Abstract
Acute ischemic stroke (AIS) is a life-threatening complication of coronavirus disease 2019 (COVID-19) infection. Increasing reports suggest an association between COVID-19 and AIS, although the underlying mechanism remains uncertain. We performed a systematic review to characterize the clinical characteristics, neuroimaging findings, and outcomes of AIS in COVID-19 patients. A literature search was performed in PubMed and Embase using a suitable keyword search strategy from 1st December 2019 to 29th May 2020. All studies reporting AIS occurrence in COVID-19 patients were included. A total of 39 studies comprising 135 patients were studied. The pooled incidence of AIS in COVID-19 patients from observational studies was 1.2% (54/4466) with a mean age of 63.4 ± 13.1 years. The mean duration of AIS from COVID-19 symptoms onset was 10 ± 8 days, and the mean NIHSS score was 19 ± 8. Laboratory investigations revealed an elevated mean d-dimer (9.2 ± 14.8 mg/L) and fibrinogen (5.8 ± 2.0 g/L). Antiphospholipid antibodies were detected in a significant number of cases. The majority of AIS neuroimaging patterns observed was large vessel thrombosis, embolism or stenosis (62.1%, 64/103), followed by multiple vascular territory (26.2%, 27/103). A high mortality rate was reported (38.0%, 49/129). We report the pooled incidence of AIS in COVID-19 patients to be 1.2%, with a high mortality rate. Elevated d-dimer, fibrinogen and the presence of antiphospholipid antibodies appear to be prominent in COVID-19 patients with concomitant AIS, but further mechanistic studies are required to elucidate their role in pathogenesis.
Electronic supplementary material
The online version of this article (10.1007/s11239-020-02228-y) contains supplementary material, which is available to authorized users.
Keywords: Antiphospholipid antibodies, COVID-19, Hypercoagulable, Ischemic stroke, Thrombosis
Highlights
Incidence of acute ischemic stroke in COVID-19 patients ranges from 0.9% to 2.7%
Stroke severity in COVID-19 patients are typically at least moderate (NIHSS score 19 ± 8), with a high prevalence (40.9%) of large vessel occlusion
Notable number of cases tested positive for antiphospholipid antibodies
A high mortality rate (38.0%) was reported
Introduction
Acute ischemic stroke (AIS) is a life-threatening complication of coronavirus disease 2019 (COVID-19) infection [1]. While much is still unknown about the novel coronavirus, the link between COVID-19 and ischemic stroke has been increasingly documented in recent literature and mass media, be it in young COVID-19 patients [2] or patients without pre-existing cardiovascular risk factors or significant comorbidities [3]. Till date, the true incidence of AIS in COVID-19 patients remains unclear. In a study of 214 patients in Wuhan, the initial epicenter of the pandemic, the incidence of stroke was 2.34% [4]. However, in a more recent study conducted in New York, the incidence was relatively lower at 0.9% [5]. Furthermore, the underlying stroke mechanism of COVID-19 remains debatable. It has been proposed that COVID-19 may induce a prothrombotic state, as supported by the elevated levels of factor VIII, fibrinogen, and d-dimer [6, 7]. Interestingly, various case series and reports also suggest a preponderance of large vessel occlusion (LVO) AIS [2].
Given the rapidly rising number of COVID-19 infections globally and AIS complications, it is important to have a more in-depth understanding of their association. Thus far, the published literature has been limited to case reports, case series and observational cohort studies. We performed a systematic review and meta-summary of the literature to evaluate patient demographics, clinical and stroke characteristics, prothrombotic workup, neuroimaging findings, treatment and outcomes of COVID-19 patients who have suffered an AIS.
Methods
Literature search strategy
We conducted the systematic review in accordance with the PRISMA guidelines. A comprehensive literature search was performed on PubMed and Embase from 1st December 2019, before the first case of COVID-19 was reported, to 29th May 2020 [1]. The search strategy consisted of different combinations of the following search terms: COVID, Coronavirus, Severe acute respiratory syndrome coronavirus 2, CoV, SARS-CoV-2, stroke, AIS, neurologic*, acute cerebrovascular*, transient ischemic attack, infarct, coagulopathy, pro-thrombotic, hypercoagulable, thrombus and clot. The search was performed by two independent reviewers (YKT & CG). During study screening for relevance, any disagreement was resolved by consensus with a senior author (BT). Studies reporting incidences of stroke in patients positive for COVID-19 were identified separately. The reference lists of these articles were also screened, and hand searched to identify further relevant studies.
Study and cohort selection
All studies (case reports, case series and observational cohort studies) that reported AIS in COVID-19 patients were included during the initial search. We subsequently excluded all studies that reported only hemorrhagic stroke, cerebral venous thrombosis and those without an English translation.
Data extraction
Relevant quantitative data were extracted by two authors (YKT & CG) in the form of absolute frequencies of events or absolute numbers when appropriate. Where available, the data included incidence rate, individual case data: patient demographics (age, gender), country of study origin, comorbidities, COVID-19 symptoms, duration of stroke from symptom onset, National Institutes of Health Stroke Scale (NIHSS), presence of LVO, patterns of stroke on neuroimaging, relevant biological markers (white cell count, absolute lymphocyte count, platelets, prothrombin time (PT), activated partial thromboplastin time (PTT), c-reactive protein (CRP), d-dimer, fibrinogen, lactate dehydrogenase (LDH), and ferritin), treatment (acute recanalisation and antithrombotic therapy) and early outcomes (hemorrhagic transformation and mortality). Neuroimaging data reported in text or image format was reviewed by two stroke neurologists (BT & LL) and classified as one of these patterns: (1) large vessel thrombosis or embolism or stenosis; (2) multiple vascular territory; (3) small vessel; (4) others.
Risk of bias assessment
The quality of the included case reports and case series was assessed using the guidelines recommended by the Johanna Briggs Institute, while the quality of the included observational cohort studies was assessed using the Newcastle–Ottawa scale. For case series, each study was assessed if there was a clear inclusion criterion, measured the condition in a reliable way, had a valid method to identify the condition, had consecutive or complete inclusion and reported patient demographics, clinical information, and outcomes. For case reports, each study was assessed if the following was well described: patient demographics, medical history, clinical conditions, diagnostic tests, treatment, post-treatment clinical condition, adverse events and takeaway lessons. For observational cohort studies, each study was assessed on their selection, comparability (if applicable) and outcome.
Statistical analysis
We present the numeric variables as mean and SD. Categorical variables are presented as percentages. Studies that reported the incidence of ischemic stroke among COVID-19 patients were pooled to generate a pooled incidence table. Individual case data were separately pooled with patient demographics, COVID-19 symptoms, stroke characteristics, treatment, and clinical outcomes presented. All data analysis was conducted using IBM SPSS Statistics 25.0 software.
Results
Study selection and grading
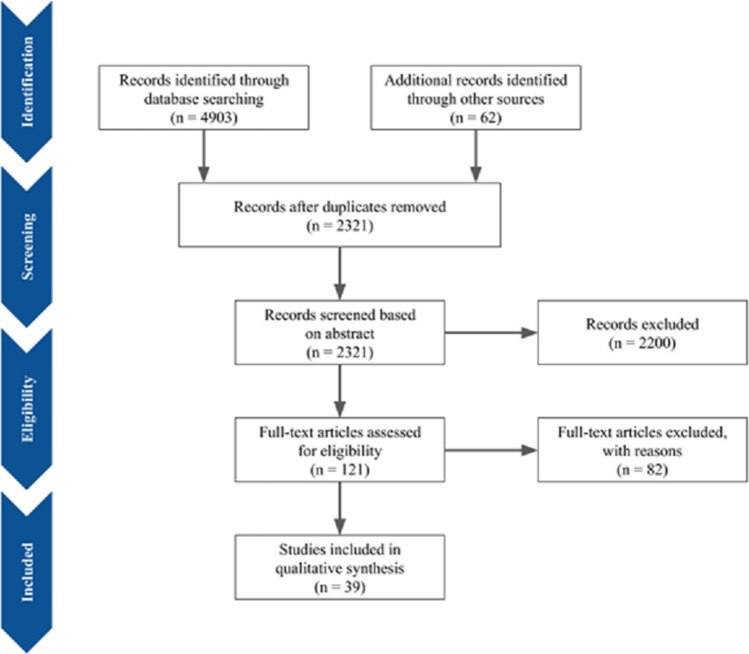
The electronic search strategy yielded 4965 studies, of which 2644 studies were removed as duplicates or not meeting criteria A further 2200 studies were excluded after an initial screen of title and abstract as they did not specifically report incidence or cases of stroke in COVID-19 patients. Lastly, 82 articles were excluded after full text review. Finally, 39 eligible studies were included in our systematic review and meta-summary. The study identification and selection process are reflected in Fig. 1 [1–5, 7–38].
Fig. 1.
PRISMA flowchart for study selection
Based on guidelines provided by the Joanna Briggs Institute, all case series studies, and the majority of case reports were assessed to be of low risk of bias. A few case reports were assessed to be moderately biased given lack of reporting completeness (Supplementary Table 1). In accordance with the Newcastle–Ottawa quality assessment scale, most observational cohort studies were assessed to be of low risk of bias.
Incidence of AIS
Five observational cohort studies reported varying incidences of AIS among COVID-19 patients ranging from 0.9% to 2.7% (Table 1). Across the five studies, there were a total of 4466 COVID-19 patients, of which AIS was reported in 54 patients, yielding an overall pooled incidence of AIS as 1.2%. All five studies were conducted in different countries (France, Netherlands, Italy, China, and the United States (US)) with varying ethnic demographics. Two studies had patient cohorts specifically consisting of patients with severe COVID-19 infection, while three included all hospitalized patients with laboratory proven COVID-19 infection.
Table 1.
Pooled Incidence of acute ischemic stroke in COVID-19 patients
| Study | Country | Patient population | Total number of COVID patients | Total number of COVID patients with stroke | Incidence (%) |
|---|---|---|---|---|---|
| Helms et al. [8] | France | Severe SARS-CoV-2 infection | 150 | 3 | 2.0 |
| Klok et al. [9] | Netherlands | Severe SARS-CoV-2 infection | 184 | 5 | 2.7 |
| Lodigiani et al. [10] | Italy | Laboratory proven COVID-19, hospitalised | 362 | 9 | 2.5 |
| Mao et al. [4] | China | Laboratory proven COVID-19, hospitalised | 214 | 5 | 2.3 |
| Yaghi et al. [5] | United States | Laboratory proven COVID-19, hospitalised | 3556 | 32 | 0.9 |
| Pooled | 4466 | 54 | 1.2 |
Clinical characteristics of COVID-19 patients with AIS
Overall, we report a total of 135 COVID-19 patients with AIS. Their demographics, COVID-19 symptoms, blood investigations and stroke characteristics are presented in Table 2. The mean age was 63.4 ± 13.1 years and the majority were male patients (62.3%, 81/130). Majority of the COVID-19 related AIS patients were reported from US (57.0%, 77/135) and China (13.3%, 18/135). Common co-morbidities included hypertension (64.5%, 78/121), diabetes mellitus (42.6%, 52/122) and hyperlipidemia (32.0%, 33/103).
Table 2.
Characteristics of COVID-19 patients with acute ischemic stroke
| Variable | Valid N | Values |
|---|---|---|
| Age (years) | 135 | 63.4 ± 13.1 |
| Male gender | 130 | 81 (62.3) |
| Country of study origin | 135 | |
| United States | 77 (57.0) | |
| Italy | 14 (10.4) | |
| France | 13 (9.6) | |
| United Kingdoms | 6 (4.4) | |
| Spain | 2 (1.5) | |
| Turkey | 4 (3.0) | |
| China | 18 (13.3) | |
| Philippines | 1 (0.7) | |
| Co-morbidities | ||
| Diabetes mellitus | 122 | 52 (42.6) |
| Hypertension | 121 | 78 (64.5) |
| Hyperlipidemia | 103 | 33 (32.0) |
| Atrial fibrillation | 94 | 17 (18.1) |
| Previous strokes | 36 | 8 (22.2) |
| Smoking | 45 | 7 (15.6) |
| COVID-19 symptoms | ||
| Fever | 102 | 65 (63.7) |
| Acute respiratory symptomsa | 96 | 73 (76.0) |
| Dyspnea | 58 | 34 (58.6) |
| Duration of stroke from symptom onset (days) | 72 | 10 ± 8 |
| NIHSS | 40 | 19 ± 8 |
| Presence of LVO | 115 | 47 (40.9) |
| Simultaneous LVO of different territories | 47 | 7 (14.9) |
| Laboratory investigations | ||
| TW (× 109/L;) | 46 | 10.2 ± 5.0 |
| Absolute lymphocyte count (× 109/L) | 37 | 0.88 ± 0.46 |
| Platelets (× 109/L) | 45 | 244.3 ± 123.9 |
| PT (s) | 22 | 14.9 ± 4.9 |
| PTT (s) | 19 | 34.1 ± 7.4 |
| CRP (mg/L) | 80 | 105.6 ± 91.1 |
| d-dimer (mg/L) | 98 | 9.2 ± 14.8 |
| Fibrinogen (g/L) | 23 | 5.8 ± 2.0 |
| LDH (U/L) | 18 | 531.6 ± 228.5 |
| Ferritin (μg/L) | 38 | 1014.4 ± 1216.2 |
| Stroke treatment | ||
| Intravenous thrombolysis | 104 | 26 (25.0) |
| Endovascular thrombectomy | 104 | 35 (33.7) |
| Antiplatelet therapy | 80 | 40 (50.0) |
| Anticoagulation | 77 | 56 (72.7) |
| Outcomes | ||
| Hemorrhagic transformation | 29 | 3 (10.3) |
| Mortality | 129 | 49 (38.0) |
ARI acute respiratory infection, COVID-19 coronavirus disease 2019, LVO large vessel occlusion, NIHSS National Institutes of Health Stroke Scale
aAcute respiratory symptoms include cough, rhinorrhea, sore throat, myalgia
The majority of patients manifested typical COVID-19 symptoms, namely fever (63.7%, 65/102), acute respiratory symptoms (76.0%, 73/96) and dyspnea (58.6%, 34/58). The mean duration of AIS from COVID-19 symptoms onset was 10 ± 8 days. Laboratory investigations showed elevated mean d-dimer (9.2 ± 14.8 mg/L) and fibrinogen levels (5.8 ± 2.0 g/L).
AIS severity and neuroimaging features
The mean NIHSS score was 19 ± 8, consistent with a LVO described in a significant number of AIS patients with COVID-19 (40.9%, 47/115). Simultaneous multiple LVO of different vascular territories was reported in 14.9% of LVO AIS patients (7/47).
Based on available neuroimaging data of 103 cases, the majority of AIS patterns was large vessel thrombosis, embolism or stenosis pattern (62.1%, 64/103), followed by multiple vascular territory (26.2%, 27/103) (Supplementary Table 2). Small vessel pattern was infrequently reported on imaging (8.7%, 9/103). There was 1 case of ophthalmic artery occlusion and 2 cases of cerebellar infarcts reported.
Significance of antiphospholipid antibodies
Information on antiphospholipid antibodies or lupus anticoagulant status was available in only 16 AIS cases (Table 3). Amongst these, 5 out of the 12 patients (41.7%) tested for lupus anticoagulant were positive. For anti-cardiolipin antibodies, 20% (2/10) tested positive for IgM and 42.9% (3/7) tested positive for IgA. No patient (0/9) tested positive for IgG anti-cardiolipin antibodies. In addition, the study by Fara et al. [14] also reported one unspecified patient with mildly elevated anti-cardiolipin antibodies. For anti-β2-glycoprotein I antibodies: 10% (1/10) of those tested were positive for IgM, 38.5% (5/13) tested positive for IgG, and 42.9% (3/7) tested positive for IgA.
Table 3.
Detection of Antiphospholipid Antibodies in concomitant AIS and COVID-19 patients
| Study | Patient no | LAC | Anti-cardiolipin Ab | Anti-B2-glycoprotein 1 Ab | Neuroimaging findings | ||||
|---|---|---|---|---|---|---|---|---|---|
| IgM | IgG | IgA | IgM | IgG | IgA | ||||
| Viguier et al. [3] | 1 | – | – | – | – | – | – | Left CCA thrombus | |
| Beyrouti et al. [12] | 2a | + | + | - | + | + | Left vertebral artery occlusion and PICA territory infarct | ||
| 3 | + | – | – | – | – | Left cerebellar and right parieto-occipital infarcts | |||
| 4 | – | – | – | – | – | Left PCA occlusion and infarct | |||
| 5 | + | – | – | – | – | Right striatocapsular infarct | |||
| 6 | + | – | – | – | – | Right M2 occlusion and insula infarct | |||
| 7 | + | – | – | – | – | Basilar thrombus and bilateral posterior circulation infarcts | |||
| Deliwala et al. [18] | 8 | – | – | – | Right MCA infarct | ||||
| Dumitrascu et al. [34] | 9 | – | – | – | – | – | – | – | Ophthalmic artery occlusion |
| Goldberg et al. [19] | 10 | + | Right MCA and bilateral ACA infarcts | ||||||
| Gunasekaran et al. [11] | 11 | – | Right MCA infarct | ||||||
| Zayet et al. [35] | 12 | – | – | + | – | Multiple vascular territory embolic pattern | |||
| 13 | – | – | – | – | Multiple vascular territory embolic pattern | ||||
| Zhang et al. [13] | 14 | – | + | + | + | Multiple vascular territory embolic pattern | |||
| 15 | – | + | + | + | Multiple vascular territory embolic pattern | ||||
| 16 | – | + | + | + | Multiple vascular territory embolic pattern | ||||
aStudy by Beyrouti et al. [12] had one patient (patient 2) who presented with medium titre values for Anti-cardiolipin Ab IgM, and low titre values for Anti-B2-glycoprotein 1 Ab IgM and IgG
ACA anterior cerebral artery, CCA common carotid artery, MCA middle cerebral artery, PCA posterior cerebral artery, PICA posterior inferior cerebellar artery
AIS treatment and outcomes
Availability of details regarding treatment varied between the different acute recanalisation therapy and antithrombotic options. For studies that reported details of treatment: 25.0% (26/104) received intravenous thrombolysis, 33.7% (35/104) received endovascular thrombectomy, 50% (40/80) received antiplatelet therapy and 72.7% (56/77) received anticoagulation treatment. Patient outcomes on hemorrhagic transformation were available in only 29 cases, of which 3 (10.3%) developing this complication. Out of the 129 patients with information on mortality, 49 (38.0%) had demised at the time that the respective reports were published.
Discussion
Our study provides a comprehensive systematic review and meta-summary of the clinical manifestations, investigations, and outcomes of COVID-19 patients with AIS. The key findings of this study are: (1) incidence of AIS in COVID-19 patients ranges from 0.9% to 2.7%; (2) AIS severity in COVID-19 patients are typically at least moderate (NIHSS score 19 ± 8), with a high prevalence (40.9%) of LVO; (3) a notable number of cases tested positive for antiphospholipid antibodies and (4) a high mortality rate (38.0%) was reported.
Since the onset of the COVID-19 pandemic, few observational studies have reported the incidence of AIS, with a pooled incidence of 1.2% (range 0.9–2.7%) [4, 5, 8–10]. The variation in incidence may be attributed to the different patient populations in which AIS outcomes were studied, for instance a relatively higher incidence was observed in studies comprising severe COVID-19 infection cases [8, 9] compared to those that included all laboratory proven hospitalized COVID-19 patients [5]. This is congruent with existing studies that reported an increased prevalence of neurological disorders in severe COVID-19 infection [4, 8], possibly mediated by the effects of cytokine storm and other complications associated with critical illness. The role of different patient demographics and ethnicities in influencing the incidence of AIS also remains uncertain. Certain countries may also be limited by testing capabilities resulting in under-reporting of COVID-19 cases and hence an overestimation of AIS incidence [39].
In a small but significant proportion of AIS patients, typical symptoms of COVID-19 were not reported on presentation (24.0% (23/96) had no acute respiratory symptoms), and respiratory COVID-19 infection was picked up incidentally on chest radiograph or computed tomography scan before testing for the virus was performed. This prompts the question as to the need for routine COVID-19 screening of AIS patients who are asymptomatic for the infection especially when the stroke mechanism is unclear or in the absence of traditional cardiovascular risk factors. In addition, it is especially difficult to detect signs of ischemic stroke in critically ill COVID-19 patients who are often intubated and heavily sedated, masking their clinical features [5]. Furthermore, social isolation policies and reluctance to go to the hospital may also contribute to under-reporting of minor strokes in asymptomatic or mild COVID-19 patients [40]. Owing to these reasons, we strongly feel that determining the true incidence of AIS in COVID-19 patients would be extremely challenging.
Although there have been some reports of young strokes in COVID-19 patients [2, 11, 41], we found that the mean age of COVID-19 patients with AIS was 63.4 ± 13.1 years, with cardiovascular risk factors being commonly seen. Studies have reported conflicting data on the timing of thromboembolic complications in COVID-19 patients [10, 12]. In this systematic review, the mean duration of AIS from onset of COVID-19 symptoms was 10 ± 8 days, suggestive of a delayed presentation. This is in agreement with the current postulations that patients with severe COVID-19 infection may develop an early hyperinflammatory state from cytokine storm followed by a prothrombotic state that is frequently complicated by both venous and arterial thromboembolism [41, 42]. However, clinicians should be wary that thrombotic sequelae such as stroke may be seen in both early and late phases of the infection [10, 12, 42, 43]. Elevated biomarkers such as d-dimer, fibrinogen, factor VIII and von Willebrand factor provide further evidence of a prothrombotic state [6, 14, 44, 45].
Antiphospholipid antibodies, which refer to any or all of the antibodies detected by enzyme-linked immunosorbent assay (ELISA) for anti-β2-glycoprotein I or anticardiolipin antibodies, IgG or IgM serotypes and the lupus-anticoagulant assays [46], were frequently reported in COVID-19 AIS patients. Anti-phospholipid antibodies increase the risk of thrombosis by inducing cellular activation, inhibiting natural anticoagulant and fibrinolytic systems, and activating the complement system [46]. While it is reported that antiphospholipid antibodies are commonly found in COVID-19 infections, the true prevalence of antiphospholipid-antibody positivity in the general population is not known and has also been detected in healthy individuals [47]. Even among patients with stroke who are younger than 50 years of age, 17% were found to have antiphospholipid antibodies [47]. Furthermore, non-criteria antiphospholipid subtypes had been tested in COVID-19 studies, such as IgA anti-β2-glycoprotein I antibodies which there is insufficient evidence of their clinical relevance [48]. Hence, the significance of antiphospholipid antibodies in the pathogenesis of AIS in COVID-19 patient remains uncertain and it may be worthwhile for future studies to repeat and trend these serological markers after the acute thrombotic setting.
COVID-19 patients appear to manifest with moderate to severe AIS, with a high prevalence of LVO. Importantly, most of these AIS demonstrated a large vessel thrombosis, embolism, stenosis or multiple vascular territory pattern on imaging, and a much lower incidence of small vessel stroke, deviating from traditional trends of AIS subtypes [49]. The presence of simultaneous multiple LVO of different vascular territories (14.9%, 7/47) was also greater than its presumed prevalence in the current literature of patients receiving thrombectomy, consistent with the postulated theory of a COVID-19 induced prothrombotic state [50]. Furthermore, AIS in COVID-19 patients suffer from worse outcomes in the form of higher hemorrhagic transformation and all-cause mortality [51]. Consistent findings were reported in a study that compared the stroke characteristics of COVID-19 positive patients with COVID-19 negative patients and historical controls. In their comparative analysis, it was observed that patients with concomitant COVID-19 suffered more severe strokes, with a higher NIHSS score, greater proportion of large vessel occlusion and higher in-hospital mortality [5]. However, we were unable to directly compare the AIS rate in matched controls at the present setting given the lack of available literature. Future studies should compare the stroke risk in an appropriately matched cohort of patients with non-COVID-19 infections.
Owing to the observations of a prothrombotic state and higher mortality in AIS patients with COVID-19 infection, recommendations have been made for the use of prophylactic or therapeutic anticoagulation [42, 52]. Nevertheless, it is difficult to establish a direct causal relationship between COVID-19 and AIS, especially in the presence of multiple cardiovascular risk factors and other stroke mechanisms, such as large artery atherosclerosis or cardioembolism due to atrial fibrillation, found in many COVID-19 patients [15].
Limitations
We acknowledge that this systematic review has several limitations. Firstly, the majority of studies included are either case series or case reports, with only a few being observational cohort studies. Accordingly, many of these studies are considered relatively lower in quality with publication and reporting bias. While observational cohorts with negligible incidence of COVID-19 related AIS may not have been reported, cases with severe stroke, LVO and dramatic neuroimaging findings could have been reported more frequently. Secondly, significant heterogeneity is expected when most of the publications are case reports or series. In addition, not all the studies included in this systematic review reported every relevant variable. These factors limit our ability to generalize the characteristics of COVID-19 related AIS. Finally, language restrictions may have affected the identification and inclusion of relevant cases. This was especially relevant as many publications related to COVID-19 related AIS may have been published in their regional language (especially Chinese, Italian, and Spanish). Perhaps, there is an urgent need to establish an international registry of COVID-19 patients manifesting with AIS, with meticulous reporting of clinical characteristics, imaging findings, treatment, and outcomes in a standardized manner. This will help further elucidate the relationship between COVID-19 and AIS and serve as a platform to provide recommendations and guidelines for the workup, treatment, and follow-up of these patients.
Conclusion
We report the pooled incidence of AIS in COVID-19 patients to be 1.2%, with a high mortality rate. Elevated d-dimer, fibrinogen and the presence of antiphospholipid antibodies appear to be prominent in COVID-19 patients with concomitant ischemic stroke, but further mechanistic studies are required to elucidate their role in the pathogenesis of AIS.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
None.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Compliance with ethical standards
Conflicts of interest
There are no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying-Kiat Tan and Claire Goh are co-first authors.
References
- 1.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, et al. (2020). COVID-19 presenting as stroke. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7187846/ [DOI] [PMC free article] [PubMed]
- 2.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. New Engl J Med. 2020;382(20):25. doi: 10.1056/nejmc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viguier A, Delamarre L, Duplantier J, Olivot J-M, Bonneville F. Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection. J Neuroradiol. 2020 doi: 10.1016/j.neurad.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;10:15. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaghi S, Ishida K, Torres J, Grory BM, Raz E, Humbert K, et al. SARS2-CoV-2 and stroke in a New York healthcare system. Stroke. 2020 doi: 10.1161/strokeaha.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;12:15. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valderrama EV, Humbert K, Lord A, Frontera J, Yaghi S. Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke. 2020 doi: 10.1161/strokeaha.120.030153. [DOI] [PubMed] [Google Scholar]
- 8.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/nejmc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klok F, Kruip M, Meer NVD, Arbous M, Gommers D, Kant K, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunasekaran K, Amoah K, Rajasurya V, Buscher MG. Stroke in a young COVID -19 patient. QJM. 2020;12:15. doi: 10.1093/qjmed/hcaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. (2020). Characteristics of ischaemic stroke associated with COVID-19. https://jnnp.bmj.com/content/early/2020/04/30/jnnp-2020-323586 [DOI] [PMC free article] [PubMed]
- 13.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al (2020) Coagulopathy and antiphospholipid antibodies in patients with Covid-19. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7161262/ [DOI] [PMC free article] [PubMed]
- 14.Fara MG, Stein LK, Skliut M, Morgello S, Fifi JT, Dhamoon MS. Macrothrombosis and stroke in patients with mild Covid-19 infection. J Thromb Haemost. 2020 doi: 10.1111/jth.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berekashvili K, Dmytriw AA, Vulkanov V, Agarwal S, Khaneja A, Turkel-Parella D, et al. (2020) Etiologic subtypes of ischemic stroke in SARS-COV-2 virus patients. https://www.medrxiv.org/content/10.1101/2020.05.03.20077206v2 [DOI] [PMC free article] [PubMed]
- 16.Saiegh FA, Ghosh R, Leibold A, Avery MB, Schmidt RF, Mouchtouris N, et al (2020) Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. https://jnnp.bmj.com/content/early/2020/04/30/jnnp-2020-323522 [DOI] [PubMed]
- 17.Chen S, Bo H, Li H, et al. A case of novel coronavirus pneumonia with acute cerebral infarction. Chin J Neurol. 2020 doi: 10.3760/cma.j.cn113694-20200214-00081. [DOI] [Google Scholar]
- 18.Deliwala S, Abdulhamid S, Abusalih MF, Al-Qasmi MM, Bachuwa G, Deliwala, et al (2020) Encephalopathy as the sentinel sign of a cortical stroke in a patient infected with coronavirus disease-19 (COVID-19). https://www.cureus.com/articles/32367-encephalopathy-as-the-sentinel-sign-of-a-cortical-stroke-in-a-patient-infected-with-coronavirus-disease-19-covid-19 [DOI] [PMC free article] [PubMed]
- 19.Goldberg MF, Goldberg MF, Cerejo R, Tayal AH (2020) Cerebrovascular disease in COVID-19. https://www.ajnr.org/content/early/2020/05/14/ajnr.A6588 [DOI] [PMC free article] [PubMed]
- 20.Luna‐Rodríguez A, Ruiz‐Lopez M (2020) Emergency room neurology in times of COVID‐19: malignant ischaemic stroke and SARS‐CoV‐2 infection. https://onlinelibrary.wiley.com/doi/10.1111/ene.14286 [DOI] [PMC free article] [PubMed]
- 21.He J, Cheng G, Xu W, Zhang L, Zeng Z (2020) Diagnosis and treatment of an elderly patient with secondary cerebral infarction caused by COVID-19. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7167313/ [DOI] [PMC free article] [PubMed]
- 22.Tunç A, Ünlübaş Y, Alemdar M, Akyüz E (2020) Coexistence of COVID-19 and acute ischemic stroke report of four cases. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7200342/ [DOI] [PMC free article] [PubMed]
- 23.Zhou B, She J, Wang Y, Ma X (2020) A case of coronavirus disease 2019 with concomitant acute cerebral infarction and deep vein thrombosis. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7188982/ [DOI] [PMC free article] [PubMed]
- 24.Salahuddin H, Castonguay AC, Zaidi SF, Burgess R, Jadhav AP, Jumaa MA. Interventional stroke care in the era of COVID-19. Front Neurol. 2020 doi: 10.3389/fneur.2020.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Wang M, Zhou Y, Chang J, Xian Y, Mao L, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. SSRN Electron J. 2020 doi: 10.2139/ssrn.3550025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morassi M, Bagatto D, Cobelli M, D’Agostini S, Gigli GL, Bnà C, Vogrig A. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020 doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhai P, Ding Y, Li Y. The impact of COVID-19 on ischemic stroke: a case report. Infect Dis. 2020;10:20. doi: 10.21203/rs.3.rs-20393/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang A, Mandigo G, Yim P, Meyers P, Lavine S (2020) Stroke and mechanical thrombectomy in patients with COVID-19: Technical observations and patient characteristics. https://jnis.bmj.com/content/early/2020/05/25/neurintsurg-2020-016220 [DOI] [PubMed]
- 29.Moshayedi P, Ryan TE, Mejia LLP, Nour M, Liebeskind DS. Triage of acute ischemic stroke in confirmed COVID-19: large vessel occlusion associated with coronavirus infection. Front Neurol. 2020 doi: 10.3389/fneur.2020.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Co CO, Yu JR, Laxamana LC, David-Ona DI. Intravenous thrombolysis for stroke in a COVID-19 positive Filipino patient, a case report. J Clin Neurosci. 2020 doi: 10.1016/j.jocn.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudilosso S, Esteller D, Urra X, Chamorro Á. Thalamic perforating artery stroke on computed tomography perfusion in a patient with coronavirus disease 2019. J Stroke Cerebrovasc Dis. 2020;29(8):104974. doi: 10.1016/j.jstrokecerebrovasdis.2020.104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papi C, Spagni G, Alexandre A, Calabresi P, Marca GD, Broccolini A. Unprotected stroke management in an undiagnosed case of severe acute respiratory syndrome coronavirus 2 infection. J Stroke Cerebrovasc Dis. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.104981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gill I, Chan S, Fitzpatrick D. COVID-19 associated pulmonary and cerebral thromboembolic disease. Radiol Case Rep. 2020 doi: 10.1016/j.radcr.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dumitrascu OM, Volod O, Bose S, Wang Y, Biousse V, Lyden PD. Acute ophthalmic artery occlusion in a COVID-19 patient on apixaban. J Stroke Cerebrovasc Dis. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.104982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zayet S, Klopfenstein T, Kovẚcs R, Stancescu S, Hagenkötter B (2020) Acute cerebral stroke with multiple infarctions and COVID-19, France, 2020. Emerg Infect Dis J. https://wwwnc.cdc.gov/eid/article/26/9/20-1791_article [DOI] [PMC free article] [PubMed]
- 36.Escalard S, Maïer B, Redjem H, Delvoye F, Hébert S, Smajda S, et al. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19. Stroke. 2020 doi: 10.1161/strokeaha.120.030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeboah K, Edgell R, Conway J, Alshekhlee A. Interventional stroke management in a COVID-19 patient. Neurology. 2020 doi: 10.1212/cpj.0000000000000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jillella DV, Janocko NJ, Nahab F, Benameur K, Greene JG, Wright WL et al (2020) Ischemic stroke in COVID-19: an urgent need for early identification and management. medRxiv. [DOI] [PMC free article] [PubMed]
- 39.Rio CD, Malani PN. COVID-19: new insights on a rapidly changing epidemic. JAMA. 2020;323(14):1339. doi: 10.1001/jama.2020.3072. [DOI] [PubMed] [Google Scholar]
- 40.Morelli N, Rota E, Terracciano C, Immovilli P, Spallazzi M, Colombi D, et al. The Baffling case of ischemic stroke disappearance from the casualty department in the COVID-19 era. Eur Neurol. 2020 doi: 10.1159/000507666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein DE, Libman R, Kirsch C, Arora R. Cerebral venous thrombosis: atypical presentation of COVID-19 in the young. J Stroke Cerebrovasc Dis. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z (2020) Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. https://onlinelibrary.wiley.com/doi/full/10.1111/jth.14817 [DOI] [PMC free article] [PubMed]
- 43.Connors JM, Levy JH (2020) Thromboinflammation and the hypercoagulability of COVID-19. https://www.ncbi.nlm.nih.gov/pubmed/32302453 [DOI] [PMC free article] [PubMed]
- 44.Rosário C, Zandman-Goddard G, Meyron-Holtz EG, D’Cruz DP, Shoenfeld Y. The hyperferritinemic syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11(1):15. doi: 10.1186/1741-7015-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shorr AF, Zilberberg MD. Going viral. Chest. 2016;150(5):991–992. doi: 10.1016/j.chest.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 46.Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013 doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 47.Garcia D, Erkan D (2018) Diagnosis and management of the antiphospholipid syndrome | NEJM. https://www.nejm.org/doi/10.1056/NEJMra1705454
- 48.Limper M, de Leeuw K, Lely AT, Westerink J, Teng YKO, Eikenboom J, Otter S, Jansen AJG, Ree M, Spierings J, Kruyt ND, van der Molen R, Middeldorp S, Leebeek FWG, Bijl M, Urbanus RT (2020) Diagnosing and treating antiphospholipid syndrome: a consensus paper. https://pubmed.ncbi.nlm.nih.gov/31012427/ [PubMed]
- 49.Ornello R, Degan D, Tiseo C, Di Carmine C, Perciballi L, Pistoia F, Carolei A, Sacco S. Distribution and temporal trends from 1993 to 2015 of ischemic stroke subtypes. Stroke. 2018;49(4):814–819. doi: 10.1161/strokeaha.117.020031. [DOI] [PubMed] [Google Scholar]
- 50.Kaesmacher J, Mosimann PJ, Giarrusso M, El-Koussy M, Zibold F, Piechowiak E, et al. Multivessel occlusion in patients subjected to thrombectomy. Stroke. 2018;49(6):1355–1362. doi: 10.1161/strokeaha.118.021276. [DOI] [PubMed] [Google Scholar]
- 51.Benussi A, Pilotto A, Premi E, Libri I, Giunta M, Agosti C, Alberici A, Baldelli E, Benini M, Bonacina S, Brambilla L, Caratozzolo S, Cortinovis M, Costa A, Piccinelli SC, Cottini E, Cristillo V, Delrio I, Filosto M, et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia. Lombardy: Neurology; 2020. [DOI] [PubMed] [Google Scholar]
- 52.Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020;18(5):1020–1022. doi: 10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.