Abstract
Introduction
There has been an increase in the global prevalence of diabetic polyneuropathy and research evidence suggests that insulin resistance plays an important role in its development and prognosis. However, there seem to be a dearth of information in understanding the likely interplay between beta endorphin, insulin resistance and pain perception especially in the setting of painful diabetic neuropathy.
Method
This study recruited 120 volunteers divided into four groups (30 per group): group 1 healthy volunteer (control); group 2 DM type 2 without neuropathy (DM group); group 3 DM type 2 with painful neuropathy (DPN group); group 4 DM type 2 without painful neuropathy (DN). All subjects were evaluated for pain threshold and neuropathy using an ischemia-induced pain model and biothesiometer respectively. Their beta-endorphin, glycated hemoglobin, fasting plasma insulin, and HOMA values were determined and means compared using ANOVA.
Result
Serum beta-endorphin is significantly reduced in DN and DPN (∗p < 0.001) compared with the control and DM group. Also, DPN and DN patients have significantly increased insulin resistance compared to those without neuropathy (∗p < 0.001; ∗p < 0.0001 respectively). There is a significant positive correlation between the pain threshold and beta-endorphin in all the groups except DN group. The correlation between beta-endorphin and insulin resistance was negative and significant in control and DM groups only. Suggestive that the fact that insulin resistance plays an important role in diabetes polyneuropathy, does not alone explain the chronic pain perception noticed in the DPN patients.
Conclusion
The present study demonstrates that diabetic neuropathy patients have a poor endogenous opioid peptide system which is associated with increased pain perception and high insulin resistance. However, insulin resistance alone does not explain the chronic pain perception noticed in the DPN patients. Thus, further study is required.
Keywords: Diabetic neuropathy, β-Endorphin, Ischemic-induced pain model, Pain threshold, Insulin resistance, Environmental science, Biological sciences, Neuroscience, Veterinary medicine, Health sciences
Diabetic neuropathy, β-Endorphin, Ischemic-induced pain model, Pain threshold, Insulin resistance, Environmental science, Biological sciences, Neuroscience, Veterinary medicine, Health sciences
1. Introduction
Pain is the most common symptom of disease known today which serves a protective function (Swieboda et al., 2013). This effect is however lost when the pain becomes a chronic medical condition as seen in patients with diabetic painful neuropathy (DNP), a common complication of diabetes mellitus (DM).
Uloko et al. (2018) reported that the prevalence of people living with diabetes in Nigeria is 5.7 % out of 174 million which is expected to hit double by 2030. According to the recent report of the International Diabetes Federation (IDF) in 2017, globally, 1 out of every 11 adults (425 million) is diabetic which is expected to hit the estimate of about 642 million in 2040.
Diabetic polyneuropathy is the most common microvascular complication of DM type 2 affecting up to 50% of cases which usually presents with one or more of “complete sensation loss, numbness, feeling like walking on cotton wool, muds or pebbles, crawling sensation, tingling or pin sensation, burning sensation, stinging sensation, electric shock-like sensation, shooting sensation, stabbing sensation especially in any of the four limbs, with most cases at the lower limbs” (Miki et al., 2013; Dyck et al., 2011; CDC, National Diabetes Fact Sheet, 2014). It is the main initiating factor for foot ulceration, Charcot neuroarthropathy and lower-extremity amputation (Boulton et al., 2005).
Diabetic painful neuropathy (DNP) is the commonest and most worrisome presentation of diabetic polyneuropathy (Abbott et al., 2011), which is defined by the International Association for the Study of Pain (IASP) as pain arising as a direct consequence of abnormalities in the somatosensory neural system of known diabetic patients. This pain is often diffuse in nature with most presenting with one or more of these characters; burning, electric shocks, shooting, stabbing or pins and needle sensations.
Insulin is a hormone that plays a pivotal role in the maintenance of glucose, lipid and protein homeostasis and controls the cellular nutrient uptake, usage, and storage, as well as a neuroprotective function (Bruce and Hanson, 2010; Grote and Wright, 2016). Insulin resistance (IR) is impairment in the function of insulin target cells such as liver cells, adipocytes and musculoskeletal cells in response to cellular insulin actions (Petersen and Shulman, 2006; Sesti, 2006). Insulin resistance develops over the years, but can be seen in young and old people alike, 23% of people with a BMI >25 kg/m2 appear to be insulin resistant (Pereira et al., 2002). IR has been strongly linked to the cause of type II DM and the severity of its various complications (Zochodne 2014, 2015). Diabetic neuropathy pathophysiology has been traced to neural dysfunctions linked to reduced central and peripheral sensory neuron insulin signaling pathways due to impaired glucose homeostasis, mitochondrial dysfunction, altered neurochemical synthesis and reduced neural maintenance capacity of insulin (Grote and Wright, 2016).
Beta-endorphin is a major central nervous system anti-nociceptive neuropeptide, which helps to modulates pain perception in the brain by acting as a strong agonist in the pain descending inhibitory system (Seo et al., 2011). Our pain behavior “perception” can be described as the balance between the down-top sensory nociceptive sensation and the top-down pain inhibitory system, its imbalance resulting in pain perception mal-behaviors seen in diabetic neuropathy. Many research works have focused more on the down-top sensory nociceptive pathway “looking at the role of various cytokines in diabetic neuropathy” (Muller et al., 2002), while nothing is known about the relationship of beta-endorphin and IR in diabetic neuropathy pathogenesis.
A long-term vision for pain management of DNP patients is not only to relieve pain but most importantly to slow down or possibly stop the progression of other micro-vascular and macro-vascular complications of DM especially DN. However, we hypothesized that beta-endorphin will be associated with pain perception threshold and IR in diabetic neuropathy, while trying to fill the dearth of information in understanding the interplay between beta endorphin, insulin resistance and pain perception especially in the pathogenesis of painful diabetic neuropathy. This study was carried out in the endocrinology “diabetic” clinic of Ekiti State University Teaching Hospital, Ado-Ekiti, Ekiti-State, Nigeria.
2. Material and methods
This study used a multi-stage sampling method, and ethical approval was obtained from the Research and Ethical Review Committee of the Ekiti State University Teaching Hospital, Ado Ekiti, Ekiti State, Nigeria. (Protocol number: EKSUTH/A67/2016/12/005).
2.1. Human subjects
This study recruited 120 volunteers using a multi-stage sampling technique, who were divided into four groups (vanVoorhis and Morgan, 2007) using purposive sampling technique in the first stage. The second stage involves the use of a stratified sampling technique, giving rise to four strata. Stratum one consists of 30 healthy volunteers who were selected in the community as the control group; Stratum two consists of 30 type II diabetes mellitus diagnosed patients without diabetic neuropathy complication, designated DM group; Stratum three consists of 30 diagnosed type II diabetes patients with polyneuropathy with painful sensation (DPN group); Stratum four consists of 30 diagnosed type II diabetes patients with diabetic polyneuropathy without painful sensation (DN group) according to the criteria established by American Diabetes Association (Boulton et al., 2005). All the diagnosed diabetic patients were selected from the diabetic outpatient clinic in Ekiti State University Teaching Hospital within September–December 2018. All subjects signed a written informed consent after the purpose, risks, clinical benefits and results usage of the study were fully discussed with them. The consent form which detailed the protocol, and purpose of the research also contained a statement that the subjects agreed to participate in the research voluntarily.
2.2. Protocol
All subjects had two contacts with the investigators “Tuesdays for recruitment based on the inclusion and exclusion criteria and Fridays for the procedure”. The control group were adults with no history of diabetes in the community, they were all compensated by performing full medical check-ups and basic chemical investigations for them free of charge. The patients that were previously diagnosed with diabetic type II in the diabetic clinic were further divided into three groups (DM, DNP, and DN) by the use of questionnaires and their Vibration Perception Threshold (VPT) as shown in Table 1.
Table 1.
Inclusion criteria for selection of subjects.
| Control Group | DM Group | DPN Group | DN Group | |
|---|---|---|---|---|
| Diagnosed DM | NO | YES | YES | YES |
| History of polyneuropathy | NO | NO | YES | YES |
| Painful sensations | NO | NO | YES | NO |
| Vibration perception threshold 16V | NO | NO | YES | YES |
Key: Diabetic without neuropathy (DM); Diabetic painful neuropathy (DNP); Diabetes neuropathy without pain (DN).
After obtaining informed written consents, participants were trained on the expectations during the study. All subjects underwent the following procedures: history taking, physical examination, blood pressure (BP) measurement, vibration perception threshold (VPT) screening, sub-maximal effort tourniquet test, and biochemical analysis. All procedures were carried out in the morning after an overnight fast. Subjects were excluded if; they were diabetic patients on insulin therapy, on body weight lowering drugs, had a history of another neurological or painful disorder, on-going infection, active smoking, psychiatric illness, and any other co-morbidities or inability to give written consent.
2.3. Vibration perception threshold test
This was determined by using a handheld biothesiometer (Digital Biothesiometer, SN: V117093366, Diabetic Foot Care, India PVT Limited, Tamilnadu, India); with a vibration output range of 0–50 V operating at a frequency of 120Hz. VPT16 was used to screen our subjects accordingly for neuropathy (Anirudhan and Pavithran, 2018). The voltage of vibration was increased until the subject could sense a vibration. The results were recorded on a paper showing the VPT scores of five points on the plantar surface of both feet. The average of the five values “in volts” was recorded for each foot (Armstrong et al., 1998) and the result of the foot with a higher average is recorded.
2.4. Ischemic pain testing model
This test was based on the method described by Plesan et al. (2000). A blood pressure cuff was placed around the non-dominant arm of the subject. With the initial systolic blood pressure known, the cuff pressure was increased to 20 mmHg above the subject's systolic pressure. After the pressure is maintained, subjects were asked to perform a handgrip exercise on an elastic ball. With their eye closed for the entire procedure to minimize distraction and time cues; they were instructed to indicate when they first noticed pain (pain threshold) and when they could no longer tolerate the pain (to a maximum of 5 min). Once pain tolerance was reached, the pressure curve was immediately deflated and when they first felt the pain and end-points were measured in seconds with the process performed 3 times while the average of the readings documented (Plesan et al., 2000).
2.5. Pain threshold assessment
Pain threshold is the point between being “about to be painful” and “just becoming painful” and the time taken for this to occur is recorded in seconds. The process is performed 3 times and the average documented.
2.6. Determination of fasting blood sugar (FBS), glycated hemoglobin (HbA1c), fasting plasma glucose, fasting insulin concentration and serum beta-endorphin concentration
To reduce the effect of circadian rhythm differences, all samples were collected at the same time each procedure day (7.45 am–8.20 am) every Friday. Blood samples (10 ml) were collected at the cubital vein for fasting blood sugar analysis and glycated hemoglobin (HbA1c) done immediately using a digital glucometer (On Call Plus II, ACON Laboratories, Inc., San Diego, USA), digital Clover A1C self-test analyzer (SN: H01C18A0800060; INFOPIA Co., Ltd, Koria. www.infopia21.com) and test cartridge (Diabetes Management Technology, INFOPIA Co.Ltd, Korea. www.infopia21.com). The remaining blood samples were stored in plain and Fluoride oxalate bottles for fasting insulin and fasting plasma glucose analysis respectively. Samples in the plain bottles were allowed to clot and then centrifuged at 3000 rpm for 10 min. Serum obtained was separated into another plain bottle and stored at -70 °C in the refrigerator until the analysis of beta-endorphin, while the fasting insulin and plasma glucose level were analyzed immediately. Fasting plasma glucose was determined by spectrophotometry using the Glucose oxidase-peroxidase method as described by Tietz (1990). The kits were purchased from Randox Laboratories Ltd, BT29 4QY, United Kingdom. While fasting insulin concentration was measured by microplate enzyme immunoassay method with Accu-bind kits purchased from Monobind Inc, Lake Forest, CA 92630, USA. Measurement of beta-endorphin was done using an enzyme immunoassay technique (ELISA) kit (Bioassay Technology Laboratory, Shanghai Korean Biotech Co. Ltd, China) as described by Leng et al. (2008).
2.7. Calculation of insulin resistance (IR), insulin sensitivity (% S) and beta-cell function (% B)
HOMA2-IR, % S and % B data were calculated using a software implementation of the HOMA2 model, HOMA2 calculator V2.2.3, released by the Diabetes Trials Unit, University of Oxford. www.dtu.ox.ac.uk/homa.
2.8. Statistical analysis
All data were expressed as mean ± SEM after Shapiro Wilk test of normality has been performed. Repeated-measures analysis of variance (ANOVA) was used to compare the differences among the four groups, followed by Tukey's multiple comparisons test and Pearson's correlation coefficient r calculation. Statistically significant differences were accepted at p < 0.05. All statistical analysis was performed with Graph Pad Prism (Version 7.01, statistical software Inc).
3. Results
3.1. Demographic features the four groups
Table 2 shows the demographic features of all the groups. There was no difference in the basic body parameters except for the body weight, DNP group has significantly higher body weight compared to the control group (p < 0.0001), while the DN group has significantly higher body weight compared to control and DM groups (p < 0.0001). Also, BMI in DNP and DN is significantly higher compared to control and DM groups (p < 0.0001). Similarly, there was a significant difference in the duration of the illness of DNP and DN compared to the DM group (p < 0.0001; p < 0.01 respectively).
Table 2.
Demographic features of the four groups.
| Control | DM | DPN | DN | R2 | ||
|---|---|---|---|---|---|---|
| Age (yrs) | 59.9 ± 1.93 | 55.6 ± 2.21 | 61.57 ± 2.05 | 58.07 ± 1.45 | ||
| Sex (% Female) | 67 | 63 | 70 | 67 | ||
| Height (m) | 1.64 ± 0.017 | 1.62 ± 0.017 | 1.6 ± 0.018 | 1.62 ± 0.011 | 0.0367 | |
| Weight (kg) | 66.3 ± 2.07 | 71.80 ± 2.07 | 78.7 ± 2.21aaa | 84.5 ± 2.1aaa,bbbb | 0.2671 | |
| BMI (kg/m2) | 24.94 ± 0.948 | 27.34 ± 0.767 | 31.27 ± 0.917aaa,b | 32.28 ± 0.91aaa,bbb | 0.276 | |
| Diabetes duration (months) | 0.0 | 41.03 ± 4.46 | 53.97 ± 4.97 | 85.13 ± 6.06bbb,ccc | 0.6125 | |
Data expressed are means ± SEM with n = 30 after Shapiro Wilk normality test has been performed. Data were analyzed by one-way ANOVA followed by Tukey's multiple post hoc test. a,b,c p < 0.05 vs Control, DM and DNP group respectively. Key: Diabetic without neuropathy (DM); Diabetic painful neuropathy (DNP); Diabetes neuropathy without pain (DN); Eta squared (R2); Body mass index (BMI).
3.2. Effect of diabetes mellitus type 2 and its complications on body physiologic parameters
As shown in Table 3, there was a significantly higher systolic and diastolic blood pressure in the DPN group compared to the control (∗p < 0.0001). Also, there was a significant difference in systolic blood pressure alone in the DPN group when compared with the DM group (∗p < 0.001), while the DN group only shows a significant difference in diastolic BP compared to the control group (∗p < 0.001).
Table 3.
Effect of diabetes mellitus type 2 and its complications on body physiologic parameters.
| Control | DM | DPN | DN | R2 | |
|---|---|---|---|---|---|
| Systolic BP (mmHg) | 119.3 ± 3.74 | 121.1 ± 3.22 | 138.7 ± 3.27aaa,bb | 131.3 ± 2.85 | 0.1646 |
| Diastolic BP (mmHg) | 74.7 ± 1.93 | 78.06 ± 1.94 | 84.13 ± 2.03aa | 84.59 ± 1.67aa | 0.1339 |
| HbA1C (%) | 4.48 ± 0.078 | 6.87 ± 0.435aaa | 8.87 ± 0.439aaa,bb | 9.45 ± 0.502aaa,bbb | 0.4496 |
| Fasting plasma glucose (mmol/l) | 4.62 ± 0.11 | 8.13 ± 0.37aaa | 10.27 ± 0.46aaa,bb | 11.64 ± 0.59aaa,bbb | 0.5778 |
| Fasting Insulin (pmol/l) | 31.76 ± 1.93 | 111.2 ± 6.52aaa | 141.6 ± 10.44aaa,b | 104 ± 7.9aaa,cc | 0.5074 |
| Vibration perception threshold (Volt)< | 9.31 ± 1.541 | 14.36 ± 1.541a | 32.25 ± 1.51aaa,bbb | 41.75 ± 1.34aaa,bbb,ccc | 0.7802 |
Data expressed are means ± SEM, n = 30 after Shapiro Wilk normality test has been performed. Data were analyzed by two-way ANOVA followed by Tukey's multiple post hoc test. a,b,c p < 0.05 vs control, DM group and DNP group respectively. Key: Diabetic without neuropathy (DM); Diabetic painful neuropathy (DNP); Diabetic neuropathy without pain (DN); Eta squared (R2); Blood Pressure (BP).
There was a significant reduction in the level of HbA1C in the control group compared to other groups (∗p < 0.0001) while there was a significant increase in the DPN and DN groups compared to the DM group (∗p < 0.01; ∗p < 0.0001 respectively).
Besides, there was a significant increase in the fasting plasma glucose in the DM, DPN and DN groups compared to the control group (∗p < 0.0001) which furthers suggested the diabetic status of these patients according to the WHO definition of diabetes. Our results also showed that fasting plasma glucose was significantly elevated in the DPN and DN (26 % and 44 % respectively) groups compared to DM group.
There was a significant increase in the fasting insulin level in the DM, DPN and DN groups compared to the control group (∗p < 0.0001). However, there was a significant elevation in the fasting insulin level in the DPN group compared to the DM and DN groups (∗p < 0.01; ∗p < 0.001 respectively).
Furthermore, there was a significant increase in the vibration perception threshold (VPT) in the DPN group compared to the control and DM group (∗p < 0.0001) while there was an increase in the VPT in the DN group compared to control, DM and DPN groups (∗p < 0.0001; ∗p < 0.001; ∗p < 0.0001 respectively).
3.3. Effect of type II diabetes mellitus and neuropathic complication on pain threshold
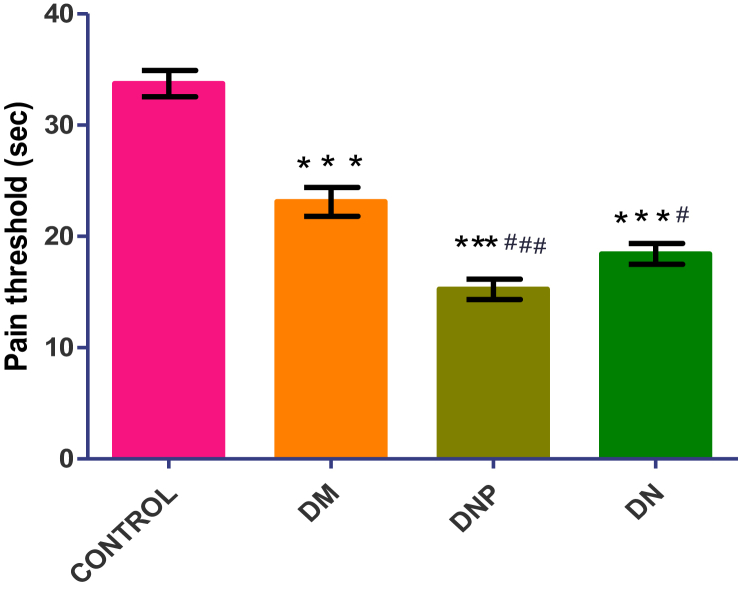
The pain threshold among the four groups is shown in Figure 1. There is a significant high pain threshold in the control group (33.73 ± 1.19 s) compared to the other groups (∗p < 0.0001) and similarly, the pain threshold of the DM group (23.1 ± 1.29 s) is significantly high compared to the DNP group (15.24 ± 0.91 s) (∗p < 0.001) and DN group (18.4 ± 0.94 s) (∗p < 0.01). All the subjects started and completed the procedure without any complications or early terminations.
Figure 1.
Shows the comparison of pain threshold of the participants across the four groups. Values are expressed as mean ± SEM with n = 30 after Shapiro Wilk normality test has been performed. Data were analyzed by one-way ANOVA followed by Tukey's multiple post hoc test. ∗p < 0.05 vs. control; #p < 0.05 vs. DM. Key: Diabetic without neuropathy (DM); Diabetic painful neuropathy (DNP); Diabetes neuropathy without pain (DN); Eta squared (R2); Body mass index (BMI). (R2 = 0.5846).
3.4. Serum beta-endorphin concentration in diabetic patients with or without neuropathy
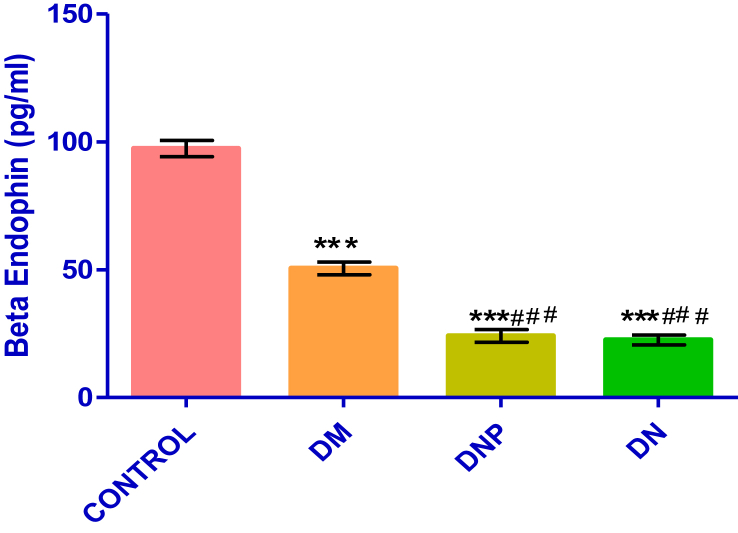
Figure 2 shows the serum concentration of beta-endorphin among the four groups.
Figure 2.
Shows the comparison of beta endorphin concentration across all the four groups. Values are expressed as mean ± SEM with n = 30 after Shapiro Wilk normality test has been performed.
There is a significantly high serum level of beta-endorphin in the control group (97.34 ± 3.12 pg/dl) compared to the other groups (∗p < 0.0001) while the DM group (50.5 ± 2.49 pg/dl) showed a similar trend when compared to the DNP group (24.11 ± 2.45 pg/dl) (∗p < 0.001) and DN group (22.5 ± 1.91 pg/dl) (∗p < 0.001).
Data were analyzed by one-way ANOVA followed by Tukey's multiple post hoc test ∗p < 0.05 vs. control; #p < 0.05 vs. DM. Key: Diabetic without neuropathy (DM); Diabetic painful neuropathy (DNP); Diabetic neuropathy without pain (DN); Eta squared (R2); (R2 = 0.831).
3.5. HOMA-2 result of all the four groups
The result of Table 4 shows a significant difference in the % beta cell (% B), % insulin sensitivity and insulin resistance (IR) the control group compared to DM, DPN, DN groups (∗p < 0.03; ∗p < 0.0001; ∗p < 0.0001 respectively). There was a significant difference in the % B of DN group compared to the DM and DNP groups (∗p < 0.0001; ∗p < 0.0001 respectively). Similarly, there was a significant difference in DPN and DN values compared to the DM group (∗p < 0.001; ∗p < 0.0001 respectively).
Table 4.
HOMA-2 parameters of diabetic patients with or without neuropathy, using fasting plasma glucose (mmol/L) and fasting insulin (uIU/mL) values.
| Control | DM | DNP | DN | R2 | |
|---|---|---|---|---|---|
| %Beta Cell | 86.8 ± 4.55 | 68.98 ± 4.419a | 61.51 ± 3.658aaa | 34.53 ± 3.031aaa,bbb,ccc | 0.437 |
| %Insulin sensitivity | 186.8 ± 9.15 | 48.54 ± 3.42aaa | 32.46 ± 2.37aaa | 31.67 ± 2.26aaa | 0.8458 |
| IR | 0.577 ± 0.03 | 2.33 ± 0.146aaa | 3.667 ± 0.306aaa,bb | 3.807 ± 0.358 aaa,bbb | 0.5679 |
Data expressed are means ± SEM, n = 30. Shapiro Wilk normality test has been performed.
Data were analyzed by two-way ANOVA followed by Tukey's multiple post hoc test. a,b,c p < 0.05 vs control, DM and DNP groups respectively. Key: Diabetic without neuropathy (DM); Diabetic painful neuropathy (DNP); Diabetic neuropathy without pain (DN); Insulin resistance (IR); Eta squared (R2).
3.6. Correlations between HbA1c, pain threshold, insulin resistance and percentage beta cells among the four groups
The results of the correlations between the HbA1c, PT, IR, and % B are presented in Table 5. There is a strong negative significant correlation between HbA1c and PT across the groups. Only the control group showed a strong correlation between beta-endorphin and IR (<0.0001∗), the DM showed a weak correlation, while DNP and DN groups had no significant relationships. Similar results were seen in the correlation of PT vs IR except in the DN group which shows no significant relationship (p = 0.16). A significant positive relationship was observed between PT and % B across the groups except in the DN group where there was a very weak relationship (p = 0.47).
Table 5.
Correlations between HbA1c, pain threshold, insulin resistance and percentage Beta cells among the four groups.
| Control (r/p) | DM (r/p) | DNP (r/p) | DN (r/p) | |
|---|---|---|---|---|
| HbA1c Vs PT | -0.42/0.021∗ | -0.69/<0.0001∗ | -0.64/0.0001∗ | -0.44/0.015∗ |
| B.Endo Vs IR | -0.68/<0.0001∗ | -0.46/0.0103∗ | -0.25/0.182 | -0.28/0.1275 |
| IR Vs PT | -0.47/0.0086∗ | -0.60/0.0004∗ | -0.56/0.0014∗ | -0.26/0.16 |
| %B Vs PT | 0.70/<0.0001∗ | 0.60/0.0004∗ | 0.401/0.028∗ | 0.14/0.478 |
Data expressed are r and p, n = 30 per group. Asterisk indicates significant correlation.
Data were analyzed by Pearson correlation test with confident interval set at 95%, ∗p < 0.05. Key: Diabetic without neuropathy (DM); Diabetic painful neuropathy (DNP); Diabetic neuropathy without pain (DN); Insulin resistance (IR); Pain threshold (PT); glycated haemoglobin concentration (HbA1c); Beta endorphin (B.Endo); Percentage of functioning beta cells (%B); Pearson correlation coefficient (r); Probability (p).
3.7. Scatter diagram showing the relationship between beta-endorphin and pain perception
The results of the correlations between the serum Beta-endorphin level and correlated PT is presented in Figure 3. There is a positive significant correlation between all the groups with the DN group showing the weakest relationship (p < 0.087) and the control group having the strongest relationship (∗p < 0.0001).
Figure 3.
Scatterplots showing the correlation between the serum beta endorphin concentration and pain threshold. Data were analyzed by Pearson correlation test with confident interval set at 95%, ∗p < 0.05. a) Control group. ∗p < 0.0001and r = 0.7261; b) DM group. ∗p < 0.0001 and r = 0.7121; c) DNP group. ∗p < 0.0001 and r = 0.7696; d) DN group. p < 0.087 and r = 0.3178.
4. Discussion
The impact of diabetic painful neuropathy is more in underdeveloped and developing countries, where most patients report very late to the hospital due to negligence, high level of poverty and illiteracy among the population. Despite controversies on the role of insulin resistance in the pathogenesis and management of DPN, insulin resistance has been reported to play a pivot role in DM type II pathogenesis (Shulman, 2000). In this present study, we considered the correlation between pain perception, beta-endorphin, and insulin resistance in diabetic neuropathy.
In this study, the HbA1c values “which is a better reflection of the body glycemic status of a diabetic patient over 2–3 months compared to the fasting blood sugar or 2 h postprandial plasma glucose” (Nathan et al., 2009) is higher in DM, DNP and DN groups compared to the control group. This confirms their relative hyperglycemic state which is the common pathway to the cause of DM and its complications (Lincoln, 2008). Also, the significantly higher weight gain in DPN and DN which translated to the significant difference seen in BMI in DPN and DN. These observations have been linked to the role of IR as a potent cause of obesity and DM type II as well as its various complications (Odegaard and Chawla, 2013).
Also, in this study, the observable decrease in pain threshold in DM, DPN and DN groups compared to the control translates to higher pain perception in these groups with the highest sensitivity noted in the DPN group. This pain sensation difference can be traced to the relative chronic inflammatory response state noted in these patients (Gutiérrez-Rodelo et al., 2017; Akintoye et al., 2018). Although, according to Veves et al. (1994) the painful symptom of diabetes polyneuropathy could present at any stage of the disease, from early to the late stage. However, Boulton et al. (2004), reported that improvement in the painful symptoms of DPN is associated with worsened sensory loss, suggesting a disease progressive phase, in which our finding further supports as the DN group has the longest duration of disease in this study. The peculiar characteristics “hyperalgesia and allodynia” associated with pain is often the main reason why many of these patients seek medical care (Ziegler et al., 2009). The decrease in pain threshold can ultimately result in anxiety, sleep disorder and other psychosocial impairments especially in DPN patients (Vinik et al., 2011), while the loss of pain sensation noticed in the DN group usually predispose them to diabetic foots ulcer and amputations (Boulton et al., 2005) which is associated with worse psychosocial impairments status.
The observed overweight, with relative hyperglycemia and hyperinsulinemia in DPN and DN patients in our study, lead to a cascade of independent/dependent (Grote and Wright, 2016) events that can result in a neuronal dysfunctional state seen in these patients. Previous studies have proved that insulin receptors are present on nociceptive neurons (Shettar and Muttagi, 2012) and Schwann cell (Eckersley, 2002; Song et al., 2003) where they promote axonal growth and neuronal survival (Huang et al., 2005) through the activation of the phosphatidylinositol-3-kinase (P13k-Akt) pathway which serves to inhibit both B-cell lymphoma 2 associated death promoter (BAD) and caspase 9 (Datta et al., 1997), thus, impaired insulin homeostasis has been implicated in diabetic polyneuropathy (Eckersley, 2002; Song et al., 2003).
Beta-endorphin, an important anti-nociceptive neuropeptide (Millan, 2002) shows a significant 52% (DPN) and 55% (DN) reduction compared to the DM group. Our report is not similar to that of Enas et al. (2017) who reported no significant difference in the serum level of β endorphin in patients with painful diabetic neuropathy, non-painful diabetic neuropathy, and the control groups. But is similar to the report of Çakir et al. (2000), who also reported no significant difference in serum β endorphin level in painful and painless diabetic neuropathy patients, but both groups were significantly lower compared to the control group. Both previous works do not account for the DM group, which our work did to separated diabetes neuropathic patients from non-neuropathic patients. However, our result showed no significant correlation between serum β endorphin and IR in diabetic neuropathy. This study then suggests that the pain perception loss in the DN group is likely due to neuronal dysfunction rather than being due to the activities of the normal endogenous opioid peptide system.
Insulin resistance results from alterations of insulin receptors and its catalytic effects associated with reduced insulin receptor substrates IRS “most especially IRS 1 an IRS 2”, increased Ser/Thr phosphorylation state, increased Tyr Phosphatase activity which dephosphorylates IRS, reduced P13K/Akt kinase activity and defects in GLUT-4 activity (Olivares-Reyes and Arellano-Plancarte, 2008). The accumulated effect of increased pro-inflammation cytokines, saturated fatty acids, and the chronic hyperinsulinemia state noticed in diabetic patients are responsible for the above molecular alterations (Olivares-Reyes, 2012). To support this hypothesis, our results show the highest IR value in the DN compared to the control, DM and DNP groups. This suggests a worse prognosis in DN patients. Likewise, our results showed that the DN patients have the poorest correlation between their serum β endorphin and pain perception. The above findings in the DN group may be due to reduced pancreatic β cell secretion, prolonged duration of this pathology and/or poor glycaemic control (Nisar et al., 2015) in these patients usually linked to late diagnosis in this part of the world (Adeloye et al., 2017).
Limitations: The study was conducted in a low resource setting therefore it lacked access to equipment that could have given better insights to the biochemical changes in each of the groups. The subjective pain perception/threshold used in this study could be complemented with molecular imaging such as positron emission tomography to better assess the opioid system and prognosticate the depletion of the endogenous opiate system. In addition, the study did not explain the possible molecular pathway responsible for the relationship between insulin resistance and beta-endorphin therefore, further studies could explore the phosphatidyl inositol-3-kinase (PI3K)/protein kinase B (AKT) signaling pathway which is known to be involved in neuronal survival and axonal growth.
5. Conclusion
The present study demonstrates that diabetic neuropathy patients have a poor endogenous opioid peptide system which is associated with increased pain perception and insulin resistance. However, insulin resistance alone does not explain the chronic pain perception noticed in the DPN patients. Thus, further study is required.
More awareness and advocacy for diabetes routine check and adequate foot care especially in the DN group to prevent various diabetic foot complications are needed in this part of the world.
Declarations
Author contribution statement
Olabode O. Akintoye, Taiwo H. Raimi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Bamidele V. Owoyele, Adesola A. Oniyide: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Oyensanmi A. Fabunmi: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Abimbola O. Akintoye, David D. Ajayi: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Ayodeji J. Ajibare: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Gbenga S. Adeleye: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Tertiary Education Trust Fund (TETFund), accessed through Institution Based Research (IBR) Funds, Ekiti State University, Ado Ekiti, Nigeria.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We appreciate the personnel of the Diabetic Clinic, Department of Internal Medicine, Ekiti State University Teaching Hospital, for their assistance in the course of this study.
References
- Abbott C.A., Malik R.A., van Ross E.R. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the UK. Diabetes Care. 2011;34:2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeloye D. Estimating the prevalence, hospitalisation and mortality from type 2 diabetes mellitus in Nigeria: a systematic review and meta-analysis. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-015424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akintoye O.O., Oniyide A.A., Owoyele B.V. A study of pain threshold, interleukins and NLR in diabetic poly neuropathy in a selected Nigerian population: Niger. J. Physiol. Sci. 2018;33(2):151–157. [Pubmed] [PubMed] [Google Scholar]
- Anirudhan A.M., Pavithran S. Vibration perception threshold values and clinical symptoms of diabetic peripheral neuropathy. J. Clin. Diagn. Res. 2018;12(5):LC20–LC23. 2018 May. [Google Scholar]
- Armstrong D.G. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch. Intern. Med. 1998;158:289–292. doi: 10.1001/archinte.158.3.289. [DOI] [PubMed] [Google Scholar]
- Boulton A.J.M., Malik R.A., Arezzo J.C. Diabetic somatic neuropathies. Diabetes Care. 2004;27(6):1458–1486. doi: 10.2337/diacare.27.6.1458. indexed in Pubmed: 15161806. [DOI] [PubMed] [Google Scholar]
- Boulton A.J., Vinik A.I., Arezzo J.C. Diabetic neuropathies: a statement by the American diabetes association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- Bruce K.D., Hanson M.A. The developmental origins, mechanisms, and implications of metabolic syndrome. J. Nutr. 2010;140:648–652. doi: 10.3945/jn.109.111179. [DOI] [PubMed] [Google Scholar]
- Çakir N., Yetkin I., Karakoç A. l-Carnitine in the treatment of painful diabetic neuropathy and its effect on plasma b-endorphin levels. Curr. Ther. Res. 2000;61(12):871–876. [Google Scholar]
- CDC, National Diabetes Fact Sheet . Centers for Disease Control and Prevention, 2011. US Department of Health and Human Services (Atlanta, GA: CDC, National Diabetes Fact Sheet); 2014. National estimates and general information on diabetes and prediabetes in the United States (2011) pp. 1–12. [Google Scholar]
- Datta S.R. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Dyck P.J., Albers J.W., Andersen H. Toronto Expert panel on Diabetic Neuropathy. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metabol. Res. Rev. 2011;27(7):620–628. doi: 10.1002/dmrr.1226. [DOI] [PubMed] [Google Scholar]
- Eckersley L. Role of the Schwann cell in diabetic neuropathy. Int. Rev. Neurobiol. 2002;50:293–321. doi: 10.1016/s0074-7742(02)50081-7. [DOI] [PubMed] [Google Scholar]
- Enas T.E. Beta-endorphin levels in both painful and painless diabetic peripheral neuropathy. Clin. Diabetol. 2017;6(5):159–171. [Google Scholar]
- Grote C.W., Wright D.E. A role for insulin in diabetic neuropathy. Front. Neurosci. 2016;10:581. doi: 10.3389/fnins.2016.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Rodelo C. Molecular mechanisms of insulin resistance. Gac. Med. Mex. 2017;153:197–209. [PubMed] [Google Scholar]
- Huang T.J., Verkhratsky A., Fernyhough P. Insulin enhances mitochondrial inner membrane potential and increases ATP levels through phosphoinositide3-kinase in adult sensory neurons. Mol. Cell. Neurosci. 2005;28:42–54. doi: 10.1016/j.mcn.2004.08.009. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation . eighth ed. 2017. IDF Diabetes Atlas.http://www.diabetesatlas.org Brussels. [Google Scholar]
- Leng S.X. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J. Gerontol. Series A: Biol. Sci. Med. Sci. 2008;63(8):879–884. doi: 10.1093/gerona/63.8.879. PMC 2562869. PMID 18772478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln N.B. Education for secondary prevention of foot ulcers in people with diabetes: a randomized controlled trial. Diabetologia. 2008;51:1954–1961. doi: 10.1007/s00125-008-1110-0. [DOI] [PubMed] [Google Scholar]
- Miki T. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail. Rev. 2013;18(2):149–166. doi: 10.1007/s10741-012-9313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M.J. Descending control of pain. Prog. Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [PubMed: 12034378] [DOI] [PubMed] [Google Scholar]
- Muller S., Matins S., Koenig W., Moghaddam H., Rathmann W., Haastert B. Impaired glucose tolerance is associated with increased serum concentrations of Interleukin 6 and coregulated acute-phase proteins but not TNF-α or its receptors. Diabetologia. 2002;45:805–812. doi: 10.1007/s00125-002-0829-2. [DOI] [PubMed] [Google Scholar]
- Nathan D.M. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisar M. Association of diabetic neuropathy with duration of type 2. Diabetes Glycemic Contr. 2015;7(8) doi: 10.7759/cureus.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard J.I., Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339(6116):172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Reyes J.A., Arellano-Plancarte A. Molecular basis of insulin actions. Rev. Edu. Bio. 2008;27:9–18. [Google Scholar]
- Olivares-Reyes J.A. Molecular basis of metabolic syndrome and insulin resistance. In: Garibay Nieto G.N., García Velasco S., editors. Obesity in Paediatric Age: Prevention and Treatment. Mexico City. 2012. pp. 185–214. [Google Scholar]
- Pereira M.A. Dairy consumption, obesity and the insulin resistance syndrome in young adults: the CARDIA Study. J. Am. Med. Assoc. 2002;287(16):208 1–9. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- Petersen K.F., Shulman G.I. Etiology of insulin resistance. Am. J. Med. 2006;119(5 Suppl 1):S10–S16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesan A. The Nmethyl-D-aspartate-receptor antagonist dextromethorphan lacks analgesic effect in a human experimental ischemic pain model. Acta Anaesthesiol. Scand. 2000;44:924–928. doi: 10.1034/j.1399-6576.2000.440805.x. [DOI] [PubMed] [Google Scholar]
- Seo Y.J., Kwon M.S., Choi S.M. Different cross-tolerance development between single and repeated immobilization stress on the antinociceptive effect induced by β-endorphin, 5-hydroxytryptamine, morphine, and WINN55,212-2, in the inflammatory mouse pain mode. Arch Pharm. Res. (Seoul) 2011;34(2):269–280. doi: 10.1007/s12272-011-0213-1. [DOI] [PubMed] [Google Scholar]
- Sesti G. Pathophysiology of insulin resistance. Best Pract. Res. Clin. Endocrinol. Metab. 2006;20(4):665–679. doi: 10.1016/j.beem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Shettar A., Muttagi G. Developmental regulation of insulin receptor gene in sciatic nerves and role of insulin on glycoprotein P0 in the Schwann cells. Peptides. 2012;36:46–53. doi: 10.1016/j.peptides.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Shulman G.I. Cellular mechanisms of insulin resistance. J. Clin. Invest. 2000;106(2):171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z. Transgenic mice overexpressing aldose reductase in Schwann cells show more severe nerve conduction velocity deficit and oxidative stress under hyperglycemic stress. Mol. Cell. Neurosci. 2003;23:638–647. doi: 10.1016/s1044-7431(03)00096-4. [DOI] [PubMed] [Google Scholar]
- Swieboda P. Assessment of pain; types, mechanism and treatment. Ann. Agric. Environ. Med. Special Issue. 2013;1:2–7. [PubMed] [Google Scholar]
- Tietz N.W. second ed. WB Saunders Co; Philadelphia, Pa: 1990. Clinical Guide to Laboratory Tests; pp. 246–250. [Google Scholar]
- Uloko A.E. Prevalence and risk factors of diabetes mellitus in Nigeria: a systemic review and meta-analysis. Diabetes Ther. 2018 doi: 10.1007/s13300-018-0441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veves A., Young M.J., Manes C. Differences in peripheral and autonomic nerve function measurements in painful and painless neuropathy: a clinical study. Diabetes Care. 1994;17(10):1200–1202. doi: 10.2337/diacare.17.10.1200. [DOI] [PubMed] [Google Scholar]
- Vinik E., Silva M.P., Vinik A.I. Measuring the relationship of quality of life and health status, including tumor burden, symptoms, and biochemical measures in patients with neuroendocrine tumors. Endocrinol Metab. Clin. N. Am. 2011;40:97–109. doi: 10.1016/j.ecl.2010.12.008. [DOI] [PubMed] [Google Scholar]
- vanVoorhis C.R.W., Morgan B.L. Understanding power and rules of thumb for determining sample sizes. Tutor. Quant. Methods Psychol. 2007;3(2):43–50. [Google Scholar]
- Ziegler D. KORA Study Group. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med. 2009;10:393–400. doi: 10.1111/j.1526-4637.2008.00555.x. [DOI] [PubMed] [Google Scholar]
- Zochodne D.W. Mechanisms of diabetic neurondamage: molecular pathways. Handb. Clin. Neurol. 2014;126:379–399. doi: 10.1016/B978-0-444-53480-4.00028-X. [DOI] [PubMed] [Google Scholar]
- Zochodne D.W. Diabetes and the plasticity of sensory neurons. Neurosci. Lett. 2015;596:60–65. doi: 10.1016/j.neulet.2014.11.017. [DOI] [PubMed] [Google Scholar]