Abstract
Cassia alata or locally known as Ketepeng Cina (Indonesia) and Gelenggang (Malaysia) has been used as a traditional medicine to treat various diseases, especially skin diseases. In addition, C. alata has been reported to have potential anti allergic, anti inflammatory, antioxidant, anticancer, antidiabetic, and antifungal. Metabolite compounds that have been isolated from C. alata include flavones, flavonols, flavonoids glycosides, alatinon, alanonal and β-sitosterol-β-D-glucoside. The compounds have been isolated mainly from the leaves. Further identification is needed to discover the secondary metabolites from other parts of the plant such as seed, flower and bark which are reported to have potent antibacterial and antifungal activity. Therefore, this article highlights the secondary metabolites and biological activity of this plant which has been shown to have pharmacological properties against selected diseases.
Keywords: Cassia alata, Phytochemical, Pharmacological activity, Disease, Natural product chemistry, Bioorganic chemistry, Pharmaceutical chemistry, Alternative medicine, Evidence-based medicine
Cassia alata; Phytochemical; Pharmacological activity; Disease; Natural product chemistry; Bioorganic chemistry; Pharmaceutical chemistry; Alternative medicine; Evidence-based medicine.
1. Introduction
Cassia alata is a plant originating from Argentina [1]. Commonly referred to as Candle brush, Candlestick, Senna alata and others [2]. In Indonesia C. alata is called as “ketepeng china”. In other part of South Asia, C. alata has become a herbs plant to treat various diseases in many countries including France [3]. C. alata root can be used to treat rheumatism and laxative [4]. Seeds and leaves have high potency as fungicides and medicine for eczema in India [5]. C. alata can be used to reduce stomach pain during pregnancy, headaches and paralysis. C. alata extracts are used in the practice of traditional herbs medicine to cure skin diseases in some countries [6]. In Thailand, C. alata leaves are used to treat constipation. This can be done with fresh leaves pounded with water, garlic, red chalk and balm and then applied to skin infected with ringworm. Besides, the boiled shoots and leaves of C. alata can be used to clean the wound and act as anti-inflammatory agent [7]. In Indonesia (especially in South Sulawesi), leaves C. alata has been used traditionally to get rid of fungus on the skin which can cause hives and others by grinding or rubbing directly on the affected skin.
Several studies have been reported the biological activity of C. alata. Crude extract of C. alata leaf has very strong antioxidant activity with IC50 value of 2.27 μg/mL [8]. n-hexane leaf extract of C. alata showed strong anti-inflammatory potential by significantly reducing rat knee swelling (CFA) [9]. C. alata leaf extract was reported that possessed good antifungal activity against Trichophyton verrucosum and Epidermophyton floccosum as well as other microbes [10]. Secondary metabolite compounds in C. alata include alkaloids, saponins, steroids, flavonoids and terpenoids [11].
2. Botany
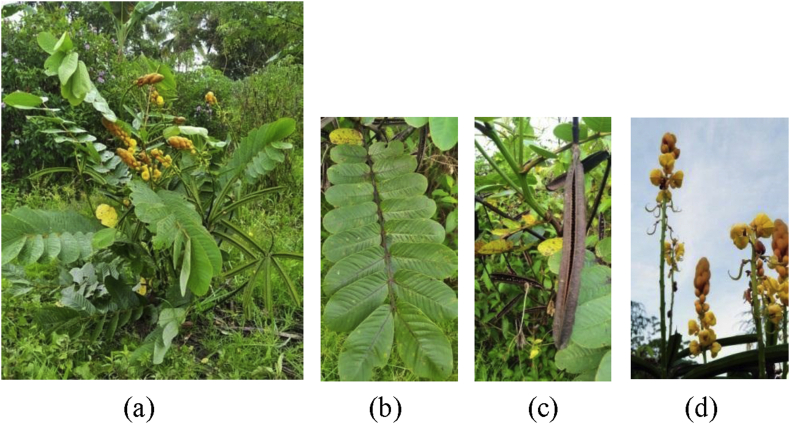
C. alata is a plant that can grow freely in the tropics. C. alata comes from the family of Fabaceae [2]. This plant has characteristic of unpleasant odor, stems erect (about 10–15 feet tall), skin of thin stems not spiked, leaves yellowish green and slightly wide. The flowers are bright yellow and form a race. The fruit is hard to resemble a brown pod when ripe and has brown seeds [1] (see Figure. 1).
Figure 1.
(a) All part of Cassia alata, (b) leaves, (c) fruit, (d) flower.
3. Isolation of compounds
3.1. Leaf
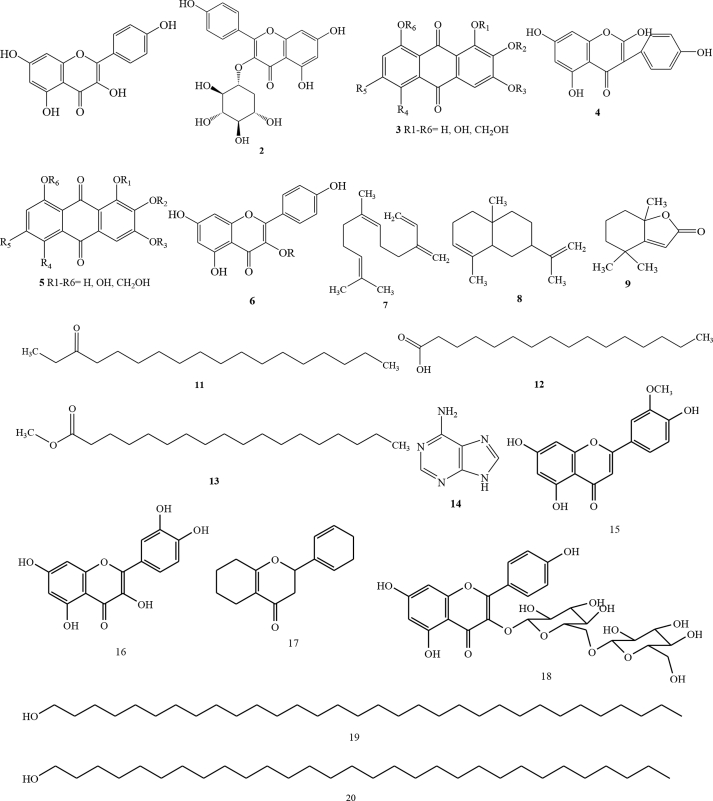
C. alata has been reported to have diverse bioactive compounds in the leaves [12, 13, 14]. Chemical constituents of C. alata leaves have been reported from Pattalung Province, Thailand [15]. The leaves were dried and macerated for three days with methanol solvent to obtain methanol extract. The methanol extract was further fractionated using a liquid vacuum gel chromatography method and eluted using chloroform:methanol to obtain 6 fractions. The fractions were separated using column chromatography of LH-20 sephadex and eluted with methanol solvent to obtain 8 fractions. Fraction VII (yellow in colour) showed strong antioxidant activity. The structure identification was performed using IR, 1H NMR dan 13C NMR. The identification results indicated as a type of flavonol, namely 1 (kaempferol) [15]. In addition, 2 (kaempferol-3-O-β-D-glucopyranoside) has also been identified using HPLC [8].
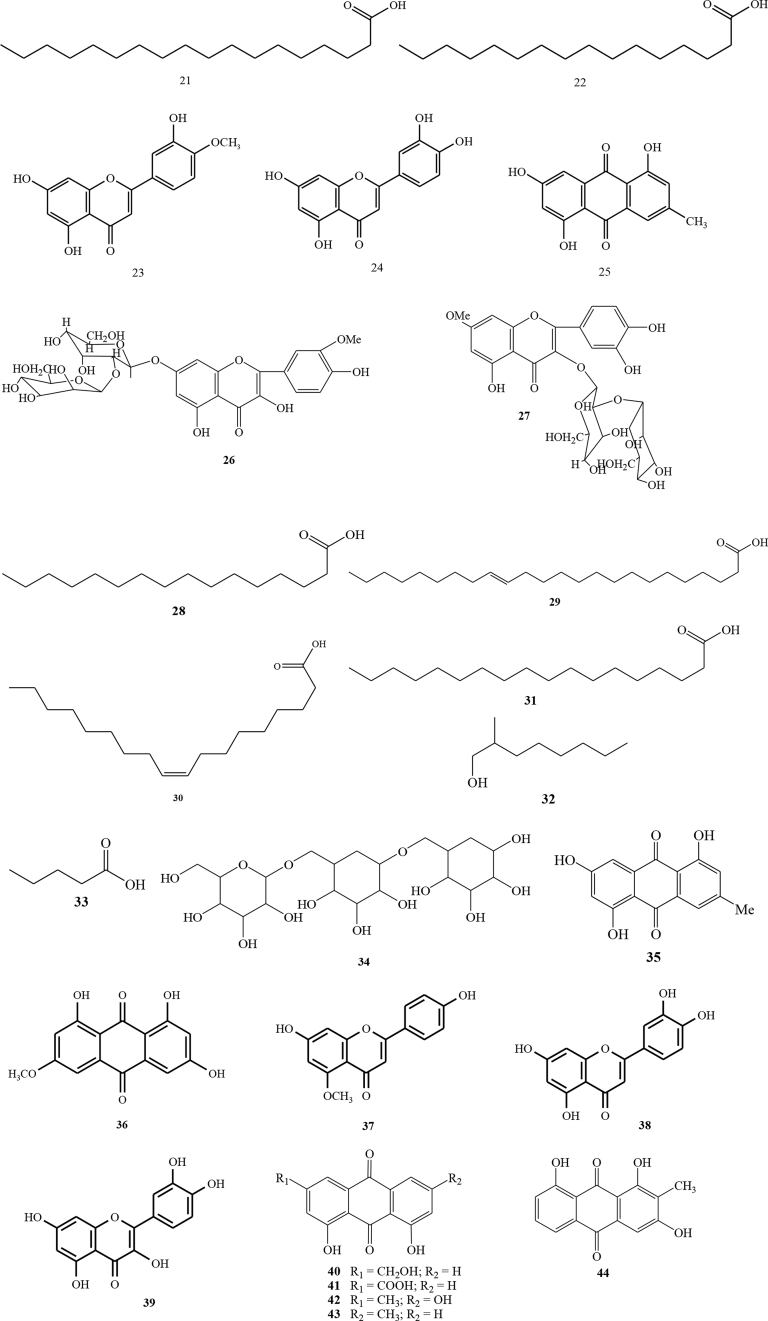
In addition to flavonols, flavone compounds have been isolated from C. alata leaf ethanol extracts in the BCSIR area at Rajshahi Campus [16, 17]. Compound 3 was a flavone compound with the name of 3,5,7,4′-tetrahydroxy flavone and 4 was 2,5,7,4′-tetrahydroxy isoflavones. The compound was obtained from an ethyl acetate fraction eluted with n-hexane and ethyl acetate that enhanced by its polarity. The resulting fraction was still have impurities, and therefore proceeded by purification with PTLC of 60G254 silica gel and eluted with the same eluent. The results showed two bright yellow bands with different Rf values [16, 17]. Other researchers also reported other types of flavonoid compounds, namely 5 (anthraquinone) and 6 (kaempferol 3-O-gentiobioside) [18]. GC/MS characterization results on C. alata leaves showed that there were 7 compounds including 7 ((6Z)-7,11-dimethyl-3-methylidenedodeca-1,6,10-triene), 8 (4a,8-dimethyl-2-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,8a-octahydronaphthalene), 9 (4,4,7a-trimethyl-5,6,7,7a-tetrahydro-1-benzofuran-2(4H)-one), 10 (3,7-dimethylocta-1,6-diene), 11 (hexadecanoic acid methyl ester), 12 (hexadecanoic acid), and 13 (octadecanoic acid methyl ester) [19]. Alkaloid compounds from C. alata leaves have also been identified, namely 14 (adenine) [20], 15 (Chrysoeriol), 16 (quercetin), 17 (5,7,4′-trihydroflavanone), 18 (kaempferol-3-O-beta-D-glucopyranosyl-(1→6)-beta-D-glucopyranoside), 19 (n-dotriacontanol), 20 (n-triacontanol), 21 (stearic acid), 22 palmitic acid [21], 23 diomestin [22], 24 (luteolin) [23], and 25 (1,3,5-trihydroxy-7-methylanthracene-9,10-dione) [24].
3.2. Seed
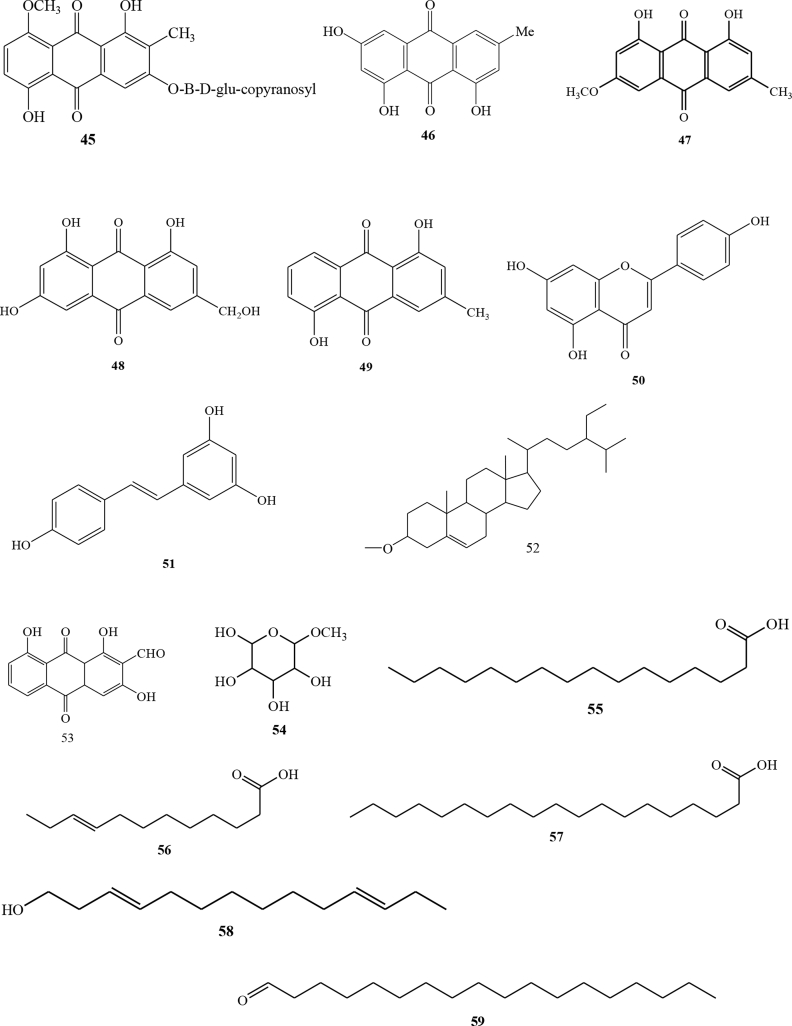
C. alata seeds were reported to have many bioactive compounds [25]. The grain of C. alata was mashed and extracted by maceration method using ethanol solvent. The obtained extract was analyzed using TLC. Fractionation was performed by using Flash Column Chromatography eluted with a solvent enhanced by its polarity resulting in two types of flavonoid glycoside compounds ie 26 chrysoeriol-7-O-(2″-O-β-D-mannopyranosyl)-β-D-allopyranoside and 27 rhamnetin-3-O-(2″-O-β-D-mannopyranosyl)-β-D-allopyranoside [26]. Chemical compounds of C. alata seeds that analyzed by GC-MS were 28 (n-hexadecanoic acid), 29 (15-tetracosenoic acid), 30 (oleic acid), 31 (octadecanoic acid), 32 (2-methyl-1-octanol, pentanoic acid), and 33 (2-ethyl-1-decanol) [27]. In addition, 34 α-D-galactopyranosyl has also been identified [28].
3.3. Stem
The chopped C. alata stem were extracted with benzene and hot ethanol. The two extracts were combined and then mixed with silica gel. The fractionation was performed using column chromatography (60–120 mesh) to obtain the compound 35 (1,5,7-trihydroxy-3-methyl-anthraquinone) with orange crystalline [29].
3.4. Twig
The chemical constituent of C. alata twigs has been reported. The characterization used 1D and 2D NMR spectra were performed on a Bruker AVANCE 300 or a Bruker FTNMR Ultra Shield 400 MHz. The isolated compounds were 36 (lunatin), 37 (7,4′-dihydroxy-5-methoxyflavone), 38 (luteolin), and 39 (trans-dihydrokaempferol) [22].
3.5. Root
Some anthraquinone compounds have been identified using high-performance liquid chromatographic method with photodiode arrays. The types of anthraquinone compounds were 40 (aloe-emodin), 41 (rhein), 42 (emodin) and 43 (chrysophanol) [30]. Others anthraquinone that have also been isolated were 44 (1,3,8-trihydroxy-2-methyl-anthraquinone), 45 (1,5-dihydroxy-8-metoxy-2-methyl-anthraquinone-3-O-β-D-(+)-glucopyranoside) [31] and 46 emodin (1,6,8-trihydroxy-3-methyl-anthraquinone) [31]. Alkaloid compounds from C. alata leaves have also been identified using 1H-NMR, 13C-NMR and MS [32]. In addition, 47 (physcion) has been identified from C. alata root [33]. Five compounds, 48 (ω-hydroxyemodin), 49 (ziganein), 50 (apigenin), and 51 (trans-resveratrol) were isolated from the C. alata roots [22].
3.6. Flower
Isolation of the compound on C. alata flower has been reported [34]. C. alata flowers were extracted with hot methanol solvent. The obtained extract was mixed with a number of silica and further dried. The extract was then fractionated with several types of eluent comparisons using chromatographic columns to obtain three types of compounds i.e stearic acid compounds 52 (alanonal) and 53 (β-sitosterol-β-D-glucoside) [34]. Furthermore, chemical compounds of flower of C. alata that analyzed by GC-MS were 54 (β-d-mannofuranoside), 55 (n-hexadecanoic acid), 56 (9-dodecenoic acid), 30 (oleic acid), 57 (nonadecanoic acid), 58 (3,11-tetradecadien-1-ol), and 59 (octadecanal) [27].
4. Pharmacological effect
Biological activity has been known contained in plants [35, 36], such as antidiabetic, antioxidant, antimicrobial, etc. [37, 38, 39]. C. alata has biological activity which is summarized in Table 1. This present review provided the biological activities of C. alata as antiallergic, anti-inflammatory, antioxidant, thrombolytic, anticancer and antitumor, antidiabetic, choleretic, analgesic, antimicrobial, antiviral, antiucler, hepatoprotective, antidepressant, antimalarial, anthelmintic, cardiovascular and anasthetic. The plant has been reported not to have side effect in clinical medicine.
Table 1.
Biological activity of C. alata.
| Part of Plant | Biological activity | Method | Result | Reference |
|---|---|---|---|---|
| Leaf | Anti allergic | In vivo (mast cell stabilization) | At 200 mg/kg (75% inhibition) while rhein and kaemferol at 76% at 5 mg/kg. | [40] |
| In vitro (lipoxygenase) | Extract hydrocalohol and rhein showed IC50 values of 90.2 and 3.9 μg/mL, respectively. | |||
| Anti inflammatory | Carrageenan-induced rat paw oedema model | The butanol fraction was the highest mean percent inhibition as 78.36% followed by ethyl acetate (58.21%) and methanol (20.89%) at 100 mg/kg as compared to Indomethacin (79.59%) at a dose of 10 mg/kg after 4 h of carrageenan injection. | [41] | |
| Concanavalin A-induced histamine, 5-lipoxygenase inhibition, cyclooxygenases (COX-1 and COX-2), | Strong inhibitory effects on Concanavalin A-induced histamine release from rat peritoneal exudate cells. The heat treated leaf extract had stronger inhibitory effects than the sun-dried leaf extract at low concentrations in the studies of Concanavalin A-induced histamine release, 5-lipoxygenase inhibition, and also inhibition of cyclooxygenases (COX-1 and COX-2), whereas K3G showed weak inhibitory effects on Concanavalin A-induced histamine release, 5-lipoxygenase, and COX-1. | [20] | ||
| Oral gavage to CFA arthritic rats (500 mg/kg, n = 6) | Extract significantly (P = 0.0032) reduced knee circumference (swelling) in the CFA arthritic rats Extract significantly (P = 0.0032) reduced knee circumference in the CFA arthritic rats. |
[9] | ||
| Antioxidant | DPPH radical scavenging | Methanol extract gave inhibition ED50 of 28.50 μg/ml, while BHT has ED50 14.17 ± 1.38 μg/ml. | [15] | |
| DPPH radical scavenging | Methanol extract gave IC50 concentration lower (54 ± 2.20) than BHT standard (72 ± 2.20). | [42] | ||
| DPPH, Nitric Oxide (NO), Deoxiribose (DOR) | Methanol extract: DPPH (% scavenging activity 58.80 ± 2.02), Nitric Oxide (NO) (% scavenging activity 35.01 ± 1.91) and Deoxiribose (DOR) (% scavenging activity 50.52 ± 0.77). |
[11] | ||
| Leaf | Antioxidant | DPPH radical scavenging Lipid peroxidation Hydroxyl radical |
DPPH (IC50 71.35 ± 0.32 μg/ml). Lipid peroxidation (IC50 38.17 ± 1.2 μg/ml). Hydroxyl radical (IC50 95.46 ± 0.79 μg/ml). |
[43] |
| Ammonium thiocyanate | Acetone extract gave % inhibition 37.02 ± 0.45 and total phenolic 23.29 + 0.89 mg/g. | [44] | ||
| DPPH radical scavenging | Essential oil 95.2% linalool (23.0%), borneol (8.6%) and pentadecanal (9.3%) were major constituents. The antioxidant activity of the oil was lower than butylated hydroxytoluene (BHT). | [46] | ||
| Anticancer and antitumor | Brine Shrimp Lethality by using shrimp larvae (Artemia salina Leach) | The cytotoxicity of ethanol extract of C. alata seed and gallic acid had LC50 value 5.29 and 4.53 ppm, respectively. | [49] | |
| In vitro MMT | The extract was fractionated and then tested against five types of human cancer cells. The results showed that isolate f6I had selectivity in MCF-7, T24, and Col 2 cells with IC50 values of 16, 17, and 17 μg/ml, respectively. | [50] | ||
| In vitro MMT | The cytotoxicity of n-hexane C. alata leaves extract showed selectivity to OV2008 cancer cells with IC50 160 μg/ml. The cytotoxicity shown by C. alata is caused by flavonoid compounds, kaempferol which was the compound of n-hexane C. alata extract. | [51] | ||
| WST-1 cytotoxicity | Hydromethanolic leaf extract of C. alata was reported cytotoxic to the K562 leukaemia cell line. | [52] | ||
| Bearing carcinomatous cells on Nude mice | At 100 and 200 mg/kg body weight, the levels of MDA decreased significantly (3.44 ± 0.76 to 1.97 ± 0.48) while the glutathione and the activities of CAT and SOD increased significantly. | [53] | ||
| Antidiabetic | In vitro by inhibitory assay against α-Glucosidase | Ethanol extract of C. alata (63.75 ± 12.81 μg/mL) was better to inhibit α-glucosidase than the standard acarbose drug (107.31 ± 12.31 μg/mL), the active fraction inhibiting α-glucosidase ie chloroform, ethyl acetate and n-butanol with IC50 of 44.25 ± 10.23, 2.95 ± 0.47 and 25.80 ± 2.4301 μg/ml respectively, and pure isolates of the n-butanol fraction (kaemferol 3-O-gentiobioside) inhibited α-glucosidase with IC50 82.5 ± 13.7 μg/ml. | [54] | |
| Streptozotocin-induced hyperglycemia in rats | The mean decreased blood sugar levels were 277.1 ± 0.8, 267.7 ± 0.9 and 259.1 ± 2.7 % at each dose of 100, 200 and 400 mg/kg. | [55] | ||
| Leaf | Antidiabetic | Blood glucose levels used albino Swiss Webster mice | Ethyl acetate extract of C. alata showed an effective result as a antidiabetic agent with percent decrease of blood glucose level (56.7%) while CMC control (38.0%). | [56] |
| Choleretics | Bile secretion of rats | The activity of choleretic extract of C. alata at 15 mg/kg had good activity than Hebucol ND. But at high doses, plants dispose to inhibit bile secretion. | [57] | |
| Analgesic | In vivo using an albino rat by the method of tail clamping, tail wagging, tail immersion and the reflexes of writhing with induction of acetic acid | The analgesic effect of C. alata was ignificantly better at doses of 400 mg/kg than at doses of 200 and 100 mg/kg. The analgesic effect produced by kaempferol 3-O-sophoroside was greatest in ±120 min. Assay writhing of acetic acid at 400 mg/kg C. alata showed a considerable increase in analgesic effect (56.4%) rather than dosage of 200 and 100 mg/kg (46% and 35.9%) while the percentage of inhibitory stretching produced by kaempferol 3-O-sophoroside was close to 100 mg/kg C. alata extract (36.9%). |
[58] | |
| Antimicrobial | Disk diffusion | Methanol extract inhibited Salmonella thypi, Proteus mirabilis, Bacillus coagulans, Micrococcus luteus. Petroleum ether extract inhibited the growth of B. coagulans. Dichloromethane extract inhibited the growth of Lactobacillus casei, Staphylococcus epidermidis, Neisseria gonorrhoeae and Trichomonas vaginalis. Ethyl acetate extracts inhibited the growth of B. coagulans and T. vaginalis. |
[59] | |
| Disk diffusion | The methanol extract of C. alata leaf inhibited Actinomyces bovis and Mucor sp. | [60] | ||
| The ethanol extract inhibited the growth of Escherichia coli bacterium and Rhizopus sp., Aspergillus niger, Saccharomyces fungi. | [61] | |||
| Agar well diffusion | The acetone extract inhibited Proteus vulgaris and Bacillus subtilis. | [62] | ||
| Disk diffusion | Water extract inhibited Stahylococcus aureus bacterium. | [63] | ||
| Leaf | Antimicrobial | Disk diffusion | The n-hexane extract had been reported to inhibit the growth of Trichopyton simii, E. floccosum, Candida albicans. Methanol extract inhibited T.metagrophytos, T.simii, Curvularia hunata, C. albicans. Ethanol extract inhibited Trichophytonrubrum,Trichophytonmetagrophytos, T.simii,Trichophytontonsuran, E. floccosum, C. hunata, C. albicans,Cryptococcus neoformans, Microsporum canis, Microsporumgypseum, Penicilliumnotatum. | [68, 68, 69, 70, 71, 72] |
| Leaf | Antiviral | In vivo with male white rats with various doses (100 mg/kg body weight, 300 mg/kg BW and 900 mg/kg BW | At 21st day dose of 300 mg/kg body weight and 900 mg/kg body weight can have an effect on shortening time bleeding, blood clots and increase the number of mice platelets white male. | [75] |
| The toxicity was measured by MTT assay | C. alata leaves extract and butanol subfraction were reported to have strong antiviral activity against DENV-2 with the IC50 0.0256 and 6.47 μg/ml and CC50 323.45 and 645.8 μg/ml, respectively. | [76] | ||
| In vitro MMT | Several extracts (methanol, chloroform, ethyl acetate, n-butanol, and aqueous) from C. alata leaves had activity against rotavirus (RV) infection | [77] | ||
| Antiucler | Pylorus ligation and ethanol induced ulcer models in experimental rats | Ethanolic extract of C. alata leaves at a dose of 150 and 300 mg/kg produced significant inhibition of the gastric lesions induced by pylorus ligation induced ulcer and ethanol induced gastric ulcer. The extract (150 mg/kg and 300 mg/kg) showed sgnificant (p < 0.05) reduction in gastric volume, free acidity and ulcer index as compared to control. | [78] | |
| Hepatoprotective | Hepatic injury in albino rats | The alcoholic extract of C. alata leaves had been reported to have hepatoprotective activity. The experiment showed that the methanol extract had activity against Paracetamol induced hepatic injury in albino rats. Additionally, pretreatment of the extract reduced the biochemical markers of hepatic injury like serum glutamate pyruvate transaminase (SGPT), serum oxaloacetate transaminase (SGOT), alkaline phosphatase (ALP), total bilirubin and gamma glutamate transpeptidas from paracetamol induced liver damage. | [79] | |
| Leaf | Antidepressant | Forced Swim Test (FST) and Tail Suspension Test (TST) | C. alata showed significant decrease in the time of immobility in both the standard drug (22.0 ± 0.26 s) and the test extract (21.25 ± 0.12 s) compared to the control group (62.5 ± 0.54 s). The leaf extracts of C. alata (200 mg/kg) showed an increased effect compared to the standard drug fluoxetine (10 mg/kg). Therefore, a significant decrease in the immobility of mice in test extract (59.6 ± 2.23 s) and a standard drug (57 ± 3.5 s) in comparison to the control group (153.4 ± 1.97 s) after administration of the standard and test extract. These results clearly indicated that aqueous leaf extracts of S. alata exhibited a strong antidepressant activity similar to that of the control drug of administration of the standard (Imipramine). | [80] |
| Leaf | Antimalarial | WHO microtest assay (Mark III) | The result showed that C. alata leaves had activity against the 3D7 strain of the Plasmodium falciparum parasite with IC50 17.270 μg/ml. While the IC50 of artesunate-amodiaquine was 0.313 μg/ml. | [81] |
| Antihelmintic | Scanning electron microscope studies (SEM) | The ethanol extract at 40 mg/ml, paralysis occurred at 1.68 ± 0.06 h which is comparable with praziquantel (1.18 ± 0.04 h) at 0.001 mg/ml. The post-paralytic time was comparatively shorter in all concentrations of C. alata, while it took more time in all concentrations of praziquantel. However, the control parasite survived up to 69.22 ± 0.23 h. | [82] | |
| Cardiovascular | DPPH and lipid peroxidation | In hyperglycemic rats, the aorta and heart shown significant increased in lipid peroxidation, decreased in total antioxidant activity (DPPH) and decrease in antioxidant catalase activity. Furthermore, administration of C. alata leaf aqueous extract to hyperglycemic rats reduced lipid peroxidation (MDA levels), increased in total antioxidant activity and antioxidant catalase activity as well as reduced in the blood glucose level. | [83] | |
| Ansthetic | ALT (Alanine Transaminase), AST (Aspartate Transaminase) and ALP (Alkaline Phosphatase) | The result showed that AST, ALT and ALP were reduced in groups 3 to 7 compared to group 2, while ALP and ALT were significantly reduced (P < 0.05) when treated with 4000 mg/kg body weight. | [84] | |
| Seed | Antioxidant | DPPH radical scavenging and ferric reducing | The antioxidant IC50 values of seed in DPPH and ferric reducing were 4.01 ± 0.11 and 0.40 ± 0.21 μmol/mg, respectively. | [23] |
| Thrombolitic | In vitro thrombolytic activity | The extract showed potent thrombolytic activity against negative control (water). | [21] | |
| Anticancer | Brine Shrimp Lethality by using shrimp larvae (Artemia salina Leach) | The cytotoxicity of ethanol extract of C. alata seed and gallic acid had LC50 value 4.31 and 4.53 ppm, respectively. | [49] | |
| Antimicrobial | Disk diffusion | The methanol extract of C. alata seeds inhibited the growth of Sarcina lutea and Klebsiella pneumonia. | [57] | |
| Stem Bark | Antimicrobial | Disk diffusion | Methanol extract inhibited S. typhi bacterium growth. Petroleum ether extract inhibited bacterial growth of B.megaterium, Spectrococcus faecalis, S. typhi. Dichloromethane extract inhibited bacterial growth of B. coagulans. Ethyl acetate extract inhibited bacterial growth B. cereus, B.megaterium, St.pneumoniae,K.pneumoniae, N. gonorrhoeae and S. typhi. | [59] |
| Disk diffusion | Ethanol and water extracts inhibited C. albicans. | [52] | ||
| Root | Antioxidant | DPPH and ABTS | Ethanol extract had IC50 value for DPPH and ABTS assays 45.18 and 39.14 μg/ml, respectively. | [48] |
| Antimicrobial | Disk diffusion | Methanol root extract inhibited B. cereus, B. subtilis, S. albus, S. aureus, S. epidermidis, St. faecalis, E. coli, S. typhi, and S. typhymurium. Petroleum, chloroform, and ethyl acetate fractions of C. alata root had activity against B. cereus, B. coagulans, B. megaterium, B. subtilis,L.casei,M.luteus, M. roseus, S. albus, S. aureus, S. epidermidis, St. faecalis, St.pneumoniae, S. mutans, Agrobacterium tumefaciens, Citrobacter freundii, Enterobacter aerogenes, E. coli,K.pneumoniae,N.gonorrhoeae,P.mirabilis, P. vulgaris, P. aeruginosa, S. typhi, S. typhymurium, Serratia marcescens. | [59] | |
| Broth dilution | The water, methanol, and chloroform extracts of C. alata had activity against S. aureus, E. coli, S. pyogenes, P. aeruginosa, and P. mirabillis. | [66] | ||
| Broth dilution | The ether and methanol extracts had activity against clinically resistant N.gonorrhoeae. | [67] | ||
| Pod | Antioxidant | DPPH radical scavenging | Methanol extract gave ED50 100.18 μg/ml, while BHT had 14.17 ± 1.38 μg/ml. | [15] |
| Flower | Antioxidant | Protective gainst carbon tetrachloride (CCl4) | After administration with CCl4, the extract more active than CCl4. In the extract, serum aspartate aminotransferase and alanine aminotransferase decreased significantly (P ≤ 0.05) in rats. | [45] |
| DPPH radical scavenging and ferric reducing | The antioxidant IC50 values of flower in DPPH and ferric reducing were 4.16 ± 0.21 and 0.33 ± 0.51 μmol/mg, respectively. | [23] | ||
| DPPH radical scavenging | Methanol extract gave ED50175.36 μg/ml, while BHT had 14.17 ± 1.38 μg/ml. | [15] | ||
| DPPH radical scavenging | Aqueous extract had EC50 823 μg/ml. | [47] | ||
| Antimicrobial | Disk diffusion | Methanol extract inhibited E. coli and C. albicans. | [73] | |
| Disk diffusion | The extracts of water, methanol, chloroform and petroleum ether of C. alata flowers at 500 mg/mL were active against S. aureus, E. coli, P. vulgaris, P. aeruginosa, B. subtilis and C. albicans. | [70] | ||
| Broth dilution technique | The crude extract C. alata flower at 500 μg/mL had activity against S. aureus,St.faecalis,M.luteus, B. subtilis and P. putida. But at concentration above 1000 mg/mL, the extract inactive against E. coli,P.vulgaris, P. aeruginosa,S.marcescens, and P. fluorescens. | [65] | ||
| Disk diffusion | Methanol extract inhibited S. typhi growth. Petroleum ether extract inhibited the growth of B. megaterium. Dichloromethane extract inhibited the growth of B. cereus and S.epidermidis. Ether extract inhibited N.gonorrhoeae. | [72] | ||
| Flower | Antimicrobial | Disk diffusion | The water, methanol and chloroform of C. alata root extracts had activity against S. aureus, E. coli, S. pyogenes, P. aeruginosa and P. mirabilis. | [71] |
| Broth dilution method | Methanol extract and fraction inhibited the growth of fungi A. brevipes, Penicillium sp., Geotricum candidum, C. utilis. | [74] | ||
| Disk diffusion | Methanol extract inhibited S. epidermidis. Chloroform extract inhibited B. subtilis. Petroleum ether extract inhibited the growth of B. coagulans. Dichloromethane extract inhibited the growth of L.casei, S. epidermidis, N.gonorrhoeae and T.vaginalis. Ethyl acetate extracts inhibited the growth of B. coagulans and T. vaginalis. | [64] |
5. Clinical effect
The therapeutic efficacy of C. alata leaf extract against Pityriusis versicolor has been reported for the first time involving humans. For the collection of clinically effective antifungal compounds from the leaves of C. alata, a simple procedure has been devised. Ten years human study indicates that the leaf extract can be reliably used as a herbal medicine to treat P. versicolor. The leaf extract has no side-effects [85]. C. alata has been reported as new treatment tools in bronchorespiratory and chemopreventive activity against various DNA damaging agents [86]. Folkloric on C. alata claims as an antimicrobial agent for treating skin infection [87]. The C. alata was found to have potential as herbal soap [88]. Furthermore, C. alata leaves gave significant effect in healing burns [89] and has been reported to against clinical isolates of Gram-positive and Gram-negative bacteria viz., Vibrio cholerae, B. subtilis, S. aureus, Stretococcus sp. and E. coli as well as against a few fungi which are mostly dermatophytes causing skin infection in human beings like, A. niger, A. flavus. A. candidus, P. patulum, C. albicans and R. stolonifer, T. mentagrophytes, T. tubrum, M. gypseum and M. canis [90, 91].
6. Toxicity studies
C. alata has been reported not to have obvious toxicity based on the experiment used Swiss albino male mice weighed 24–28 g. At 3,000 mg/kg body weight of alcoholic C. alata leaves extract did not change in the general behavior of the test animals [92]. Aqueous dried leaf extract of C. alata was reported not to have toxic at 250, 500 and 1000 mg/kg based on the experiment used male albino rats (80–100 g). The histopathology of the liver and kidney did not reveal any pathological changes [93]. Furthermore, the aqueous extract of C. alata flower has been reported safe with administered orally in rat based on the experiment used albino wistar rats of either sex (150–180 g). The histological sections of the liver, lung, kidney, spleen and heart did not show any remarkable changes [94]. But, the alkaloids that isolated from C. alata at 250–1000 mg/kg reported changes plasma membrane of the liver and kidney [95]. Additionally, emodin, kaempferol, aloe-emodin, and rhein were reported caused subtle hepatorenal toxicity [96].
7. Conclusions
C. alata plant is a herbal medicine that has been used in Asian countries. Several secondary metabolite compounds from the plant have been isolated from parts of leaves, seeds, stems and flowers. The results of research on the biological activity of C. alata which has been reported by some researchers gives scientific fact that this plant has pharmacological aspects. However, research on isolation of secondary metabolite compounds is still needed to be continued to investigate potentially pharmacological compounds.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This paper is partially funded by the Indonesian Ministry of Research, Technology and Higher Education under WCU Program managed by Institut Teknologi Bandung.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Gilman E.F., Watson D.G. Southern Group of State Foresters; 1993. Cassia Alata Candlebrush; pp. 1–3. [Google Scholar]
- 2.Lim T.K. “Senna alata” edible medicinal and non-medicinal plants. 2014;7:841–859. [Google Scholar]
- 3.Hennebelle T., Bernard W., Henry J., Sevser S., Francois B. Senna alata. Fitoterapia. 2009;80(7):389–393. doi: 10.1016/j.fitote.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Reezal I., Somchit M.N., Rahim M.A. The Regional Symposium on Environment and Natural Resources. 2002. In vitro anti-fungal properties of Cassia alata (Gelenggang Besar) pp. 654–659. [Google Scholar]
- 5.Shiddamallayya N., Yasmeen A., Gopakumar K. Medicobotanical survey of Kumar pavatha Kukke Subramanya, Manglore, Karnataka. Indian J. Tradit. Knowl. 2010;9:96–99. [Google Scholar]
- 6.Alalor C.A., Igwilo C.I., Jeroh E. Evaluation of the anti-bacterial properties of aqueous and methanol extracts of Cassia alata. J. Pharm. Allied Health Sci. 2012;2:40–46. [Google Scholar]
- 7.Monkheang P., Sudmoon R., Tanee T. Species diversity, usages, molecular markers and barcode of medicinal Senna species (Fabaceae, Caesalpinioideae) in Thailand. J. Med. Plants Res. 2011;5:6073–6181. [Google Scholar]
- 8.Saito S., Silva G., Santos R.X., Gosmann G., Pungartnik C., Bredel M. Astragalin from Cassia alata induces DNA adducts in vitro and repairable DNA Damage in the yeast Saccharomyces cerevisiae. Int. J. Mol. Sci. 2012;13:2846–2862. doi: 10.3390/ijms13032846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis A., Levy A. “Antiinflammatory activities of Cassia alata leaf extract in complete Freund’s adjuvant arthritis in rats. W. Indian Med. J. 2011;60:615–621. [PubMed] [Google Scholar]
- 10.Sule W.F., Okondo I.O., Joseph T.A. In vitro antifungal activity of Senna alata Linn. Crude Leaf Extract. Res. J. Biol. Sci. 2010;5(3):275–284. [Google Scholar]
- 11.Akinmoladun A.C., O. Efere M., Farombi E.O. Evaluation of antioxidant and free radical scavenging capacities of some nigerian indigenous medicinal plants. J. Med. Food. 2010;13:444–451. doi: 10.1089/jmf.2008.0292. [DOI] [PubMed] [Google Scholar]
- 12.Alam M.T., Karim M.M., Khan A.N. Antibacterial activity of different organic extracts of achyranthes aspera and Cassia alata. J. Sci. Res. 2009;1(2):393–398. [Google Scholar]
- 13.Veerachari U., Bopaiah D.R.A.K. Phytochemical investigation of the ethanol, methanol and ethyl acetate leaf extracts of six Cassia species. Int. J. Pharma Bio Sci. 2012;3(2):260–270. [Google Scholar]
- 14.Kundu S., Roy S., Lyndem M.L. Cassia alata l: potential role as anthelmintic agent against Hymenolepis diminuta. Parasitol. Res. 2012;111:1187–1192. doi: 10.1007/s00436-012-2950-6. [DOI] [PubMed] [Google Scholar]
- 15.Panichayupakaranant P., Kaewsuwan S. Bioassay-guided isolation of the antioxidant constituent from Cassia alata L. leaves Songklanakarin. J. Sci. Technol. 2004;26:103–107. [Google Scholar]
- 16.Rahaman M.S., Hasan A.J.M.M., Ali M.Y., Ali M.U. A flavone from the leaves of Cassia alata Bangladesh. Counc. Sci. Ind. Res. 2006;41:93–96. [Google Scholar]
- 17.Rahman M.S., Ali M.Y., Ali M.U. In vitro screening of two flavonoid compounds isolated from cassia alata L. leaves for fungicidal activities. J. Biol. Sci. 2008;16:142–193. [Google Scholar]
- 18.Adiana M.A., Mazura M.P. Study on Senna alata and its different extracts by Fourier Transform Infrared Spectroscopy and two-dimensional correlation infrared spectroscopy. J. Mol. Struct. 2011;991:84–91. [Google Scholar]
- 19.Igwe O.U., Onwu F.K. Leaf essential oil of senna alata linn from south east Nigeria and its antimicrobial activity. Int. J. Res. Pharm. Chem. 2015;5(1):27–33. [Google Scholar]
- 20.Moriyama H., Iizuka T., Nagai M., Miyataka H., Toshio S. Anti-inflammatory activity of Heat-treated Cassia alata leaf extract and its flavonoid glycoside. Yakugaku Zasshi. 2003;123:607–611. doi: 10.1248/yakushi.123.607. [DOI] [PubMed] [Google Scholar]
- 21.Liu A., Xu L., Yang S. Studies on chemical constituents from leaves of Cassia alata. Zhongguo Zhongyao Zazhi. 2009;34(7):861–863. [PubMed] [Google Scholar]
- 22.Promgool T., Pancharoen O., Deachathai S. Antibacterial and antioxidative compounds from Cassia alata Linn. Songklanakarin J. Sci. Technol. 2014;36(4):459–463. [Google Scholar]
- 23.Tatsimo S.J.N., Jean-de-Dieu Tamokou, Tsague V.T., Lamshoft M., Sarkar P., Bag P.K., Spiteller M. Anti bacter ial-guided isolation of constituents from Senna alata leaves with a particular reference against Multi Drug Resistant Vibrio cholerae and Shigella flexneri. Int. J. Brain Cognit. Sci. 2017;11(1) [Google Scholar]
- 24.Prasenjit M., Tanaya G., Sumanta G., Basudeb B., Kumar M.P. Isolation and characterization of a compound from the leaves of Cassia alata Linn. Chem. Res. 2016:1–7. [Google Scholar]
- 25.Mannan A., Kawser Md J., Abu ahmed A.M. Assessment of antibacterial, thrombolytic and cytotoxic potential of Cassia alata seed oil. J. Appl. Pharmaceut. Sci. 2011;1(9):56–59. [Google Scholar]
- 26.Gupta D., Singh J. Flavonoid glycosides from Cassia alata. Phytochmistry. 1991;30:2761–2763. doi: 10.1016/0031-9422(91)85140-u. [DOI] [PubMed] [Google Scholar]
- 27.Isah A., Abdullahi M., Tsado M.J. Evaluation of phytochemical, anti-nutritional and antioxidant potentials of flower and seed methanol extracts of Senna alata L. grown in Nigeria. Am. J. Appl. Chem. 2015;3(3):93–100. [Google Scholar]
- 28.Gupta D.S., Jann B. Structure of a galactomannan from Cassia alata seed. Carbohydr. Res. 1987;162:271–276. [Google Scholar]
- 29.Hemlata, Kalidhar S.B. Alatinone, an anthraquinone from Cassia alata. Phytochemstry. 1993;32:1616–1617. [Google Scholar]
- 30.Chatsiriwej N., Wungsintaweekul J., Panichayupakaranant P. Anthraquinone production in Senna alata root cultures. Pharmaceut. Biol. 2006;44:416–420. [Google Scholar]
- 31.Tiwari R.D., Yadava O.P. Structural study of the quinone pigments from the roots of Cassia alata. Planta Med. 1971;19(4):299–305. doi: 10.1055/s-0028-1099645. [DOI] [PubMed] [Google Scholar]
- 32.Husain K., Jamal J.A., Abu Safran N.A. Pharmacognostical analysis and preliminary studies of the chemical constituents from the roots of Senna alata Linn. Malays. J. Sci. 2005;24:137–141. [Google Scholar]
- 33.Fernand V.E., Dinh D.T., Washington S.J., Fakayode S.O., Losso J.N., van Ravenswaay R.O., Warner I.M. Determination of pharmacologically active compounds in root extracts of Cassia alata L. by use of high performance liquid chromatography. Talanta. 2008;74:896–902. doi: 10.1016/j.talanta.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yadav S.K. Isolation and characterization of chemical compounds from flowers of Cassia alata. Der Pharma Chem. 2013;5:59–62. [Google Scholar]
- 35.Hidayati D.M., Ersam, Shimizu T.K., Fatmawati S. Antioxidant activity of syzygium polyanthum Extracts. Indonesian J. Chem. 2017;17(1):49–53. [Google Scholar]
- 36.Yuliana, Fatmawati S. Senyawa metabolit sekunder dan aspek farmakologi Alocasia machrorrhizos. Akta Kimia Indonesia. 2018;3:141–158. [Google Scholar]
- 37.Lulan T.Y.K., Fatmawati S., Santoso M., Ersam T. Antioxidant capacity of some selected medicinal plants in east Nusa Tenggara, Indonesia: the potential of Sterculia quadrifida R.Br. Free Radic. Antioxidants. 2018;8(2) [Google Scholar]
- 38.Fatmawati S., Ersam T., Shimizu K. The inhibitory activity of aldose reductase in vitro by constituents of Garcinia mangostana Linn. Phytomedicine. 2014;22(1):49–51. doi: 10.1016/j.phymed.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Putri D.A., Ulfi A., Purnomo A.S., Fatmawati S. Antioxidant and antibacterial activities of Ananas comosus peel extracts. Malaysian J. Fund. Appl. Sci. 2018;14(2):307–311. [Google Scholar]
- 40.Singh B., Janhavi R.N., Ram A.V. The hydroalcoholic extract of Cassia alata (Linn.) leaves and its major compound rhein exhibits antiallergic activity via mast cell stabilization and lipoxygenase inhibition. J. Ethnopharmacol. 2012;141:469–473. doi: 10.1016/j.jep.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Patrick-Iwuanyanwu K.C., Onyeike E.N., Dar A. Anti-inflammatory effect of crude methanolic extract and fractions of Ring worm plant Senna alata (L. Roxb) leaves from Nigeria. Der Pharm. Sin. 2011;2:9–16. [Google Scholar]
- 42.Chatterjee S., Sabyasachi C., Dey K.K., Sikha D. Study of antioxidant activity and immune stimulating potency of the ethnomedicinal plant Cassia alata (L.) Roxb. Med. Aromatic Plants. 2013;2:1–6. [Google Scholar]
- 43.Sarkar B., Khodre S., Patel P., Mandaniya M. “HPLC analysis and antioxidant potential of plant extract of Cassia alata,” vol. Asian J. Pharmaceut. Sci. Technol. 2014;4:4–7. [Google Scholar]
- 44.Okoro I.O., Osagie A., Asibor E.O. Antioxidant and antimicrobial activities of polyphenols from ethnomedicinal plants of Nigeria. Afr. J. Biotechnol. 2010;9:2989–2993. [Google Scholar]
- 45.Wegwu M.O., Ayalogu E.O., Sule O.J. Antioxidant protective effects of Cassia alata in rats exposed to carbon tetrachloride. J. Appl. Sci. Environ. Manag. 2005;9:77–80. [Google Scholar]
- 46.Agnaniet H., Bikanga R., Bessiere J.M., Menut C. Aromatic plants of tropical central africa. part XLVI. Essential oil constituents of Cassia alata (L.) from Gabon. J. Essent. Oil Res. 2005;17:410–412. [Google Scholar]
- 47.Bhuvaneswari S., Deepa S., Sripriya N., Prameela L., Prakash N.K.U. Antioxidant activity and phytochemistry of various flowers from Tawil Nadu, India. Int. J. Res. Pharm. Sci. 2014;5(1):40–45. [Google Scholar]
- 48.Ita B.N., Ndukwe G.I. Antioxidant activity of Senna alata root. J. Nat. Prod. Res. 2017;3(1):94–99. [Google Scholar]
- 49.Awal M.A., Nahar A., Hossain M.S. Brine shrimp toxicity of and seed extract of Cassia alata Linn. and their antibacterial potency. J. Med. Sci. 2004;4:188–193. [Google Scholar]
- 50.Olarte E.I., Annabelle A.H., Irene M.V., Sonia D.J. In vitro antitumor properties of an isolate from leaves of Cassia alata L. Asian Pac. J. Cancer Prev. APJCP. 2013;14:3191–3196. [PubMed] [Google Scholar]
- 51.Levy A.S., Shanna-kay C. Cytotoxic activity of hexane extracts of Psidium guajava L (Myrtaceae) and Cassia alata L (Caesalpineaceae) in Kasumi-1 and OV2008 Cancer cell lines. Trop. J. Pharmaceut. Res. 2012;11:201–207. [Google Scholar]
- 52.Adebesin O., Okpuzor J., Iroanya O., Adenekan S. Antioxidant and cytotoxic properties of Senna alata and Senna podocarpa leaf extracts. Planta Med. 2013;79(13) 1136-1136. [Google Scholar]
- 53.Pieme C.A., Peniap V.N., Nkegoum B., Ngogang J. In vivo antioxidant and potential antitumor activity of aqueous ethanol extract of leaves of Senna alata (L.) roxb (ceasalpiniaceae) on bearing carcinomatous cells. Int. J. Pharmacol. 2008;4:245–251. [Google Scholar]
- 54.Varghese G.K., Lekshmi V.B., Solomon H. Antidiabetic components of Cassia alata leaves: identification through α-glucosidase inhibition studies. Pharmaceut. Biol. 2012;51:345–349. doi: 10.3109/13880209.2012.729066. [DOI] [PubMed] [Google Scholar]
- 55.Palanichamy S., Nagaraja S., Devasagayam M. Effect of Cassia alata leaf extract on hyperglycemic rats. J. Ethnophawnacology. 1988;22:81–90. doi: 10.1016/0378-8741(88)90233-4. [DOI] [PubMed] [Google Scholar]
- 56.Villaseñor I.M., Canlas A.P., Pascua M.P., Sabando M.N., Soliven L.A. Bioactivity studies on Cassia alata Linn. leaf extracts. Phytother Res. 2002;16:S93–S96. doi: 10.1002/ptr.768. [DOI] [PubMed] [Google Scholar]
- 57.Assane M., Traore M., Bassene E., Sere A. Choleretic effects of Cassia alata Linn in the rat. Dakar Med. 1993;38:73–77. [PubMed] [Google Scholar]
- 58.Palanichamy S., Nagarajan S. Analgesic activity of Cassia alata leaf extract and kaempferol 3-O- sophoroside. J. Ethnopharmacol. 1990;29:73–78. doi: 10.1016/0378-8741(90)90099-f. [DOI] [PubMed] [Google Scholar]
- 59.Khan M.R., Kihara M., Omoloso A.D. Antimicrobial activity of Cassia alata. Fitoterapia. 2001;72:561–564. doi: 10.1016/s0367-326x(00)00335-x. [DOI] [PubMed] [Google Scholar]
- 60.Makinde A.A., Igoli J.O., Ta’Ama L., Shaibu S.J., Garba A. “Antimicrobial activity of Cassia alata”. Afr. J. Biotechnol. 2007;6:1509–1510. [Google Scholar]
- 61.Oyowale J.A., Olatunji G.A., Oguntoye S.O. Antifungal and antibacterial activities of an alcoholic extract of Senna alata leaves. J. Appl. Sci. Environ. Manag. 2005;9(3):105–107. [Google Scholar]
- 62.Sharma P., Pandey D., Rizvi A.F., Gupta A.K. Antimicrobial activity of Cassia alata from Raipur region against clinical and MTCC isolates. Int. J.Curr. Microbiol. Appl. Sci. 2015;4(1):330–339. [Google Scholar]
- 63.Somchit M.N., Reezal I., Nur I.E., Mutalib A.R. In vitro antimicrobial activity of ethanol and water extracts of Cassia alata. J. Ethnopharmacol. 2003;84:1–4. doi: 10.1016/s0378-8741(02)00146-0. [DOI] [PubMed] [Google Scholar]
- 64.Idu M., Omonigho S.E., Igeleke C.L. Preliminary investigation on the phytochemistry and antimicrobial activity of Senna alata L. flower. Pakistan J. Biol. Sci. 2007;10(5):806–809. doi: 10.3923/pjbs.2007.806.809. [DOI] [PubMed] [Google Scholar]
- 65.Adedayo O., Anderson W.A., Moo-Young M., Snieckus V., Patil P.A., Kolawole D.O. Phytochemistry and antibacterial activity of Senna alata Flower. Pharmaceut. Biol. 2001;39(6):408–412. [Google Scholar]
- 66.El-Mahmood A.M., Doughari J.H. Phytochemical screening and antibacterial evaluation of the leaf and root extracts of Cassia alata Linn. African J.Pharm. Pharmacol. 2008;2(7):124–129. [Google Scholar]
- 67.Otto R.B.D., Ameso S., Onegi B. Assessment of antibacterial activity of crude leaf and root extracts of Cassia alata against Neisseria gonorrhea. Afr. Health Sci. 2014;14(4):840–848. doi: 10.4314/ahs.v14i4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nivas R.K., Boominathan M. Antimicrobial evaluation of selected south indian medicinal plants against Streptococcus pneumoniae. Int. J.Curr. Microbiol. Appl. Sci. 2015;4(3):835–840. [Google Scholar]
- 69.Ponnusamy K., Chelladurai P., Ramasamy M., Waheeta H. In vitro antifungal activity of indirubin isolated from a South Indian ethnomedicinal plant Wrightia tinctoria R. Br. J. Ethnopharmacol. 2010;132:349–354. doi: 10.1016/j.jep.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 70.Ranganathan S., Balajee S.S.M. Anti-Cryptococcus activity of combination of extracts of Cassia alata and Ocimum sanctum. Mycoses. 2000;43:299–301. doi: 10.1046/j.1439-0507.2000.00581.x. [DOI] [PubMed] [Google Scholar]
- 71.Wuthi-udomlert M., Kupittayanant P., Gritsanapan W. In vitro evaluation of antifungal activity of anthraquinone derivatives of Senna alata. J. Health Res. 2010;24:117–122. [Google Scholar]
- 72.Ibrahim D., Halim O. Antimicrobial activity of Cassia alata from Malaysia. J. Ethnopharmacol. 1995;45:151–156. doi: 10.1016/0378-8741(94)01200-j. [DOI] [PubMed] [Google Scholar]
- 73.Timothy S.Y., Wazis C.H., Adati R.G., Maspalma I.D. Antifungal activity of aqueous and ethanolic leaf extracts of Cassia alata Linn. J. Appl. Pharmaceut. Sci. 2012;2:182–185. [Google Scholar]
- 74.Adedayo O., Anderson W.A., Moo-Young M., Kolawole D.O. Antifungal properties of some components of Senna alata flower. Pharmaceut. Biol. 1999;37:369–374. [Google Scholar]
- 75.Oktavia S., Pebryantika S., Dharma S. Pengaruh pemberian ekstrak etanol daun ketepeng cina (cassia alata l.) terhadap waktu pendarahan, pembekuan darah dan jumlah trombosit mencit putih jantan. Jurnal Farmasi Higea. 2015;7(1):1–9. [Google Scholar]
- 76.Angelina M., Hanafi M., Syatna F.D., Wirawati T., Ratnasari S.S., Dewi B.E. Antiviral effect of sub fraction Cassia alata leaves extract to dengue virus serotype-2 strain new Guinea c in human cell line huh-7 it-1. IOP Conf. Ser. Earth Environ. Sci. 2017;101:1–10. [Google Scholar]
- 77.Shaheen M., Mostafa S., El-Esnawy N. In vitro and in vivo anti-rotaviral activity of C. alata. J. Res. Appl. Sci. 2015;2(3):63–71. [Google Scholar]
- 78.Babu S., V V., Narayana S.V., S M., N. Naik D., Geethanjali B., Yamini K., Sultana B N., Malothu R. Evaluation of antiulcer activity of Cassia alata Linn leaves. Int. J. Pharm. Therapeut. 2012;3(2):1–4. [Google Scholar]
- 79.Anandan R., Jayakar B., Manavalan R. Hepatoprotective activity of the alcoholic extract of the dried leaves of Cassia alata, Linn. J. Pharm. Res. 2009;2(6):1107–1109. [Google Scholar]
- 80.Pamulaparthi A., Prathap V.R., Banala M., Nanna R.S. Experimental evaluation of antidepressant and antianxiety activities of aqueous leaf extracts of Senna alata (L.) roxb. using in vitro animal models. Int. J. Curr. Pharmaceut. Res. 2016;8(4):60–63. [Google Scholar]
- 81.Yaw V.B., Samuel O.A., Gyan B.A., Bertha L. In vitro antimalarial activity of the ethanol extracts of Afzelia africana and Cassia alata commonly used as herbal remedies for malaria in Ghana. Int. J. Novel Res. 2015;2(6):10–16. [Google Scholar]
- 82.Kundu S., Roy S., Lyndem L.M. Cassia alata L: potential role as anthelmintic agent against Hymenole pisdiminuta. Parasitol. Res. 2012;111:1187–1192. doi: 10.1007/s00436-012-2950-6. [DOI] [PubMed] [Google Scholar]
- 83.Ishak R., Abu I.F., Lajis H.M., Ambia K.M., Noah R.M. Effects of Cassia alata treatment towards cardiovascular oxidative stress in hyperglycemic rats. Int. J. Pharmaceut. Sci. Rev. Res. 2015;34(42):254–258. [Google Scholar]
- 84.Onyegeme-Okerenta B.M., Nwosu T., Wegwu M.O. Investigation of the effect of aqueous leaf extract of Senna alata (l) roxb on palm oil-induced hyperlipidaemic plasma lipid in wister rats. Clini. Exp. Med. Sci. 2017;5(1):19–34. [Google Scholar]
- 85.Damodaran S., Venkataraman S. A study on the therapeutic efficacy of Cassia alata, Linn. leaf extract against Pityriasis versicolor. J. Ethnopharmacol. 1994;42:19–23. doi: 10.1016/0378-8741(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 86.Ouedraogo M., Da F.L., Fabre A., Konate K., Dibala C.I., Carreyre H., Thibaudeau S., Coustard J.M., Vandebrouck C., Bescond J., Belemtougri R.G. Evaluation of the bronchorelaxant, genotoxic, and antigenotoxic effect of Cassia alata L. Evid. base Compl. Alternative Med. 2013;11 doi: 10.1155/2013/162651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oladele A., Dairo B.A., Elujoba A.A., Oyelami A.O. “Management of superficial fungal infections with Senna alata (“alata”) soaps: a preliminary report. African J.Pharm. Pharmacol. 2010;4(3) [Google Scholar]
- 88.Oladele A.T., Elujoba A.A., Oyolami A.O. Clinical studies of three herbal soaps in the management of superficial fungal infection. Res. J. Med. Plant. 2012;6(1):56–64. [Google Scholar]
- 89.Nasution S.L.R., Putri M., Hulu W., Girsang E., Nasution A.N. Optimalization of Senna alata leaves extract for healing burns. J. Global Trends Pharmaceut. Sci. 2019 [Google Scholar]
- 90.Chatterjee S., Chatterjee S., Duta S. A survey on VAM association in three different species of Cassia and determination of antimicrobial property of these phytoextracts. J. Med. Plants Res. 2010;4:286–292. [Google Scholar]
- 91.Ibrahim D., Osman H. Antimicrobial activity of Cassia alata from Malaysia. J. Ethnopharmacol. 1995;45:151–156. doi: 10.1016/0378-8741(94)01200-j. [DOI] [PubMed] [Google Scholar]
- 92.Roy S., Ukil B., Lyndem L.M. Acute and sub-acute toxicity studies on the effect of Senna alata in Swiss Albino mice. Cogent Biology. 2016;2:1–11. [Google Scholar]
- 93.Ugbogu A.E., Okezie E., Uche-Ikonne C., Duru M., Atasie O.C. Toxicity evaluation of the aqueous stem extracts of Senna alata in wistar rats. Am. J. Biomed. Res. 2016;4(4):80–86. [Google Scholar]
- 94.Igbe I., Edusuyi O. Toxicity profile of aqueous extract of Cassia alata flower in Wistar rats. J. Pharmacy Bioresour. 2016;13(2):92–102. [Google Scholar]
- 95.Yakubu M.T., Musa I.F. Liver and kidney functional indices of pregnant rats following the administration of the crude alkaloids from Senna alata (Linn. Roxb) Leaves. Iranian J. Toxicol. 2012;6(16):615–625. [Google Scholar]
- 96.Yagi S.M., Tigani S.E., Adam S.E.I. Toxicity of Senna obtusifolia fresh and fermented leaves (kawal), Senna alata leaves and some products from Senna alata on rats. Phytoterpy Res. 1998;12:324–330. [Google Scholar]