateful for her participation in the BIH Charité – Junior Clinician and Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health.
Funding information
This research received no specific grant from any funding organisation in the public, commercial, or not-for-profit sectors.
References
- 1.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16:341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng K.I., Gao F., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;154244 doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reilly J.J., El-Hamdouchi A., Diouf A., Monyeki A., Somda S.A. Determining the worldwide prevalence of obesity. Lancet. 2018;391:1773–1774. doi: 10.1016/S0140-6736(18)30794-3. [DOI] [PubMed] [Google Scholar]
- 4.Rosenquist K.J., Pedley A., Massaro J.M., Therkelsen K.E., Murabito J.M., Hoffmann U. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc Imaging. 2013;6:762–771. doi: 10.1016/j.jcmg.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tong Y., Udupa J.K., Torigian D.A. Optimization of abdominal fat quantification on CT imaging through use of standardized anatomic space: a novel approach. Med Phys. 2014;41 doi: 10.1118/1.4876275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S.J., Liu J., Yao J., Kanarek A., Summers R.M., Pickhardt P.J. Fully automated segmentation and quantification of visceral and subcutaneous fat at abdominal CT: application to a longitudinal adult screening cohort. Br J Radiol. 2018;91:20170968. doi: 10.1259/bjr.20170968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Team R.C. 2013. R: a language and environment for statistical computing. [Google Scholar]
- 8.Wickham H. 2017. Tidyverse: easily install and load the ‘tidyverse’. R package version. 2017;1. [Google Scholar]
- 9.Hill J.J. Obesity: an emerging threat. J Natl Black Nurses Assoc. 2018;29:36–39. [PubMed] [Google Scholar]
- 10.Dhurandhar N.V., Bailey D., Thomas D. Interaction of obesity and infections. Obes Rev. 2015;16:1017–1029. doi: 10.1111/obr.12320. [DOI] [PubMed] [Google Scholar]
- 11.Reilly S.M., Saltiel A.R. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 12.Muscogiuri G., Pugliese G., Barrea L., Savastano S., Colao A. Comentary: obesity: the “Achilles heel” for COVID-19? Metabolism. 2020;108:154251. doi: 10.1016/j.metabol.2020.154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farb M.G., Gokce N. Visceral adiposopathy: a vascular perspective. Horm Mol Biol Clin Investig. 2015;21:125–136. doi: 10.1515/hmbci-2014-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritter A., Friemel A., Fornoff F., Adjan M., Solbach C., Yuan J. Characterization of adipose-derived stem cells from subcutaneous and visceral adipose tissues and their function in breast cancer cells. Oncotarget. 2015;6:34475–34493. doi: 10.18632/oncotarget.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park Y.S., Kwon H.T., Hwang S.S., Choi S.H., Cho Y.M., Lee J. Impact of visceral adiposity measured by abdominal computed tomography on pulmonary function. J Korean Med Sci. 2011;26:771–777. doi: 10.3346/jkms.2011.26.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe M., Risi R., Tuccinardi D., Baquero C.J., Manfrini S., Gnessi L. Obesity and SARS-CoV-2: a population to safeguard. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3325. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Sideleva O., Suratt B.T., Black K.E., Tharp W.G., Pratley R.E., Forgione P. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012;186:598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020 doi: 10.1002/path.5471. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hruby A., Hu F.B. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33:673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalligeros M., Shehadeh F., Mylona E.K., Benitez G., Beckwith C.G., Chan P.A. Association of obesity with disease severity among patients with COVID-19. Obesity. 2020;28:1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietz W., Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity. 2020;28(6):1005. doi: 10.1002/oby.22818. www.obesityjournal.org [DOI] [PubMed] [Google Scholar]
- 23.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa415. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camhi S.M., Bray G.A., Bouchard C., Greenway F.L., Johnson W.D., Newton R.L. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011;19:402–408. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng M., Qi Y., Deng L., Wang H., Xu Y., Li Z. Obesity as a potential predictor of disease severity in young COVID-19 patients: a retrospective study. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395:1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demerath E.W., Sun S.S., Rogers N., Lee M., Reed D., Choh A.C. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity (Silver Spring) 2007;15:2984–2993. doi: 10.1038/oby.2007.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Despres J.P., Couillard C., Gagnon J., Bergeron J., Leon A.S., Rao D.C. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000;20:1932–1938. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y., Guo D., Niu Z., Wang Y., Fu G., Zhou Y. Prediction of the risk of laparoscopy-assisted gastrectomy by comparing visceral fat area and body mass index. Gastroenterol Res Pract. 2018;2018:1359626. doi: 10.1155/2018/1359626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox C.S., Massaro J.M., Hoffmann U., Pou K.M., Maurovich-Horvat P., Liu C.Y. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 34.Winfield R.D., Mellnick V.M., Chamieh J., Nohra E., Tan W.H., Ramirez R. Adipose tissue location and contribution to postinjury hypercoagulability. J Trauma Acute Care Surg. 2016;81:79–85. doi: 10.1097/TA.0000000000001096. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen G.K., Mellnick V.M., Yim A.K., Salter A., Ippolito J.E. Synergy of sex differences in visceral fat measured with CT and tumor metabolism helps predict overall survival in patients with renal cell carcinoma. Radiology. 2018;287:884–892. doi: 10.1148/radiol.2018171504. [DOI] [PubMed] [Google Scholar]
- 36.Yoshizumi T., Nakamura T., Yamane M., Islam A.H., Menju M., Yamasaki K. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–286. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 37.Nemoto M., Yeernuer T., Masutani Y., Nomura Y., Hanaoka S., Miki S. Development of automatic visceral fat volume calculation software for CT volume data. J Obes. 2014;2014:495084. doi: 10.1155/2014/495084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuk J.L., Church T.S., Blair S.N., Ross R. Does measurement site for visceral and abdominal subcutaneous adipose tissue alter associations with the metabolic syndrome? Diabetes Care. 2006;29:679–684. doi: 10.2337/diacare.29.03.06.dc05-1500. [DOI] [PubMed] [Google Scholar]