Abstract
Currently and according to the growing worldwide interest in the revaluation of agricultural by-products, the use of legumes waste presents great potential to obtain bioactive compounds. In this context, an extract rich in phenolic compounds was obtained from Vigna unguiculata (cowpea) pods by optimizing the high-intensity ultrasound conditions (10 min and 36% of amplitude) using response surface methodology. Then, the extract was encapsulated in Ca(II)-alginate beads with the addition of arabic or guar gums or cowpea isolated proteins. A complete morphological study by image analysis and microstructural evaluation by SAXS has been carried out. Results showed that beads containing alginate and alginate-guar gum have the highest loading efficiency of total phenolic compounds (47 ± 5%) and antioxidant activity (44 ± 3%). However, the coupled effect of the cowpea extract and the isolated proteins (at it higher concentration) increased the antioxidant capacity of the beads due to the contribution of the phenolic compounds and the amino acids with anti-radical activity, reaching a value of 67 ± 3 % of inhibition of ABTS.+. Finally, the microstructural analyses revealed that cowpea pod extract increased the interconnectivity of the rods due to the presence of trivalent cations, conferring versatility, and larger coordination to the network. Also, it was observed that the addition of cowpea proteins produced more interconnected bigger and fewer compacts rods than beads containing only alginate, increasing 12 and 49 % the interconnection and the size, respectively, and decreasing 10 % their compactness. This research demonstrated the use of cowpea sub-products as a source of bioactive compounds that further modulate the microstructure of the hydrogel network, and the outstanding potential for being incorporated in techno-functional foods by using Ca(II)-alginate as a carrier.
Keywords: Food science, Food analysis, Food technology, Legumes, Crop, Antioxidants, Encapsulation, Ca(II)-alginate beads, Microstructure, Small-angle X-ray scattering
Food Science; Food Analysis; Food Technology; legumes; crop; antioxidants; encapsulation; Ca(II)-alginate beads; microstructure; small-Angle X-ray scattering
1. Introduction
Cowpea (Vigna unguiculata) belongs to the Fabaceae family. It is considered as a very nutritious multi-purpose crop, which is mostly consumed in their dry form, even though it can also be ingested as a fresh green vegetable (immature seeds, leaves, and pods) [1, 2]. The plant shows excellent tolerance to heat and drought, which makes it a crop of high adaptability considering climate change [3]. Cowpea production extends worldwide, although its primary production is concentrated in sub-Saharan Africa, Asia, Central and South America, the Mediterranean region, and Southern United States [2]. During 2014, the world production of dried cowpea was around 5.589.216 metric tons, being Nigeria, the main producer. Argentina has great production, having reached 406 tons in 2008 and reaching a fifth place with the highest worldwide production in 2009. Unfortunately, nowadays, there are no updated statistics [4, 5]. Within the framework of a bioeconomy, cowpeas are frequently produced by small and medium farmers for personal consumption and/or trade-in northeastern Argentina. Although the weather and environmental conditions of this region are ideal for large-scale cultivation, extensive bioprospecting work is needed to promote its nutritional, technological uses, and applications.
Following the FAO recommendations and the growing interest in the revaluation of agricultural by-products as abundant, bio-renewable, and low-cost resources, the use of cowpea waste presents great potential [6]. In this context, dried cowpea pods are considered as lignocellulosic biomass, which contain polymerized sugar as cellulose and hemicellulose [7] and which have high concentrations of phenolic compounds, which tend to increase with the maturity of the plant while decreasing concentration in tannins [8]. According to the research carried out by Awika & Duodu [3], cowpea has a high content of phenolic acids, flavonoids (flavonols, tannins, anthocyanins), and bioactive peptides, although their content varies depending on the phenotype and variety. On the other hand, in a recent study, Avanza et al. [9] have shown that cowpea pods have a high content of total phenolic compounds (43.8 ± 0.3 mgequivalents of gallic acid g−1) and a high antioxidant activity (0.460 ± 0.004 mmolequivalents of trolox g−1); moreover, they established that their extraction increased significantly if they are assisted by technologies such as extraction by pressurized liquids or high-intensity ultrasound.
In this context, ultrasound-assisted extraction (UAE) of phenolic compounds represents a very promising tool, which has already been applied in legume matrices. Hayta & Isçimen [10] have shown that the optimal condition for phenolic compounds extraction in chickpeas is using a solid/liquid ratio of 0.4, a total treatment time of 20.17 min, and amplitude of 36.16%. Ryu & Koh [11] optimized the extraction of anthocyanins, total phenolic compounds, and antioxidant activity of black soybeans using an amplitude of 81.4% for 8.59 min. The importance of polyphenolic compounds lies in their anti-cancer, anti-hypertensive, anti-inflammatory, anti-diabetic properties, and in that they prevent cardiovascular diseases [3].
Despite the great benefits that these compounds provide to health, their industrialization represents a challenge since they are generally susceptible to the external environment, processing, and gastrointestinal conditions [12]. In this context, the encapsulation of phenolic compounds in biopolymer matrices represents a great possibility in their incorporation and consumption in different food systems [13]. The effectiveness of Ca(II)-alginate hydrogels in the stabilization and controlled release of biomolecules has been widely demonstrated [14, 15, 16].
The present research is focused on giving added-value to cowpea wastes from the optimization of extraction of the phenolic compounds from the pod by the application of high-intensity ultrasound and its subsequent encapsulation in Ca(II)-alginate for their conservation and stability. Microstructural analysis by SAXS was also performed to establish structural-functional relationships on the matrix and the effect of the added excipients.
2. Materials and methods
2.1. Extract: obtention and optimization
2.1.1. Raw material
Cowpea pods variety Colorado harvested in 2018 were provided by Estación Experimental El Sombrero Corrientes (Instituto Nacional de Tecnología Agropecuaria-INTA), Argentina. Pods were sun-dried and stored in a hermetic vessel at 10 °C until processing. Cowpea pods were grounded in a high-speed mill (ARCANO FW100, Argentina) and passed through a 60 mesh sieve or 250 μm, to obtain uniform particle size flour [17]. The pod flour was maintained at 4 °C until used.
2.1.2. Experimental design
To obtain the optimum conditions for UAE of phenolic compounds, a response surface methodology (RSM) was carried out using Design Expert® (Design Expert, Stat-Ease Inc., Minneapolis, MN). A hexagonal design (or Doehlert design or Doehlert Uniform Shell design) has been used to develop the RSM [18]. It consisted of 9 runs, as shown in Figure 1. The effects of two independent variables: extraction time (X1, min) and ultrasound extraction power (amplitude; X2, %), on the total phenolic compounds content and the antioxidant activity were studied. The coded and corresponding actual values are shown in Table 1, using five and three levels for each variable, respectively. The experimental data were fitted to a second-order polynomial model to obtain the regression coefficients. Regression analysis was performed with the following equation:
| (1) |
where is the response (dependent variable); is the intercept, and , and corresponds to the linear, quadratic, and cross-product regression coefficients. and are the independent variables, and is the number of tested variables.
Figure 1.
Doehlert experimental design showing the 9 runs.
Table 1.
Coded and actual values of the experimental design for the optimization of the extraction (assisted by ultrasound) of phenolic compounds content and antioxidant capacity of cowpea pods.
| Std | Run | Coded Value |
Real Value |
||
|---|---|---|---|---|---|
| time (min) | US Amplitude (%) | time (min) | US amplitude (%) | ||
| 2 | 1 | -0.5 | 1 | 12.5 | 80 |
| 6 | 2 | -0.5 | -1 | 12.5 | 20 |
| 5 | 3 | 0.5 | -1 | 17.5 | 20 |
| 7 | 4 | 0 | 0 | 15.0 | 50 |
| 8 | 5 | 0 | 0 | 15.0 | 50 |
| 4 | 6 | 1 | 0 | 20.0 | 50 |
| 9 | 7 | 0 | 0 | 15.0 | 50 |
| 3 | 8 | 0.5 | 1 | 17.5 | 80 |
| 1 | 9 | -1 | 0 | 10.0 | 50 |
Design Expert software's graphical and numerical optimization technique was used to optimize the response following the criterion of desirability. Three runs of the optimum solution were conducted and introduced on the model for the point prediction.
2.1.3. Ultrasound-assisted extraction
Before RSM, the optimal relation of pod flour/distilled water and the effect of the maceration and ultrasound post-treatment were analyzed, as shown in Table 2, selecting 1:15 as the optimal ratio with no post-treatment. Then, a solution with a relation of 1:15 pod flour/distilled water was placed into 100 mL beaker and stirred for 10 min at room temperature. Subsequently, high-intensity ultrasound was applied using an ultrasound probe (220-B, CV334 model, Sonics, USA) with 13 mm tip diameter connected to a high-intensity ultrasonic processor with temperature control (VCX500, Sonics, USA). The probe was immersed into the solution at a frequency of 20 kHz, and a maximum power of 500 W. The treatments were performed according to the conditions obtained from the experimental design (times: 10, 12.5, 15, 17.5 and 20 min; amplitudes: 20, 50 and 80%) for the independent variables. The time and temperature ranges were selected in preliminary experiments and considering Hayta & Isçimen's work [10]. The overheating of the sample was prevented by covering the beaker with ice. After the ultrasonic treatment, the solution was centrifuged at 6000 rpm (7647 g) and 25 °C for 15 min. Then, it was filtered through filter paper on a Buchner funnel under vacuum condition, and the supernatants were collected and freeze-dried (model Christ Alpha 1–4 LO, Martin Christ, Osterode am Harz, Germany).
Table 2.
Phenolic compounds content and TEACABTS•+ for cowpea pods subjected to different extraction treatments.
| Treatment | Relation cowpea pod flour/distilled water | Phenolic compounds content (mgGAE/mL) | TEACABTS•+ (mmol/L) |
|---|---|---|---|
| US (80% amplitude/15 min) | 1:10 | 0.36 ± 0.01b∗ | 8.5 ± 0.3b |
| US (80% amplitude/15 min) | 1:15 | 0.37 ± 0.01ab | 9.7 ± 0.3a |
| US (80% amplitude/15 min) | 1:20 | 0.33 ± 0.01c | - |
| US (80% amplitude/15 min) + Maceration (1 h) | 1:10 | 0.42 ± 0.04a | 5.5 ± 0.3c |
Standard deviation values are included. Different letters on the columns (a−c) indicate significant differences (p < 0.05).
2.1.4. Extract characterization
Phenolic compounds content for cowpea extracts was determined as follows: an aliquot of 800 μL of distilled water, 125 μL of the Folin Ciocalteu reagent (Biopack®, Zárate, Buenos Aires, Argentina) and 125 μL of a solution of Na2CO3 (20% w/v) was added to 50 μL of the sample. After 30 min of reaction at 40 °C in the dark, the absorbance at 765 nm was measured. Results were expressed as mg gallic acid equivalents/mL (mgGAE/mL) through a calibration curve [19].
Antioxidant activity was determined as Trolox equivalents antioxidant capacity assay (TEAC), using the method described by Re et al. [20] with some modifications. The ABTS•+ radical was produced by reacting 7 mM ABTS and 2.45 mM potassium persulfate in the dark at room temperature (25 °C) during 16 h before use. The aqueous ABTS•+ solution was diluted with 5 mM phosphate buffer (pH 7.4) to an absorbance of 0.70 (±0.02) at 734 nm. The samples (10 μL, 5 different concentrations from 0.2 to 3.33 % v/v) and 1 mL of ABTS•+ solution were mixed in a microcentrifuge tube. After incubation for 45 min, 300 μL of the mixture was transferred into a 96-well microplate. The absorbance of initial and endpoints was measured at 734 nm in a microplate spectrophotometer reader (Multiskan GO Microplate Spectrophotometer, Thermo Scientific™, Vantaa, Finland). Trolox was used as a standard reference, and the results were expressed as TEAC values (mmol of Trolox/L extract). These values were obtained from five different concentrations of each extract that were tested in the assay giving a linear response between 20% and 80% of the blank absorbance.
Protein content in extracts was measured by the Kjeldahl method (N x 6.25, AOAC) [21]. Iron was determined by using a microwave plasma-atomic emission spectrometry (model 4100 MP-AES, Agilent Technologies, Melbourne, Australia).
All determinations were done in triplicate.
2.2. Encapsulation procedure
2.2.1. Materials
Sodium alginate (Cargill S.A., San Isidro, Buenos Aires, Argentina), molecular weight of 1.97·105 g/mol, mannuronate/guluronate ratio of 0.6, and free of proteins or other metal cations impurities that can affect microstructure; arabic gum (AG, from Biopack, Zárate, Buenos Aires, Argentina), molecular weight of 250.000 g/mol and purity of 99%; guar gum (GG, from Cordis S.A., Villa Luzuriaga, Buenos Aires, Argentina), molecular weight of 220.000 g/mol, mannose/galactose ratio of 1.8 and protein content of 2.1 ± 0.3 g/100 g dry gum. Cowpea isolated protein was obtained according to Peyrano et al. [22] method at a pH of extraction of 10.
2.2.2. Gel beads generation
Ca(II)-alginate beads were generated by ionotropic gelation (drop method) [16, 23]. Five different systems with extract (E) were obtained: alginate (EA); alginate-arabic gum (EAAG); alginate-guar gum (EAGG); and two alginate-cowpea protein at alginate:protein 2:1 (EAP2:1) or 1:1 (EAP1:1) ratios. Control systems without extract for each system for comparison purposes. Due to the pH of the extract solution is ≈ 5.5, all the solutions were prepared in 0.1 M acetate buffer pH 5.5, to obtain an electrostatic interaction between the alginate and the extract. A peristaltic pump model BT50-1J-JY10 (Baoding Longer Precision Pump Co, Ltd, China) was used to drop 5 mL of the solutions into 50 mL of the gelling solution. For the preparation of the beads for all systems, 1.5% (w/v) alginate solution containing the extract was dropped into 2.5% (w/v) CaCl2 solution (prepared in 0.1 M acetate buffer pH 5.5). 0.25% (w/v) of arabic or guar gums were added to the sodium alginate solution for EAAG and EAGG, respectively. For EAP1:1 and EAP2:1, cowpea protein isolates were added to the solution at alginate:protein ratios of 1:1 and 2:1, respectively [24]. The operating conditions followed for the encapsulation process were previously described in Traffano-Schiffo et al. [16].
2.2.3. Beads characterization
2.2.3.1. Phenolic compounds content of the beads
Phenolic compounds content was measured following the same protocol described above in extract characterization (section 2.1.). However, 40 beads were previously dissolved with 200 μL of sodium citrate 20 % (w/v) and maintained under stirring at room temperature (25 °C) during at least 40 min. Loading efficiency of phenolic compounds on the beads was estimated as follows [23]:
| (2) |
where corresponds to total phenolic compounds content in the beads or the extract. The was normalized by the size and number of beads, considering a volume of a sphere ( for each system, where corresponds to the half of Feret's diameter.
2.2.3.2. Antioxidant activity
The activity of the antioxidants was determined by detecting their ability to scavenge the ABTS•+ free radical [20]. Firstly, 250 μL of sodium citrate 20% (w/v) was added to 40 beads and maintained under stirring at room temperature during at least 40 min to dissolve the Ca(II)-alginate beads. The measurement was conducted following Aguirre Calvo et al. [23]. The percentage of inhibition was calculated against control and compared to a gallic acid standard curve and expressed as mg gallic acid equivalents (GAE)/mL [25].
The ABTS remaining activity was calculated as follows [23]:
| (3) |
where, corresponds to the antioxidant activity (beads or extract). Antioxidant activity of the beads was normalized as was described for .
2.2.3.3. Digital image analysis
Morphology (area, Feret's diameter, and circularity) of at least 40 beads was analyzed through digital images by ImageJ software (http://rsbweb.nih.gov/ij/) [26].
2.2.3.4. Microstructure
Microstructure analysis was carried out by small-angle X-ray scattering (SAXS) at the LNLS SAXS1 beamline in Campinas (Brazil), working at λ = 0.1488 nm and a vector (q) range between 0.142 nm−1< q < 5.035 nm−1. All the Ca(II)-alginate beads analyzed showed isotropic scattering and were modeled as a fractal system composed of rod-like structures. Five parameters were analyzed, as fully described in Traffano-Schiffo et al. [16] and Aguirre Calvo et al. [27]. All measurements were made in triplicate. Parameters α1, α2 and α3 were evaluated from the slope of the scattering intensity averaged along with azimuthal angles versus the scattering vector q in the log-log scale by using log-log line fitting with Prism 6 (GraphPad Software Inc., San Diego, CA, USA). Kratky plot was conducted as [16] using the same software.
2.2.3.5. Physico-chemical determinations
Moisture was obtained gravimetrically drying the beads in a vacuum oven for 48 h at 96 ± 2 °C. Water activity (aw) was determined by a dew point Hygrometer Decagon (Aqualab®, series 3 TE, Decagon Devices, Pullman, WA, USA), using a unique sample holder to reduce the size of the chamber. A calibration curve was carried out with salts with known aw [28]. Measurements were performed in triplicate.
2.3. Statistical analyses
One-way ANOVA with Tukey's post-test was conducted using Prism 6 (GraphPad Software Inc.) to determine significant differences among means.
3. Results and discussion
3.1. Ultrasound-assisted extraction
The effect of the time and the amplitude of the high-intensity ultrasound treatment on phenolic compounds extraction and extract antioxidant capacity was studied. The objective was to maximize the antioxidant properties of the resulting solution. The nine experiments performed following a surface response methodology (Doehlert design) of two factors produced phenolic compound contents ranging from 0.23 to 0.29 mgGAE/mL. The antioxidant capacity, expressed as TEAC, ranged from 6.85 to 12.38 mmol/L. Three-dimensional response surface and two-dimensional contour plots, which correspond to the graphical representations of the regression are shown in Figures 2 and 3, revealing a strong relationship between the factors and the response values.
Figure 2.
Response surface plots of cowpea pod flour showing the effect of the ultrasound on a. phenolic compounds content and b. antioxidant activity expressed as TEACABTS.+.
Figure. 3.
Countor plots of desirability, phenolic compounds content and TEACABTS.+ as a function of time and ultrasound (US) amplitude.
Figures 2 and 3 show that the highest phenolic compounds extraction was obtained at low amplitudes, being the maximum between 20 to 55%, while the maximum antioxidant activity was obtained at the lowest time (10 min). ANOVA was used to analyze the significance and the suitability of the adjusted models (Table 3). It should be taking into account that F-value compares the mean square with the residual mean square, and p-values were used as a tool to check the significance of each coefficient, which might indicate the pattern of interaction between the variables [29]. The results obtained for phenolic compounds content model were: F-value of 331.28, the p-value of 0.0003, a determination coefficient (R2) equal to 0.9982 and a very low coefficient of the variation (CV = 0.625%), which indicate a very high significance of the model and degree of precision, with a good deal of reliability of the experimental values. The linear coefficients ( and ) and the quadratic terms (and ) were significant. Similar results were obtained for TEACABTS.+, also with a high significance of the model (F-value = 23.91; p-value = 0.0127, = 0.9755 and CV = 4.93%); however, even though the linear coefficient () for the amplitude of the US treatment was not significant (p-value = 0.9737), the quadratic term was (0.0395), indicating that it also influenced the antioxidant activity of the extract. The validity of models was also confirmed using the lack of fit testing, where the values obtained for two responses were not significant (p > 0.05).
Table 3.
ANOVA for response surface models: estimated regression model of the relationship between response variables (phenolic compounds content and TEAC) and independent variables ( and ).
| Sum of squares | df∗ | Mean square | F-value | p-value | ||
|---|---|---|---|---|---|---|
|
Phenolic compounds content (mgGAE/mL) | ||||||
| Model | 0.0047 | 5 | 0.0009 | 331.28 | 0.0003 | significant |
| - time | 0.0002 | 1 | 0.0002 | 68.74 | 0.0037 | |
| - US amplitude | 0.0026 | 1 | 0.0026 | 901.16 | <0.0001 | |
| 1.44✕10−06 | 1 | 1.44✕10−06 | 0.5040 | 0.5290 | ||
| 0.0003 | 1 | 0.0003 | 97.36 | 0.0022 | ||
| 0.0019 | 1 | 0.0019 | 662.83 | 0.0001 | ||
| Residual | 8.55✕10−06 | 3 | 2.85✕10−06 | |||
| Lack of Fit | 9.09✕10−07 | 1 | 9.09✕10−07 | 0.2379 | 0.6740 | not significant |
| Pure Error | 7.64✕10−06 | 2 | 3.82✕10−06 | |||
|
Total |
0.0047 |
8 |
||||
|
TEACABTS.+(mmol/L) | ||||||
| Model | 21.29 | 5 | 4.26 | 23.91 | 0.0127 | significant |
| - time | 4.39 | 1 | 4.39 | 24.65 | 0.0157 | |
| - US amplitude | 0.0002 | 1 | 0.0002 | 0.0013 | 0.9737 | |
| 0.6519 | 1 | 0.6519 | 3.66 | 0.1517 | ||
| 11.42 | 1 | 11.42 | 64.11 | 0.0041 | ||
| 2.18 | 1 | 2.18 | 12.25 | 0.0395 | ||
| Residual | 0.5343 | 3 | 0.1781 | |||
| Lack of Fit | 0.0686 | 1 | 0.0686 | 0.2947 | 0.6416 | not significant |
| Pure Error | 0.4657 | 2 | 0.2328 | |||
| Total | 21.82 | 8 | ||||
df, degree of freedom.
The fitted quadratic models for phenolic compounds content and TEAC are given in Eqs. (4) and (5), respectively (for coded factors).
| (4) |
| (5) |
Table 4 shows the values predicted from model, and the experimental data fitted in Figures 2 and 3 obtained for the nine experiments. The error percentage was also included, showing low values overall as a consequence of the good fitting (Table 3).
Table 4.
Actual values of the experimental design, model values calculated from model, and experimental values (phenolic compounds content and TEACABTS.+).
| Run | Factors |
Phenolic compounds content (mgGAE/mL) |
TEACABTS•+ (mmol/L) |
|||||
|---|---|---|---|---|---|---|---|---|
| time (min) | US amplitude (%) | Experimental | Model | Error % | Experimental | Model | Error % | |
| 1 | 12.5 | 80 | 0.2333 | 0.2329 | 0.2 | 7.83 | 7.94 | 1 |
| 2 | 12.5 | 20 | 0.2852 | 0.2848 | 0.1 | 8.65 | 8.76 | 1 |
| 3 | 17.5 | 20 | 0.2751 | 0.2755 | 0.1 | 6.85 | 6.74 | 2 |
| 4 | 15.0 | 50 | 0.2903 | 0.2884 | 0.7 | 7.58 | 7.98 | 5 |
| 5 | 15.0 | 50 | 0.2864 | 0.2884 | 0.7 | 8.52 | 7.98 | 7 |
| 6 | 20.0 | 50 | 0.2655 | 0.2651 | 0.2 | 9.75 | 9.86 | 1 |
| 7 | 15.0 | 50 | 0.2885 | 0.2884 | 0.1 | 7.85 | 7.98 | 2 |
| 8 | 17.5 | 80 | 0.2256 | 0.2261 | 0.2 | 7.64 | 7.53 | 1 |
| 9 | 10.0 | 50 | 0.2809 | 0.2813 | 0.1 | 12.38 | 12.28 | 1 |
The optimal extraction condition for the highest extraction of phenolic compounds and antioxidant activity was 10 min and 36% of US-amplitude (Table 5), the representative's solution of the zone observed for the desirability function (Figure 3). According to the validation of the optimal condition, the best condition is consistent with the results obtained by Hayta, & İşçimen [10] for chickpea (20 min and 36% of amplitude) but employing lower time.
Table 5.
Model and experimental values obtained for the validation of the experimental design.
| Run | Factors |
Desirability | Phenolic compounds content (mgGAE/mL) |
TEAC (mmol/L) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| time (min) | US amplitude (%) | Experimental | Model | Error % | Experimental | Model | Error % | ||
| 1 | 10 | 36 | 0.983 | 0.3228 | 0.2873 | 12 | 10.49 | 12.38 | 15 |
The extract was also fully characterized, showing a yield of 6.7 ± 0.4, 7.82 ± 0.02% of protein, and 18.7 ± 0.7 mgiron/kgdried sample.
3.2. Cowpea phenolic compounds encapsulation
Ca(II)-alginate beads were successfully prepared and are showed in Figure 4. Ca(II)-alginate beads are hydrogels containing the phenolic compounds and water. Water is a small molecule that can diffuse through the matrix affecting the structure of the hydrogel and leading the contraction or the expansion of the beads [30]. Besides, the interaction of the biopolymers within the matrix can change water availability. Table 6 shows the aw and the water content for all the studied systems, obtaining aw values between 0.948 and 0.957, which are consistent with other published works [26, 28]. The aw of EA is significantly lower compared with the beads containing gums, probably due to the presence of the hydroxyl groups in the structure of the biopolymers (arabic and guar gums), which are available to interact with water molecules; while the beads containing protein showed intermediate values, which could be related to the hydrogen bonds established by cowpea protein and water balanced by hydrophobic interactions of the protein [24].
Figure 4.
Formulations used for Ca(II)-alginate beads generation and image analysis (original image and outline from particle analysis).
Table 6.
Water activity (aw) and water content (xw) of the beads.
| Systems | aw | xw (kgw/kgT) |
|---|---|---|
| EA | 0.948 ± 0.003b∗ | 0.96 ± 0.003a |
| EAAG | 0.957 ± 0.004a | 0.960 ± 0.003ab |
| EAGG | 0.957 ± 0.003a | 0.956 ± 0.003bc |
| EAP2:1 | 0.956 ± 0.003ab | 0.955 ± 0.003c |
| EAP1:1 | 0.953 ± 0.003ab | 0.938 ± 0.003d |
E, extract; A, alginate; AG, arabic gum; GG, guar gum and P, cowpea protein. Standard deviation values are included. Different letters on the columns (a−d) indicate significant differences (p < 0.05).
The water content of the beads ranged from 0.938 to 0.962 kgW/kgT. Beads containing cowpea isolated protein showed significantly lower water content, probably due to the preference of the polypeptides of cowpea to interact with each other than with water [24] or to interact with the alginate and/or the extract.
The morphology of the beads was also studied, and the results are in Figure 5. Feret's diameter of the beads with cowpea pods extracts (Figure 5a) was higher than the obtained in previous works with encapsulated enzymes, reaching values close to 1.7 mm [28, 31], but this increment is related to their higher alginate content used in present work. Regarding the circularity and the area of the beads, systems that contain proteins showed significantly higher values in both parameters comparing with the other systems (with alginate and alginate/gums). This behavior could be due to the isolated cowpea proteins, which mainly contain legumins (11S-hexameric) and vicilins (7S-trimeric), both globular proteins with a molecular weight between 950 and 1300 Da [32], generating bigger beads with more uniform shape.
Figure 5.
a. Feret's diameter, b. circularity and c. area of beads with different compositions. E, extract; A, alginate; AG, arabic gum; GG, guar gum and P, cowpea protein. Standard deviation values are included. Different letters on the columns (a–c) indicate significant differences (p < 0.05).
One of the main objectives of this research was to encapsulate the optimum cowpea extract to retain high phenolic compounds content and antioxidant activity in the hydrogel. Thus, the loading efficiencies of total phenolic compounds and antioxidant activity in the beads with different compositions are shown in Figure 6.
Figure 6.
a. Loading efficiency of total phenolic compounds (L.E.TP) and b. remaining antioxidant activity of ABTS of encapsulated cowpea extract. E, extract; A, alginate; AG, arabic gum; GG, guar gum and P, cowpea protein. Standard deviation values are included. Different letters on the columns (a–c) indicate significant differences (p < 0.05).
As can be seen, EA and EAGG showed the highest loading efficiencies for both parameters, while EAAG, EAP2:1, and EAP1:1 showed no significant differences between them. It was previously observed that the addition of GG to Ca(II)-alginate beads produced higher entrapment of betacyanin and phenolic compounds extracted from beet stem and leaves [23]. However, these authors observed even a diminish of the phenolic compound encapsulation with the addition of arabic gum, which is probably related to the chemical characteristics of the extracted phenolic compounds. It was demonstrated that the use of US allows not only the extraction of more phenolic compounds concerning water extraction but also phenolic compounds which are not extracted during maceration due to the increase of cell wall permeability by the cavitation effects [33]; in this sense, the use of AG and proteins probably enhance their encapsulation due to the intrinsic characteristics of these excipients. Besides, the higher efficiencies produced by AG and GG are possible related to the stabilization of the microstructure of the beads at several levels, as further analysis may show, as already observed Traffano-Schiffo et al. [16] for Ca(II)-alginate beads containing lactase.
On the other hand, it is important to highlight that the antioxidant activity for beads containing isolated proteins at 1:1 ratio (EAP1:1) without subtraction of blank beads was significantly higher than other systems, reaching a value of 67 ± 3 expressed as the percentage of inhibition of the radical ABTS.+. This is because this blank beads (without extract) showed a very high % of inhibition of ABTS.+ of 30 ± 2 (AP1:1), while the others systems showed blank values of 4.8 ± 0.9, 4.8 ± 0.6, 5.0 ± 0.8 and 11.2 ± 0.4 % ABTS.+ inhibition for A, AAG, AGG, and AP2:1, respectively, confirming the bioactive potential of cowpea proteins. It should be noted that the beads have been generated at a pH = 5.5, and at this point, the isolated proteins show a 50% solubility [34]. Thus, this condition can produce changes in tertiary and quaternary structures of the proteins, leaving the protons of the amino acids exposed and available to be reduced by radical ABTS.+, supporting the antioxidant activity of the legume proteins. The antioxidant capacity of cowpea isolated protein was previously demonstrated by researches such as Rodrigues Marques et al. [35] and Xiong et al. [36].
Figure 7a shows the intensity plots versus the scattering vector (q) obtained by SAXS for Ca(II)-alginate beads containing cowpea extract. The Log-log SAXS profile should be divided into three regions to understand the microstructural information. At low q values, from the slope (blue lines) is possible to obtain parameter α1, which indicates the interconnectivity of the rods. At intermediate values of q, α2 determines the compactness within the rods, and at high values of q, α3 characterizes the connectivity between associated polymer chains forming dimers. Figure 7b shows the Kratky plot where the cross-sectional radius of the rods or R1 can be obtained. The size of the polymer dimers basic units or R2 was obtained from the crosspoint between α2 and α3 [16].
Figure 7.
a. log-log SAXS profile plots of different formulations of Ca(II)-alginate beads containing cowpea extract. Parameters α1, α2 and α3 were evaluated from the slope of the scattering intensity at low and high values of q, respectively. b. Kratky plots, where the radii of gyration of the rods (parameters R1) was obtained. E, cowpea extract; A, alginate; AG, arabic gum; GG, guar gum; P, cowpea protein; 2:1 and 1:1 corresponds to alginate:protein ratio.
The interconnectivity of the rods (α1) for all the formulations with/without cowpea extract is shown in Figure 8. All the blank beads showed a significantly lower α1 than the corresponding system with the extract. Thus, the extract favored the interconnectivity of the rods, probably due to the presence of trivalent cations, particularly iron, which can interconnect the structure via pre-coordination of carboxylic groups from three alginate chains. Similar results have been obtained by Aguirre Calvo et al. for beet extracts containing Fe3+ [23]. However, the system that contained cowpea isolated protein in a 1:1 ratio with alginate did not exhibit this behavior, changing from 1.93 ± 0.01 in the control system to 1.78 ± 0.05 in the system with the cowpea extract (Figure 8). The high concentration of the protein strongly affected the alginate network by increasing the interconnectivity of rods. This microstructural change also impacted in the morphology of the beads, giving beads of greater size and circularity (Figure 5). AG and GG did not produce any changes at this level, as previously observed [28].
Figure 8.
The fractal dimension of the rod network or parameter α1 of the microstructure of Ca(II)-alginate beads with/without extract, derived from log-log SAXS profiles. Standard deviation values are included. E, cowpea extract; A, alginate; AG, arabic gum; GG, guar gum; P, cowpea protein; 2:1 and 1:1 corresponds to alginate:protein ratio. Different lowercase letters on the columns (a–b) indicate significant differences between each system and its blank (p < 0.05). Different capital letters on the columns (A–B) indicate significant differences between different systems with cowpea extract (p < 0.05).
Figure 9 shows the degree of compactness and the size of the rods (α2 and R1, respectively). As can be appreciated, the presence of the extract in the formulation of the beads significantly increased the compactness and the size (cross-sectional radius) of the rods, which is in concordance with the presence of trivalent cations in the extract and with the results obtained for α1 parameter. As was explained by Sonego et al. [37] and reported by Aguirre Calvo et al. [23], trivalent cations confer to the nanostructure of the hydrogels more versatility and larger coordination environment, resulting in the expansion of the chains within the hydrogel and the ramification of the network. Regarding the size of the rods, the values obtained for EA, EAAG, EAGG, and EAP2:1 were between 8.53 and 8.8 nm, while the formulation with the higher isolated protein concentration reached 13.2 nm. Therefore, EAP1:1 exhibited lower compactness and a higher cross-sectional radius, which may be due to some steric hindrance within the network by the globular structure of the proteins. These results are in agreement with the previous results obtained by the macrostructural analysis (Figure 5). AG and GG reduced rod size and compactness, as previously observed [28], which is related to the steric hindrance along alginate chains produced by the gums [28]. These changes affect loading efficiency (Figure 6) as previously observed Traffano-Schiffo and co-workers with lactase [28], as related to the extent of interaction between the excipients (being involved inside the rods, or outside of them) and the encapsulated agent.
Figure 9.
Microstructure parameters of Ca(II)-alginate beads with/without extract. a. Fractal dimension at distances lower than the characteristic size of the rods or parameter α2 of the microstructure derived from log-log SAXS profiles. b. Rod cross-sectional radius (R1) deduced from the maxima obtained on Kratky plots. Standard deviation values are included. E, cowpea extract; A, alginate; AG, arabic gum; GG, guar gum; P, cowpea protein; 2:1 and 1:1 corresponds to alginate:protein ratio. Different lowercase letters on the columns (a–b) indicate significant differences between each system and its blank (p < 0.05). Different capital letters on the columns (A–D) indicate significant differences between different systems with cowpea extract (p < 0.05).
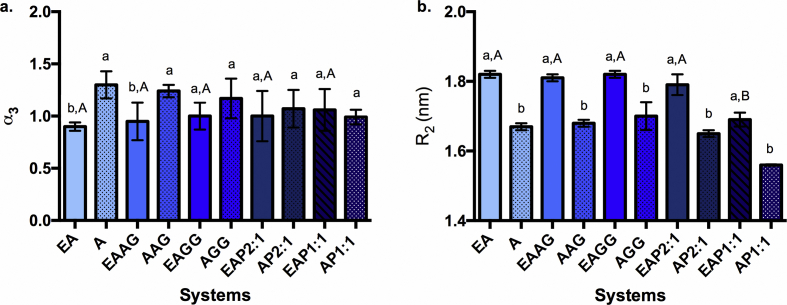
At higher scattering intensity, the information of the smaller units can be obtained: α3 and R2, which respectively characterizes the connectivity between associated polymer chains and the size of the polymer dimers or basic units, are shown in Figure 10. Once again, there was a marked effect for the presence of the extract by increasing R2, showing that its presence modulates the nanostructure of the dimers, as previously observed, Aguirre Calvo and co-workers [23], which is compatible with the hypothesis of the extract being intercalated within the dimers. A decrease in their compactness was observed in two systems (A and AAG) while the others remain unmodified, which should be further clarified by performing more measurements since the SD obtained at this scale for this parameter are quite high and may hide the observed trend. The most relevant change regarding the inclusion of excipients was the significantly smaller R2 for EAP1:1 than for the other systems. Therefore, considering the data at different scales, the addition of cowpea isolated protein (at high concentrations) produces structures with small basic units of dimers but highly interconnected, conforming bigger rods as well as beads.
Figure 10.
Microstructure parameters of Ca(II)-alginate beads with/without extract. a. Fractal dimension at distances lower than R2 or parameter α3 of the microstructure derived from log-log SAXS profiles. b. Characteristic size of the Ca(II)-alginate dimers (R2). Standard deviations values are included. E, cowpea extract; A, alginate; AG, arabic gum; GG, guar gum; P, cowpea protein; 2:1 and 1:1 corresponds to alginate:protein ratio. Different lowercase letters on the columns (a–b) indicate significant differences between each system and its blank (p < 0.05). Different capital letters on the columns (A–B) indicate significant differences between different systems with cowpea extract (p < 0.05).
4. Conclusions
In the present research article, high-intensity ultrasound-assisted extraction of phenolic compounds from cowpea waste was performed using a Doehlert experimental design with two factors, showing that the best condition of the US-treatment was 10 min and 36% of amplitude.
The optimum cowpea pods extract was successfully encapsulated in Ca(II)-alginate by the ionotropic gelation method. Beads containing isolated cowpea protein showed higher area and circularity probably because of legumins and vicilins globular proteins. A deep microstructural analysis of the beads has been performed by SAXS. The results showed that beads containing cowpea protein at the highest alginate:protein ratio had the biggest rods which are highly interconnected; however, they are less compact than the other systems. Cowpea pod extract favors the interconnectivity of the rods due to the presence of Fe3+, which confers versatility and a larger coordination environment to the network.
EA and EAGG systems showed the highest loading efficiency of total phenolic compounds and antioxidant activity. However, taking into account the results obtained for the percentage of inhibition of the radical ABTS.+, the formulation containing cowpea isolated protein at the higher alginate:protein ratio (1:1) was significantly higher than the others. The analysis of the control beads without extract showed that isolated cowpea proteins present a high antioxidant capacity, demonstrating its great potential as a bioactive ingredient for Ca(II)-alginate beads.
This research highlighted the usefulness of the cowpea sub-products as a source of bioactive compounds, which can be incorporated in techno-functional foods by using Ca(II)-alginate network as a carrier.
Declarations
Author contribution statement
Maria V. Traffano-Schiffo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Tatiana R. Aguirre Calvo: Performed the experiments; Analyzed and interpreted the data.
María V. Avanza: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Patricio R. Santagapita: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Brazilian Synchrotron Light Laboratory (LNLS, Brazil, proposal SAXS1-20190143 and SAXS1-20190073), Universidad de Buenos Aires (UBACyT 20020130100610BA), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT PICT 2017-0569 and 2017-1744), Consejo Nacional de Investigaciones Científicas y Técnicas (RESOL-2019-574-APN-DIR#CONICET, code 229 20180100023 CO) and Secretaría General de Ciencia y Técnica of UNNE (PI: 16F017 RES 970/16 CS).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Collado E., Klug T.V., Artés-Hernández F., Aguayo E., Artés F., Fernández J.A., Gómez P.A. Quality changes in nutritional traits of fresh-cut and then microwaved cowpea seeds and pods. Food Bioprocess Technol. 2019;12(2):338–346. [Google Scholar]
- 2.Lazaridi E., Ntatsi G., Fernández J.A., Karapanos I., Carnide V., Savvas D., Bebeli P.J. Phenotypic diversity and evaluation of fresh pods of cowpea landraces from Southern Europe. J. Sci. Food Agric. 2017;97(13):4326–4333. doi: 10.1002/jsfa.8249. [DOI] [PubMed] [Google Scholar]
- 3.Awika J.M., Duodu K.G. Bioactive polyphenols and peptides in cowpea (Vigna unguiculata) and their health promoting properties: a review. J. Func. Foods. 2017;38:686–697. [Google Scholar]
- 4.Avanza M.V., Acevedo B., Chaves M., Añon M. Nutritional and anti-nutritional components of four cowpea varieties under thermal treatments: principal component analysis. LWT-Food Sci. Technol. 2013;51(1):148–157. [Google Scholar]
- 5.Saka J.O., Agbeleye O.A., Ayoola O.T., Lawal B.O., Adetumbi J.A., Oloyede-Kamiyo Q.O. Assessment of varietal diversity and production systems of cowpea (Vigna unguiculata (L.) Walp.) in Southwest Nigeria. J. Agric. Rural Dev. Tropics Subtropics. 2019;119:43–52. [Google Scholar]
- 6.FAO . Food and Agriculture Organization of the United Nation (FAO); Rome: 2017. FAOSTAT Online Statistical Services: Crop Production Data.http://www.fao.org/faostat/en/#data/QC Available at: [Google Scholar]
- 7.Onyelucheya C.M., Nwabanne T.J., Onyelucheya O.E., Onuoha O.E. Dilute acid hydrolysis of cowpea hulls: a kinetic study. Int. J. Adv. Sci. Eng. Inf. Technol. 2016;6(4):451–455. [Google Scholar]
- 8.Chikagwa-Malunga S.K., Adesogan A.T., Szabo N.J., Littell R.C., Phatak S.C., Kim S.C., Krueger N.A. Nutritional characterization of Mucuna pruriens: 3. Effect of replacing soybean meal with Mucuna on intake, digestibility, N balance and microbial protein synthesis in sheep. Anim. Feed Sci. Technol. 2009;148(2-4):107–123. [Google Scholar]
- 9.Avanza M.V., Alvarez Rivera G., Mendiola J., Ibañez E. XCII Congreso Cytal 2019 y XXI Congreso Alaccta 2019. Buenos Aires, Argentina. 2019. Obtención de ingredientes bioactivos mediante tecnologías verdes. [Google Scholar]
- 10.Hayta M., İşçimen E.M. Optimization of ultrasound-assisted antioxidant compounds extraction from germinated chickpea using response surface methodology. LWT. 2017;77:208–216. [Google Scholar]
- 11.Ryu D., Koh E. Optimization of ultrasound-assisted extraction of anthocyanins and phenolic compounds from black soybeans (Glycine max L.) Food Analy.l Meth. 2019:1–8. [Google Scholar]
- 12.Aguirre Calvo T.R., Molino S., Perullini M., Rufián-Henares J.A., Santagapita P.R. Effect of in vitro digestion-fermentation of Ca(II)-alginate beads containing sugar and biopolymers over global antioxidant response and short chain fatty acids production. Food Chem. 2020 doi: 10.1016/j.foodchem.2020.127483. In press. [DOI] [PubMed] [Google Scholar]
- 13.Traffano-Schiffo M.V., Castro-Giraldez M., Fito P.J., Santagapita P.R. Encapsulation of lactase in Ca (II)-alginate beads: effect of stabilizers and drying methods. Food Res. Int. 2017;100:296–303. doi: 10.1016/j.foodres.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 14.da Silva Carvalho A.G., da Costa Machado M.T., Barros H.D.D.F.Q., Cazarin C.B.B., Junior M.R.M., Hubinger M.D. Anthocyanins from jussara (Euterpe edulis Martius) extract carried by calcium alginate beads pre-prepared using ionic gelation. Powder Technol. 2019;345:283–291. [Google Scholar]
- 15.Gorbunova N., Bannikova A., Evteev A., Evdokimov I., Kasapis S. Alginate-based encapsulation of extracts from beta Vulgaris cv. beet greens: stability and controlled release under simulated gastrointestinal conditions. LWT. 2018;93:442–449. [Google Scholar]
- 16.Traffano-Schiffo M.V., Castro-Giraldez M., Fito P.J., Perullini M., Santagapita P.R. Gums induced microstructure stability in Ca (II)-alginate beads containing lactase analyzed by SAXS. Carbohydr. Polym. 2018;179:402–407. doi: 10.1016/j.carbpol.2017.09.096. [DOI] [PubMed] [Google Scholar]
- 17.Singh B., Singh N., Thakur S., Kaur A. Ultrasound assisted extraction of polyphenols and their distribution in whole mung bean, hull and cotyledon. J. Food Sci. Technol. 2017;54(4):921–932. doi: 10.1007/s13197-016-2356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chagas Moreira G., de Souza Dias F. Mixture design and Doehlert matrix for optimization of the ultrasonic assisted extraction of caffeic acid, rutin, catechin and trans-cinnamic acid in Physalis angulata L. and determination by HPLC DAD. Microchem. J. 2018;141:247–252. [Google Scholar]
- 19.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299(197):152–178. [Google Scholar]
- 20.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9-10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 21.AOAC . fifteenth ed. Association of Official Analytical Chemists; Washington: 1990. Official Methods of Analysis. [Google Scholar]
- 22.Peyrano F., de Lamballerie M., Avanza M.V., Speroni F. Calorimetric study of cowpea protein isolates. Effect of calcium and high hydrostatic pressure. Food Biophys. 2017;12(3):374–382. [Google Scholar]
- 23.Aguirre Calvo T.R., Santagapita P.R., Perullini M. Functional and structural effects of hydrocolloids on Ca (II)-alginate beads containing bioactive compounds extracted from beetroot. LWT. 2019;111:520–526. [Google Scholar]
- 24.Peyrano F., de Lamballerie M., Speroni F., Avanza M.V. Rheological characterization of thermal gelation of cowpea protein isolates: effect of processing conditions. LWT. 2019;109:406–414. [Google Scholar]
- 25.Wootton-Beard P.C., Moran A., Ryan L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin–Ciocalteu methods. Food Res. Int. 2011;44(1):217–224. [Google Scholar]
- 26.Aguirre Calvo T., Santagapita P. Physicochemical characterization of alginate beads containing sugars and biopolymers. J. Qual. Reliab. Eng. 2016;2016 [Google Scholar]
- 27.Aguirre Calvo T.R., Perullini M., Santagapita P.R. Encapsulation of betacyanins and polyphenols extracted from leaves and stems of beetroot in Ca (II)-alginate beads: a structural study. J. Food Eng. 2018;235:32–40. [Google Scholar]
- 28.Traffano-Schiffo M.V., Aguirre Calvo T.R., Castro-Giraldez M., Fito P.J., Santagapita P.R. Alginate beads containing lactase: stability and microstructure. Biomacromolecules. 2017;18(6):1785–1792. doi: 10.1021/acs.biomac.7b00202. [DOI] [PubMed] [Google Scholar]
- 29.Rahmani M., Ghasemi E., Sasani M. Application of response surface methodology for air assisted-dispersive liquid-liquid microextraction of deoxynivalenol in rice samples prior to HPLC-DAD analysis and comparison with solid phase extraction cleanup. Talanta. 2017;165:27–32. doi: 10.1016/j.talanta.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 30.Zazzali I., Aguirre Calvo T.R., Ruíz-Henestrosa V.M.P., Santagapita P.R., Perullini M. Effects of pH, extrusion tip size and storage protocol on the structural properties of Ca (II)-alginate beads. Carbohydr. Polym. 2019;206:749–756. doi: 10.1016/j.carbpol.2018.11.051. [DOI] [PubMed] [Google Scholar]
- 31.Santagapita P.R., Mazzobre M.F., Buera M.P. Invertase stability in alginate beads: effect of trehalose and chitosan inclusion and of drying methods. Food Res. Int. 2012;47(2):321–330. [Google Scholar]
- 32.Shevkani K., Kaur A., Kumar S., Singh N. Cowpea protein isolates: functional properties and application in gluten-free rice muffins. LWT-Food Sci. Technol. 2015;63(2):927–933. [Google Scholar]
- 33.Wang C., Tallian C., Su J., Vielnascher R., Silva C., Cavaco-Paulo A., Guebitz G.M., Fu J. Ultrasound-assisted extraction of hemicellulose and phenolic compounds from bamboo bast fiber powder. PloS One. 2018;13(6) doi: 10.1371/journal.pone.0197537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horax R., Hettiarachchy N.S., Chen P., Jalaluddin M. Functional properties of protein isolate from cowpea (Vigna unguiculata L. Walp.) J. Food Sci. 2004;69(2):119–121. [Google Scholar]
- 35.Rodrigues Marques M., Freitas R.A.M.S., Carlos A.C.C., Siguemoto É.S., Fontanari G.G., Arêas J.A.G. Peptides from cowpea present antioxidant activity, inhibit cholesterol synthesis and its solubilisation into micelles. Food Chem. 2015;168:288–293. doi: 10.1016/j.foodchem.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 36.Xiong S., Yao X., Li A. Antioxidant properties of peptide from cowpea seed. Int. J. Food Prop. 2013;16(6):1245–1256. [Google Scholar]
- 37.Sonego J.M., Santagapita P.R., Perullini M., Jobbágy M. Ca (II) and Ce (III) homogeneous alginate hydrogels from the parent alginic acid precursor: a structural study. Dalton Trans. 2016;45(24):10050–10057. doi: 10.1039/c6dt00321d. [DOI] [PubMed] [Google Scholar]