Highlights
-
•
Risk-taking peaked in mid adolescence such that mid adolescents took more risks than early and later adolescents.
-
•
Reward sensitivity, measured with the Reward Positivity, predicted risk-taking only in late but not early adolescence.
-
•
Developmental stage is important to consider when investigating how neural response to rewards contributes to risk-taking.
Keywords: Adolescence, Risk-taking, Reward sensitivity, EEG
Abstract
Risk-taking peaks in adolescence and reflects, in part, hyperactivity of the brain’s reward system. However, it has not been established whether the association between reward-related brain activity and risk-taking varies across adolescence. The present study investigated how neural reward sensitivity is associated with laboratory risk-taking in a sample of female adolescents as a function of age. Sixty-three female adolescents ages 10–19 completed the Balloon Analogue Risk Task, a laboratory measure of risk-taking behavior, as well as a forced choice monetary gambling task while an electroencephalogram (EEG) was recorded. This gambling task elicits the reward positivity (RewP), a frontocentral event-related potential component that is sensitive to feedback signaling reward. We observed a negative quadratic association between age and risk-taking, such that those in early and late adolescence had lower relative risk-taking compared to mid-adolescence, with risk-taking peaking at around 15 years of age. In predicting risk-taking, we observed an interaction between age and RewP, such that reward-related brain activity was not associated with risk-taking in early adolescence but was associated with a greater propensity for risk in later adolescence. These findings suggest that for females, neural response to rewards is an important factor in predicting risk-taking only in later adolescence.
1. Introduction
Adolescence involves a host of interacting neurobiological, hormonal, and behavioral changes that occur during a period of increasing autonomy. These changes make adolescence a time of enormous growth, but also a vulnerable period, marked by an increase in the incidence of psychopathology (Breslau et al., 2017; Costello et al., 2011; Kessler et al., 2005) and a rise in risky behavior (Bjork and Pardini, 2015; Braams et al., 2015; Burnett et al., 2010; Casey and Jones, 2010; Dahl, 2004; Sherman et al., 2018; Spear, 2000; though see Defoe et al., 2015). Some forms of risk-taking are adaptive in that they serve the developmental needs of adolescence, namely increasing independence and forming more significant extra-familial relationships, processes which rely on some degree of risk and exploration (Spear, 2000). In excess, however, adolescent risk-taking is also associated with adverse consequences (Dahl, 2004; Resnick et al., 1997).
These behavioral changes may be supported by concomitant structural changes to dopaminergic reward circuitry and increased neural sensitivity to rewards (Galván, 2010; Romer et al., 2017). There is substantial evidence, primarily from functional magnetic resonance imaging (fMRI) studies, that adolescents show increased neural sensitivity to reward receipt compared to adults and children and that this increased reward sensitivity peaks in mid adolescence (Braams et al., 2015; Galván, 2013; Van Leijenhorst et al., 2010a, b; see however Bjork, 2004; Bjork et al., 2010). This increased sensitivity to, and valuation of, rewards in adolescence appears to result in increased motivation to pursue rewarding stimuli such as drugs, alcohol, and novel social interactions (Doremus-Fitzwater et al., 2010). Consistent with this, greater reward sensitivity at the neural (Chein et al., 2011; Van Leijenhorst et al., 2010a) and behavioral level (Duell et al., 2016; Lejuez et al., 2003) has also been associated with greater laboratory risk-taking (Blankenstein et al., 2018) and self-reported risk-taking across all ages (Galván et al., 2007), though the opposite pattern has also been observed (Schneider et al., 2011). However, in spite of the significant neurobiological changes that transpire during adolescence (Asato et al., 2010; Lebel and Beaulieu, 2011; Luna et al., 2001; Ostby et al., 2009), it is unclear how associations between reward sensitivity and risk-taking might change across this developmental stage.
Although the majority of the literature on the neurobiology of risk-taking and reward processing in adolescence has relied on fMRI, event-related electroencephalogram (EEG) studies can serve as an important complement. In particular, the event-related potential (ERP) component known as the Reward Positivity (RewP) has been established as an index of reward sensitivity. This frontocentral ERP typically occurs 250−350 ms after rewarding feedback, is more positive to gains than losses, and is thought to originate in the medial prefrontal cortex (mPFC) and striatum (Becker et al., 2014; Carlson et al., 2011; Foti et al., 2015, 2011). Moreover, there is evidence that the RewP derived from monetary reward tasks primarily tracks reward rather than performance accuracy (Gehring and Willoughby, 2002). Because it is a very early and relatively obligatory response to rewarding feedback, the RewP can be conceptualized as an index of initial responsiveness to reward (NIMH RDoC, 2018), and indeed, the RewP is associated with self-reported reward responsiveness, behavioral reward sensitivity (Bress and Hajcak, 2013), and self-reported liking of received rewards (Angus et al., 2015).
Studies vary in their description of the developmental trajectory of the RewP across adolescence, perhaps because the magnitude of the component reflects the coordinated activity of multiple brain regions, each developing at different rates. Although some data from both longitudinal (Kujawa et al., 2018) and cross-sectional studies (Bowers et al., 2018; Lukie et al., 2014) have suggested the magnitude of the RewP is stable across adolescence, other studies have found age-related differences in the RewP, although they do not all agree on the direction of change (Arbel et al., 2018; Burani et al., 2019; Crowley et al., 2013; Hämmerer et al., 2013; Zottoli and Grose-Fifer, 2012).
Studies that have examined links between the RewP and risk-taking are equally mixed; for instance, in adults, a larger RewP has been associated with positive urgency (Ait Oumeziane and Foti, 2016) and sensation seeking (Novak et al., 2016). These two traits increase across adolescence (Littlefield et al., 2016) and predict heightened substance use and risky sexual behavior in both adolescents (MacPherson et al., 2010; Sargent et al., 2010) and young adults (Zapolski et al., 2009). Some research also suggests that young adults characterized by higher rates of risky real-world behaviors, like problem gamblers, have a larger RewP (Hewig et al., 2010; Oberg et al., 2011). Yet several studies have found a smaller RewP in adolescents at risk for substance abuse (Crowley et al., 2009; see however Morie et al., 2018) as well as in adolescents exhibiting problematic internet use (Yau et al., 2015). Combined, these data suggest it will be critical to investigate whether and how the association between reward sensitivity and risk-taking changes across development.
The goal of the present study is therefore to determine whether reward sensitivity is uniformly or differentially predictive of risk-taking across adolescence. To this end, we examined associations between the magnitude of the RewP and risk-taking behavior in the Balloon Analogue Risk Task (BART; Lejuez et al., 2003) in a sample of adolescents ages 10–19. Because this study was a secondary analysis of a predominantly female sample, we focused the present analysis on female participants. We hypothesized that neural reward sensitivity measured by the RewP would be predictive of risk-taking across adolescence, but most strongly associated in mid adolescence, when reward sensitivity should be at its highest.
2. Methods
2.1. Participants
Participants included in the present analysis were drawn from two studies in which they completed overlapping procedures. A complete list of additional variables available in the two samples can be found in supplemental Table 1. Participants in both studies were recruited through local schools and the community through fliers and internet postings. All participants were screened to ensure they had never experienced a loss of consciousness greater than 10 min and had no known neurological problems.
Table 1.
Sample characteristics and correlation matrix describing associations between age, RewP and average pumps in the BART. P-values for Pearson’s correlation coefficients are all > 0.2 with df = 61.
| M (SD) | 1 | 2 | 3 | |
|---|---|---|---|---|
| 1. Age | 14.37 (2.25) | -- | ||
| 2. RewPresid | 0 (.99) | −.14 | -- | |
| 3. Pumps | 52.06 (13.28) | .16 | .16 | -- |
Of the 146 adolescents enrolled, 63 female participants completed all procedures and were included in the final sample for analysis. Age was calculated in years by subtracting the participant’s date of birth from their date of participation and rounding to two decimal places. Date of birth was missing for two participants and age in whole years was used. Participant ages ranged from 10.0–19.09 years with a mean of 14.37 years (SD = 2.25). The sample was 82.7 % Caucasian, 1.6 % Chinese, 1.6 % African American, 1.6 % Native/First Nations, 3.2 % Hispanic, and 3.2 % other, with 20.7 % not providing racial and/or ethnic information.
Before commencing the study, we received written informed parental consent and assent from all participants under age 18 and written informed consent from participants ages 18 and older. Every participant was compensated $25/hour for her participation. All procedures were approved by McGill University’s Research Ethics Board.
2.2. Procedure
All participants completed an online demographics questionnaire either at home or during their lab visit. Among other computerized tasks, participants then completed a monetary reward task while continuous EEG was recorded, as well as the auto-pump BART (described below; Pleskac et al., 2008) in a randomized order.
2.3. Reward response
To capture neural response to reward receipt, participants completed a simple forced-choice guessing task, which is known to elicit the RewP (Proudfit, 2015). On each trial, participants were presented with two doors and were told that one was holding a prize. They were instructed to choose a door by clicking the left or right mouse button. After a 1000 ms fixation cross, either a green arrow pointing up indicating gain or a red arrow pointing down indicating loss was displayed for 2000 ms, followed by a 1500 ms fixation cross. The participant would then be prompted to click to proceed to the next trial. Participants were given five practice trials to ensure they understood the task. After the practice, there were two blocks of 20 trials each, where 50 % of trials resulted in a win (reward) and 50 % resulted in a loss in random order. On win trials, participants earned 50 cents while on loss trials they lost 25 cents; this discrepancy is intended to equalize the subjective value of the feedback and allow rewards to accumulate (Proudfit, 2015; Tversky and Kahneman, 1992). Participants were told at the outset that they were playing for real money and immediately following the task all participants received $3.00 in winnings.
2.4. Risk taking
An adapted BART task, the BART auto pump (Pleskac et al., 2008), was used to measure risk-taking. This task is one of the few risk-taking tasks that reliably relates to reported real-world risk-taking (Sherman et al., 2018) and greater risk-taking in this task has been associated with greater drug and alcohol use, criminal activity, or not wearing a seatbelt (Lejuez et al., 2003). Another advantage of this task is that is that some risk-taking is necessary to perform well. This adds ecological validity as that is also the case for key developmental tasks of adolescence like making new friends and developing independence (Spear, 2000).
In the task, participants earned money by inflating a series of balloons, but lost earnings if they popped a balloon through over-inflation. On each of 30 trials, the participant chose to inflate each balloon a number of “pumps,” ranging from 1 to 128, by entering their selected number into a box on the screen. Each pump could earn the participant one point. Each balloon also had an unknown explosion point, ranging from 1−128. If the number of pumps selected by the participant was less than the balloon’s explosion point, the balloon would inflate, and points would accumulate in the “bank” displayed on the screen. If the number of pumps selected by the participant exceeded the explosion point, the balloon would disappear with a loud popping sound and the participant would earn no points for that trial. On the subsequent trial, the actual explosion point of the previous balloon (and not the number of pumps the participant selected) was displayed on one side of the screen. Participants were told that across all balloons the most advantageous number of pumps was 64, but that individual explosion points for balloons vary. Participants were also told they would receive a dollar amount proportional to their task winnings. All participants received $2.00 directly after the task, independent of performance; they were not debriefed on this minor deception due to planned re-recruitment of this sample and repetition of this task. The average of the number of pumps participants opted to make across all balloons was used as an index of risk-taking (Pleskac et al., 2008).
2.5. Electroencephalographic recording and data processing
During the doors guessing task, continuous EEG was recorded with a BrainVision actiCHamp system (Brain Products, Munich, Germany) and a 32-electrode cap arranged based on the standard 10/20 layout with the ground electrode at Fpz. To correct for eye movements, the electrooculogram (EOG) was collected with facial electrodes placed 1 cm above and below one eye and 1 cm to the outside of both eyes. Data was recorded using a sampling rate of 1000 Hz.
Offline analyses were conducted using BrainVision Analyzer software (Brain Products). A Butterworth Zero Phase bandpass filter attenuated frequencies below 0.1 Hz and above 30 Hz (with a slope of 24 dB/oct for each) in the unsegmented data. After filtering, trials were segmented 200 ms before and 1000 ms after feedback (the upward or downward arrow). Segmented data were referenced offline to an average of TP9 and TP10, the left and right mastoids. Eye-blinks and ocular movements were removed from the data using the EOG based on a modification of the algorithm described in Gratton et al. (1983). Artifacts were rejected using a semi-automatic procedure in which channels were automatically eliminated from a trial if they included a voltage step of > 50 μV between sample points, a voltage difference of > 175 μV within a 400 ms interval, or activity < 0.5 μV in a 100 ms interval. Remaining artifacts were removed from the data via visual inspection.
All gain trials were averaged together, as were loss trials, and mean activity from -200 ms – 0 ms was used as a baseline and subtracted from each subsequent data point. The RewP was subsequently scored as the mean activity between 250−350 ms following feedback at electrode Cz. Split-half reliability for neural response to gain and loss at Cz was calculated for odd and even numbered trials using the Spearman-Brown coefficient. This coefficient was 0.75 for loss trials and 0.71 for gain trials.
2.6. Data analysis
We conducted a sensitivity analysis using G*Power to establish the smallest effect size we had at least 80 % power to detect. With a total sample size of 63, 3 predictors in a multiple regression, and α error probability set to 0.05, the smallest effect size we could detect was f2 = 0.13, or R2 = 0.12.
All subsequent analyses were conducted in SPSS version 24 (IBM Corp., Armonk NY) or in R (R Core Team). A one-way (Feedback: Gain vs. Non-Gain) repeated-measures ANOVA was conducted to verify that gains elicited a larger RewP than losses. Next, because we were interested in neural response unique to reward, we calculated a standardized residual from a regression of response to loss predicting response to gain. This residual, which has better psychometric properties than a simple difference score (Ethridge and Weinberg, 2018), was used in all analyses as a measure of reward sensitivity and will be referred to as the RewPresid. The internal consistency of this RewPresid was 0.46 based on the Spearman-Brown coefficient.
Following this, Pearson’s correlations between age, RewPresid, and average pumps were computed to assess any bivariate linear relationships between them. To assess the relationship between risk-taking and tangible outcomes in the BART, we tested linear and quadratic associations of average pumps predicting points earned. Next, we tested for two additional quadratic associations: one predicting average BART pumps from age (Braams et al., 2015) and a second predicting RewPresid from age, each controlling for linear effects, to probe nonlinear developmental trajectories. To determine the effects of age and RewP on risk-taking, we conducted a linear regression predicting average pumps in the BART from mean-centered age and RewPresid in the first step and added an interaction term between age and the RewPresid in the second step. We conducted a chi-square likelihood ratio test using the lmtest package in R (Zeileis and Hothorn, 2002) to assess whether step two from this regression better fit the data than step one. Finally, we performed a simple slopes analysis at the mean age ± 1SD to decompose the interaction.
3. Results
3.1. Neural response to reward and loss
A repeated measure analysis of variance (ANOVA) was conducted to assess the effect of feedback on neural responses in the time-window of the RewP. There was a main effect of feedback type (F(1,62) = 37.98, p < .001, η2 = .38), confirming that, as expected, monetary rewards elicited a larger RewP than monetary losses (Fig. 1).
Fig. 1.
A. Average waveforms to gain and loss at electrode Cz from all participants. The dotted line represents a simple difference of gain - loss. Time 0 represents the onset of feedback stimuli. B. Scalp distribution demonstrating the difference in neural activity to gain - loss between 250-350 ms. Red indicates a greater positivity to gain than loss. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.2. Risk-taking across adolescence
In the BART, the average number of pumps across subjects was 52.06 (SD = 13.28), below the indicated optimal number of 64. There was both a positive linear (b = 6.68, t = 4.93, p < 0.001) and a negative quadratic (b = -0.281, t = -3.47, p = 0.001) association between average pumps and points earned such that participants who took more risks earned more points, but earnings peaked at 64 pumps as indicated and declined thereafter.
There was no significant linear association between age and average number of pumps (b = .94, t = 1.26, p = .21); but there was a significant quadratic effect of age (b = -0.87, t = -3.08, p = .003), with average risk-taking peaking around age 15 (Fig. 2). The average number of pumps was 51.12 (SD = 13.06) in adolescents aged 10–13, 55.17 (SD = 12.29) in adolescents aged 14–16, and 51.31 (SD = 15.34) in adolescents aged 17-19.
Fig. 2.
Quadratic association between age and average pumps in the BART. Average risk-taking peaked between 14-16 years.
3.3. Neural response to reward and risk-taking across adolescence
Pearson’s correlations between age, RewPresid and average pumps are presented in Table 1. Age was neither linearly (b = -0.07 t = -1.16, p = .25) nor quadratically (b = -0.021 t = -0.95, p = .35) significantly associated with the RewPresid. To assess the association between neural response to reward and risk-taking across adolescence, we conducted a linear regression with average pumps as the dependent variable and RewP and age as independent variables. In the second step, we added an interaction between RewP and age (Table 2).
Table 2.
Two-step regression predicting risk-taking measured by average pumps in the BART. An interaction term between RewP and age is added in step two.
| Step | Predictor | b | b 95 % CI | β | p | R2 | F | p |
|---|---|---|---|---|---|---|---|---|
| 1 | .06 | 1.96 | .15 | |||||
| Intercept | 52.06 | 48.756−55.36 | — | <.001 | ||||
| Age | 1.11 | −.39 – 2.60 | .19 | .14 | ||||
| RewPresid | 2.57 | −.82 – 5.95 | .19 | .14 | ||||
| 2 | .13 | 2.97 | .04 | |||||
| Intercept | 52.69 | 49.44 – 55.94 | — | <.001 | ||||
| Age | 1.55 | .04 – 3.05 | .26 | .05 | ||||
| RewPresid | 3.43 | .05 – 6.81 | .26 | .04 | ||||
| RewPresid x Age | 1.96 | .16 – 3.76 | .28 | .03 |
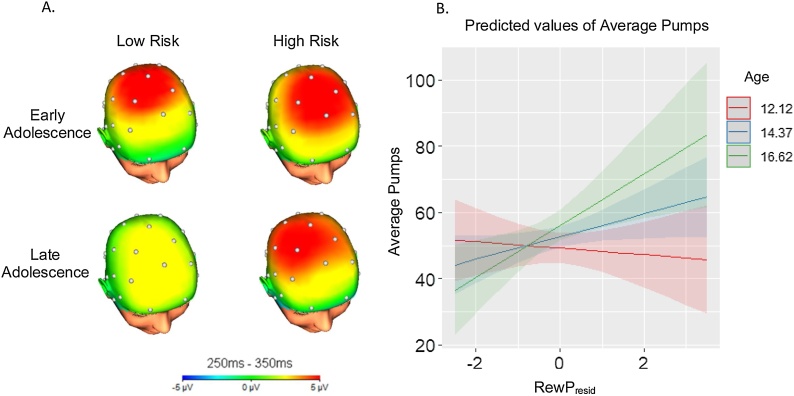
There was no main effect of age or RewP in the first step, but in the second step, age significantly moderated the association between the RewP and risk-taking (R2 change = .07, p = .03). A likelihood ratio test indicated that including the interaction term resulted in a better fitting model (χ2 = 4.86, p = .03). Simple slopes analysis at the mean age ± 1SD indicated that the RewP did not significantly predict risk-taking at younger ages (simple slope at age 12.12 = -0.97, t = -0.42, p = 0.67), but did predict risk-taking in mid and late adolescence (simple slope at age 14.37 = 3.43, t = 2.03, p = .05; simple slope at age 16.62 = 7.83, t = 2.67, p = .01; Figs. 3A and B).
Fig. 3.
A. Scalp distributions of the RewP showing activity to gain minus loss, made for presentation purposes. Early adolescence includes ages 10-14, while late adolescence includes ages 15-19. Low and high risk-taking are defined by a median split of average pumps in the BART. Whereas early adolescents low and high on risk-taking show similar magnitudes of their RewP, individuals who engage in riskier behavior in late adolescence show a larger RewP than those lower on risk-taking. B. Simple slopes of the standardized RewP residual predicting average pumps in the BART at the mean age ± 1 SD. Age interacted with RewP such that the RewP became a stronger predictor of risk-taking with age. Only the slopes at age 14.37 and 16.62 were statistically significant.
4. Discussion
Adolescence has been characterized as a time of heightened reward sensitivity and increased risk-taking. The present study tested whether the RewP, an electrophysiological measure of reward sensitivity, was associated with risk-taking, and if so, whether that association changed across adolescence. Our results demonstrated that although reward sensitivity did not differ across ages, greater reward sensitivity was associated with greater risk-taking only later in adolescence, suggesting that reward sensitivity’s link with risk-taking varies across development.
Prevailing theories of adolescent risk-taking, such as imbalance or dual systems models, posit that adolescent risk-taking emerges due to heightened reward sensitivity while executive control is still developing (Casey et al., 2008; Crone and Dahl, 2012; Ernst et al., 2006; Shulman et al., 2016; Steinberg, 2007; Van Leijenhorst et al., 2010b). The present results are somewhat consistent with this model, in that risk-taking in this sample was highest in the same age range in which reward sensitivity and risk-taking became positively linked (i.e., between 14 and 16). However, because the magnitude of the RewP appears to reflect contributions from both subcortical and prefrontal regions (Carlson et al., 2011; Foti et al., 2015), we cannot attribute these results to the relative maturation of striatal or prefrontal regions. Moreover, unlike striatal response to rewards, which appears to have an inverted u-shaped developmental trajectory from childhood to emerging adulthood (Braams et al., 2015; Galván, 2013), the RewP was not significantly associated—either linearly or quadratically—with the age of our participants, consistent with some prior cross-sectional and prospective research that the RewP remains stable across adolescent development (e.g. Kujawa et al., 2018; Lukie et al., 2014). Nonetheless, our results suggest that age is an important factor in understanding associations between neural reward response and risk-taking behavior. Inconsistencies in the literature attempting to link these constructs may be, in part, due to neglecting to account for developmental factors in analyses.
Additionally, while the association between the RewP and risk-taking became stronger after age 14, the amount of overall observed risk-taking behavior diminished after age 15. Therefore, the positive association with the RewP increased as risk-taking decreased, such that older adolescents who show larger neural responses to reward within 300 ms of feedback continue to engage in higher levels of risk-taking while those with reduced activity in this time-window show reduced risk-taking relative to their peers. Perhaps this pattern emerges because normative increases in risk-taking—as seen in mid-adolescence—are supported by a broad range of processes and are less influenced by individual differences in reward sensitivity. As the behavior becomes less normative, however, individual differences in initial reward responsiveness may exert themselves more, such that those with greater sensitivity to reward remain incentivized to take risks with greater frequency, relative to those who are less reward sensitive. However, as our sample was cross-sectional, longitudinal studies will be needed to assess whether the RewP prospectively predicts changes in risk-taking across adolescence.
The present study replicated previous findings that risk-taking peaks in mid-adolescence (Braams et al., 2015; Burnett et al., 2010). However, other studies do not find this pattern of risk-taking across development (Defoe et al., 2015). One potential reason for mixed results is that increased adolescent risk-taking occurs particularly in the context of emotional arousal, mediated by increased striatal activation to rewards (Blakemore and Robbins, 2012; Figner et al., 2009). This emotional arousal may occur through presence of peers (Chein et al., 2011), emotional priming (Burnett et al., 2010), or task characteristics that increase incremental feedback or immediacy of feedback (Figner et al., 2009) The auto-pump BART shares characteristics with other emotionally arousing or “hot” tasks. These include receiving immediate feedback and potentially inducing counterfactual emotions by viewing the explosion point of the previous balloon. Therefore, our results are consistent with existing literature observing a peak in adolescent risk-taking in emotionally charged tasks.
Although risk-taking can be dangerous, especially when it involves drugs or alcohol, a certain inclination toward positive types of risk can be advantageous (Steinberg, 2008). For example, a willingness to take a risk is required for forming new relationships. Because peers become more important in adolescence (Doremus-Fitzwater et al., 2010; Foulkes and Blakemore, 2016; Kandel, 1986), a deficit in willingness to take risks to obtain rewards may be a liability that feeds into social difficulties. Greater willingness to take risks can also facilitate exploration, which is increasingly important as adolescents gain independence from their caregivers (Cohen, 1980; Spear, 2000; Steinberg, 2005). Consequently, very low levels of risk-taking are not always more adaptive than high risk taking. Consistent with this, in the present study, the mean number of pumps in the BART was 52.06, and only eleven participants had a mean number of pumps greater than or equal to 64, the optimal number across all trials. Accordingly, less risk-taking was associated with earning fewer points in the task. Because 64 was communicated to the participants as the most advantageous choice over the whole task, results from our sample may better reflect how reward sensitivity relates to risk-aversion (Huggins et al., 2019) as opposed to heightened propensity for risk.
Our sample also bears further consideration: This study only included female participants. Yet, there is evidence that males tend to take more risks than females (Lewis et al., 2019; Van Leijenhorst et al., 2008), possibly even more in adolescence relative to adulthood (Byrnes et al., 1999). That combined with different rates of development between males and females (Marceau et al., 2011) suggests that the present results may not generalize to males. Future research should recruit larger samples that are evenly distributed by sex to evaluate whether our findings replicate in males or whether there are sex differences in how the RewP predicts risk-taking across adolescence.
Another limitation of the present study is that individuals mature at different rates such that age is not a perfect measure of development. The addition of a pubertal development scale may have provided a more precise indication of how reward sensitivity and risk-taking change across adolescent development. Further, pubertal hormone levels (i.e. testosterone, estradiol) affect reward processing and risk-taking in adolescents. For example, in adolescent females, higher levels of endogenous testosterone are associated with greater risk-taking (Op de Macks et al., 2011) and reduced striatal reactivity to reward outcome (Forbes et al., 2010) while higher levels of estradiol are also associated with reduced striatal reactivity to reward outcome (Ladouceur et al., 2019). Understanding the role of pubertal hormones may therefore be crucial to understanding how associations between reward sensitivity and risk-taking develop across adolescence.
While overly high reward sensitivity and risk-taking may have consequences for externalizing disorders, blunted reward sensitivity and low risk-taking may have consequences for internalizing disorders (Bjork and Pardini, 2015; Goff et al., 2013; Huggins et al., 2019; Telzer et al., 2013). Therefore, another important future direction is to examine neural reward sensitivity and risk-taking in both healthy subjects and subjects with a range of internalizing and externalizing psychopathology.
Prior literature has linked an adolescent peak in risk-taking with heightened neural reward responsiveness (Romer et al., 2017; Van Leijenhorst et al., 2010a), though there is still substantial heterogeneity in the literature (Defoe et al., 2019; Sherman et al., 2018). Our findings build on the existing literature by addressing the question of how a very early psychophysiological measure of reward sensitivity differentially predicts risk-taking across adolescent development. These findings have important implications for better understanding adolescent risk-taking by challenging the assumption that reward sensitivity and risk-taking are uniformly related across adolescence, a period with significant neurobiological and behavioral change.
5. Conclusion
This study employed a novel analysis testing an interaction between neural reward sensitivity and age to predict risk-taking in adolescent females. Our results suggest that age is an important factor to consider when investigating how reward sensitivity relates to risk-taking in adolescence. In particular, our results indicate that as risk-taking diminished at older ages, the association with the RewP grew stronger and more positive. This implies that as risk-taking decreases in later adolescence, those with greater reward sensitivity continue to take risks while those with blunted reward sensitivity do not. A more cautious response style is not always optimal, neither in the BART nor in real life. A more nuanced understanding of optimal reward sensitivity and risk levels will be necessary to recognize their implications for emergent adolescent psychopathology and adaptive functioning.
Financial disclosures
This research was funded through grants by the Social Sciences and Humanties Research Council of Canada (insight grants 435-2014-0974 and 430-2017-00386) and the Natural Sciences and Engineering Research Council of Canada (NSERC RGPIN-2017-04329) to AW and/or MD. The funding sources did not have involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100808.
Contributor Information
Clara Freeman, Email: clara.freeman@mail.mcgill.ca.
Melanie Dirks, Email: melanie.dirks@mcgill.ca.
Anna Weinberg, Email: anna.weinberg@mcgill.ca.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Ait Oumeziane B., Foti D. Reward-related neural dysfunction across depression and impulsivity: a dimensional approach. Psychophysiology. 2016;53:1174–1184. doi: 10.1111/psyp.12672. [DOI] [PubMed] [Google Scholar]
- Angus D.J., Kemkes K., Schutter D.J.L.G., Harmon-Jones E. Anger is associated with reward-related electrocortical activity: evidence from the reward positivity. Psychophysiology. 2015;52:1271–1280. doi: 10.1111/psyp.12460. [DOI] [PubMed] [Google Scholar]
- Arbel Y., McCarty K.N., Goldman M., Donchin E., Brumback T. Developmental changes in the feedback related negativity from 8 to 14 years. Int. J. Psychophysiol. 2018;132:331–337. doi: 10.1016/j.ijpsycho.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato M.R., Terwilliger R., Woo J., Luna B. White matter development in adolescence: a DTI study. Cereb. Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M.P.I., Nitsch A.M., Miltner W.H.R., Straube T. A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. J. Neurosci. 2014;34:3005–3012. doi: 10.1523/JNEUROSCI.3684-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 2004;24:1793–1802. doi: 10.1523/jneurosci.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Pardini D.A. Who are those “risk-taking adolescents”? Individual differences in developmental neuroimaging research. Dev. Cogn. Neurosci. 2015;11:56–64. doi: 10.1016/j.dcn.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J., Robbins T.W. Decision-making in the adolescent brain. Nat. Neurosci. 2012;15:1184–1191. doi: 10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- Blankenstein N.E., Schreuders E., Peper J.S., Crone E.A., van Duijvenvoorde A.C.K. Individual differences in risk-taking tendencies modulate the neural processing of risky and ambiguous decision-making in adolescence. Neuroimage. 2018;172:663–673. doi: 10.1016/j.neuroimage.2018.01.085. [DOI] [PubMed] [Google Scholar]
- Bowers M.E., Buzzell G.A., Bernat E.M., Fox N.A., Barker T.V. Time-frequency approaches to investigating changes in feedback processing during childhood and adolescence. Psychophysiology. 2018;55:1–13. doi: 10.1111/psyp.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., van Duijvenvoorde A.C.K., Peper J.S., Crone E.A. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. J. Neurosci. 2015;35:7226–7238. doi: 10.1523/jneurosci.4764-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau J., Gilman S.E., Stein B.D., Ruder T., Gmelin T., Miller E. Sex differences in recent first-onset depression in an epidemiological sample of adolescents. Transl. Psychiatry. 2017;7:e1139. doi: 10.1038/tp.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress J.N., Hajcak G. Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology. 2013;50:610–616. doi: 10.1111/psyp.12053. [DOI] [PubMed] [Google Scholar]
- Burani K., Mulligan E.M., Klawohn J., Luking K.R., Nelson B.D., Hajcak G. Longitudinal increases in reward-related neural activity in early adolescence: evidence from event-related potentials (ERPs) Dev. Cogn. Neurosci. 2019;36:100620. doi: 10.1016/J.DCN.2019.100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S., Bault N., Coricelli G., Blakemore S.J. Adolescents’ heightened risk-seeking in a probabilistic gambling task. Cogn. Dev. 2010;25:183–196. doi: 10.1016/j.cogdev.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes J.P., Miller D.C., Schafer W.D. Gender differences in risk taking: a meta-analysis. Psychol. Bull. 1999;125:367–383. doi: 10.1037/0033-2909.125.3.367. [DOI] [Google Scholar]
- Carlson J.M., Foti D., Mujica-Parodi L.R., Harmon-Jones E., Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage. 2011;57:1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J.M., Albert D., O’Brien L., Uckert K., Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev. Sci. 2011;14:1–10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Adolescent independence and adolescent change. Youth Soc. 1980;12:107–124. [Google Scholar]
- Costello E.J., Copeland W., Angold A. Trends in psychopathology across the adolescent years: what changes when children become adolescents, and when adolescents become adults? J. Child Psychol. Psychiatry Allied Discip. 2011;52:1015–1025. doi: 10.1111/j.1469-7610.2011.02446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Crowley M.J., Wu J., Crutcher C., Bailey C.A., Lejuez C.W., Mayes L.C. Risk-taking and the feedback negativity response to loss among at-risk adolescents. Dev. Neurosci. 2009;31:137–148. doi: 10.1159/000207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley M.J., Wu J., Hommer R.E., South M., Molfese P.J., Fearon R.M.P., Mayes L.C. A developmental study of the feedback-related negativity from 10–17 years: age and sex effects for reward versus non-reward. Dev. Neuropsychol. 2013;38:595–612. doi: 10.1080/87565641.2012.694512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R.E. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote Address. Ann. N. Y. Acad. Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Defoe I.N., Dubas J.S., Figner B., Van Aken M.A.G. A meta-analysis on age differences in risky decision making: adolescents versus children and adults. Psychol. Bull. 2015;141:48–84. doi: 10.1037/a0038088. [DOI] [PubMed] [Google Scholar]
- Defoe I.N., Semon Dubas J., Romer D. Heightened adolescent risk-taking? Insights from lab studies on age differences in decision-making. Policy Insights from Behav. Brain Sci. 2019;6:56–63. doi: 10.1177/2372732218801037. [DOI] [Google Scholar]
- Doremus-Fitzwater T.L., Varlinskaya E.I., Spear L.P. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duell N., Steinberg L., Chein J., Al-Hassan S.M., Bacchini D., Lei C., Chaudhary N., Di Giunta L., Dodge K.A., Fanti K.A., Lansford J.E., Malone P.S., Oburu P., Pastorelli C., Skinner A.T., Sorbring E., Tapanya S., Tirado L.M.U., Alampay L.P. Interaction of reward seeking and self-regulation in the prediction of risk taking: a cross-national test of the dual systems model. Dev. Psychol. 2016;52:1593–1605. doi: 10.1037/dev0000152. [DOI] [PubMed] [Google Scholar]
- Ernst M., Pine D.S., Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge P., Weinberg A. Psychometric properties of neural responses to monetary and social rewards across development. Int. J. Psychophysiol. 2018;132:311–322. doi: 10.1016/j.ijpsycho.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Figner B., Mackinlay R.J., Wilkening F., Weber E.U. Affective and deliberative processes in risky choice: age differences in risk taking in the columbia card task. J. Exp. Psychol. Learn. Mem. Cogn. 2009;35:709–730. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Ryan N.D., Phillips M.L., Manuck S.B., Worthman C.M., Moyles D.L., Tar J.A., Sciarillo S.R., Dahl R.E. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(2):e1–5. doi: 10.1097/00004583-201002000-00010. 162-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D., Weinberg A., Dien J., Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Hum. Brain Mapp. 2011;32:2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D., Weinberg A., Bernat E.M., Proudfit G.H. Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clin. Neurophysiol. 2015;126:1338–1347. doi: 10.1016/j.clinph.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes L., Blakemore S.-J. Is there heightened sensitivity to social reward in adolescence? Curr. Opin. Neurobiol. 2016;40:81–85. doi: 10.1016/J.CONB.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Galván A. Adolescent development of the reward system. Front. Hum. Neurosci. 2010;4:1–9. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A. The teenage brain. Curr. Dir. Psychol. Sci. 2013;22:88–93. doi: 10.1177/0963721413480859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A., Hare T., Voss H., Glover G., Casey B.J. Risk-taking and the adolescent brain: who is at risk? Dev. Sci. 2007;10:8–14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Willoughby A.R. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Goff B., Gee D.G., Telzer E.H., Humphreys K.L., Gabard-Durnam L., Flannery J., Tottenham N. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G., Coles M.G.H., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983:672–681. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hämmerer D., Li S.-C., Völkle M., Müller V., Lindenberger U. A lifespan comparison of the reliability, test-retest stability, and signal-to-noise ratio of event-related potentials assessed during performance monitoring. Psychophysiology. 2013;50:111–123. doi: 10.1111/j.1469-8986.2012.01476.x. [DOI] [PubMed] [Google Scholar]
- Hewig J., Kretschmer N., Trippe R.H., Hecht H., Coles M.G.H., Holroyd C.B., Miltner W.H.R. Hypersensitivity to reward in problem gamblers. Biol. Psychiatry. 2010;67:781–783. doi: 10.1016/j.biopsych.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Huggins A.A., Weinberg A., Gorka S.M., Shankman S.A. Blunted neural response to gains versus losses associated with both risk‐prone and risk‐averse behavior in a clinically diverse sample. Psychophysiology. 2019;56:e13342. doi: 10.1111/psyp.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D.B. Processes of peer influences in adolescence. In: Silbereisen R.K., Eyferth K., Rudinger G., editors. Development as Action in Context: Problem Behavior and Normal Youth Development. Springer; Berlin Heidelberg, Berlin, Heidelberg: 1986. pp. 203–227. [DOI] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kujawa A., Carroll A., Mumper E., Mukherjee D., Kessel E.M., Olino T., Hajcak G., Klein D.N. A longitudinal examination of event-related potentials sensitive to monetary reward and loss feedback from late childhood to middle adolescence. Int. J. Psychophysiol. 2018;132:323–330. doi: 10.1016/j.ijpsycho.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C.D., Kerestes R., Schlund M.W., Shirtcliff E.A., Lee Y., Dahl R.E. Neural systems underlying reward cue processing in early adolescence: the role of puberty and pubertal hormones. Psychoneuroendocrinology. 2019;102:281–291. doi: 10.1016/j.psyneuen.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31:10937–10947. doi: 10.1523/jneurosci.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez C.W., Aklin W.M., Zvolensky M.J., Pedulla C.M. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J. Adolesc. 2003;26:475–479. doi: 10.1016/S0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lewis Gemma, Srinivasam R., Roiser J., Blakemore S.-J., Flouri E., Lewis Glyn. Risk taking to obtain reward: gender differences and associations with emotional and depressive symptoms in a nationally representative cohort of UK adolescents. bioRxiv. 2019:644450. doi: 10.1101/644450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield A.K., Stevens A.K., Ellingson J.M., King K.M., Jackson K.M. Changes in negative urgency, positive urgency, and sensation seeking across adolescence. Pers. Individ. Dif. 2016;90:332–337. doi: 10.1016/J.PAID.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukie C.N., Montazer-Hojat S., Holroyd C.B. Developmental changes in the reward positivity: an electrophysiological trajectory of reward processing. Dev. Cogn. Neurosci. 2014;9:191–199. doi: 10.1016/j.dcn.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Thulborn K.R., Munoz D.P., Merriam E.P., Garver K.E., Minshew N.J., Keshavan M.S., Genovese C.R., Eddy W.F., Sweeney J.A. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- MacPherson L., Magidson J.F., Reynolds E.K., Kahler C.W., Lejuez C.W. Changes in sensation seeking and risk-taking propensity predict increases in alcohol use among early adolescents. Alcohol. Clin. Exp. Res. 2010;34:1400–1408. doi: 10.1111/j.1530-0277.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K., Ram N., Houts R.M., Grimm K.J., Susman E.J. Individual differences in boys’ and girls’ timing and tempo of puberty: modeling development with nonlinear growth models. Dev. Psychol. 2011;47:1389–1409. doi: 10.1037/a0023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morie K.P., Wu J., Landi N., Potenza M.N., Mayes L.C., Crowley M.J. Feedback processing in adolescents with prenatal cocaine exposure: an electrophysiological investigation. Dev. Neuropsychol. 2018;43:183–197. doi: 10.1080/87565641.2018.1439945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak B.K., Novak K.D., Lynam D.R., Foti D. Individual differences in the time course of reward processing: stage-specific links with depression and impulsivity. Biol. Psychol. 2016;119:79–90. doi: 10.1016/J.BIOPSYCHO.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Oberg S.A.K., Christie G.J., Tata M.S. Problem gamblers exhibit reward hypersensitivity in medial frontal cortex during gambling. Neuropsychologia. 2011;49:3768–3775. doi: 10.1016/J.NEUROPSYCHOLOGIA.2011.09.037. [DOI] [PubMed] [Google Scholar]
- Op de Macks Z.A., Moor B.G., Overgaauw S., Güroğlu B., Dahl R.E., Crone E.A. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Dev. Cogn. Neurosci. 2011;1:506–516. doi: 10.1016/J.DCN.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y., Tamnes C.K., Fjell A.M., Westlye L.T., Due-Tonnessen P., Walhovd K.B. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J. Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleskac T.J., Wallsten T.S., Wang P., Lejuez C.W. Development of an automatic response mode to improve the clinical utility of sequential risk-taking tasks. Exp. Clin. Psychopharmacol. 2008;16:555–564. doi: 10.1037/a0014245. [DOI] [PubMed] [Google Scholar]
- Proudfit G.H. The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology. 2015;52:449–459. doi: 10.1111/psyp.12370. [DOI] [PubMed] [Google Scholar]
- Resnick M.D., Bearman P.S., Blum R.W., Bauman K.E., Harris K.M., Jones J., Tabor J., Beuhring T., Sieving R.E., Shew M., Ireland M., Bearinger L.H., Udry J.R. Protecting adolescents from harm: findings from the national longitudinal study on adolescent health. JAMA. 1997;278:823–832. doi: 10.1001/jama.278.10.823. [DOI] [PubMed] [Google Scholar]
- Romer D., Reyna V.F., Satterthwaite T.D. Beyond stereotypes of adolescent risk taking: placing the adolescent brain in developmental context. Dev. Cogn. Neurosci. 2017;27:19–34. doi: 10.1016/j.dcn.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent J.D., Tanski S., Stoolmiller M., Hanewinkel R. Using sensation seeking to target adolescents for substance use interventions. Addiction. 2010;105:506–514. doi: 10.1111/j.1360-0443.2009.02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S., Peters J., Bromberg U., Brassen S., Miedl S.F., Banaschewski T., Barker G.J., Conrod P., Flor H., Garavan H., Heinz A., Ittermann B., Lathrop M., Loth E., Mann K., Martinot J., Nees F., Paus T., Rietschel M., Robbins T.W., Smolka M.N., Spanagel R., Ströhle A., Struve M., Schumann G., Büchel C. Risk taking and the adolescent reward system: a potential common link to substance abuse. Am. J. Psychiatry. 2011;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- Sherman L., Steinberg L., Chein J. Connecting brain responsivity and real-world risk taking: strengths and limitations of current methodological approaches. Dev. Cogn. Neurosci. 2018;33:27–41. doi: 10.1016/j.dcn.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman E.P., Smith A.R., Silva K., Icenogle G., Duell N., Chein J., Steinberg L. The dual systems model: review, reappraisal, and reaffirmation. Dev. Cogn. Neurosci. 2016 doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cogn. 2005;9:69–74. doi: 10.1016/J.TICS.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: new perspectives from brain and behavioral science. Curr. Dir. Psychol. Sci. 2007;16:55–59. doi: 10.1111/j.1467-8721.2007.00475.x. [DOI] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Galván A. Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Dev. Cogn. Neurosci. 2013;3:45–52. doi: 10.1016/j.dcn.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A., Kahneman D. Advances in prospect theory: cumulative representation of uncertainty. J. Risk Uncertain. 1992;323:297–323. doi: 10.1007/Bf00122574. [DOI] [Google Scholar]
- Van Leijenhorst L., Westenberg P.M., Crone E.A. A developmental study of risky decisions on the cake gambling task: age and gender analyses of probability estimation and reward evaluation. Dev. Neuropsychol. 2008;33:179–196. doi: 10.1080/87565640701884287. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L., Moor B.G., Op de Macks Z.A., Rombouts S.A.R.B., Westenberg P.M., Crone E.A. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L., Zanolie K., Van Meel C.S., Westenberg P.M., Rombouts S.A.R.B., Crone E.A. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb. Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Yau Y.H.C., Potenza M.N., Mayes L.C., Crowley M.J. Blunted feedback processing during risk-taking in adolescents with features of problematic Internet use. Addict. Behav. 2015;45:156–163. doi: 10.1016/j.addbeh.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolski T.C.B., Cyders M.A., Smith G.T. Positive urgency predicts illegal drug use and risky sexual behavior. PsyPsychology Addict. Behav. 2009;23:348–354. doi: 10.1037/a0014684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottoli T.M., Grose-Fifer J. The feedback-related negativity (FRN) in adolescents. Psychophysiology. 2012;49:413–420. doi: 10.1111/j.1469-8986.2011.01312.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.