Abstract
Background:
Total knee arthroplasty (TKA) can cause excessive blood loss requiring allogenic transfusions. Tranexamic acid (TXA) has been increasingly used for lowering blood loss. The present study aimed to compare the efficacy of intravenous (IV) and intra-articular (IA) administrations of TXA in TKA patients who receive aspirin as chemoprophylaxis and uses no drain post-operative.
Methods:
In this prospective randomized clinical trial, 49 TKA patients were intravenously given 15 mg/kg dose of TXA, and 49 patients intraarticularly received 15 mg/kg of TXA. Demographic information, pre-operative and post-operative hemoglobin values of the patients were used for assessing total perioperative blood loss by GOOD & NADLER formulae.
Results:
There was not any significant difference between the IV TXA and IA TXA groups concerning blood loss (P=0.102). However, the decrease in hemoglobin level at 48 hours post-operation compared to the preoperative level in the IV TXA group was significantly higher than that in the IA TXA group (-2.3 ±0.8 vs. -1.9 ±1.0 g/dL; P=0.038). No blood transfusion was needed, and the deep venous thrombosis and pulmonary embolization were not observed in either of the groups (P>0.05).
Conclusion:
Our study showed that during TKA, the IA TXA is equally safe and effective as its IV infusion concerning decreased blood loss and adverse effects. The use of TXA during TKA is safe for patients who receive less potent chemoprophylaxis agents such as aspirin.
Key Words: lood loss, ntra-articular, Intravenous, TKA, Tranexamic acid
Introduction
Total knee arthroplasty (TKA) is an advanced orthopedic surgery mostly performed in osteoarthritis, rheumatoid arthritis, and abnormalities caused by the destruction of the articular surfaces (1). While the primary objective of a TKA is relieving the disease-associated pain, it is also used to correct deformations, restore the optimal motion range of the joint, and ameliorate its function. However, TKA can be accompanished by a significant amount of perioperative blood loss demanding allogenic blood transfusion (2, 3). Blood transfusion, especially in elderly people, is not without consequences including the, the risk of blood-borne infections, cardiovascular impairments, and immunological reactions (4-7). There is a relationship between blood transfusion and increased risk of periprosthetic joint infection (8). In addition to particular challenges in the management of the patient, it would impose an additional cost on the patient and healthcare system (9, 10).
There are several methods for preserving blood loss and reducing the chance of blood transfusion in orthopedic surgical procedures (11). One of these strategies is the preoperative application of an antifibrinolytic medication (12). Synthetic Tranexamic acid (TXA) has increasingly drawn the attention of surgeons for blood loss reducing in TKA patients (13-15). To date, different studies among different populations have confirmed high efficacy, safety, and cost-effectiveness of TXA in diminishing blood loss and thereby with lessening allogenic blood transfusion frequency and related risks in TKA as well as in many other orthopedic surgeries (15-17).
Numerous randomized controlled trials have established the efficacy of intravenous (IV) infusion of TXA in lowering blood loss without serious complications during TKA (13-15, 18). Previously, direct intra-articular (IA) injection of TXA has been taken into consideration in several trials for effective management of blood preservation in TKA patients (19-22). Many studies supported that both IA and IV TXA are effective in reducing total blood loss following TKA (19, 22-24) without increasing the complications such as DVT (24). On the other hand, the studies which had compared the efficacy of IA and intravenous TXA used low molecular weight heparin (LMWH) as a venous thromboembolism (VTE) prophylaxis (19, 20, 25) and one study used either LMWH or Aspirin for chemoprophylaxis (22). There is an attitude that maybe using TXA can increase the risk of thrombosis (26) and previous studies used LMWH anticoagulant for VTE prophylaxis (19, 20, 25). However, few studies have compared the effect of IV and IA TXA in patients undergoing TKA and receiving aspirin for chemoprophylaxis (22-25). Pharmacologically TXA is not a procoagulant, and the administration of TXA should not increase the risk of VTE (27). Heller et al. revealed that TXA reduces bleeding without increasing the risk of VTE in patients who receive Aspirin as a VTE prophylaxis following total joint arthroplasty (TJA) (28). Also, most studies which compare IA and IV TXA following TKA, used closed suction drainage in the postoperative period. This, in turn, causes variation in IA TXA based on the rate of drainage. We have not used drains in our patients, which may lead to more reliable IA TXA dosage.
We conceive this study align with the previous studies in comparing the efficacy of IA Versus IV TXA on reducing postoperative total blood loss but, unlike most of the previous studies, our patients received aspirin for VTE chemoprophylaxis and did not receive closed suction drainage in the postoperative period.
Materials and Methods
Experiment design
All patients with no history of bleeding disorders, specific blood clotting problems, and allergy to TXA, referring to our institution from September 2015 to June 2016 for unilateral TKA surgery by the same prosthesis (Scorpio® NRG, Stryker) due to knee DJD were eligible for inclusion in this prospective Randomized clinical trial. Patients with no history of allergy to TXA, deep venous thrombosis (DVT), pulmonary thromboembolism, diabetes, clotting or hematologic problems, stroke, heart failure, cardiovascular disorders, peripheral vascular surgery, and severe pulmonary or renal disorders were excluded from the study. All participants signed an informed written consent. The extra cost was imposed on the patients due to the study. Also, the ethical committee of our institution approved the trial design and procedure.
The patients were randomly and consecutively allocated to either the experiment group (n=49) receiving IA TXA, or the control group (n=49) receiving IV TXA. Patient’s demographic characteristics (age, gender, and BMI) as well as preoperative hemoglobin and hematocrit were recorded [Table 1]. All patients were given aspirin 325 mg/BID as chemoprophylaxis postoperatively. All operations were performed with tourniquet control. In the experiment group, TXA was administrated 15mg/kg IA at the end of the surgery after watertight capsular closure, while the control group was given 15 mg/kg TXA intravenously (Caspian Tamin Co, Rasht, Iran), respectively, 10 minutes before the surgical incision. All patients underwent TKA surgery by a single surgeon. No postoperative drain was used in these patients.
Table 1.
Patients’ demographic features and baseline laboratory values in the present study. Data are presented as mean ± standard deviation (SD) for numerical variable or as frequency (with number and percentage) for categorical variables
| Variable | Intra-Venous TXA (N=49) | Intra-Articular TXA (N=49) | P value | |||
|---|---|---|---|---|---|---|
| Demographic features | Age (year) | 69.6 ± 8.9 | 69.5 ± 6.5 | 0.965 ┼ | ||
| Weight (kg) | 81.7 ± 18.1 | 77.4 ± 9.5 | 0.434 ץ | |||
| Height (m) | 157.7 ± 14.5 | 158.1 ± 9.6 | 0.835 ץ | |||
| Body Mass Index (kg/m2) | 31.2 ± 4.4 | 30.6 ± 3.9 | 0.485 ┼ | |||
| Sex, Female vs. Male | 40 (91%) vs. 4 (9%) | 49 (92%) vs. 4 (8%) | 0.534 § | |||
| Side, Right vs. Left | 23 (52%) vs. 21 (48%) | 26 (49%) vs. 27 (51%) | 0.456 § | |||
| Laboratory | Hemoglobin (g/dL) | 13.3 ± 1.3 | 13.0 ± 1.3 | 0.249 ┼ | ||
| Hematocrit (%) | 40.3 ± 3.6 | 39.3 ± 4.0 | 0.179 ┼ | |||
┼ t test, ץ Mann-Whitney U test, and § Fisher‘s exact test
All patients were monitored for three consecutive days after surgery. The post-operative concentrations of hemoglobin and hematocrit were regularly assessed by routine CBC test on days one, two, and three, following the surgery. The indications for blood transfusions in our institution are patients with symptoms related to anemia or asymptomatic patients with hemoglobin level<8 mg/dL. All the patients were followed prospectively for at least two years. The number of blood transfusions and any intra- or post-operative complications associated with the surgery or the TXA infusion were recorded as well. The total amount of blood loss for each patient was estimated by GOOD & NADLER formulae as follows (29, 30):
BV stands for the estimated total blood volume (L) H for height (meter), W for body weight (Kg), Hgbi for hemoglobin concentration prior to surgery in g/dL, Hgbe for the lowest hemoglobin concentration during hospitalization period in g/dL, and Hgbt for total amount of allogeneic hemoglobin transfused in g.
Where;
Statistical Analysis
Data were analyzed by GraphPad Prism version 5 software for Windows (San Diego, CA, USA). The results were showed as mean ±standard deviation (SD) for binary variables or as frequency (with number and percentage) for categorical ones. The independent t-test or Mann-Whitney U test were used for comparing the mean of numerical values between the groups. Also, the mean laboratory values before and after the surgery were compared by a paired t-test. Fisher’s exact test was used to examine the differences between the frequency of categorical variables. A P<0.05 was considered as statistically significant within the interval confidence of 95%.
Tehran University of Medical Science’s Institutional Review Board (IRB) reviewed and approved method of the study and declared that there were no ethical concern and conflict of interest in the study (Approval code: IR.TUMS.VCR.REC.1396.2879). The study was retrospectively registered in Iranian Registry of Clinical Trials (IRCT) (IRCT20160809029286N3).
Results
The mean follow-up duration was 30 months (24 to 36). Demographic features and preoperative hemoglobin and hematocrit values of the groups are given in Table 1. No significant difference was seen in the mean age (P=0.965), weight (P=0.434), height (P=0.835), BMI (P=0.485), gender distribution (P=0.534), surgical side (P=0.456), and also preoperative levels of hemoglobin (P=0.249) and hematocrit (P=0.179) between two groups.
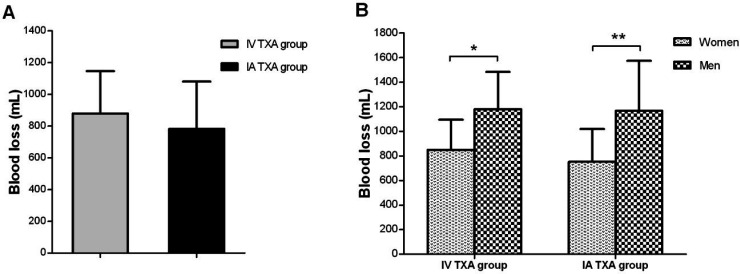
None of the patients in the IV TXA or IA TXA groups did require blood transfusion. The DVT and pulmonary embolism were not observed in any of the groups. The amount of blood loss was calculated by GOOD & NADLER formulae as described above. There was no significant difference between the IV TXA and IA TXA groups concerning blood loss after the surgery [P=0.102; Figure 1A]. Also, no significant difference was determined in the mean blood loss between the two groups by gender [Figure 1B]. However, the amount of blood loss in men was significantly higher compared with women in both groups (P=0.016 for the IV TXA group and P=0.006 for the IA TXA group).
Figure 1.
Comparisons of the amount of blood loss; A) between the intra-articular (IA) TXA and intravenous (IV) TXA groups, and B) concerning gender. Data are presented as mean ± standard deviation (SD). The signs * and ** represent the difference with P<.
Furthermore, the hemoglobin levels in both IV TXA and IA TXA patients were similarly decreased at 24, 48 and 72 hours post operation; no significant difference was observed between the two groups [P>0.05; Figure 2A]. There was not any significant difference between groups for hematocrit values of 24, 48 and 72 hours post operation [P>0.05; Figure 2B; Table 2]
Figure 2.
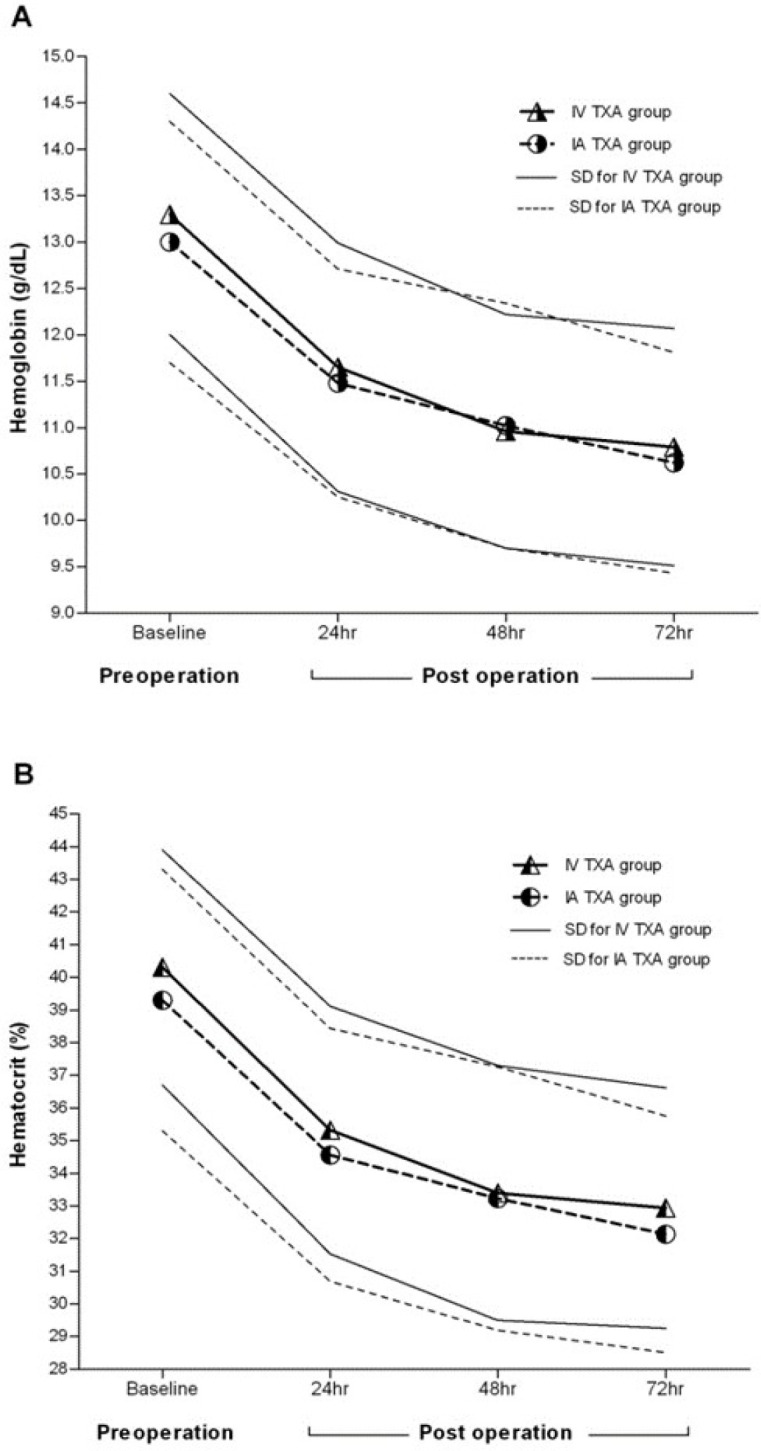
Changes in concentrations of hemoglobin (A) and hematocrit (B) according to the time. Data are presented as mean ± standard deviation (SD).
Table 2.
Patients’ demographic features and baseline laboratory values of the study groups. Data are presented as mean ± standard deviation (SD) for numerical variable or as frequency (with number and percentage) for categorical variables
| Variable | Intra-Venous TXA (N=49) | Intra-Articular TXA (N=49) | P value | |
|---|---|---|---|---|
| Post-operative hemoglobin change from preoperative value (g/dL) | 24hr | -1.6 ± 0.9 | -1.7 ± 2.1 | 0.805 ┼ |
| 48hr | -2.2 ± 0.9 | -1.9 ± 0.9 | 0.105 ┼ | |
| 72hr | -2.5 ± 0.8 | -2.3 ± 1.2 | 0.126 ץ | |
| Post-operative hematocrit change from preoperative value (%) | 24hr | -5.0 ± 2.5 | -4.6 ± 3.1 | 0.476 ┼ |
| 48hr | -6.9 ± 2.5 | -5.9 ± 3.1 | 0.090 ┼ | |
| 72hr | -7.4 ± 2.5 | -7.2 ± 3.4 | 0.516 ץ | |
┼ t test, and ץ Mann-Whitney U test.
Discussion
Perioperative blood loss is a significant challenge in TKA patients. It may result in numerous postoperative complications and even in death (31). Among several strategies to decrease blood loss and blood transfusion following TKA TXA has shown the most promising outcomes.
Similarly, Our results are in line with the previous studies in comparing the efficacy of IA vs. IV TXA in controlling the total blood loss following TKA. However, two studies revealed that IV rout was superior to IA administration of TXA (7, 32), while, one study showed vise versa findings (21) and three studies concluded that IV and IA had the same efficacy [Table 3] (19, 20, 25, 33).
Table 3.
IV vs Topical TXA Studies
| Study | No | IV | IA | Closed suction drain | VTE prophylaxis |
|---|---|---|---|---|---|
| Soni et al 2014 | 40 | 10mg/kg | 3 g | yes | LMWH |
| Patel et al 2014 | 47 | 10mg/kg | 2 g | yes | LMWH |
| Gomez et al 2015 | 39 | 15mg/kg | 3 g | yes | LMWH |
| Chen et al 2016 | 50 | 1.5 g | 1.5 g | yes | LMWH |
| Goyal et al 2017 | 85 | 1 g | 3 g | No | LMWH or ASA |
| Current Study | 49 | 15 mg/kg | 15 mg/kg | No | ASA |
The TXA has been shown to decrease perioperative blood loss, hemoglobin drop, and the need for blood transfusion in patients undergoing TKA (34). However, the most effective and safe route of TXA administration is still under debate. Pharmacologically, TXA is not a procoagulant (27) and its TXA should not increase the risk of VTE. Heller et al. revealed that TXA reduces bleeding without increasing the risk of VTE in patients who receive Aspirin as a VTE prophylaxis following TJA, (28).
In this study, we compared the effect of IV and IA administration of TXA on the blood loss, hemoglobin drop, and TXA adverse effects in patients who receive aspirin as an anticoagulant for VTE prophylaxis and no postoperative drain following TKA surgery. We observed equal effect using either IV or IA TXA in decreasing postoperative blood loss. We also found that the amount of blood loss varied by gender so that men suffered significantly more blood loss compared to the women, regardless of the administration route. Also, none of the groups needed a perioperative blood transfusion and experienced serious complications such as systemic DVT or pulmonary embolism.
These findings are consistent with many previous studies in which no significant difference was reported between the efficacy of IV infusion and IA injection of TXA in TKA patients; the IA TXA has been demonstrated to be same effective as IV regimen in lowering blood loss and allogeneic blood transfusion during TKA (5, 6, 33, 35-37). Ueno et al. reported no significant difference in reducing postoperative blood loss between IV and IA routes in unilateral TKA patients (36). In a study by Gomez-Barrena et al., no significant difference in the drain blood loss was reported at 24 and 48 hours post TKA operation between IV and IA administration of TXA concerning (20). Patel et al. also demonstrated no significant difference between the efficacy of IV and IA approaches in terms of perioperative blood loss and the lowest hemoglobin level; however, blood transfusion was needed in one of their patients receiving IA TXA (33).
There is some evidence that suggested using IA TXA has potent effects than the IV route in TKA patients. Aggarwal et al., showed that IA TXA was better than IV TXA in terms of lowering blood loss, hemoglobin drop, and allogeneic blood transfusion frequency in patients who underwent bilateral TKA (38). Seo et al. (21), Aguilera et al. (39), and Digas et al. (40) similarly found that while both IV and IA administration of TXA decreased drained blood loss compared to the control, the blood loss, hemoglobin drop, and transfusion rate were much lower in the IA group than in the IV group. Also, the transfusion rate was significantly lower in the IA TXA receiving patients compared to the IV TXA patients in the study of Hamlin and colleagues (41). These variations between the different studies regarding the efficacy of IA and IV routes might be due in part to the differences in the dose of the administrated TXA.
There are some more advantages for IA TXA. It was observed that IA TXA not only decreases the perioperative blood loss and transfusion but also may contribute to alleviating the knee joint swelling after TKA (42). Also, IA TXA might be more cost-effective, as, a direct IA injection of TXA with a lower dose might have the same effect of a higher IV dose. Finally, IA injection can theoretically result in minimal if any systemic adverse effects such as thromboembolic events, and finally reduces recovery and hospitalization period and costs (43, 44).
One of the great features of the present study was that it did not use drain during the TKA similar to the studies of Yang et al. and Goyal et al. (22, 45). The use of drain may increase the transfusion frequency and interfere with the real estimation of hemoglobin drop at 48 hours following the TKA (46). Also, unlike many previous studies that have used total drain output values for calculation of total blood loss, it allows the appropriate IA injection of TXA (20, 21, 37, 40) (45). The present study recruited GOOD & NADLER formulae, which deals explicitly with BMI of each as well as pre- and post-operative and hemoglobin values, is not influenced by the operation condition and thereby gives more reliable results compared to the drain-based outputs regarding the estimated volume of blood loss. In conclusion, no use of drain in TKA patients can reduce postoperative total blood loss.
Another feature of this study was that all patients received aspirin 325 mg/bid as VTE chemoprophylaxis. Many Previous studies have used LMWH anticoagulant as a VTE prophylaxis following TKA (19, 20, 25) and one study used either LMWH or aspirin(22). There is an attitude that TXA maybe can increase the risk of thrombosis after surgery (26). Heller et al. showed that TXA reduces bleeding with no additional risk of VTE in patients who receive aspirin as VTE prophylaxis following TJA (28). Our study confirmed that both IV and IA TXA routes could be safely administered for patients undergoing TKA and receive ASA as chemoprophylaxis.
The present study suffers some significant limitations. First, it did not include any control given IV or IA saline. Second, the sample size allocated to each group was inevitably too small to reach a more citable and extendable conclusion for larger populations. Third, we only evaluated the DVT clinically, while, PE symptoms did not survey for it using imaging. Fourth, there were not any significant differences between groups in our study. However, our study may suffer from type B error, which may be due to an insufficient number of patients in each group.
Our study corroborated that safity and effects of the IA TXA as same sa IV TXA in decreasing blood loss and the systemic thromboembolic adverse effects TKA in patients undergoing TKA and receiving aspirin as thromboprophylaxis and no suction drain following surgery. However, considering the topical injection of TXA, which possibly avoids any unpredictable systemic complications as well as the lower dosage of the drug used for achieving a comparable outcome, the IA route might be considered safer and more cost-effective than the IV route in TKA patients who received aspirin as a VTE prophylaxis and no drain postoperatively. More studies are needed to confirm this conclusion.
Conflict of Interests:
The authors declare no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Lin ZX, Woolf SK. Safety, efficacy, and cost-effectiveness of tranexamic acid in orthopedic surgery. Orthopedics. 2016;39(2):119–30. doi: 10.3928/01477447-20160301-05. [DOI] [PubMed] [Google Scholar]
- 2.Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123(5):687–96. doi: 10.1016/j.thromres.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on blood loss and transfusion rate in primary total knee arthroplasty. J Arthroplasty. 2013;28(7):1080–3. doi: 10.1016/j.arth.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Quintero JI, Cárdenas LL, Navas M, Bautista MP, Bonilla GA, Llinás AM, et al. Primary joint arthroplasty surgery: is the risk of major bleeding higher in elderly patients? A retrospective cohort study. J Arthroplasty. 2016;31(10):2264–8. doi: 10.1016/j.arth.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19(3):281–7. doi: 10.1016/j.arth.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710–5. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarzaeem MM, Razi M, Kazemian G, Moghaddam ME, Rasi AM, Karimi M. Comparing efficacy of three methods of tranexamic acid administration in reducing hemoglobin drop following total knee arthroplasty. J Arthroplasty. 2014;29(8):1521–4. doi: 10.1016/j.arth.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Rasouli MR, Gomes LS, Parsley B, Barsoum W, Bezwada H, Cashman J, et al. Blood conservation. J Arthroplasty. 2014;29(2 Suppl):65–70. doi: 10.1016/j.arth.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Keyhani S, Esmailiejah AA, Abbasian MR, Safdari F. Which route of tranexamic acid administration is more effective to reduce blood loss following total knee arthroplasty? Arch Bone Jt Surg. 2016;4(1):65–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenblatt MA. Strategies for minimizing the use of allogeneic blood during orthopedic surgery. Mt Sinai J Med. 2002;69(1-2):83–7. [PubMed] [Google Scholar]
- 11.Lemaire R. Strategies for blood management in orthopaedic and trauma surgery. J Bone Joint Surg Br. 2008;90(9):1128–36. doi: 10.1302/0301-620X.90B9.21115. [DOI] [PubMed] [Google Scholar]
- 12.Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132–8. [PubMed] [Google Scholar]
- 13.Camarasa M, Ollé G, Serra-Prat M, Martín A, Sánchez M, Ricós P, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576–82. doi: 10.1093/bja/ael057. [DOI] [PubMed] [Google Scholar]
- 14.Molloy DO, Archbold HA, Ogonda L, McConway J, Wilson RK, Beverland DE. Comparison of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomised controlled trial. J Bone Joint Surg Br. 2007;89(3):306–9. doi: 10.1302/0301-620X.89B3.17565. [DOI] [PubMed] [Google Scholar]
- 15.Vigna-Taglianti F, Basso L, Rolfo P, Brambilla R, Vaccari F, Lanci G, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545–51. doi: 10.1007/s00590-013-1225-y. [DOI] [PubMed] [Google Scholar]
- 16.Badeaux J, Hawley D. A systematic review of the effectiveness of intravenous tranexamic acid administration in managing perioperative blood loss in patients undergoing spine surgery. J Perianesth Nurs. 2014;29(6):459–65. doi: 10.1016/j.jopan.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Melvin JS, Stryker LS, Sierra RJ. Tranexamic acid in hip and knee arthroplasty. J Am Acad Orthop Surg. 2015;23(12):732–40. doi: 10.5435/JAAOS-D-14-00223. [DOI] [PubMed] [Google Scholar]
- 18.MacGillivray RG, Tarabichi SB, Hawari MF, Raoof NT. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty: a prospective, randomized double blind study. J Arthroplasty. 2011;26(1):24–8. doi: 10.1016/j.arth.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Chen JY, Chin PL, Moo IH, Pang HN, Tay DKJ, Chia SL, et al. Intravenous versus intra-articular tranexamic acid in total knee arthroplasty: a double-blinded randomised controlled noninferiority trial. Knee. 2016;23(1):152–6. doi: 10.1016/j.knee.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937–44. doi: 10.2106/JBJS.N.00060. [DOI] [PubMed] [Google Scholar]
- 21.Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869–74. doi: 10.1007/s00167-012-2079-2. [DOI] [PubMed] [Google Scholar]
- 22.Goyal N, Chen DB, Harris IA, Rowden NJ, Kirsh G, MacDessi SJ. Intravenous vs intra-articular tranexamic acid in total knee arthroplasty: a randomized, double-blind trial. J Arthroplasty. 2017;32(1):28–32. doi: 10.1016/j.arth.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Lee SY, Chong S, Balasubramanian D, Na YG, Kim TK. What is the ideal route of administration of tranexamic acid in TKA? A randomized controlled trial. Clin Orthop Relat Res. 2017;475(8):1987–96. doi: 10.1007/s11999-017-5311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mi B, Liu G, Zhou W, Lv H, Liu Y, Zha K, et al. Intra-articular versus intravenous tranexamic acid application in total knee arthroplasty: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2017;137(7):997–1009. doi: 10.1007/s00402-017-2683-1. [DOI] [PubMed] [Google Scholar]
- 25.Soni A, Saini R, Gulati A, Paul R, Bhatty S, Rajoli SR. Comparison between intravenous and intra-articular regimens of tranexamic acid in reducing blood loss during total knee arthroplasty. J Arthroplasty. 2014;29(8):1525–7. doi: 10.1016/j.arth.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 26.Myers SP, Kutcher ME, Rosengart MR, Sperry JL, Peitzman AB, Brown JB, et al. Tranexamic acid administration is associated with an increased risk of post-traumatic venous thromboembolism. J Trauma Acute Care Surg. 2019;86(1):20–7. doi: 10.1097/TA.0000000000002061. [DOI] [PubMed] [Google Scholar]
- 27.Åstedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol. 1987;22(137):22–5. [PubMed] [Google Scholar]
- 28.Heller S, Secrist E, Shahi A, Chen AF, Parvizi J. Tranexamic acid can be administered to arthroplasty patients who receive aspirin for venous thromboembolic prophylaxis. J Arthroplasty. 2016;31(7):1437–41. doi: 10.1016/j.arth.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 29.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596–9. doi: 10.1093/bja/aeg111. [DOI] [PubMed] [Google Scholar]
- 30.Nadler SB, Hidalgo JU, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–32. [PubMed] [Google Scholar]
- 31.Kirksey M, Chiu YL, Ma Y, Della Valle AG, Poultsides L, Gerner P, et al. Trends in in-hospital major morbidity and mortality after total joint arthroplasty: United States 1998-2008. Anesth Analg. 2012;115(2):321–7. doi: 10.1213/ANE.0b013e31825b6824. [DOI] [PubMed] [Google Scholar]
- 32.Maniar RN, Kumar G, Singhi T, Nayak RM, Maniar PR. Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res. 2012;470(9):2605–12. doi: 10.1007/s11999-012-2310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528–31. doi: 10.1016/j.arth.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577–85. doi: 10.1302/0301-620X.93B12.26989. [DOI] [PubMed] [Google Scholar]
- 35.Nawabi DH. Topical tranexamic acid was noninferior to intravenous tranexamic Acid in controlling blood loss during total knee arthroplasty. J Bone Joint Surg Am. 2015;97(4):343. doi: 10.2106/JBJS.9704.ebo103. [DOI] [PubMed] [Google Scholar]
- 36.Ueno M, Sonohata M, Fukumori N, Kawano S, Kitajima M, Mawatari M. Comparison between topical and intravenous administration of tranexamic acid in primary total hip arthroplasty. J Orthop Sci. 2016;21(1):44–7. doi: 10.1016/j.jos.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Uğurlu M, Aksekili MA, Çağlar C, Yüksel K, Şahin E, Akyol M. Effect of topical and intravenously applied tranexamic acid compared to control group on bleeding in primary unilateral total knee arthroplasty. J Knee Surg. 2017;30(02):152–7. doi: 10.1055/s-0036-1583270. [DOI] [PubMed] [Google Scholar]
- 38.Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016;31(7):1442–8. doi: 10.1016/j.arth.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 39.Aguilera X, Martínez-Zapata MJ, Hinarejos P, Jordan M, Leal J, Gonzalez J, et al. Topical and intravenous tranexamic acid reduce blood loss compared to routine hemostasis in total knee arthroplasty: a multicenter, randomized, controlled trial. Arch Orthop Trauma Surg. 2015;135(7):1017–25. doi: 10.1007/s00402-015-2232-8. [DOI] [PubMed] [Google Scholar]
- 40.Digas G, Koutsogiannis I, Meletiadis G, Antonopoulou E, Karamoulas V, Bikos C. Intra-articular injection of tranexamic acid reduce blood loss in cemented total knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(7):1181–8. doi: 10.1007/s00590-015-1664-8. [DOI] [PubMed] [Google Scholar]
- 41.Hamlin BR, DiGioia AM, Plakseychuk AY, Levison TJ. Topical versus intravenous tranexamic acid in total knee arthroplasty. J Arthroplasty. 2015;30(3):384–6. doi: 10.1016/j.arth.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Ishida K, Tsumura N, Kitagawa A, Hamamura S, Fukuda K, Dogaki Y, et al. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop. 2011;35(11):1639–45. doi: 10.1007/s00264-010-1205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shemshaki H, Nourian SM, Nourian N, Dehghani M, Mokhtari M, Mazoochian F. One step closer to sparing total blood loss and transfusion rate in total knee arthroplasty: a meta-analysis of different methods of tranexamic acid administration. Arch Orthop Trauma Surg. 2015;135(4):573–88. doi: 10.1007/s00402-015-2189-7. [DOI] [PubMed] [Google Scholar]
- 44.Wong J, Abrishami A, El Beheiry H, Mahomed NN, Davey JR, Gandhi R, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503–13. doi: 10.2106/JBJS.I.01518. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Lv YM, Ding PJ, Li J, Ying-Ze Z. The reduction in blood loss with intra-articular injection of tranexamic acid in unilateral total knee arthroplasty without operative drains: a randomized controlled trial. Eur J Orthop Surg Traumatol. 2015;25(1):135–9. doi: 10.1007/s00590-014-1461-9. [DOI] [PubMed] [Google Scholar]
- 46.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th Edition) Chest. 2008;133(6):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]