Abstract
Animals have evolved multiple systems, including genetic and epigenetic systems, to respond accordingly to heat exposure and heat acclimation. Heat exposure greatly affects immunity, changes metabolic processes, and poses a serious threat to animals. Heat acclimation is induced by repeated organism exposure to heat stress to dissipate heat. This review focuses on genetic modulation via heat shock transcription factors and calcium as two important factors and compares the changes in HSPs under heat stress and heat acclimation. Epigenetic regulation summarizes the role of HSPs in DNA methylation and histone modifications under heat stress and heat acclimation. These genetic and epigenetic modifications protect cells from thermal damage by mediating the transcriptional levels of heat-responsive genes. This review highlights recent advances in the genetic and epigenetic control of animal thermal responses and their interactions.
Keywords: heat exposure, genetic mechanism, epigenetic regulation, immunity, heat acclimation
Introduction
Terrestrial and aquatic organisms are subjected to environmental stressors (abiotic and biotic factors), such as pathogen attacks and heat stress (Kultz, 2003; Norouzitallab et al., 2014). The intensity of thermal stress is expected to increase in the coming decades because of climate change (Ishita et al., 2010). When temperature is above the prescriptive zone of animals, their core body temperature increases, resulting in hyperthermia and altering various biological functions, such as breaking down biological regulatory mechanisms, deteriorating health, declining immunity, and increasing mortality (Logan and Buckley, 2015; Han et al., 2016; Nyboer and Chapman, 2017; Negrón-Pérez et al., 2019). Heat-shock response (HSR) was originally discovered as a transcriptional response to elevated temperature shock (Ritossa, 1996). Its discovery led to the identification of heat shock proteins and heat shock factor 1 (HSF1). Accumulating evidence shows that HSF1, the central player in HSR, is mediated according to specific cellular requirements through cell-autonomous and non-autonomous signals (Li et al., 2017). HSF1 enhances transcriptional activity, especially the molecular chaperones heat-shock protein family (HSP), which helps protect the structural stability of refolding or degrading intracellular proteins (Yura et al., 1993; Goldberg, 2003). Heat acclimation is induced by repeated organism exposure to heat stress to dissipate heat and reduce heat illness (Carter et al., 2005; Hung et al., 2005; Magalhães et al., 2010). In addition to mammals, scholars are also studying heat acclimation and thermal death in insects (Bowler and Hollingsworth, 1965; Hoffmann et al., 2003; Weldon et al., 2011; Allen et al., 2012). Results suggested that heat acclimation has an important relationship with HSR. Heat acclimation invokes HSR, whereas HSR promotes the development of heat acclimation (Yamada et al., 2007; Mcclung et al., 2008; Magalhães et al., 2010).
In addition, calcium, as the hallmark of heat acclimation, is an important second intracellular messenger that can convert extracellular stimuli into intracellular signals, as well as regulate cell development, survival, differentiation, and gene expression by affecting signaling pathways (Berna-Erro et al., 2012). Calcium and HSF1 also play a key role in the stress response of biological cells under heat stress, such as endoplasmic reticulum (ER) stress, reactive oxygen species (ROS) stress (Ryu et al., 2013), and apoptosis (Manucha et al., 2011). Therefore, in this review, we discussed the genetic mechanisms of HSR and heat acclimation mainly focusing on HSF1 and calcium. The core mechanisms HSF1, calcium, and critical genes involved in HSR are summarized.
Genetic regulation or environmental exposure alone often cannot fully explain the process of thermoregulation. Epigenetics is a regulatory system for environmental stress without change in the DNA arrangement, and it plays a vital role in genome stability, nuclear organization, transcription, and imprinting (Bartels et al., 2018). To date, various epigenetic modifications, including DNA methylation, histone modifications, and non-coding RNA, have been studied in which the transcription level, not the DNA sequence, is changed (Arányi et al., 2005; Huang and Fan, 2010). DNA methylation, which frequently occurs at the CpG dinucleotide in eukaryotes, is presented among the most extensively studied epigenetic regulatory mechanisms. Genome-wide methylation profiling has been recently conducted for economically important animals (Khavari et al., 2010; Almamun et al., 2014; Smallwood et al., 2014; Almamun et al., 2015). In particular, numerous genes undergo DNA methylation under heat stress, which affects the expression of many genes. Dynamic changes in DNA methylation present a specific pattern in tissues or cell types. Epigenetic mechanisms may play an important role in the formation of heat acclimation and the changes in mitochondrial respiratory chain (OXPHOS) (Dai et al., 2018). However, few studies have been conducted on the relationships between genetic and epigenetic regulations under heat stress. This review discusses several important physiological processes, briefly introduces the genetic and epigenetic mechanisms of animal responses to heat, and emphasizes the relationships between these mechanisms.
Genes Modulate Animal Responses to Heat Stress
Heat and Oxidative Stress
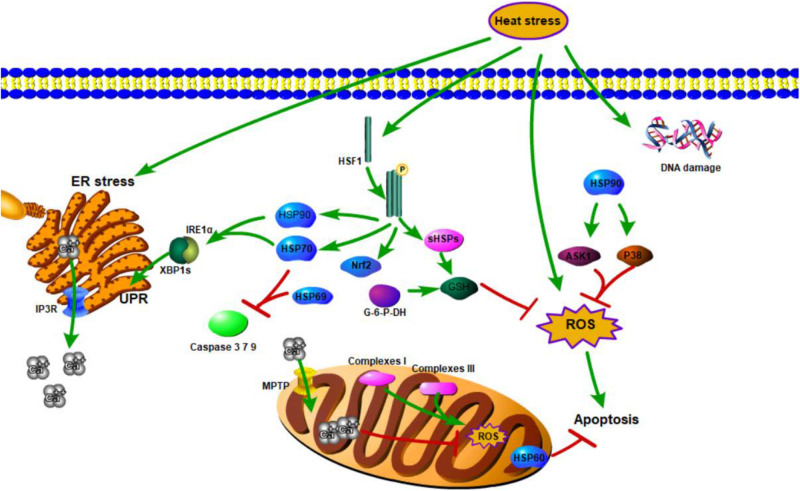
Previous studies have reported that heat stress induces ROS production, which, in turn, produces oxidative stress (Ryu et al., 2013). Two time-dependent oxygen free radicals have been identified under heat stress in human umbilical vein endothelial cells: O2– and H2O2 (Li et al., 2014). Under heat stress, O2– noticeably increases immediately, and then H2O2 increases, indicating that the generation of primary ROS mainly depends on excess O2 after heat stress.
The role of HSP genes under oxidative stress, the production of HSF1, is well known (Lee et al., 1999; Chuang et al., 2002). The role of small HSPs (sHSPs, molecular weight 8.5–40 kDa) and the HSP60, HSP70, and HSP90 protein families in protective stress response to heat stress has been studied. The HSP70 family abolishes heat-induced ROS production (Sreedhar et al., 2002; Belhadj et al., 2014). HSP70 suppresses mitochondrial damage by reducing ROS level in rat histiocytoma (Sreedhar et al., 2002). HSP90 reduces ROS damage by inhibiting ASK1-p38 activation induced by H2O2 in human umbilical vein endothelial cells (Zhang et al., 2005). HSP60, as a typical mitochondrial protein in eukaryotes, inhibits apoptosis through the outer mitochondrial membrane under heat stress (Song et al., 2018).
Certain sHSPs, such as HSPB1 (HSP25/27) and HSPB5 (αB-crystallin), are known to participate in oxidative stress reactions that reduce the level of oxidative damage by maintaining the redox state of cells (Mehlen et al., 1996). sHSP overexpression leads to increased antioxidant glutathione (GSH) concentration and increased glucose 6-phosphate dehydrogenase (G-6-P-DH) activity, which contribute to the formation of reduced GSH in murine L929 fibrosarcoma cells (Mehlen et al., 1996).
Under oxidative stress, HSF1 has been shown to synergize with nuclear factor erythroid 2-related factor 2 (Nrf2) to bind to promoters and transcriptionally upregulate several target gene expression, such as sequestosome-1 (SQSTM1, also known as p62) and heme oxygenase-1 (HMOX1) (Jain et al., 2010; Samarasinghe et al., 2014; Watanabe et al., 2016), activating transcription factor 3 (ATF3) (Kyu-Han et al., 2009; Takii et al., 2010), HSPA1A/B/L (HSP70), and HSPB1 (HSP25/27) (Mehlen et al., 1996). Therefore, HSF1 and HSP can induce a variety of cytoprotective mechanisms and protect related genes as a response to oxidative stress.
Moreover, heat stress increases the metabolic rate. The organism consumes more oxygen, which leads to electron leakage in the respiratory chain and an increase in ROS levels. Complex I and complex III is the main source of ROS; the former increases ROS through glutathione, the latter through ubisemiquinone radical intermediate (QH) at the QO site of complex III (Turrens, 1997; Julie et al., 2002; Muller et al., 2003; Taylor et al., 2003), while calcium stimulates Krebs cycle enzymes and oxidative phosphorylation in the mitochondria to promote ATP synthesis (Brookes, 2004; Görlach et al., 2015). Under normal circumstances, calcium reduces ROS from complexes I and III, and when these complexes are inhibited, calcium can enhance ROS production (Görlach et al., 2015). The three-dimensional conformational changes in the respiratory chain complex may be regulated by calcium to increase ROS level (Brookes, 2004). Although calcium plays an important role in affecting changes in ROS content, the specific mechanisms underlying this process have not been elucidated.
Heat and Endoplasmic Reticulum Stress
The ER is a dynamic organelle whose functions include protein folding, calcium buffering, and lipid and carbohydrate metabolism (Song et al., 2018). Under heat stress, diverse cellular stresses, such as perturbations in calcium homeostasis, redox imbalance, and protein folding defects, cause misfolded and unfolded proteins to accumulate in the ER lumen, which triggers the unfolded protein response (UPR) (Qian et al., 2011; Zhang et al., 2014; Zhang S. et al., 2017). UPR activates the genes encoding the ER resident protein required for protein folding (Hetz, 2012). This regulatory response transmits signals from the ER lumen to the nucleus via the inositol-requiring protein 1α/spliced X-box binding protein 1 (IER1α/XBP1) by the conserved intracellular signaling pathway of UPR (Barna et al., 2018). The UPR signals through the sensor IRE1α, which controls the splicing of the mRNA encoding the transcription factor XBP1. Together with UPR, HSR protects eukaryotic cells from the damage caused by protein toxicity. Experiments have demonstrated that HSP72 (HSPA1A) and HSP90 (HSPC1), two HSF1-regulated chaperones, interact with IRE1α cytoplasmic domains to enhance the complex of IRE1α/XBP1 at the ER, and this process, in turn, affects UPR in cell culture (Marcu et al., 2003; Gupta et al., 2010; Ahmed and Averill-Bates, 2015). ATPase domain of Hsp72 binds to the monomeric and nonphosphorylated cytoplasmic tail of IRE1α, in order to protect IRE1α from ER stress-mediated phosphorylation and oligomerization of IRE1α. In addition, increased XBP1 protein is required for enhanced cell survival induced by Hsp72 under ER stress conditions. Furthermore, Hsp90 has been shown to increase the half-life of IRE1α by binding to the cytoplasmic domains. The molecular mechanisms by which HSP72 and HSP90 inhibits ER stress need more experiments to explore.
Aside from HSF1, calcium has been identified to be associated with ER stress. Under heat stress, the accumulation of unfolded proteins in the ER causes calcium to leak from the ER into the cytoplasm by Bak/Bax, then, causes the ER to induce apoptosis (Bhandary et al., 2012). The calcium releasing is mainly through redox sensitivity of IP3R. In addition, cytoplasmic calcium overload can lead to cytotoxicity, which can lead to the activation of endogenous or mitochondrial-dependent apoptotic pathways (Crompton et al., 2002).
Heat and Apoptotic Pathway
The mitochondria are central integrators and transducers of pro-apoptotic signals (Crompton et al., 2002). Recent evidence suggests that the heat-shock proteins HSP90, HSP70, and sHSPs can inhibit heat-induced cell death by intervening at various steps of the mitochondrial-dependent apoptotic pathway (Roufayel and Kadry, 2019). Both sHSPs (HSP27) and HSP70 inhibit the release of cytochrome c and, consequently, the intrinsic pathway of apoptotic cell death in cell culture (Samali et al., 2001; Klein and Brüne, 2002; Sancho, 2003). Further experiments found that HSP70 and HSP90 may inhibit apoptosis through the caspase 3 pathway because ROS-mediated caspase 3 activation partially depends on the downregulation of HSP70 and HSP90 in L-02 hepatocytes (Xiao et al., 2012). Moreover, HSP69s and HSP20 mediate ROS through caspase 7 and caspase 9 to further inhibit apoptosis in Plutella xylostella sperm and ovary cells (Zhang L. J. et al., 2017).
In addition to HSPs acting directly on the apoptosis pathway, HSP70-enhanced miR-23a prevents NOXA (Bcl-2 family proteins), leading to Bax (Bcl-2 family proteins) activation and cytochrome c releasing from the mitochondria, thereby preventing heat-induced apoptosis in cell culture (Roufayel and Kadry, 2019). The HSP90–Akt–ASK1 complex decreases H2O2-induced ASK1–p38/JNK signaling and cell apoptosis in human umbilical vein endothelial cells (Zhang et al., 2005). By contrast, HSF1 overexpression can trigger apoptotic cell death programs in a Tdag-51-dependent manner (T-cell death-related genes) (Barna et al., 2018). Under heat stress, HSF1 directly activates Tdag51-related regulatory regions to activate its transcription in HeLa cells (Takaki et al., 2006). However, several studies have reported that TDAG51 may have both pro- and anti-apoptotic functions depending on the cellular context and circumstances. With regard to the pro-apoptotic function of TDAG51, available evidence supports the notion that TDAG51 expression is closely associated with enhanced apoptosis (Park et al., 1996; Lama et al., 2003). With regard to the anti-apoptotic function of TDAG51, TDAG51 may be involved in modulating the expression of antioxidant enzymes or non-enzymatic components to eliminate excess ROS generation as a response to oxidative stress, thereby reducing apoptosis in mouse embryonic fibroblasts (MEFs) (Park et al., 2013). Further studies must address the regulatory mechanisms of the physiological importance of TDAG51 expression in oxidative stress response.
No direct evidence supports the supposition that calcium mediates TDAG51 under heat stress. However, in mesenchymal transition (EMT) response (during embryonic development in which cells lose epithelial characteristics and gain mesenchymal properties), calcium imbalance can mediate TDAG51 upregulation and prime the cells for mesenchymal transformation in human renal cubular epithelial cells (HRPTECs) (Carlisle et al., 2012), suggesting that calcium may regulate the expression of Tdag51 in the same manner under heat stress. Investigations on the effects of calcium on the apoptotic pathway showed that heat stress may induce apoptosis through the calcium-mediated mitochondrial apoptosis pathway, such as IP3R-regulated cytoplasmic calcium elevation, which further affects Apaf-1, caspase 9, and caspase 3 activations, thereby mediating apoptosis in rabbit corneal cells (Hsu et al., 2011). In addition, the redox sensitivity of IP3R and mitochondria permeability transition pore (MPTP) increases ER calcium releasing and mitochondrial calcium loading (Bhandary et al., 2012). A brief summary of studies on complex regulatory mechanisms, such as oxidative stress, calcium signaling, UPR, or their combinations produced by organisms under thermal stress, is shown in Figure 1. The regulatory effects of HSF1 and calcium under heat stress are summarized in Table 1.
FIGURE 1.
Genetic mechanisms of animal responses to heat.
TABLE 1.
Regulatory effects of heat shock factor 1 (HSF1) and Calcium (Ca) under heat stress.
| Oxidative stress | ER stress | Apoptotic pathway | |
| HSF1 | Synergized with nuclear factor erythroid 2-related factor 2 (Nrf2) to regulate sequestosome-1 (SQSTM1)/p62, heme oxygenase-1 (HMOX1), activating transcription factor 3 (Atf3), heat-shock protein family (Hsp)70 and Hsp25/27 | Over-expressed HSF1 triggered apoptosis in a Tdag-51-dependent manner | |
| Hsp90 | Hsp90 inhibited hydrogen peroxide (H2O2)-induced ASK1–p38 activation | Interact with inositol-requiring protein 1α (IRE1α) to regulate unfolded protein response (UPR) | Hsp90–Akt–ASK1 complex decreased cell apoptosis |
| Hsp70 | Abolished heat-induced reactive oxygen species (ROS) production, but the mechanism was unknown | Interact with IRE1α to regulate UPR | HSP70-enhanced miR-23a prevented the release of cytochrome c |
| Small Hsps (sHsps) | Increased glutathione (GSH) and G6PD contributed to reduced state; abolished ROS by TNFα | Interrupted apoptosis by preventing the release of cytochrome c | |
| Calcium | Reduced ROS by regulating complexes I and III | Leaked from the endoplasmic reticulum (ER) into the cytoplasm, which causes ER to induce apoptosis | Cytoplasmic calcium elevation activated Apaf-1, caspase 9 and caspase 3 |
Heat Stress and Heat Acclimation
Understanding the survival mechanisms and mitigation strategies of organisms to heat stress is important. Heat acclimation accelerates the rate and alters the magnitude of HSPs expression upon stress application. Under normal conditions, a higher HSP70 level is observed in heat-acclimated organisms than in the non-acclimated rat heart (Horowitz et al., 1997). Compared with the normal group, there are larger HSP reserves in acclimation rats in normal environment, which may contribute to delayed thermal injury (Maloyan et al., 1999). An elevated HSP level may involve a pathway different from that involved in heat stress. Studies on the effects of HSP family on heat acclimation showed that HSP70, HSP90, and HSP110 are required for immediate fish survival at high temperatures, whereas HSP60, HSP70, and HSP78 are needed for their long-term survival at high temperatures (Purohit et al., 2014). mRNA expression of HSP70 from Cnaphalocrocis medinalis increases in all five generations of heat selection, but HSP90 increases only in the first two generations in rice leaf folder larvae (Gu et al., 2019). These results indirectly prove the point and also support the premise that HSP70 plays the most important role in HSR and heat acclimation.
Epigenetic Mechanisms of Animal Responses to Heat Stress and Heat Acclimation
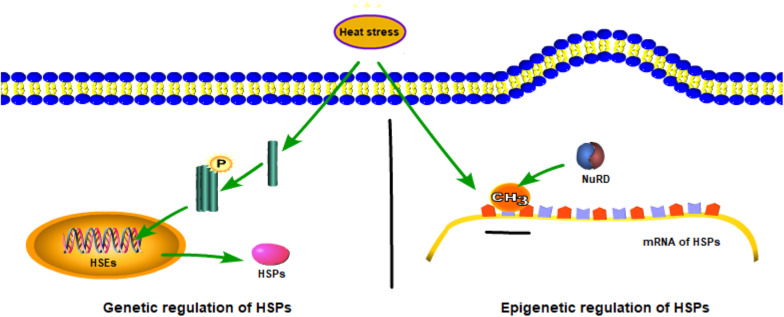
Epigenetic regulation changes the gene expression or expression speed through DNA methylation, histone modifications, and other regulatory pathways. In this discussion, HSP is taken as an example (Figure 2). In the genetic mechanism of HSP synthesis, HSF1 becomes trimerized and phosphorylated under heat stress and binds to HSR DNA elements in the nucleus, thereby, activating the transcriptional expression of HSP (Kukreti et al., 2013). By contrast, in the epigenetic regulation of HSP (taken HSP70 as an example), the HSP70 promoter is hypermethylated after acclimation to high temperatures by a reduction in both POU class 2 homeobox 1 (POU2F1, also known as octamer-binding transcription factor-1, is a ubiquitous transcription factor that plays a key role in the regulation of genes related to inflammation and cell cycles) binding and recruitment of the nucleosome remodeling deacetylase (NuRD, combining both deacetylase and remodeling enzymatic activities in a single macromolecular complex) chromatin-remodeling complex, thereby affecting HSP70 expression (Matsuda et al., 2006). In addition to this, epigenetic regulation has been studied through different modifications under environmental stresses. Then, we will discuss the DNA methylation and histone methylation under heat stress.
FIGURE 2.
Genetic and epigenetic mechanisms of heat-shock protein family (HSP) synthesis.
Epigenetic Mechanisms of DNA Methylation Under Heat Stress
DNA methylation is a way of epigenetic modification that regulates gene expression. In general, methylation occurs in the promoter region and blocks protein binding, thereby, inhibiting gene transcription (Kuroda et al., 2009). Methylation of the promoter regions of HSP 90 alpha, HSP 90 beta, and HSP 70 is negatively associated with their mRNA expressions in control and heat-treated Naked Neck chicken (Vinoth et al., 2018). However, such an inverse relationship under heat stress cannot be detected in Punjab broiler-2 chicken (Vinoth et al., 2018). Therefore, establishing the link between methylation and gene expression is difficult.
Aside from regulating gene expression, methylation can also form epigenetic memory. DNA methylation is a dynamic process during development and cell differentiation, and several DNA methylation patterns may remain in the form of epigenetic memory (Kim and Costello, 2017). Epigenetic regulation of HSP70 expression via alterations in the CpG methylation profile of a distal promoter region that affects POU2F1 recruitment and H3 deacetylation may reflect heat stress-related epigenetic memory (Kisliouk et al., 2017). Moreover, DNA methylation is a stable epigenetic mark that can be inherited through multiple cell divisions rather than through germline or germ cells (Dor and Cedar, 2018).
Epigenetic Mechanisms of Histone Methylation Under Heat Stress
Apart from DNA methylation, histone modifications can also affect the transcriptional expression of individual genes by affecting chromosomal domains (Suzuki and Bird, 2008). Studies have focused on the histone modifications of H2B and H3 (Matsuda et al., 2006). The H2B methylation amino acid residue has been identified as methylproline at the N-terminus of H2B (Desrosiers and Tanguay, 1988). Heat shock not only increases the level of H2B methylation but also decreases the level of H3 methylation. Several specific methylated amino acid residues of H3, such as H3 at lysine 4 (H3K4) and H3 at lysine 9 (H3K9), have been identified in rat astrocyte and cortical neuronal cultures (Marinova et al., 2011). H3K4 methylation is correlated with activation of gene transcription, whereas H3K9 methylation is linked to gene repression (Marinova et al., 2011). At the same time, H3 methylation can be inherited. Analysis of the expression profile of Caenorhabditis elegans showed that temperature-induced expression of endogenously inhibited repeats can be inherited for multiple generations through the trimethylation of histone H3 lysine 9 (Klosin et al., 2017).
Epigenetic Mechanisms of Heat Acclimation
The molecular program is the key to the occurrence of heat acclimation. In heat acclimation, accumulating evidence indicates that epigenetic mechanisms are powerful participants in these processes. Epigenetic mechanisms affect DNA accessibility to transcription factors, thereby regulating gene expression and controlling the phenotype. Another important group of epigenetic markers involved in epigenetic mechanisms is miRNAs with miR-297 upregulation in the heat-acclimated Rattus norvegicus (Horowitz, 2014; Tetievsky et al., 2014). In addition, decreased miR-1 and miR-206 levels promote HSP70 synthesis (Kwon et al., 2005; Kukreti et al., 2013; Radom-Aizik et al., 2013). In addition to methylation modification, the other posttranslational modifications (phosphorylation and acetylation) in histones play an important role in cytoprotective acclimatory memory. After studying the heat shock element (HSE) binding site of the promoters of HSP70 and HSP90, early histone H3 phosphorylation contributes to histone H4 acetylation, then the constitutively acetylated histone H4 and preserved euchromatin state lead to acclimatory memory (Tetievsky and Horowitz, 2010).
Studies on oxidative phosphorylation have shown that metabolic rate is a major indicator of heat acclimation (Rolfe and Brown, 1997; Seebacher et al., 2009, 2010). Under different conditions of heat stress and heat acclimation, numerous indicators, such as the metabolism of an organism, are widely different. Studies on metabolic capacity have demonstrated that cytochrome c oxidase (COX-respiratory complex IV) sensitivity/activation increases under heat stress (Seebacher, 2009; Seebacher et al., 2009). These results were consistent with a preference of cells for OXPHOS under heat stress (Sajjanar et al., 2019). In contrast, the expression of cytochrome c oxidase, citrate synthase, and proliferator-activated receptor γ coactivator 1α-a (PGC-1α) decreases in heat-acclimated organisms (Horowitz, 2014). Combined with larger HSP reserves in acclimation organism discussed above and that HSP70 was found to inhibit OXPHOS, we make a hypothesis that HSP70 inhibits OXPHOS in the acclimation organism and simultaneously enhances glycolysis to compensate the ATP unbalance (Wang et al., 2012). The OXPHOS complex exhibits different regulatory mechanisms under heat stress and heat acclimation. Another explanation states that heat acclimation reduces the thermal sensitivity of respiration of freshwater and marine animals to temperature changes that, in turn, decreases mitochondrial energy metabolism (Seebacher et al., 2014).
Another hallmark of heat acclimation is high mitochondrial calcium content (Assayag et al., 2012). Calcium content, metabolic rate, and ROS levels are closely related (Sohal and Allen, 1985; Cairns, 2009). Existing evidence shows that different mitochondrial metabolic states cause calcium to induce different effects on mitochondrial ROS levels (Adam-Vizi and Starkov, 2010). Under heat stress, calcium homeostasis is mediated by the chloride intracellular channels (CLICs) through the ryanodine receptor gene (RYR) in the ER membrane, whereas both the CLIC2 and RYR genes are identified as differentially methylated genes (Board et al., 2004). Aside from this pathway, methylated pyruvate kinase M2 (PKM2) isoforms can also alter the influx of calcium from the ER to the mitochondria by suppressing IP3R in tumors in MDA-MB-231 cells (Liu et al., 2017). Here, we make a hypothesis that DNA methylation may regulate the transport of calcium in various organs by methylating specific genes, thereby contributing to the development of heat acclimation.
Limitation
Although numerous studies have been conducted on the genetic and epigenetic mechanisms involved in HSR, several issues and questions remain to be elucidated. Many members of the HSP family play different roles. The current researches on HSP70 are relatively comprehensive, and the research of other members is relatively scarce. In addition, the experimental conditions set by these studies were different. They performed either in vivo or in vitro experiments, used different cell cultures, and utilized different organs of different species. It is hard to form a complete network regulation structure, and more experiments need to be supplemented and studied on the model organisms. Heat acclimation is a survival mechanism in organisms. At present, it is more based on the length of time to divide the heat acclimation into short- (<7 days), medium- (8–14 days), and long-term (>15 days) acclimation, which were summarized by Garrett et al. (2011). At present, many studies have shown that many genes are related to heat acclimation, but there is no clear statement, such as which genes or pathways play a key role in it during the formation of heat acclimation. For calcium, its main role is to change the microenvironment of cells by flowing between ER, cytoplasm, and mitochondria. Calcium plays an auxiliary role in oxidative stress, ER stress, and apoptosis, but a clear regulatory mechanism still needs a lot of experimental proof, such as affecting three-dimensional conformation or releasing responsive proteins. In addition, other components, such as miRNAs, have not been extensively investigated.
Conclusion
Heat greatly affects animal metabolism. Several heat sensors, including HSF1 and calcium, have been reported. As HSF1 production, HSPs facilitate the synthesis and ensure the structural stability of other intracellular proteins. The primary role of HSPs is to improve heat acclimation and form long-term memory. These two biological processes may be two separate processes and have different requirements for HSP production. Calcium, as a secondary messenger, functions by flowing between the ER, cytoplasm, and the mitochondria and is regulated by IP3R and MPTP or methylated genes, such as CLIC2, RYRs, and PKM2. Under heat stress, HSPs in the mitochondria and cytoplasm protect damaged proteins, reduce ROS in cells, and change metabolic processes, thereby further promoting the formation of heat acclimation. HSPs are the common regulatory pathway between immunity stress and heat acclimation. Immunity stress enables organisms to resist heat stress, whereas heat acclimation allows them to adapt to their environment. Further researches should also focus on specific genetic and epigenetic regulatory mechanisms involved in animal thermal response.
Author Contributions
CL, WZ, and JW wrote the manuscript. CL and WZ contributed to the reagents, materials, and analysis tools. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was financially supported by the National Key R&D Program of China (2018YFD0900603), National Natural Science Foundation of China (31522059, 41576139, and 31902399), and the K.C. Wong Magna Fund in Ningbo University.
References
- Adam-Vizi V., Starkov A. A. (2010). Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J. Alzheimer’s Dis. 20(Suppl. 2), S413–S426. 10.3233/JAD-2010-100465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed B., Averill-Bates D. A. (2015). Thermotolerance induced at a mild temperature of 40 °C alleviates heat shock-induced ER stress and apoptosis in HeLa cells. Biochim. Biophys. Acta Mol. Cell Res. 1 52–62. 10.1016/j.bbamcr.2014.09.016 [DOI] [PubMed] [Google Scholar]
- Allen J. L., Clusella-Trullas S., Chown S. L. (2012). The effects of acclimation and rates of temperature change on critical thermal limits in Tenebrio molitor (Tenebrionidae) and Cyrtobagous salviniae (Curculionidae). J. Insect Physiol. 58 669–678. 10.1016/j.jinsphys.2012.01.016 [DOI] [PubMed] [Google Scholar]
- Almamun M., Levinson B. T., Gater S. T., Schnabel R. D., Arthur G. L., Davis J. W., et al. (2014). Genome-wide DNA methylation analysis in precursor B-cells. Epigenetics 9 1588–1595. 10.4161/15592294.2014.983379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almamun M., Levinson B. T., Swaay A. C. V., Johnson N. T., Taylor K. H. (2015). Integrated methylome and transcriptome analysis reveals novel regulatory elements in pediatric acute lymphoblastic leukemia. Epigenet. Off. J. Dna Methyl. Soc. 10 882–890. 10.1080/15592294.2015.1078050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arányi T., Faucheux B. A., Khalfallah O., Vodjdani G., Biguet N. F., Mallet J., et al. (2005). The tissue-specific methylation of the human tyrosine hydroxylase gene reveals new regulatory elements in the first exon. J. Neurochem 94 129–139. 10.1111/j.1471-4159.2005.03173.x [DOI] [PubMed] [Google Scholar]
- Assayag M., Saada A., Gerstenblith G., Canaana H., Shlomai R., Horowitz M. (2012). Mitochondrial performance in heat acclimation-a lesson from ischemia/reperfusion and calcium overload insults in the heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303:R870. 10.1152/ajpregu.00155.2012 [DOI] [PubMed] [Google Scholar]
- Barna J., Csermely P., Vellai T. (2018). Roles of heat shock factor 1 beyond the heat shock response. Cel. Mol. Life Sci. 75 2897–2916. 10.1007/s00018-018-2836-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A., Han Q., Nair P., Stacey L., Gaynier H., Mosley M., et al. (2018). Dynamic DNA methylation in plant growth and development. Int. J. Mol. Sci. 19 E2144. 10.3390/ijms19072144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhadj S. I., Najar T., Ghram A., Dabbebi H., Ben Mrad M., Abdrabbah M. (2014). Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperther. 30 513–523. 10.3109/02656736.2014.971446 [DOI] [PubMed] [Google Scholar]
- Berna-Erro A., Woodard G. E., Rosado J. A. (2012). The versatility and universality of calcium signalling. J. Cell Mol. Med. 1 11–21. 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Bhandary B., Marahatta A., Kim H.-R., Chae H.-J. (2012). An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int. J. Mol. Sci. 14 434–456. 10.3390/ijms14010434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board P. G., Coggan M., Watson S., Gage P. W., Dulhunty A. F. (2004). CLIC-2 modulates cardiac ryanodine receptor Ca2+ release channels. Int. J. Biochem. Cell Biol. 36 1599–1612. 10.1016/j.biocel.2004.01.026 [DOI] [PubMed] [Google Scholar]
- Bowler K., Hollingsworth M. J. (1965). The effect of inbreeding on temperature acclimatization in Drosophila Subobscura. Genet. Res 10 1–12. 10.1017/S0016672300003931 [DOI] [PubMed] [Google Scholar]
- Brookes P. S. (2004). Calcium, ATP and ROS : a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 287 C817–C833. 10.1152/ajpcell.00139.2004 [DOI] [PubMed] [Google Scholar]
- Cairns R. B. (2009). The logic of chromatin architecture and remodelling at promoters. Nature 461 193–198. 10.1038/nature08450 [DOI] [PubMed] [Google Scholar]
- Carlisle R. E., Heffernan A., Brimble E., Liu L., Jerome D., Collins C. A., et al. (2012). TDAG51 mediates epithelial-to-mesenchymal transition in human proximal tubular epithelium. Am. J. Physiol. Ren. Physiol. 303 F467–F481. 10.1152/ajprenal.00481.2011 [DOI] [PubMed] [Google Scholar]
- Carter R., Cheuvront S. N., Williams J. O., Kolka M. A., Stephenson L. A., Sawka M. N., et al. (2005). Epidemiology of hospitalizations and deaths from heat illness in soldiers. Med. Sci. Sports Exerc. 37 1338–1344. 10.1249/01.mss.0000174895.19639.ed [DOI] [PubMed] [Google Scholar]
- Chuang Y. Y., Chen Y., Gadisetti, Chandramouli V. R., Cook J. A., Coffin D., et al. (2002). Gene expression after treatment with hydrogen peroxide, menadione, or t-butyl hydroperoxide in breast cancer cells. Cancer Res. 62 6246–6254. 10.1046/j.1523-5394.2002.106009.x [DOI] [PubMed] [Google Scholar]
- Crompton M., Barksby E., Johnson N., Capano M. (2002). Mitochondrial intermembrane junctional complexes and their involvement in cell death. Biochimie 84 143–152. 10.1016/s0300-9084(02)01368-8 [DOI] [PubMed] [Google Scholar]
- Dai T. M., Lü Z. C., Wang Y. S., Liu W. X., Hong X. Y., Wan F. H. (2018). Molecular characterizations of DNA methyltransferase 3 and its roles in temperature tolerance in the whitefly, Bemisia tabaci Mediterranean. Insect. Mol. Biol. 27 123–132. 10.1111/imb.12354 [DOI] [PubMed] [Google Scholar]
- Desrosiers R., Tanguay R. M. (1988). Methylation of Drosophila histones at proline, lysine, and arginine residues during heat shock. J. Biol. Chem. 263 4686–4692. [PubMed] [Google Scholar]
- Dor Y., Cedar H. (2018). Principles of DNA methylation and their implications for biology and medicine. Lancet 392 777–786. 10.1016/S0140-6736(18)31268-6 [DOI] [PubMed] [Google Scholar]
- Garrett A. T., Rehrer N. J., Patterson M. J. (2011). Induction and decay of short-term heat acclimation in moderately and highly trained athletes. Sports Med. 41 757–771. 10.2165/11587320-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Goldberg L. A. (2003). Protein degradation and protection against misfolded or damaged proteins. Nature 426 895–899. 10.1038/nature02263 [DOI] [PubMed] [Google Scholar]
- Görlach A., Bertram K., Hudecova S., Krizanova O. (2015). Calcium and ROS: a mutual interplay. Redox Biol. 6 260–271. 10.1016/j.redox.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L.-L., Li M.-Z., Wang G.-R., Liu X.-D. (2019). Multigenerational heat acclimation increases thermal tolerance and expression levels of Hsp70 and Hsp90 in the rice leaf folder larvae. J. Therm. Biol. 81 103–109. 10.1016/j.jtherbio.2019.02.024 [DOI] [PubMed] [Google Scholar]
- Gupta S., Deepti A., Deegan S., Lisbona F., Hetz C., Samali A. (2010). HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1alpha-XBP1 signaling through a physical interaction. PLoS Biol. 8:e1000410. 10.1371/journal.pbio.1000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q., Keesing J. K., Liu D. (2016). A review of sea cucumber aquaculture, ranching, and stock enhancement in China. Rev. Fish. Sci. 24 326–341. 10.1080/23308249.2016.1193472 [DOI] [Google Scholar]
- Hetz C. (2012). The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13 H2410–H2418. 10.1038/nrm3270 [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A., Srensen J. G., Loeschcke V. (2003). Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 28 175–216. 10.1016/s0306-4565(02)00057-8 [DOI] [Google Scholar]
- Horowitz M. (2014). Heat acclimation, epigenetics, and cytoprotection memory. Compr. Physiol. 4 199–230. 10.1002/cphy.c130025 [DOI] [PubMed] [Google Scholar]
- Horowitz M., Maloyan A., Shlier J. (1997). HSP 70 kDa dynamics in animals undergoing heat stress superimposed on heat acclimation. Ann. N. Y. Acad. Sci. 813 617–619. 10.1111/j.1749-6632.1997.tb51755.x [DOI] [PubMed] [Google Scholar]
- Hsu Y. L., Yu H.-S., Lin H.-C., Wu K.-Y., Yang R.-C., Kuo P.-L. (2011). Heat shock induces apoptosis through reactive oxygen species involving mitochondrial and death receptor pathways in corneal cells. Exp. Eye Res. 93 405–412. 10.1016/j.exer.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Huang K., Fan G. (2010). DNA methylation in cell differentiation and reprogramming: an emerging systematic view. Regenerat. Med. 5 531–544. 10.2217/rme.10.35 [DOI] [PubMed] [Google Scholar]
- Hung C. H., Chang N. C., Cheng B. C., Lin M. T. (2005). Progressive exercise preconditioning protects against circulatory shock during experimental heatstroke. Shock 23 426–433. 10.1097/01.shk.0000159557.95285.96 [DOI] [PubMed] [Google Scholar]
- Ishita A., de Vos R. C., Bones A. M., Hall R. D. (2010). Plant molecular stress responses face climate change. Trends Plant Sci. 15 664–674. 10.1016/j.tplants.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Jain A., Lamark T., Sjottem E., Bowitz Larsen K., Atesoh Awuh J., Overvatn A., et al. (2010). p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 285 22576–22591. 10.1074/jbc.M110.118976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julie S.-P., Buckingham J. A., Roebuck S. J., Brand M. D. (2002). Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 277 44784–44790. 10.1074/jbc.M207217200 [DOI] [PubMed] [Google Scholar]
- Khavari D. A., Sen G. L., Rinn J. L. (2010). DNA methylation and epigenetic control of cellular differentiation. Cell Cycle 9 3880–3883. 10.4161/cc.9.19.13385 [DOI] [PubMed] [Google Scholar]
- Kim M., Costello J. (2017). DNA methylation: an epigenetic mark of cellular memory. Exp. Mol. Med. 49 322–330. 10.1038/emm.2017.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisliouk T., Cramer T., Meiri N. (2017). Methyl CpG level at distal part of heat-shock protein promoter HSP70 exhibits epigenetic memory for heat stress by modulating recruitment of POU2F1-associated nucleosome-remodeling deacetylase (NuRD) complex. J. Neurochem. 141 358–372. 10.1111/jnc.14014 [DOI] [PubMed] [Google Scholar]
- Klein S. D., Brüne B. (2002). Heat-shock protein 70 attenuates nitric oxide-induced apoptosis in RAW macrophages by preventing cytochrome c release. Biochem. J. 362 635–641. 10.1042/0264-6021:3620635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosin A., Casas E., Hidalgo-Carcedo C., Vavouri T., Lehner B. (2017). Transgenerational transmission of environmental information in C. elegans. Science 356 320–323. 10.1126/science.aah6412 [DOI] [PubMed] [Google Scholar]
- Kukreti H., Amuthavalli K., Harikumar A., Sathiyamoorthy S., Feng P. Z., Anantharaj R., et al. (2013). Muscle-specific microRNA1 (miR1) targets heat shock protein 70 (HSP70) during dexamethasone-mediated atrophy. J. Biol. Chem. 288 6663–6678. 10.1074/jbc.M112.390369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kultz D. (2003). Evolution of the cellular stress proteome: from monophyletic origin to ubiquitous function. J. Exp. Biol. 206 3119–3124. 10.1242/jeb.00549 [DOI] [PubMed] [Google Scholar]
- Kuroda A., Rauch T. A., Todorov I., Ku H. T., Al-Abdullah I. H., Kandeel F., et al. (2009). Insulin gene expression is regulated by DNA methylation. PLoS One 4:e6953. 10.1371/journal.pone.0006953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C., Han Z., Olson E. N., Srivastava D. (2005). MicroRNA1 influences cardiac differentiation in Drosophila and regulates Notch signaling. Proc. Natl. Acad. Sci. U.S.A. 102 18986–18991. 10.1073/pnas.0509535102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyu-Han K., Jae-Yeon J., Young-Joon S., Kyu-Won K. (2009). Expression of stress-response ATF3 is mediated by Nrf2 in astrocytes. Nucleic Acids Res. 38 48–59. 10.1093/nar/gkp865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama G., Ferraraccio F., Iaccarino F., Luongo I., Marte A., Rambaldi P. F., et al. (2003). Pelviureteral junction obstruction: correlation of renal cell apoptosis and differential renal function. J. Urol. 169 2335–2338. 10.1097/01.ju.0000067385.26560.7c [DOI] [PubMed] [Google Scholar]
- Lee J., Bruce-Keller A. J., Kruman Y., Chan S. L., Mattson M. P. (1999). 2-deoxy-d-glucose protects hippocampal neurons against excitotoxic and oxidative injury: evidence for the involvement of stress proteins. J. Neurosci. Res. 57 48–61. [DOI] [PubMed] [Google Scholar]
- Li J., Labbadia J., Morimoto R. I. (2017). Rethinking HSF1 in stress, development, and organismal health. Trends Cell Biol. 27 895–905. 10.1016/j.tcb.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Tan H., Gu Z., Liu Z., Geng Y., Liu Y., et al. (2014). Heat stress induces apoptosis through a Ca2+-mediated mitochondrial apoptotic pathway in human umbilical vein endothelial cells. PLoS One 9:e111083. 10.1371/journal.pone.0111083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Ma F., Wang Y., Hao L., Zeng H., Jia C., et al. (2017). PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis. Nat. Cell Biol. 19 1358–1370. 10.1038/ncb3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C. A., Buckley B. A. (2015). Transcriptomic responses to environmental temperature in eurythermal and stenothermal fishes. J. Exp. Biol. 218 1915–1924. 10.1242/jeb.114397 [DOI] [PubMed] [Google Scholar]
- Magalhães Fde C, Amorim F. T., Passos R. L., Fonseca M. A., Oliveira K. P., Lima M. R., et al. (2010). Heat and exercise acclimation increases intracellular levels of Hsp72 and inhibits exercise-induced increase in intracellular and plasma Hsp72 in humans. Cell Stress Chaperones 6 885–895. 10.1007/s12192-010-0197-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloyan A., Aaron P., Michal H. (1999). Heat acclimation increases the basal HSP72 level and alters its production dynamics during heat stress. Am. J. Physiol. 276 1506–1515. 10.1007/s002320010015 [DOI] [PubMed] [Google Scholar]
- Manucha W., Kurbán F., Mazzei L., Benardón M. E., Bocanegra V., Tosi M. R., et al. (2011). eNOS/Hsp70 interaction on rosuvastatin cytoprotective effect in neonatal obstructive nephropathy. Eur. J. Pharmacol. 650 487–495. 10.1016/j.ejphar.2010.09.059 [DOI] [PubMed] [Google Scholar]
- Marcu M. G., Doyle M., Bertolotti A., Ron D., Neckers L. (2003). Heat shockprotein 90 modulates the unfolded protein response by stabilizing IRE1. Mol. Cell. Biolo. 22 8506–8513. 10.1128/MCB.22.24.8506-8513.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinova Z., Leng Y., Leeds P., Chuang D.-M. (2011). Histone deacetylase inhibition alters histone methylation associated with heat shock protein 70 promoter modifications in astrocytes and neurons. Neuropharmacology 60 1109–1115. 10.1016/j.neuropharm.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Nakagawa S. Y., Nakano T., Asaumi J. I., Jagetia G. C., Kawasaki S. (2006). Effects of KNK437 on heat-induced methylation of histone H3 in human oral squamous cell carcinoma cells. Int. J. Hyperthermia 22 729–735. 10.1080/02656730601074375 [DOI] [PubMed] [Google Scholar]
- Mcclung J. P., Hasday J. D., He J., Montain S. J., Cheuvront S. N., Sawka M. N., et al. (2008). Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294 R185–R191. 10.1152/ajpregu.00532.2007 [DOI] [PubMed] [Google Scholar]
- Mehlen P., Kretz-Remy C., Préville X., Arrigo A. P. (1996). Human HSP27, Drosophila HSP27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFα-induced cell death. EMBO J. 15 2695–2706. 10.1002/j.1460-2075.1996.tb00630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F. L., Roberts A. G., Bowman M. K., Kramer D. M. (2003). Architecture of the Qo site of the cytochrome bc1 complex probed by superoxide production. Biochemistry 42 6493–6499. 10.1021/bi0342160 [DOI] [PubMed] [Google Scholar]
- Negrón-Pérez V. M., Fausnacht D. W., Rhoads M. L. (2019). Invited review: management strategies capable of improving the reproductive performance of heat-stressed dairy cattle. J. Dairy Sci. 102 10695–10710. 10.3168/jds.2019-16718 [DOI] [PubMed] [Google Scholar]
- Norouzitallab P., Baruah K., Vandegehuchte M., Van Stappen G., Catania F., Vanden Bussche J., et al. (2014). Environmental heat stress induces epigenetic transgenerational inheritance of robustness in parthenogenetic Artemia model. Faseb J. 28 3552–3563. 10.1096/fj.14-252049 [DOI] [PubMed] [Google Scholar]
- Nyboer E. A., Chapman L. J. (2017). Elevated temperature and acclimation time affect metabolic performance in the heavily exploited Nile perch of Lake Victoria. J. Exp. Biol. 220 3782–3793. 10.1242/jeb.163022 [DOI] [PubMed] [Google Scholar]
- Park C. G., Lee S. Y., Kandala G., Lee S. Y., Choi Y. (1996). A novel gene product that couples tcr signaling to fas(cd95) expression in activation-induced cell death. Immunity 4 583–591. 10.1016/S1074-7613(00)80484-7 [DOI] [PubMed] [Google Scholar]
- Park E. S., Kim J., Ha T. U., Choi J. S., Soo Hong K., Rho J. (2013). Tdag51 deficiency promotes oxidative stress-induced apoptosis through the generation of reactive oxygen species in mouse embryonic fibroblasts. Exp. Mol. Med. 45 35–43. 10.1038/emm.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit G. K., Mahanty A., Suar M., Sharma A. P., Mohanty S. (2014). Investigating hsp Gene expression in liver of channa striatus under heat stress for understanding the upper thermal acclimation. J. Biomed. Biotechno. 2014 1–10. 10.1155/2014/381719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z., Zhou B., Ann D., Parviz M., Liu Y. (2011). Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am. J. Respir. Cell Mol. Biol. 45 498–509. 10.1165/rcmb.2010-0347OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radom-Aizik S., Zaldivar F. P., Nance D. M., Haddad F., Cooper D. M., Adams G. R. (2013). Growth inhibition and compensation in response to neonatal hypoxia in rats. Pediatr. Res. 74 111–120. 10.1038/pr.2013.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa F. (1996). Discovery of the heat shock response. Cell Stress Chaperones 1 97–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe D. F., Brown G. C. (1997). Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 77 731–758. 10.0000/PMID9234964 [DOI] [PubMed] [Google Scholar]
- Roufayel R., Kadry S. (2019). Examination of the role of miR-23a in the development of thermotolerance. Curr. Mol. Med. 20 194–201. 10.2174/1566524019666191021111028 [DOI] [PubMed] [Google Scholar]
- Ryu D. S., Yang H., Lee S. E., Park C.-S., Jin Y.-H., Park Y. S. (2013). Crotonaldehyde induces heat shock protein 72 expression that mediates anti-apoptotic effects in human endothelial cells. Toxicol. Lett. 223 116–123. 10.1016/j.toxlet.2013.09.010 [DOI] [PubMed] [Google Scholar]
- Sajjanar B., Siengdee P., Trakooljul N., Liu X., Ponsuksili S. (2019). Cross-talk between energy metabolism and epigenetics during temperature stress response in c2c12 myoblasts. Int. J. Hyperthermia 36 776–784. 10.1080/02656736.2019.1639834 [DOI] [PubMed] [Google Scholar]
- Samali A., Robertson J. D., Peterson E., Manero F., Zeijl L. V., Paul C., et al. (2001). Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones 6 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarasinghe B., Wales C. T. K., Taylor F. R., Jacobs A. T. (2014). Heat shock factor 1 confers resistance to Hsp90 inhibitors through p62/SQSTM1 expression and promotion of autophagic flux. Biochem. Pharmacol. 87 445–455. 10.1016/j.bcp.2013.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho P. (2003). Differential effects of catalase on apoptosis induction in human promonocytic cells. Relationships with heat-shock protein expression. Mol. Pharmacol. 63 581–589. 10.1124/mol.63.3.581 [DOI] [PubMed] [Google Scholar]
- Seebacher F. (2009). Responses to temperature variation: integration of thermoregulation and metabolism in vertebrates. J. Exp. Biol. 212 2885–2891. 10.1242/jeb.024430 [DOI] [PubMed] [Google Scholar]
- Seebacher F., Brand M. D., Else P. L., Guderley H., Hulbert A. J., Moyes C. D. (2010). Plasticity of oxidative metabolism in variable climates: molecular mechanisms. Physiol. Biochem. Zool. 83 721–732. 10.1086/649964 [DOI] [PubMed] [Google Scholar]
- Seebacher F., Murray S. A., Else P. L. (2009). Thermal acclimation and regulation of metabolism in a reptile (Crocodylus porosus): the importance of transcriptional mechanisms and membrane composition. Physiol. Biochem. Zool. 82 766–775. 10.1086/605955 [DOI] [PubMed] [Google Scholar]
- Seebacher F., White C. R., Franklin C. E. (2014). Physiological plasticity increases resilience of ectothermic animals to climate change. Na. Clim. Change 5 61–66. 10.1038/nclimate2457 [DOI] [Google Scholar]
- Smallwood S. A., Lee H. J., Angermueller C., Krueger F., Saadeh H., Peat J., et al. (2014). Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat. Methods 11 817–820. 10.1038/nmeth.3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal R. S., Allen R. G. (1985). Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. Basic Life Sci. 35 75–104. 10.1007/978-1-4899-2218-2_4 [DOI] [PubMed] [Google Scholar]
- Song S., Tan J., Miao Y., Zhang Q. (2018). Crosstalk of ER stress-mediated autophagy and ER-phagy: involvement of UPR and the core autophagy machinery. J. Cell. Physiol. 233 3867–3874. 10.1002/jcp.26137 [DOI] [PubMed] [Google Scholar]
- Sreedhar A. S., Pardhasaradhi B. V. V., Khar A., Srinivas U. K. (2002). A cross talk between cellular signaling and cellular redox state during heat-induced apoptosis in a rat histiocytoma. Free Radic. Biol. Med. 32 221–227. 10.1016/s0891-5849(01)00796-1 [DOI] [PubMed] [Google Scholar]
- Suzuki M. M., Bird A. (2008). DNA methylation landscapes: provocative insights from epigenomics. Nat. Rev. Genet. 9 465–476. 10.1038/nrg2341 [DOI] [PubMed] [Google Scholar]
- Takaki E., Ichikawa H., Rho J., Nakai A., Hayashida N., Inouye S., et al. (2006). A novel HSF1-mediated death pathway that is suppressed by heat shock proteins. Embo J. 25 4773–4783. 10.1038/sj.emboj.7601370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takii R., Inouye S., Fujimoto M., Nakamura T., Shinkawa T., Prakasam R., et al. (2010). Heat shock transcription factor 1 inhibits expression of IL-6 through activating transcription factor 3. J. Immunol. 184:1041. 10.4049/jimmunol.0902579 [DOI] [PubMed] [Google Scholar]
- Taylor E. R., Hurrell F., Shannon R. J., Lin T. K., Hirst J., Murphy M. P. (2003). Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J. Biol. Chem. 278 19603–19610. 10.1074/jbc.m209359200 [DOI] [PubMed] [Google Scholar]
- Tetievsky A., Assayag M., Ben-Hamo R., Efroni S., Cohen G., Abbas A., et al. (2014). Heat acclimation memory: do the kinetics of the deacclimated transcriptome predispose to rapid reacclimation and cytoprotection. J. Appl. Physiol. 117 1262–1277. 10.1152/japplphysiol.00422.2014 [DOI] [PubMed] [Google Scholar]
- Tetievsky A., Horowitz M. (2010). Posttranslational modifications in histones underlie heat acclimation-mediated cytoprotective memory. J. Appl. Physiol. 109 1552–1561. 10.1152/japplphysiol.00469.2010 [DOI] [PubMed] [Google Scholar]
- Turrens J. F. (1997). Superoxide production by the mitochondrial respiratory chain. Biosci. Rep. 17 3–8. 10.1023/a:1027374931887 [DOI] [PubMed] [Google Scholar]
- Vinoth A., Thirunalasundari T., Shanmugam M., Uthrakumar A., Suji S., Rajkumar U. (2018). Evaluation of DNA methylation and mRNA expression of heat shock proteins in thermal manipulated chicken. Cell Stress Chaperones 23 235–252. 10.1007/s12192-017-0837-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Schumann U., Liu Y., Prokopchuk O., Steinacker J. M. (2012). Heat shock protein 70 (Hsp70) inhibits oxidative phosphorylation and compensates ATP balance through enhanced glycolytic activity. J. Appl. Physiol. 113 1669–1676. 10.1152/japplphysiol.00658.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Tsujimura A., Taguchi K., Tanaka M. (2016). HSF1 stress response pathway regulates autophagy receptor SQSTM1/p62-associated proteostasis. Autophagy 13 133–148. 10.1080/15548627.2016.1248018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon C. W., Terblanche J. S., Chown S. L. (2011). Time-course for attainment and reversal of acclimation to constant temperature in two ceratitis species. J. Therm. Biol. 36 479–485. 10.1016/j.jtherbio.2011.08.005 [DOI] [Google Scholar]
- Xiao F., Li Y., Dai L., Deng Y., Zou Y., Li P., et al. (2012). Hexavalent chromium targets mitochondrial respiratory chain complex I to induce reactive oxygen species-dependent caspase-3 activation in L-02 hepatocytes. Int. J. Mol. Med. 30 629–635. 10.3892/ijmm.2012.1031 [DOI] [PubMed] [Google Scholar]
- Yamada P. M., Amorim F. T., Moseley P., Robergs R., Schneider S. M. (2007). Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J. Appl. Physiol. 103 1196–1204. 10.1152/japplphysiol.00242.2007 [DOI] [PubMed] [Google Scholar]
- Yura T., Nagai H., Mori H. (1993). Regulation of the heat-shock response in bacteria. Annu. Rev. Microbiol. 47 321–350. 10.1146/annurev.mi.47.100193.001541 [DOI] [PubMed] [Google Scholar]
- Zhang B., Wang X. Q., Chen H. Y., Liu B. H. (2014). Involvement of the Nrf2 pathway in the regulation of pterostilbene-induced apoptosis in HeLa cells via ER Stress. J. Pharmacol. Sci. 126 216–229. 10.1254/jphs.14028fp [DOI] [PubMed] [Google Scholar]
- Zhang L. J., Chen J. L., Yang B. L., Kong X. G., Bourguet D., Wu G. (2017). Thermotolerance, oxidative stress, apoptosis, heat-shock proteins and damages to reproductive cells of insecticide-susceptible and -resistant strains of the diamondback moth Plutella xylostella. Bull. Entomol. Res. 107 513–526. 10.1017/S0007485317000049 [DOI] [PubMed] [Google Scholar]
- Zhang S., Zheng H., Chen Q., Chen Y., Wang S., Lu L., et al. (2017). The lectin chaperone calnexin is involved in endoplasmic reticulum stress response by regulating Ca2+ homeostasis in Aspergillus nidulans. Appl. Environ. Microbiol. 83 673–617. 10.1128/AEM.00673-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Luo D., Miao R., Bai L., Ge Q., Sessa W. C., et al. (2005). Hsp90–Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene 24 3954–3963. 10.1038/sj.onc.1208548 [DOI] [PubMed] [Google Scholar]