Abstract
Background:
Of the pharmacological modalities for knee osteoarthritis (OA), intra-articular injections including ozone (O3) and hyaluronic acid (HA) are commonly used for reducing pain and improving function. In this systematic review and meta-analysis, we aimed to compare the effect of O3 versus HA in reducing pain and increasing function in patients with knee OA.
Methods:
After searching databases, we included 6 randomized controlled trials on patients with knee OA that compared the effects of intra-articular injection of ozone versus HA. The primary outcome was visual analogue scale (VAS) of pain. The secondary outcome was Western Ontario and McMaster Universities Arthritis Index (WOMAC) score.
Results:
There was a total of 237 patients in the HA group and 230 patients in the Ozone group. Of 6 studies, 4 were in English, 1 was in Persian, and 1 was in German language. The overall Standardized Mean Difference (SMD) for VAS pain did not show a significant difference between the groups although it favored HA injection (1.27 [95%CI: (-0.12)-2.66]). Total WOMAC score showed a significant difference over the time favoring HA injection (4.5 [95%CI: 1.1-8]). However, no single time point showed any significant difference between groups.
Conclusion:
This meta-analysis showed no significant difference between HA and ozone in reducing pain and improving function in patients with knee OA, although the overall results favored HA over ozone. Since previous studies have shown comparable results between HA and placebo, ozone seems to fall in the same category with more placebo effect rather than a real disease-modifier.
Key Words: Hyaluronic acid, Knee osteoarthritis, Ozone, Pain, WOMAC score
Introduction
Of the pharmacological therapies for knee osteoarthritis (OA), intra-articular injections are becoming more popular because of their efficacy in reducing pain and some improving function without serious side effects (1). Hyaluronic acid (HA) is reported to increase viscoelastic function, act like a lubricant to maintain the cartilage matrix integrity, and have good anti-inflammatory effects (2). In comparison to corticosteroids, HA injection has been shown to result in more persistent therapeutic effects lasting almost 12 times longer than corticosteroid injections (3, 4). Nevertheless, the latest evidence-based guideline released in 2013 by the American Academy of Orthopaedic Surgeons, did not recommend HA in patients with symptomatic knee OA with strong recommendation due to lack of superiority over placebo (5).
Ozone therapy has recently become popular in the treatment of OA. Ozone (O3) gas was discovered and has been utilized since 1800s. Ozone therapy with a history of being practiced for disease treatment and disinfection in more than a century has shown considerable potential with its minimal side effects, safety and proven consistent effectiveness. During the first world war (1914-18) doctors already knowing about the O3’s antibacterial properties, discovered the hemodynamic and anti-inflammatory properties of O3 too (6).
Oxygen ozone consists of pure O2 and O3 which is made by O2 passing through a high voltage gradient in a medical generator. The natural chemical properties of O3 tend to be the main reason for anti-inflammatory and pain-reducing effects of O2O3 (1, 7, 8). The efficacy of O3 in the management of knee OA has been shown to some extent (9). A randomized clinical trial found superior results over corticosteroid injection in a 6 month follow up based on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) (10). Also another study showed higher efficacy of ozone over placebo in relieving pain and improving function after 8 weeks (11). However, there is still a dearth of data regarding the efficacy of ozone in compare to other modalities including HA.
In this paper, we aimed to perform a systematic review and meta-analysis of all randomized clinical trials (RCTs) comparing intra-articular injection of ozone versus HA in terms of improvement in the visual analog scale (VAS) and WOMAC scores in patients with knee OA.
Materials and Methods
In this study we used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) guidelines (12).
Search strategy
Four main databases including PubMed/Medline, ISI web of knowledge, Cochrane Library and Scopus were searched using the appropriate key words. Grey literature in The Grey Literature Report and OpenGrey were also searched. To avoid missing any relevant articles, we searched the Scientific Information Database (SID) – the main webpage of scientific articles in Persian language – with Persian keywords. The main key words were “joint disease” or “arthritis” or “osteoarthritis” or “arthrosis” combined with “ozone” or “O3” or “O2” or “oxygen” and “hyaluronic acid” or “HA”. We adapted the search strategy for each database. They were searched to find out all randomized controlled trials comparing the effect of intra-articular injection of ozone versus hyaluronic acid (HA) in the treatment of arthritis in the adult population. There was no language or time limitation in selecting articles. The last search was done on August 12th, 2018.
Selection criteria (Inclusion and Exclusion criteria)
We included all randomized controlled trials (RCTs) that compared the effects of intra-articular injection of ozone versus HA on pain in adult patients with arthritis. We only included the original articles, while other search results such as book chapters, reviews, and conference papers were excluded. We also removed duplicate studies using title and abstract screening [Figure 1].
Figure 1.
PRISMA flow diagram for selection of the studies is illustrated
Quality Assessment and Data extraction
We assessed the quality of the selected RCTs using the Jadad score (13). Two separate authors (J.J and M.E) evaluated the studies. This measure applies 5 questions that cover the three main domains of randomization, blinding, and drop-outs. Jadad score ranges from 0 to 5 with higher score showing higher quality (14). Scores of 3 or higher is considered appropriate.
Two authors (N.T and J.J) independently extracted data using a pre-defined data collection form. The following data was collected: the first author’s surname, year of publication, country, age of the patients, sample size, duration of the follow up, and the therapeutic outcomes.
Data Synthesis
To calculate the effect size, we used the change in measurements as the Mean Difference (MD) (measured mean value at the time of follow up – measured mean value at baseline). To pool the data, we used the Standardized Mean Difference (SMD) for the analysis. One study had provided the correlation coefficient for the mean change in scores (4) with which we calculated the Standard Deviation of the mean difference for both experimental and control groups in the other 5 studies using the below theorem where E stands for the experimental group. Similarly, it is calculated for the control group (15).
Outcome measures
The primary outcome was the visual analogue scale (VAS) of pain ranging from 0-10 with 0 being no pain and 10 being the worst pain. All 6 studies had reported the VAS Score. Secondary outcome was Western Ontario and McMaster Universities Arthritis Index (WOMAC) score. Only 4 Studies had reported this score. One study had reported Oxford Knee Score and 1 study had reported Short Form 12 (SF-12). We avoided standardizing and pooling the scores to maintain the value of WOMAC scoring. Moreover, WOMAC score was subdivided to its domains including pain, stiffness, and function.
We performed subgroup analysis at different time points separately. As there were only six relevant articles with different follow up times, for a better conclusion, we pooled the follow-up times of 2 weeks and 1 month together, 2 and 3 months together, and 6 and 12 months together categorizing as 1 month and less, 2-6 months, 6 months and more, respectively. If two measurements were provided in one categorization, we used the latest one in the analysis.
To address the proportion of sampling error versus the true effect, we assessed the heterogeneity using the chi-square-based Cochran’s Q and I2 index. I2 describes the percentage of total variation across studies that are due to heterogeneity rather than chance. I2 more than 50% is considered heterogeneous where it is recommended to use the random-effects model in the analysis. Otherwise, the fixed effect model can be carried out. Effect sizes are described as standardized mean difference (SMD) and 95% confidence intervals (95%CI). For sensitivity analysis we used the leave-one-out method, which removes one study and repeats the analysis.
We carried out the meta-analysis using the OpenMeta software (16).
Results
Study characteristic
We included 6 studies with a total of 237 patients in the HA group (76 men and 161 women) and 230 patients in the Ozone group (85 men and 145 women). Of 6 studies, 4 were in English, 1 was in Persian, and 1 was in German. The mean age of the HA and Ozone groups were 59.8 and 59.1, respectively. Three studies used Ozone (O3) while 2 studies used the mixed of O2-O3 and 1 study used O2 to inject in the osteoarthritic knees [Table 1-3].
Table 1.
Design details of the included studies
| Author, year of publication | Country | Sample size | % female | Mean age |
Outcome
measurement |
Assessment point |
|---|---|---|---|---|---|---|
| Raeissadat et al. 2018 (4) | Iran | HA:74 Ozone:67 |
HA:77% Ozone:75% |
HA:61.1±6.3 Ozone: 58.1±6.4 |
VAS,WOMAC (total, pain, function, stiffness) scores |
T0:baseline T1:6 months |
| Duymus et al. 2016 (19) | Turkey | HA:34 Ozone:35 |
HA:97.1% Ozone:88.6% |
HA: 60.3 ± 9.1 Ozone: 59.4 ± 5.7 |
VAS,WOMAC (total, pain, function, stiffness) scores |
T0:baseline T1:1 month T2:3 months T3:6 months T4:12 months |
| Giombini et al. 2016 (17) | Italy | HA:23 O2O3:23 Combined:24 |
HA:52% O2O3:47% |
HA: 64±4.52 O2O3:68±5.36 |
VAS score | T0:baseline T1:post treatment T2:2 months |
| Invernizzi et al. 2017 (9) | Italy | HA:20 O2O3:22 |
HA:65% O2O3:72.7% |
HA: 70.7 ± 5.4 O2O3: 70.3 ± 6.5 |
VAS score | T0:baseline T1:2 weeks T2:1 month |
| Auerbach et al. 2002 (18) | Germany | HA:56 O2:53 |
HA:51.7% O2:50.9% |
HA: 48.0 (17-78) O2: 46.5(18-80) |
VAS,WOMAC (pain, function, stiffness) scores |
T0:baseline T1:2 weeks T2:3 months T3:6 months T4:12 months |
| Momenzadeh et al. 2014 (23) | Iran | HA:30 Ozone:30 |
HA:60% Ozone:66.6% |
HA: 67.53±11.18 Ozone: 68.57±9.29 |
VAS,WOMAC (total) scores |
T0:baseline T1:1 month T2:2 months |
Table 3.
Results of the included studies
| Author, year of publication | Results |
|---|---|
| Raeissadat et al. 2018 (4) | VAS HA → T0:7.1±3.2; T1: 3.0±2.4. VAS Ozone → T0:7.6±2.8; T1: 2.6±2.0. WOMAC total HA → T0: 40.8±9.0; T1: 17.1±4.2. WOMAC total Ozone → T0: 38.5±8.0; T1: 20.4±5.0. WOMAC pain HA → T0: 8.8±4.0 ; T1: 2.9±1.6. WOMAC pain Ozone → T0: 3.2±1.6; T1: 3.2±1.6. WOMAC Stiffness HA → T0: 2.1±1.6; T1: 1.1±0.8. WOMAC Stiffness Ozone → T0: 2.3±2.4; T1: 1.1±1.6. WOMAC function HA → T0: 27.6±6.6; T1: 13.1±3.2. WOMAC function Ozone → T0: 29.2±7.0; T1: 16.1±4.2. |
| Duymus et al. 2016 (19) | VAS HA → T0: 8.3 ± 0.4; T1: 2.6 ± 1.2; T2: 3.1 ± 0.9; T3: 4.3 ± 1.3; T4: 6.8 ± 0.1. VAS Ozone → T0: 7.2 ± 1.1; T1: 3.5 ± 1.5; T2: 5.7 ± 1.2; T3: 7.3 ± 1.03; T4: 7.6 ± 1.1. WOMAC total HA → T0: 77.0 ± 2.5; T1: 33.2 ± 12.2; T2: 35.3 ± 10.5; T3: 44.5 ± 6.6; T4: 69.3 ± 4.3. WOMAC total Ozone → T0: 76.0 ± 11.9; T1: 31.1 ± 12.9; T2: 53.1 ± 15.9; T3: 76.6 ± 10.7; T4: 77.0 ± 10.1. WOMAC pain HA →T0: 16.6 ± 1.1; T1: 6.1 ± 2.4; T2: 7.00 ± 1.74; T3: 9.7 ± 1.6; T4: 14.2 ± 1.1. WOMAC pain Ozone → T0: 16.0 ± 2.7; T1: 6.6 ± 3.5; T2: 11.1 ± 3.4; T3: 16.0 ± 2.9; T4: 16.2 ± 2.8. WOMAC Stiffness HA → T0: 6.0 ± 0.8; T1: 2.7 ± 1.1; T2: 3.2 ± 1.0; T3: 3.8 ± 1.1; T4: 5.4 ± 0.7. WOMAC Stiffness Ozone →T0: 6.4 ± 1.0; T1: 2.7 ± 1.6; T2: 4.2 ± 1.3; T3: 6.4 ± 1.0; T4: 6.5 ± 0.1. WOMAC function HA →T0: 54.3 ± 1.8 ; T1: 24.3 ± 9.5; T2: 25.1 ± 8.9; T3: 30.1 ± 5.7; T4: 49.6 ± 3.3. WOMAC function Ozone →T0: 53.5 ± 8.7; T1: 21.7 ± 8.6; T2: 387 ± 12.2; T3: 54.1 ± 7.3; T4: 54.2 ± 7.9. |
| Giombini et al. 2016 (17) | VAS HA →T0: 6.93±0.68; T1: 4.58±0.44; T2: 2.40±0.48. VAS O2O3 →T0: 6.71±0.87; T1: 3.98±1.10; T2: 2.40±1.41. |
| Invernizzi et al. 2017 (9) | VAS HA →T0: 6.3839±0.8472; T1: 4.2857±0.89285; T2: 3.0357±0.69195. VAS O2O3 →T0: 7.0089±0.55805; T1: 4.553±0.57779; T2: 4.1332±0.3778. |
| Auerbach et al. 2002 (18) | VAS HA → T0: 29.03±30.46; T1: 18.33±24.80; T2: 21.33±29.18; T3: 20.38±30.80; T4: 14.52±24.11. VAS O2 → T0: 28.89±28.73; T1: 16.92±24.42; T2: 14.43±23.95; T3: 17.44±27.90; T4: 14.43±24.47. WOMAC pain HA → T0: 8.2±4.2; T1: 5.3±3.7; T2: 5.7±4.2; T3: 4.6±4.8; T4: 4.5±4.2. WOMAC pain O2 → T0: 6.8±4.7; T1: 4.0±4.0; T2: 4.3±3.8 ; T3: 4.0±3.9; T4: 3.7±3.9. WOMAC Stiffness HA → T0: 2.7±1.9; T1: 2.2±1.8; T2: 2.3±1.8; T3: 2.6±2.0; T4: 2.2±1.9. WOMAC Stiffness O2 → T0: 2.7±2.3; T1: 2.0±2.1; T2: 1.8±1.8 ; T3: 1.7±1.8; T4: 1.7±2.1. WOMAC function HA →T0: 22.5±15.0; T1: 17.7±13.5; T2: 16.6±13.2; T3: 16.7±16.3; T4: 15.1±15.8. WOMAC function O2 →T0: 20.6±15.5 ; T1: 14.4±13.8 ; T2: 13.8±13.9; T3: 12.6±13.5; T4: 11.1±14.2. |
| Momenzadeh et al. 2014 (23) | VAS HA → T0: 8.07±0.74; T1: 5.00±1.31; T2: 2.97±1.24. VAS Ozone → T0: 8.30±0.79; T1: 5.43±1.43; T2: 2.63±1.12. WOMAC total HA → T0: 60.33±10; T1: 42.13±10.92; T2: 26.60±9.78. WOMAC total Ozone → T0: 67.62±8.26; T1: 42.20±9.84; T2: 24.80±8.54. |
Quality Assessment
According to Jadad scoring most of the studies had appropriate quality. Only two studies were of score less than three [Table 4] (13, 17, 18).
Table 4.
Quality assessment of the included studies using the Jadad scale
| ID | Was the study described as randomized?* | Was the study described as a double blind?* | Was there a description of withdrawal and dropouts?* | The randomization scheme described and appropriate* | The method of double blinding described and appropriate* | The randomization scheme described and inappropriate** | The method of double blinding described and inappropriate** | Total score |
|---|---|---|---|---|---|---|---|---|
| Raeissadat et al. 2018 (4) | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Duymus et al. 2016 (19) | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 3 |
| Giombini et al. 2016 (17) | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| Invernizzi et al. 2017 (9) | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 3 |
| Auerbach et al. 2002 (18) | 1 | 0 | 1 | 0 | 0 | -1 | 0 | 1 |
| Momenzadeh et al. 2014 (23) | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 3 |
Test of Heterogeneity
We performed statistical test for heterogeneity to determine if the scoring was the same in all studies. Cochran Q result rejected the null hypothesis that there is no heterogeneity between studies (P<0.001). Moreover, I2 revealed that almost 98% of the variation across the studies was because of heterogeneity rather than a sampling error and chance. Considering the presence of heterogeneity, we used random-effects model to conduct the meta-analysis.
Visual Analogue Scale
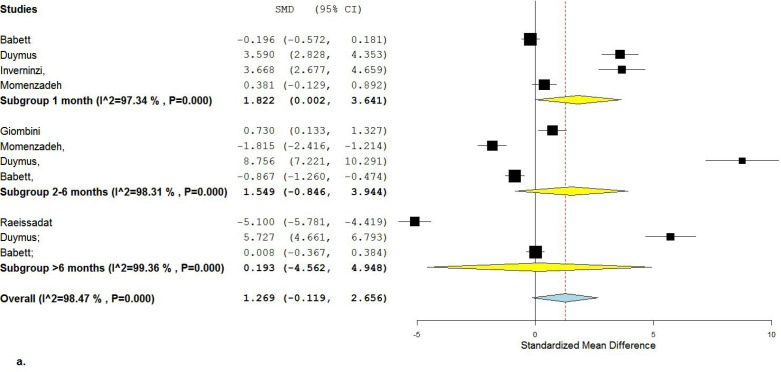
Based on the random-effect model considering all 6 studies, the overall Standardized Mean Difference (SMD) for VAS pain was 1.27 [95%CI: (-0.12)-2.66], which did not show a significant difference between the groups although it favored the HA injection [Figure 2 a; b].
Figure 2 a.
Forest plot of VAS score favoring HA is shown
Figure 2 b.
Result of VAS score sensitivity analysis by using leave-one-out analysis is shown
After subgroup analysis, SMD for VAS pain was 1.82 [95%CI: 0.002-3.64] after 1 month (4 studies included), 1.55 [95%CI: (-0.85)-3.94] after 2-5 months (4 studies included), and 0.193 [95%CI: (-4.56)-4.95] after 6-12 months (3 studies included). The only significant difference between Ozone and HA was shown after 1-month follow-up favoring HA injection.
Cochran Q statistics rejected the null hypothesis showing 98% heterogeneity between studies for the overall VAS (P<0.001; I2=98).
WOMAC, Total score
Based on the random-effect model, Standardized Mean Difference (SMD) for the overall WOMAC-Total score over time was 4.13 [95%CI: 0.94-7.32], which showed a significant difference over the time favoring HA injection. Considering the leave-one-out analysis, there was no significant difference in the total WOMAC score if the 6-month follow-up of Raeissadat study was omitted from the analysis [Figure 3 a,b] (4).
Figure 3 a.
Forest plot of WOMAC-total score favoring HA injection over time is shown
Figure 3 b.
Result of WOMAC-total score sensitivity analysis by using leave-one-out analysis is shown
After subgroup analysis separating follow-up time points, the SMD was (-1.4) [95%CI: (-3.9)-1.1] after 1 month (including 2 studies), (-0.14) [95%CI: (-6.3)-6.0] after 2-5 months (including 2 studies), and 15.63 [95%CI: (-8.21)-39.47] after 6-12 months (including 2 studies), all of which showed no significant difference at any individual time point between HA and Ozone groups.
Cochran Q statistics rejected the null hypothesis showing 99% heterogeneity between studies for the overall WOMAC scores (P<0.001; I2=99).
WOMAC, Function domain
Based on the random-effect model, Standardized Mean Difference (SMD) for the WOMAC-Function score over time was 1.38 [95%CI: 0.008-2.76], which showed a significant difference over time favoring HA injection [Figure 4 a; b].
Figure 4 a.
Forest plot of WOMAC-function score favoring HA injection is shown
Figure 4 b.
Result of WOMAC-function score sensitivity analysis by using leave-one-out analysis is shown
After subgroup analysis separating the follow-up time points, the SMD was (-0.33) [95%CI: (-0.62)-(-0.033)] after 1 month (including 2 studies), 1.28 [95%CI: (-1.6)-4.2] after 2-5 months (including 2 studies), and 2.62 [95%CI: (-0.99)-6.24] after 6-12 months (including 3 studies). The only significant difference at any time was in the 1-month follow-up favoring Ozone injection.
Cochran Q statistics rejected the null hypothesis showing 98% heterogeneity between studies for the overall WOMAC-Function scores (P<0.001; I2=98).
WOMAC, Pain domain
Based on the random-effect model, Standardized Mean Difference (SMD) for the overall WOMAC-Pain score over time was 1.7 [95%CI: 0.55-2.9], which showed a significant difference over time favoring HA injection. Considering the leave-one-out analysis, there was no significant difference in the overall WOMAC-Pain score if the 3-month follow-up of Duymus study was omitted from the analysis [Figure 5 a; b] (19).
Figure 5 a.
Forest plot of WOMAC-pain score favoring HA injection is shown
Figure 5 b.
Result of WOMAC-pain score sensitivity analysis by using leave-one-out analysis is shown
After subgroup analysis separating the follow-up time points, the SMD was 0.18 [95%CI: (-0.11)-(0.48)] after 1 month (including 2 studies), 4.7 [95%CI: (-4.5)-13.9] after 2-5 months (including 2 studies), and 1.3 [95%CI: (-0.93)-3.5] after 6-12 months (including 3 studies), all of which showed no significant difference at any individual time point between HA and Ozone groups.
Cochran Q statistics rejected the null hypothesis showing 98% heterogeneity between studies for the overall WOMAC-Pain scores (P<0.001; I2=98).
WOMAC, Stiffness domain
Based on the random-effect model, Standardized Mean Difference (SMD) for the overall WOMAC-Stiffness score over time was (-0.46) [95%CI: (-1.7)-0.79], which showed no significant difference over time [Figure 6 a; b].
Figure 6 a.
Forest plot of WOMAC-stiffness score is shown
Figure 6 b.
Result of WOMAC-stiffness score sensitivity analysis by using leave-one-out analysis is shown
After subgroup analysis separating the follow-up time points, the SMD was (-0.75) [95%CI: (-1.6)-0.104] after 1 month (including 2 studies), 1.04 [95%CI: (-3.06)-5.1] after 2-5 months (including 2 studies), and -1.24 [95%CI: (-3.8)-1.3] after 6-12 months (including 3 studies), all of which showed no significant difference at any individual time point between HA and Ozone groups.
Cochran Q statistics rejected the null hypothesis showing 98% heterogeneity between studies for the overall WOMAC-Stiffness scores (P<0.001; I2=98).
Discussion
In this systematic review and meta-analysis, we studied randomized controlled trials, which compared the effects of intra-articular (IA) injection of ozone (or oxygen) versus hyaluronic acid (HA) on pain and function in adult patients with arthritis.
The results showed that VAS score, which was measured in all six studies, did not show any significant difference between HA and ozone. The only difference found was at 1-month follow-up still favoring HA. All 6 studies showed significant improvement in pain with both HA and ozone. However, their effectiveness must be inferred cautiously because one can argue that HA has been shown to have no more effect than intra-articular injection of placebo (20).
Considering the leave-one-out analysis, the result in VAS and WOMAC score did not change after omitting Auerbach study in which they have used O2 (18). It is assumed that O2O3 breakdown turns into two products including reactive oxygen species (ROS) and lipid oxidation products (LOP). These products decrease the release of proteolytic enzymes and proinflammatory cytokines and increase the synthesis of chondrocyte, fibroblasts, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (21).
Duymus et al. and Invernizzi et al. reported more rapid pain relief with ozone, but the pain relief with HA stayed longer. This was also shown by continuing decline in VAS score after 1 month in the HA group while the VAS score started to increase after 1 month in the ozone group (9, 19). The rapid effect of ozone is attributed to the anti-inflammatory effect by declining the inflammatory cytokines and the following intra-articular edema (4). HA is also thought to have this effect while reducing cartilage damage. This has been shown by Listerat et al. where they reported slower progression of OA after HA injection (22). Pasquali et al. also reported reduction of inflammation with structural repairing changes six month after injection (23). Giombini et al. included the third group receiving both HA and ozone injection. This group showed higher improvement at 2 months in compare to HA and ozone groups (17). The rationale behind a combined injection is still debatable and is not suggested by this meta-analysis.
In our analysis after omitting the study by Momenzadeh et al., overall VAS pain showed a significant difference over the time favoring HA. This could be due to inclusion of grades I and II of knee OA in this study while the other 5 studies included grades II and III (24).
Total WOMAC score, which was measured in four studies, showed a significant difference between the two groups over the time favoring HA. However, after omitting the Raeissadat et al. study, there would be no significant difference (4). This result can be attributed to the large sample size of their study. Interestingly, they did not find any significant difference in their own study. In the analysis of the three domains of WOMAC, changes in pain and function domains showed significant differences favoring HA while changes in stiffness domain showed no significant difference.
There are also limitations in our study. Because of the limited number of the studies, we had to include all RCTs although the quality of some studies is not high enough. Also, available RCTs did not study the long-term effect of the two modalities. Probably the cost benefit study of each modality can be assessed to better help in decision-making. The strength of our study is that we managed to find the full texts and include all 6 available studies in different languages. A similar systematic review was published recently including only 4 studies of our review that were in English (25).
This systematic review and meta-analysis showed no significant difference between HA and ozone in reducing pain and improving function in patients with knee OA, although the overall results favored HA more than ozone. Since previous studies have shown comparable results between HA and placebo, ozone seems to fall in the same category with more placebo effect than a real disease-modifier. The monetary burden of ozone injection on the patients and the government should be weighed against the amount and longevity of improvement.
Disclosure statement: The authors report no conflicts of interest.
Table 2.
Characteristics of the included studies
|
Author,
year of publication |
Grade of Osteoarthritis | BMI | Duration of Treatment | Complications |
|---|---|---|---|---|
| Raeissadat et al. 2018 (4) | HA → Grade II/III:40/34 Ozone → Grade II/III: 37/30 |
HA: 28.6±1.65 Ozone: 26.8±1.95 |
In both groups weekly injections were performed for 3 consecutive weeks | no major complications mild flare reaction after the first injection |
| Duymus et al. 2016 (19) | HA → Grade II/III: 23/12 Ozone → Grade II/III: 24/10 |
HA: 28.4 ± 3.6 Ozone: 27.6 ± 4.4 |
HA: single dose Ozone: for 4 weeks |
only mild and very short-term side effects (pain, heat and redness) |
| Giombini et al. 2016 (17) | HA → Grade II/III: 13/10 O2O3 → Grade II/III: 11/12 |
HA: 24.4±4.5 O2O3: 27.7±4.9 |
In both groups once a week for 5 consecutive weeks | no complications |
| Invernizzi et al. 2017 (9) | HA → Grade II/III: 14/6 O2O3 → Grade II/III: 13/7 |
HA: 26.8 ± 1.7 O2O3: 27.1 ± 1.9 |
In both groups once per week (q1wk) for 4 consecutive weeks | Only one case of fatal septic shock after intramuscular-paravertebral O2O3 injection |
| Auerbach et al. 2002 (18) | HA → Grade II/III: 20/20 O2 → Grade II/III: 22/23 |
HA: 16,8 O2: 16,4 |
HA: 5 times O2: once a week for 3 weeks |
no complications |
| Momenzadeh et al. 2014 (23) | Grade I,II | No report | In both groups weekly injections were performed for 3 weeks | No complications |
Acknowledgments
We thank Clinical Research Development Unit of Ghaem Hospital for participation in data analysis.
References
- 1.Bocci V, Borrelli E, Zanardi I, Travagli V. The usefulness of ozone treatment in spinal pain. Drug design, development and therapy. 2015;9:2677–85. doi: 10.2147/DDDT.S74518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller LE, Block JE. US-Approved Intra-Articular Hyaluronic Acid Injections are Safe and Effective in Patients with Knee Osteoarthritis: Systematic Review and Meta-Analysis of Randomized, Saline-Controlled Trials. Clinical medicine insights Arthritis and musculoskeletal disorders. 2013;6:57–63. doi: 10.4137/CMAMD.S12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang KV, Hung CY, Aliwarga F, Wang TG, Han DS, Chen WS. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: a systematic review and meta-analysis. Archives of physical medicine and rehabilitation. 2014 ;95(3):562–75. doi: 10.1016/j.apmr.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Raeissadat SA, Rayegani SM, Moridnia M, Dehgolan SR. INTRA ARTICULAR OZONE OR HYALURONIC ACID INJECTION: WHICH ONE IS SUPERIOR IN PATIENTS WITH KNEE OSTEOARTHRITIS? A 6-MONTH RANDOMIZED CLINICAL TRIAL. Annals of the Rheumatic Diseases. 2017 Jun;76:1547–8. doi: 10.2147/JPR.S142755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. The Journal of the American Academy of Orthopaedic Surgeons. 2013 ;21(9):571–6. doi: 10.5435/JAAOS-21-09-571. [DOI] [PubMed] [Google Scholar]
- 6.Elvis AM, Ekta JS. Ozone therapy: A clinical review. Journal of natural science, biology, and medicine. 2011;2(1):66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy K, Elias G, Steppan J, Boxley C, Balagurunathan K, Victor X, et al. Percutaneous Treatment of Herniated Lumbar Discs with Ozone: Investigation of the Mechanisms of Action. Journal of vascular and interventional radiology : JVIR. 2016 ;27(8):1242–50. doi: 10.1016/j.jvir.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Zanardi I, Borrelli E, Valacchi G, Travagli V, Bocci V. Ozone: A Multifaceted Molecule with Unexpected Therapeutic Activity. Current medicinal chemistry. 2016;23(4):304–14. doi: 10.2174/0929867323666151221150420. [DOI] [PubMed] [Google Scholar]
- 9.Invernizzi M, D S, Carda S, Grana E, Picelli A, Smania N, et al. Safety of Intra-Articular Oxygen-Ozone Therapy Compared to Intra-Articular Sodium Hyaluronate in Knee Osteoarthritis: A Randomized Single Blind Pilot Study. 2017 [Google Scholar]
- 10.Kr S, Pramanik R, Das P, Pratim Das P, Kumar A, Roy J, et al. Role of intra-articular ozone in osteo-arthritis of knee for functional and symptomatic improvement. 2018 [Google Scholar]
- 11.Lopes de Jesus CC, Dos Santos FC, de Jesus LMOB, Monteiro I, Sant’Ana MSSC, Trevisani VFM. Comparison between intra-articular ozone and placebo in the treatment of knee osteoarthritis: A randomized, double-blinded, placebo-controlled study. PloS one. 2017;12(7):e0179185–e. doi: 10.1371/journal.pone.0179185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009 ;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled clinical trials. 1996 ;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Lundh A, Gotzsche PC. Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC medical research methodology. 2008 Apr;8:22. doi: 10.1186/1471-2288-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J, Green Se. Cochrane Handbook for Systematic Reviews of Interventions version 510 [updated March 2011] The Cochrane Collaboration. 2011 [Google Scholar]
- 16.Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. 2012;49(5):15. [Google Scholar]
- 17.Giombini A, Menotti F, Di AC, Giovannangeli F, Rizzo M, Moffa S, et al. Comparison between intrarticular injection of hyaluronic acid, oxygen ozone, and the combination of both in the treatment of knee osteoarthrosis %J Journal of biological regulators and homeostatic. agents. 2016;30(2):621–5. [PubMed] [Google Scholar]
- 18.Auerbach B, Melzer C. [Cross-linked hyaluronic acid in the treatment of osteoarthritis of the knee--results of a prospective randomized trial] Zentralblatt fur Chirurgie. 2002 ;127(10):895–9. doi: 10.1055/s-2002-35137. [DOI] [PubMed] [Google Scholar]
- 19.Duymus TM, Mutlu S, Dernek B, Komur B, Aydogmus S, Kesiktas FN. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee surgery, sports traumatology, arthroscopy. 2017;25(2):485–92. doi: 10.1007/s00167-016-4110-5. [DOI] [PubMed] [Google Scholar]
- 20.Jevsevar DS, Shores PB, Mullen K, Schulte DM, Brown GA, Cummins DS. Mixed Treatment Comparisons for Nonsurgical Treatment of Knee Osteoarthritis: A Network Meta-analysis. The Journal of the American Academy of Orthopaedic Surgeons. 2018;26(9):325–36. doi: 10.5435/JAAOS-D-17-00318. [DOI] [PubMed] [Google Scholar]
- 21.Borrelli E. disc herniation and knee arthritis as chronic oxidative stress diseases: the therapeutic role of oxygen ozone therapy. 2015;4:161–66. [Google Scholar]
- 22.Listrat V, Ayral X, Patarnello F, Bonvarlet JP, Simonnet J, Amor B, et al. Arthroscopic evaluation of potential structure modifying activity of hyaluronan (Hyalgan) in osteoarthritis of the knee. Osteoarthritis and cartilage. 1997 ;5(3):153–60. doi: 10.1016/s1063-4584(97)80010-6. [DOI] [PubMed] [Google Scholar]
- 23.Pasquali Ronchetti I, Guerra D, Taparelli F, Boraldi F, Bergamini G, Mori G, et al. Morphological analysis of knee synovial membrane biopsies from a randomized controlled clinical study comparing the effects of sodium hyaluronate (Hyalgan) and methylprednisolone acetate (Depomedrol) in osteoarthritis. Rheumatology (Oxford, England) 2001 ;40(2):158–69. doi: 10.1093/rheumatology/40.2.158. [DOI] [PubMed] [Google Scholar]
- 24.Momenzadeh S, Poorfarrokh M, Hashemi M, Barikani A. Comparison of intra-articular oxygen-ozone and hyaluronic acid prolotherapy on pain and disability of osteoarthritis patients. Research-in-Medicine. 2014;38(1):32–6. [Google Scholar]
- 25.Li Q, Qi X, Zhang Z. Intra-articular oxygen-ozone versus hyaluronic acid in knee osteoarthritis: A meta-analysis of randomized controlled trials. International journal of surgery (London, England). 2018 Aug;58:3–10. doi: 10.1016/j.ijsu.2018.08.007. [DOI] [PubMed] [Google Scholar]