Abstract
Background
Erectile dysfunction in men is a common underestimated complication of diabetes mellitus, which is becoming a significant public health problem both in developing and developed countries. Erectile dysfunction threatens the well-being of clients, hence determining its risk factors and controlling it at an early stage is vital to preventing serious consequences and the burden of the disease. Therefore, this study aimed to systematically evaluate erectile dysfunction risk factors in patients with diabetes mellitus in Africa.
Methods
PubMed, Web of Science, Scopus, African Journals Online, Wiley Online Library and Google Scholar were searched and complemented by manual searches. Egger's regression test was used to determine publication bias. The I2 statistic was used to check heterogeneity between the studies. DerSimonian and Laird random-effects model was applied to estimate pooled effect size, odds ratios, and 95% confidence interval across studies. STATA version 14 statistical software was used for the meta-analysis.
Result
Overall, 17 studies with 6002 study participants were included to identify risk factors of erectile dysfunction among diabetic patients. Duration of diabetes mellitus >10 years (AOR = 2.63; 95% CI 1.27, 5.43), age >40 years (AOR = 1.24; 95% CI: 1.03, 1.51), peripheral neuropathy (AOR = 2.34; 95% CI: 1.51, 10.72), no physical exercise (AOR = 1.63; 95% CI: 1.49, 1.78), testosterone level <8 nmol/l (AOR = 2.83; 95% CI: 1.06, 12.86), and peripheral vascular disease (AOR = 2.85, 95% CI: 1.54–5.27) were significantly associated with erectile dysfunction among diabetic patients.
Conclusions
This study found that long duration of diabetes mellitus, age >40 years, testosterone deficiency, peripheral neuropathy, not involved in physical exercise, peripheral vascular disease, were significantly associated with increased risk of erectile dysfunction among diabetic patients Therefore, situation-based interventions and country context-specific preventive strategies should be developed to decrease the risk factors of erectile dysfunction among patients with diabetes mellitus.
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; DM, diabetes mellitus; ED, erectile dysfunction; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Keywords: Erectile dysfunction, Impotence, Sexual dysfunction, Diabetes mellitus, Africa
Background
Diabetes is a major public health problem, and one of the common chronic non-communicable diseases in the world [1]. About 629 million people worldwide are projected to develop diabetes mellitus (DM) by the year 2045, with the highest estimates in developing countries [2]. The burden of DM in Africa has been increasing, and which substantially affects Africa's health care systems [3]. Erectile dysfunction (ED) in men is a common underestimated complication of DM [4]. Globally, about 322 million adult men are estimated to develop ED by the year 2025 [5]. ED is the inability to achieve and maintain an erection sufficient to permit satisfactory sexual intercourse [6]. ED in a man with diabetes is currently considered to be the most reliable indicator of organ-related and vascular complications [7].
Though the debate about the mechanism of ED among diabetic patients is on-going, evidence suggests that it is often related to a complex mechanism involving several factors including arterial impairment, poor glycemic control, neurologic damage, autonomic neuropathy, and hormone imbalance [4], [8], [9], [10], [11]. Though ED in patients with DM is a highly preventive challenge, it highly threatens the well-being of clients by increased psychological distress [12], [13], decreased quality of life [14], [15], and increased risk of cardiovascular disease [16], [17].
The prevalence of ED in patients with diabetes varies widely in different studies across the globe. For instance, it has been reported 35.8% in Italy [18], 64.6% in Japan [19], 65.4% in Korean [20], 86.1 in Saudi Arabia [21], 38.9% in India [22]. Besides, one review in Africa found that the pooled prevalence of erectile dysfunction was 71.45% among diabetic patients [23]. Though the influencing factors for ED are multifactorial and complex. A review of several studies showed that autonomic neuropathy [24], increased adipose mass[25], increased blood pressure [26], [27], [28], [29], cardiovascular disease [26], smoking [26], [27], [28], [29], [30], low education level [27], HbA1c >7% [27], [31], [32], age >50 years [22], [27], [30], [32], [33], increasing duration of diabetes [33], presence of depressive symptoms [22], high income [32], fat-rich diets [34], and other diabetic complications [35], were all found to be statistically associated with ED among DM.
Identification of associated factors is a significant consideration in decreasing the risk and magnitude of ED. Therefore, several studies have shown that determining risk factors can be used as benchmarks to design appropriate prevention measure through lifestyle modification, such as exercise and weight loss, cessation of smoking, and appropriate glycemic control through diet [36], [37], [38]. However, contributing factors related to ED are not been well known. Therefore, this study aimed to systematically evaluate the influencing factors of ED in DM patients using evidence-based medicine. This report provides a scientific basis for a better understanding of the causes of DM complicated with ED and preventive strategies.
Methods
Data sources and search strategy
First, Joanna Briggs Institute, and PROSPERO databases were searched to ensure whether a systematic review and meta-analysis was previously carried out or for the presence of ongoing research related to the current topic. A preliminary search was conducted in the English databases such as PubMed, Google Scholar, African Journals Online, Scopus, Web of Science, and the Cochrane Library articles published from inception to April 16, 2020. In addition, a manual search of grey literature and other related articles were deployed to identify additional relevant research. Endnote X 8.1 reference manager software was used to collect and organize search outcomes and for removal of duplicate articles. The searches were restricted to full texts, free articles, human studies, and English language publications. The search terms used a combination of relevant medical subject headings (MeSH) and phrases: “erectile dysfunction”, “sexual function”, “sexual dysfunction”, “impotence”, “diabetes mellitus”, “diabetes”, “risk factors”, “determinates”, “predictors”, “Africa”, and “names of each Africa countries”. Boolean operators like “AND” and “OR” were used to combine search terms. Mainly, to fit the advanced PubMed database, the following search strategy was used in (Supplementary file 1).
Eligibility criteria
The inclusion criteria were: (1) observational studies investigating ED risk factors in patients with DM; (2) studies published in peer-reviewed journals or grey literature; (3) articles published in English from inception to April 16, 2020; and (4) studies from Africa countries and include male participants >18 years of age. Excluded studies if: (1) they were not fully accessible; (2) overlapping data; (3) they possessed a low-quality score as per the stated criteria; (4) reported outcome measures was not odds ratios (ORs) or relative risks (RRs) with 95% confidence intervals; (5) case series, letters, comments and editorials; and (6) included only females.
Study selection
Following the search, all identified citations were uploaded into EndNote version 8.1 and duplicates were removed. Titles and abstracts were then screened by two independent reviewers (WSS & YAA) for assessment against the inclusion criteria for the review. Potentially relevant studies were retrieved in full, and their citation details imported into STATA software. The full text of selected citations was assessed in detail against the inclusion criteria by two independent reviewers (HAA& TYA). Reasons for exclusion of full text studies that did not meet the inclusion criteria were recorded and reported in the systematic review. Any disagreements that arose between the reviewers at each stage of the study selection process were resolved through discussion.
Data extraction and quality assessment
Two (HAA &YAA) investigators independently performed data extraction. The Joanna Briggs Institute (JBI) tool used for the data extraction [39]. For each included study, we extracted data including the name(s) of the author(s), year of publication, study countries, study design, sample size, data collection year, sampling technique, diagnostic criteria used for ED, and risk factor data including the definition of risk factors, ORs, RRs, and corresponding confidence intervals (CIs). Newcastle-Ottawa Scale (NOS) was used to examine the methodological quality of each selected articles[40]. Based on the previous literature reports, studies were included in the analysis if they scored ≥5 out of 10 points in three domains of ten modified NOS components [40]. Moreover, any disagreements at the time of data abstraction and quality assessment resolved by discussion and consensus (Supplementary file 2). Furthermore, the risk of bias of selected articles was assessed using the risk of bias tool for prevalence studies developed by Hoy et al. [41]. The risk of bias within the articles chosen papers were classified as either low, moderate, or high. Two authors independently carried out the risk of bias assessment of the included studies.
Statistical analysis
We used random-effects DerSimonian and Laird model to assess the pooled risk estimates reported as odds ratios and 95% confidence intervals [42]. All of the results were reported as odds ratio (ORs) with 95% confidence intervals to compare dichotomous variables. Heterogeneity across the included studies was checked using the chi-square based Cochran's Q test and the I2 statistical test [43]. To investigate the sources of heterogeneity sensitivity and subgroup analysis were performed. The meta-analysis performed using the STATA version 14 statistical software for Windows [44].
Data synthesis and reporting
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was used to report the pooled risk estimates of ED in patients with DM [45]. All processes of study screening, selection, and inclusions are described using a flow diagram. Finding was presented using forest plots and summary tables.
Results
Selection of the studies
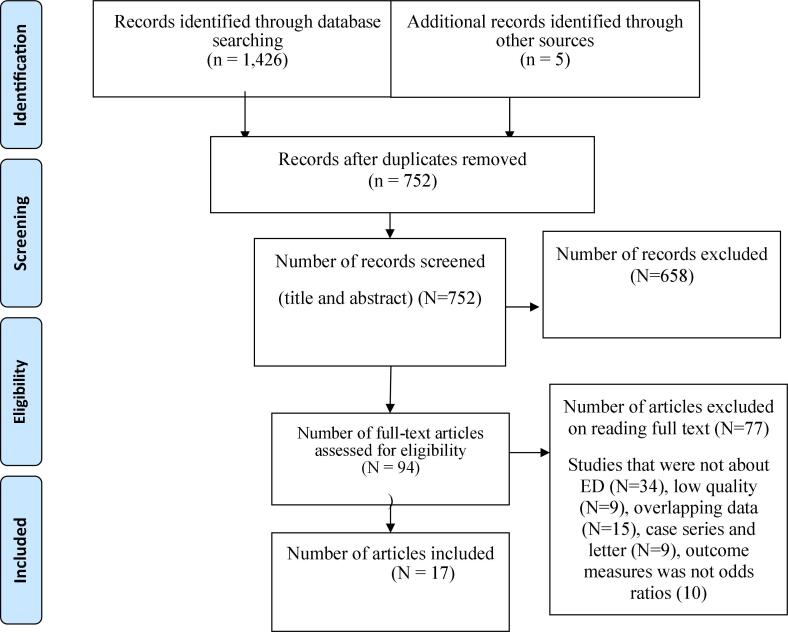
Initial database searching recovered 1,431 unique records, which were catalogued in citation management software (EndNote X8.1). Of these 1,426 studies were retrieved from PubMed (3 2 4), Scopus (28), Google Scholar (5 1 0), Web of Science (30), Wiley Online Library (1 2 7), and African Journals Online (4 0 7). On the other hand, the remaining 5 articles were found through manual search. Out of them, 679 duplicate records were identified and removed. Following removal of duplicate studies, the titles and abstracts were evaluated, and 658 studies were excluded based on the pre-specified inclusion criteria. Then, 94 studies were included for further assessment. After reviewing the full text, based on the pre-defined criteria and quality assessment, only 17 articles were included for the final analysis (Fig. 1).
Fig. 1.
Flowchart of study selection process.
Baseline characteristics of the included studies
A total of 17 studies with 6,002 study participants were included to identify risk factors of erectile dysfunction among diabetic patients. Regarding to study design, all included studies were cross-sectional the number of participants per study ranging from 70 to 1,600. Risk factors of ED patients with DM were obtained from various countries in Africa. Five of the studies included in this review were from Nigeria [46], [47], [48], [49], [50], four from Ethiopia [51], [52], [53], [54], three from Ghana [32], [55], [56], one each from South Africa [57], Egypt [31], Kenya [58], Zimbabwe [59], and Tanzania [60]. Concerning the sampling technique 12 of the studies [31], [32], [46], [47], [48], [49], [50], [54], [55], [56], [58], [59] used consecutive sampling to select study participants. However, one study [60] did not report the sampling method. Different diagnostic criteria were used to assess ED in DM patients. These consisted of 11 studies used the International Index of Erectile Function tool [31], [46], [47], [48], [49], [50], [51], [52], [58], [59], [60]. Two of the studies used the Golombok Rust Inventory of Sexual Satisfaction criteria [32], [55]. Only one study used the Sexual Health Inventory for Men items [57] and the remaining one [51] used changes in Sexual Functioning Questionnaire. Concerning the type of DM reported risk factors of ED, ten [32], [51], [52], [53], [54], [55], [57], [58], [59], [60] studies were included both type 1 and type 2 DM. The quality score of included studies, based on the Newcastle-Ottawa quality score assessment, was moderate to high for all 17 articles assessed (Table 1).
Table 1.
Baseline characteristics of the included studies.
| Authors [reference] | Publication Year |
Study Country |
Sample Size | Diagnostic criteria for ED | Risk factors | Measure of association |
Sampling Technique |
Type of DM | Quality Score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Effect size | p-value | |||||||||
| Ugwu et al [48] | 2016 | Nigeria | 160 | IIEF-5 | Duration of DM P. vasulardisease HbA1c ≥ 7% Testosterone Autonomic neuropathy |
aOR = 1.14(1.02–1.28) aOR = 3.8(1.28,11.67) aOR = 7.1(2.49,20.37) aOR = 6.6(2.61–16.83) aOR = 3.5(1.82–6.79) |
0.024 0.016 <0.001 <0.001 <0.001 |
Consecutive | Type2 | 6 |
| Obi et al. [49] | 2016 | Nigeria | 300 | IIEF-5 | AGE SBP |
Mean = 60.7 ± 10.3 Mean = 135.23 ± 21.49 |
0.048 0.003 |
Consecutive | Type2 | 7 |
| BELLO KS, et al. [50] | 2017 | Nigeria | 311 | IIEF | Age Divorced Unemployment HTN SBP |
X2 = 29.382 X2 = 7.446 X2 = 17.503 X2 = 11.440 X2 = 5.089 |
<0.001 0.026 0.001 0.001 0.032 |
Consecutive | Type2 | 8 |
| Amidu et al. [55] | 2013 | Ghana | 300 | GRISS | Age SBP DBP duration of DM |
r = 0.39 r = 0.23 r = 0.19 r = 0.26 |
0.001 0.001 0.01 0.001 |
Consecutive | Type 1&2 |
6 |
| Worku et al. [53] | 2010 | Ethiopia | 305 | NR | Age Duration of DM |
0.045 0.012 |
Systematic random sampling | Type 1&2 | 7 | |
| Asefa et al. [54] | 2019 | Ethiopia | 423 | CSFQ-14 | Age lack of education divorced depression comorbidity not exercise |
aOR: 3.9(2.32–6.85) aOR: 3.2(1.60–6.39) aOR: 5.3(2.35–11.86) aOR: 4.0(2.32–7.10) aOR: 2.0(1.18–3.58) aOR: 1.62(1.47–1.77) |
0.001 0.001 0.02 0.001 0.01 0.037 |
Consecutive | Type 1&2 |
6 |
| Annani-Akollor et al. [56] | 2019 | Ghana | 1600 | NR | Age Married Read and write |
0.04 0.03 0.01 |
Consecutive | Type2 | 7 | |
| El Saghier et al. [31] | 2015 | Egypt | 70 | IIEF-5 | Age BMI kg/m2 WC cm SBP mmHg DBP mmHg HBA1c% Testosterone |
aOR = 0.68 (0.58,0.80) aOR = 0.55(0.41,0.72) aOR = 0.89 (0.84,0.94) aOR = 0.81(0.74, 0.89) aOR = 0.73 (0.62,0.85) OR = 0.02(0.004,0.114 aOR = 1.4 (1.21, 1.63) |
0.001 0.001 0.001 0.001 0.001 0.001 0.001 |
Consecutive | Type2 | 7 |
| Kemp et al. [57] | 2015 | South Africa | 150 | SHIM | Age Body mass index P. neuropathy On diuretic |
aOR = 1.1(1.05, 1.17) aOR = 1.09(1.01, 1.18) aOR = 1.22(1.04,1.45) aOR = 5.26(1.89,14.6) |
0.001 0.049 0.018 0.002 |
Simple random sampling | Type 1&2 | 7 |
| Likata et al. [58] | 2012 | Kenya | 350 | IIEF | Age P. education 2nd education Duration of DM |
aOR = 4.8(1.1,22.2) aOR = 8.3 (2.1,33.8) aOR = 12.9(3.1,52.8) aOR = 4.7 (1.9,11.6) |
0.039 0.001 0.003 0.001 |
Consecutive | Type 1&2 |
7 |
| Mutagaywa et al. [60] | 2014 | Tanzania | 312 | IIEF | Age p. neuropathy p. vascular disease Monofilament test couldn’t feel |
aOR = 7.1(1.2–40.7) aOR = 5.9(1.6–21.3) aOR = 2.5(1.2–5.3) aOR = 4.9(2.6–9.5) |
0.001 0.001 0.001 0.001 |
Unspecified | Type 1&2 | 7 |
| Olarinoye et al. [46] | 2006 | Nigeria | 77 | IIEF | Age Duration of DM |
0.04 0.04 |
Consecutive | Type2 | 7 | |
| Owiredu et al. [32] | 2011 | Ghana | 300 | GRISS | Age Income Obese No exercise |
r = 0.39 r = 0.20 aOR = 10.4(2.3–47.6) aOR = 2.0(1.1–3.6) |
0.001 0.01 0.003 0.023 |
Consecutive | Type 1&2 |
7 |
| Pasipanodya et al. [59] | 2018 | Zimbabwe | 348 | IIEF | Age SBP On HTN drug HbA1C > 7% |
0.009 0.009 0.007 0.02 |
Consecutive | Type 1&2 | 6 | |
| Seid et al. [51] | 2017 | Ethiopia | 249 | IIEF | Age > 60 duration of dm high monthly income |
aOR = 15(3.21, 70.16) aOR = 3.7(1.2,11.05) aOR = 0.28(0.13,0.61) |
0.001 0.001 0.015 |
Systematic random sampling | Type 1&2 |
8 |
| Ugwumba et al. [47] | 2018 | Nigeria | 325 | IIEF | Age FBS HBA1c Duration of DM BMI |
aOR = 1.05(1.03,1.08) aOR = 1.03(1.01,1.08) aOR = 5.9(4.01,7.07) aOR = 2.7(1.48,5.23) aOR = 2.22(1.17,3.36) |
0.02 0.001 0.001 0.002 <0.001 |
Consecutive | Type2 | 7 |
| Walle et al. [52] | 2018 | Ethiopia | 422 | IIEF | Age Duration of DM Dm complication |
aOR = 7.1(2.61,19.45) aOR = 3.9 (1.06,17.36) aOR = 5.2(2.04–13.42) |
<0.05 <0.05 <0.05 |
Systematic Random sampling |
Type 1&2 |
6 |
NR; not reported; GRISS: Golombok Rust Inventory of Sexual Satisfaction; IIEF: International Index of Erectile Function; SHIM: Sexual Health Inventory for Men; CSFQ-14: Changes in Sexual Functioning Questionnaire-fourteen items; DM: diabetes mellitus.
Review findings
Risk factors of erectile dysfunction
Seventeen studies reported risk factors associated with ED in diabetic patients. However, in most the studies the method to assess this association was varied. In the majority of studies, correlates of ED included systolic and diastolic high blood pressure, long duration of diabetes, older age, glycosylated haemoglobin, autonomic neuropathy, and obesity [31], [32], [46], [47], [48], [49], [51], [52], [53], [57], [58], [59].
Association of age and duration of diabetes on erectile dysfunction
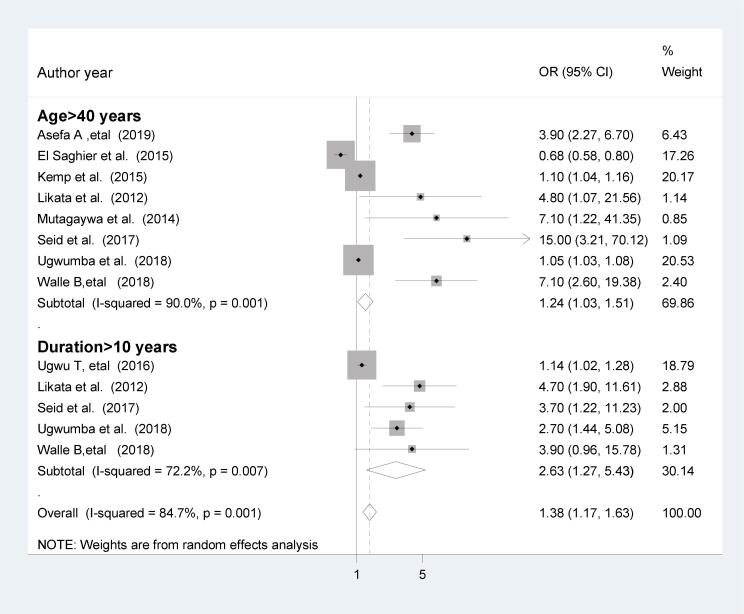
The majority of the reports described the effects of age on ED in patients with DM; all 16 studies were cross-sectional. Of these reports, only those that described the data in terms of the odds ratio, relative risk and categorical variables were included, therefore eight cross -sectional studies were included. In the present analysis, the pooled effect of eight studies [31], [47], [51], [52], [54], [57], [58], [60] showed that being over 40 years was statistically associated with ED in patients with DM (OR = 1,24; 95%CI; 1.03, 1.51). The heterogeneity test (I2 = 90%) showed significant evidence of variation across studies. Therefore, the random-effects model was used to pool the results. On the other hand, eight of the studies reported the effects of duration of diabetes on erectile dysfunction. Of these, only studies reported data in terms of odds ratio, relative risk and categorical variable were included. Therefore, five cross-sectionall studies were included in the final analysis. The pooled effects of five studies [47], [48], [51], [52], [58] showed that a duration of diabetes for >10 years was statistically associated with ED in patients with DM (OR = 2.63; 95%CI; 1.27, 5.43). The heterogeneity test (I2 = 72.2%) shows moderate evidence of variation across studies (Fig. 2).
Fig. 2.
The pooled effect of age and duration of diabetes on erectile dysfunction.
Other influencing factors
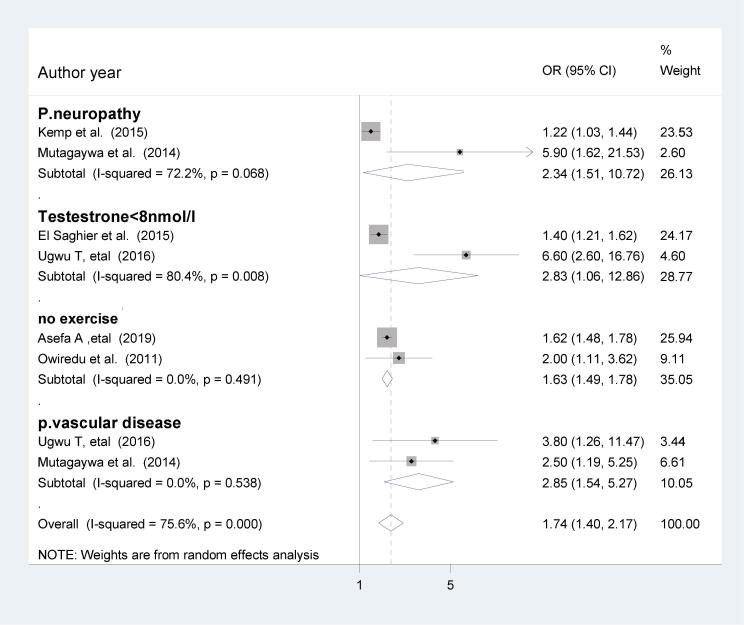
In the present review, those who had peripheral neuropathy were 2.34 times more likely to develop ED compared to those who had no peripheral neuropathy (OR = 2.34; 95%CI; 1.51, 10.72). In addition, those who had testosterone level <8 nmol/l were nearly 3 times more likely to develop ED in patients with DM (OR = 2.83; 95%CI; 1.06, 12.86). Similarly, those who had not involved in the physical exercise were 63% more likely to develop ED compared to those who had physical exercise (OR = 1.63; 95%CI; 1.49, 1.78). Furthermore, those who had the peripheral vascular disease were nearly 3 times more likely to develop ED compared to those who had no peripheral vascular disease (OR = 2.85; 95%CI; 1.54, 5.27), (Fig. 3).
Fig. 3.
The effect of peripheral neuropathy, testosterone, exercise and vascular disease on ED.
Glycemic control and risk of ED
According to our current systematic review, four observational studies reported the effect of glycosylated haemoglobin on ED. Most of the reports were from Nigeria [47], [48]; one from Zimbabwe [59], and Egypt [31]. The results indicated that patients with glycosylated haemoglobin >7% had increased occurrence of ED.
Hypertension and risk of ED
Hypertension was significantly associated with ED. In a study conducted in Egypt, Type 2 DM patients with systolic hypertension and diastolic hypertension were more likely to have erectile dysfunction [31]. Besides, similar several other studies also reported on the effects of hypertension on ED [49], [50], [55], [59]. Moreover, one study showed that hypertensive patients with diuretics treatment were nearly 5 times increased the risk of ED [57]. Likewise, in a study conducted in Zimbabwe reported that those patients with antihypertensive therapy had higher risk of ED [59].
Body mass index (BMI) and risk of ED
Most studies reported that being overweight or obese was a strong predictor of erectile dysfunction. For instance, BMI >30 kg/m2 level was associated with ED in patients with DM 1.09 (95%CI: 1.01–1.18), and a 2.22 (95%CI: 1.17–3.36; P < 0.001) [47], [57]. In addition, a 1 kg/m2 increase in body mass index level has been found to be associated with erectile dysfunction (OR 0.55, 95%CI: 0.41–0.72; P = 0.001)[31].
Discussion
The current systematic review and meta-analysis included 17 relevant studies published from inception up to 4/15/2020 on ED in people with DM residing in Africa. This study aimed to synthesize evidence on the risk factors of ED in patients with DM in Africa. Based on the pooled analysis of the adjusted odds ratio of studies, old age, duration of DM, BMI ≥30 kg/m2, deficient in testosterone level, peripheral neuropathy, peripheral vascular disease, and not involved in the physical exercise were associated with ED of diabetic patients.
The results of this study showed that the increased duration of the disease had a statistically significant effect on ED in patients with DM. This finding is consistent with previous research conducted in Israel [61], Korea [20], Sir Lanka [62], and Kuwait [27]. Therefore, we are suggest that early screening for the primary disease is essential to prevent and delay the progression of ED.
The current study revealed that age >40 years leads to 24% more likelihood of developing erectile dysfunction. This finding is in support of previous studies conducted in Turkey [63], China [64], Korea [20], Belgium [13], and Kuwait [27]. Our study suggests that assessment of ED could be a part of a more comprehensive prediction of patients' with advanced age. Also, screening among such a highly selected population may help identify those that would most benefit from pharmaceutical interventions and life-style changes [65].
In the present review, those who had testosterone levels <8 nmol/l were nearly 3 times more likely to develop ED in patients with DM. This finding was supported by studies done on Bulgaria [66], and USA [67]. Testosterone could enhance copulation through dopamine release in the medial preoptic area perhaps through up-regulation of NO synthesis [68]. Therefore evidence showed that testosterone therapy might moderately improve sexual desire and erectile function in men with Type 2 diabetes [69]. Besides, men with ED, hypoactive sexual desire and retarded ejaculation should be screened for the testosterone deficiency and treated accordingly [70].
Many studies have demonstrated that exercise can help prevent ED in patients with DM [19], [20], [61]. In the present finding, we found that those who were not involved in the physical exercise were 63% more likely to develop ED compared to those who had physical exercise. Therefore, evidence showed that physical activity protected against ED. An assessment of the association between ED and physical activity in population-based studies with meta-analysis, demonstrated that higher physical activity was seen to lower the risk of ED [71].
In the present review, those who had peripheral neuropathy were 2.34 times more likely to develop ED compared to those who had no peripheral neuropathy. This finding was consistent with previous reports in Korea [20] and Germany [72]. Though we did not identify previous studies which support our findings, in the present meta-analysis those who had peripheral vascular disease were nearly 3 times more likely to develop ED compared to those who had no peripheral vascular disease.
Previous studies have demonstrated that hypertension is highly associated with ED among diabetic patients [27], [62], [73], [74]. Likewise, we did observe such a relationship in this study. The present review was also reported in the effect of hypertension on ED [49], [50], [55], [59]. Mean blood pressures, both systolic and diastolic, were significantly associated with ED among diabetic patients.
Many studies have revealed that a BMI ≥30 kg/m2 is strongly associated with ED in patients with DM [27], [61], [75], [76], [77]. Though the present study would not pool the effect of BMI on ED, the review showed that BMI >30 kg/m2 level was substantially associated with ED in patients with DM [47], [57]. In addition, a 1 kg/m2 increase in body mass index level is associated with ED [31].
Several studies [20], [61], [78], [79] found a positive association between poor glycaemic control and the magnitude of ED in diabetic men. In this study, four observational studies [31], [47], [48], [59] reported the effect of glycosylated haemoglobin on ED. The results indicated that glycosylated haemoglobin >7% increased the occurrence of ED. In addition, meta-analysis evidence demonstrated that the risk of ED is higher in type 2 diabetic men with poor glycaemic control than those with good control [80]. Furthermore, the currently reported risk factors for diabetes-related erectile dysfunction include lack of education [51], divorced [50], [51], depression [51], comorbidity [51], autonomic neuropathy [48], type 2 diabetes mellitus [51], unemployment [50], wait circumstance [31].
The implications of these findings are clear: findings should guide health care professionals to increase patient awareness about diabetes-related ED, to monitor and maintain glycaemic control closely. Furthermore, identifying risk factors may help health care professionals treat DM patients with ED during their clinical care. This systematic review is not free of limitations. First, the results may not be generalizable, as no data were found for all of the African countries. Second, we only used English language articles, although our target was Africa which could be in several other languages such as Spanish, French, or Portuguese. Third, all included studies were cross-sectional study design, cause-effect relationships, therefore, cannot be reflected in this review.
Conclusion
This study found that long duration of DM, age >40 years, testosterone deficiency, peripheral neuropathy, not involved in physical exercise, and peripheral vascular disease were significantly associated with increased risk of ED among diabetic patients. The findings provide a scientific basis for a further understanding of the risk factors of ED in patients with DM and serve as a baseline for preventive strategies. Therefore, situation-based interventions and country context-specific preventive strategies should be developed to decrease the risk factors of ED among patients with DM.
Implication for practice
Based on the evidence in this review, health professionals are recommended to inform diabetes patient about the risk of developing erectile dysfunction. Comorbidities enhance the risk of the problem and should be evaluated appropriately and treated. Health care professionals should treat DM patients with ED during their clinical care.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Availability of data and materials: All relevant data are with in the paper and its supporting information files. There is no separate data set to share.
Funding: Not applicable.
Authors’ contributions
WSS, HAA, and TYA developed the protocol and were involved in the design, the selection of the studies, data extraction, statistical analysis, and the development of the initial drafts of the manuscript. YAA, PMP and TYA were involved in data extraction, quality assessment, statistical analysis, and revising the manuscript. HAA, WSS, PMP and YAA prepared the final draft of the manuscript. All authors read and approved the final draft of the document.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcte.2020.100232.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Roglic G. WHO Global report on diabetes: a summary. Int J Noncommun Dis. 2016;1(1):3. [Google Scholar]
- 2.IDF: Diabetes Atlas 8th edition 2017.
- 3.Mbanya J.-C., Sobngwi E. Diabetes microvascular and macrovascular disease in Africa. J Cardiovasc Risk. 2003;10(2):97–102. doi: 10.1097/01.hjr.0000060842.48106.78. [DOI] [PubMed] [Google Scholar]
- 4.Msc AMIM: Erectile Dysfunction in Diabetes: An Overview, Int J Innov Stud Med Sci 2019;3(1):13–14.
- 5.Ayta I., McKinlay J., Krane R. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84(1):50–56. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 6.Consensus N. Development Panel on Impotence. NIH Consensus Conference. Impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 7.Colson M., Cuzin B., Faix A., Grellet L., Huyghes E. Current epidemiology of erectile dysfunction, an update. Sexologies. 2018;27(1):e7–e13. [Google Scholar]
- 8.Heaton J.P., Adams M.A. Causes of erectile dysfunction. Endocrine. 2004;23(2–3):119–123. doi: 10.1385/ENDO:23:2-3:119. [DOI] [PubMed] [Google Scholar]
- 9.Lue T.F. Erectile dysfunction. N Engl J Med. 2000;342(24):1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 10.Wiles P. Erectile impotence in diabetic men: aetiology, investigation, and management. Diabet Med. 1992;9(10):888–892. doi: 10.1111/j.1464-5491.1992.tb01726.x. [DOI] [PubMed] [Google Scholar]
- 11.Dunsmuir W., Holmes S. The aetiology and management of erectile, ejaculatory, and fertility problems in men with diabetes mellitus. Diabet Med. 1996;13(8):700–708. doi: 10.1002/(SICI)1096-9136(199608)13:8<700::AID-DIA174>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen S., Peng D., Xu X., Gao J., Dai F., Zuo C. Assessment of erectile dysfunction and associated psychological distress in Chinese men with type 2 diabetes mellitus. Int J Impot Res. 2017;29(5):210–214. doi: 10.1038/ijir.2017.25. [DOI] [PubMed] [Google Scholar]
- 13.Enzlin P., Mathieu C., Van den Bruel A., Vanderschueren D., Demyttenaere K. Prevalence and predictors of sexual dysfunction in patients with type 1 diabetes. Diabetes Care. 2003;26(2):409–414. doi: 10.2337/diacare.26.2.409. [DOI] [PubMed] [Google Scholar]
- 14.Idung A.U., Abasiubong F., Udoh S.B., Akinbami O.S. Quality of life in patients with erectile dysfunction in the Niger Delta region, Nigeria. J Mental Health. 2012;21(3):236–243. doi: 10.3109/09638237.2012.664300. [DOI] [PubMed] [Google Scholar]
- 15.Malavige L., Jayaratne S., Kathriarachchi S., Sivayogan S., Ranasinghe P., Levy J. Erectile dysfunction is a strong predictor of poor quality of life in men with type 2 diabetes mellitus. Diabet Med. 2014;31(6):699–706. doi: 10.1111/dme.12412. [DOI] [PubMed] [Google Scholar]
- 16.Guo W., Liao C., Zou Y., Li F., Li T., Zhou Q. Erectile dysfunction and risk of clinical cardiovascular events: a meta-analysis of seven cohort studies. J Sex Med. 2010;7(8):2805–2816. doi: 10.1111/j.1743-6109.2010.01792.x. [DOI] [PubMed] [Google Scholar]
- 17.Hodges L., Kirby M., Solanki J., O'Donnell J., Brodie D. The temporal relationship between erectile dysfunction and cardiovascular disease. Int J Clin Pract. 2007;61(12):2019–2025. doi: 10.1111/j.1742-1241.2007.01629.x. [DOI] [PubMed] [Google Scholar]
- 18.Fedele D., Coscelli C., Santeusanio F., Bortolotti A., Chatenoud L., Colli E. Parazzini F: Erectile dysfunction in diabetic subjects in Italy. Gruppo Italiano Studio Deficit Erettile nei Diabetici. Diabetes Care. 1998;21(11):1973–1977. doi: 10.2337/diacare.21.11.1973. [DOI] [PubMed] [Google Scholar]
- 19.Minami H., Furukawa S., Sakai T., Niiya T., Miyaoka H., Miyake T. Physical activity and prevalence of erectile dysfunction in Japanese patients with type 2 diabetes mellitus: The Dogo Study. J Diabetes Investigation. 2018;9(1):193–198. doi: 10.1111/jdi.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho N., Ahn C., Park J., Ahn T., Lee H., Park T. Prevalence of erectile dysfunction in Korean men with Type 2 diabetes mellitus. Diabet Med. 2006;23(2):198–203. doi: 10.1111/j.1464-5491.2005.01789.x. [DOI] [PubMed] [Google Scholar]
- 21.El-Sakka A.I., Tayeb K.A. Erectile dysfunction risk factors in noninsulin dependent diabetic Saudi patients. J Urol. 2003;169(3):1043–1047. doi: 10.1097/01.ju.0000050080.21839.f9. [DOI] [PubMed] [Google Scholar]
- 22.Dan A., Chakraborty K., Mondal M., Neogi R., Chatterjee S., Makhal M. Erectile dysfunction in patients with diabetes mellitus: Its magnitude, predictors and their bio-psycho-social interaction: a study from a developing country. Asian J Psychiatry. 2014;7:58–65. doi: 10.1016/j.ajp.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Shiferaw W.S., Akalu T.Y., Aynalem Y.A. Prevalence of erectile dysfunction in patients with diabetes mellitus and its association with body mass index and glycated hemoglobin in africa: a systematic review and meta-analysis. Int J Endocrinol. 2020;2020:5148370. doi: 10.1155/2020/5148370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quadri R., Veglio M., Flecchia D., Tonda L., De Lorenzo F., Chiandussi L. Autonomic neuropathy and sexual impotence in diabetic patients: analysis of cardiovascular reflexes/autonome neuropathie und sexuelle impotenz bei diabetikern: analyse der cardiovasculären reflexe. Andrologia. 1989;21(4):346–352. [PubMed] [Google Scholar]
- 25.Fabian U.A., Charles-Davies M.A., Fasanmade A.A., Olaniyi J.A., Oyewole O.E., Owolabi M.O. Male sexual dysfunction, leptin, pituitary and gonadal hormones in nigerian males with metabolic syndrome and type 2 diabetes mellitus. J Reprod Infertility. 2016;17(1):17. [PMC free article] [PubMed] [Google Scholar]
- 26.Berrada S., Kadri N., Mechakra-Tahiri S., Nejjari C. Prevalence of erectile dysfunction and its correlates: a population-based study in Morocco. Int J Impot Res. 2003;15(S1):S3. doi: 10.1038/sj.ijir.3900968. [DOI] [PubMed] [Google Scholar]
- 27.Al-Hunayan A., Al-Mutar M., Kehinde E.O., Thalib L., Al-Ghorory M. The prevalence and predictors of erectile dysfunction in men with newly diagnosed with type 2 diabetes mellitus. BJU Int. 2007;99(1):130–134. doi: 10.1111/j.1464-410X.2006.06550.x. [DOI] [PubMed] [Google Scholar]
- 28.De Klerk H., De Villiers P., Isaacs S. Prevalence and characteristics of erectile dysfunction in black and mixed race primary care populations of the Cape Flats and Helderberg Basin area of the Western Cape, South Africa. South African Family Practice. 2003;45(1):14–20. [Google Scholar]
- 29.Seyam R., Albakry A., Ghobish A., Arif H., Dandash K., Rashwan H. Prevalence of erectile dysfunction and its correlates in Egypt: a community-based study. Int J Impot Res. 2003;15(4):237. doi: 10.1038/sj.ijir.3901000. [DOI] [PubMed] [Google Scholar]
- 30.Bortolotti A., Fedele D., Chatenoud L., Colli E., Coscelli C., Landoni M. Cigarette smoking: a risk factor for erectile dysfunction in diabetics. Eur Urol. 2001;40(4):392–397. doi: 10.1159/000049805. [DOI] [PubMed] [Google Scholar]
- 31.El Saghier EO, Shebl SE, Fawzy OA, Eltayeb lM, Bekhet LM, Gharib A: Androgen deficiency and erectile dysfunction in patients with type 2 diabetes. Clin Med Insights: Endocrinology and Diabetes 2015, 8:CMED. S27700. [DOI] [PMC free article] [PubMed]
- 32.Owiredu W.K., Amidu N., Alidu H., Sarpong C., Gyasi-Sarpong C.K. Determinants of sexual dysfunction among clinically diagnosed diabetic patients. Reprod Biol Endocrinol. 2011;9(1):70. doi: 10.1186/1477-7827-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bacon C.G., Hu F.B., Giovannucci E., Glasser D.B., Mittleman M.A., Rimm E.B. Association of type and duration of diabetes with erectile dysfunction in a large cohort of men. Diabetes Care. 2002;25(8):1458–1463. doi: 10.2337/diacare.25.8.1458. [DOI] [PubMed] [Google Scholar]
- 34.Kassier S., Veldman F. When science meets culture: the prevention and management of erectile dysfunction in the 21st century. South African J Clin Nutr. 2014;27(1):7–12. [Google Scholar]
- 35.Malavige L.S., Levy J.C. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6(5):1232–1247. doi: 10.1111/j.1743-6109.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 36.McVARY K.T., Carrier S., Wessells H. Smoking and erectile dysfunction: evidence based analysis. J Urol. 2001;166(5):1624–1632. [PubMed] [Google Scholar]
- 37.Esposito K., Giugliano F., Di Palo C., Giugliano G., Marfella R., D'Andrea F. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291(24):2978–2984. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 38.Gupta B.P., Murad M.H., Clifton M.M., Prokop L., Nehra A., Kopecky S.L. The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: a systematic review and meta-analysis. Arch Intern Med. 2011;171(20):1797–1803. doi: 10.1001/archinternmed.2011.440. [DOI] [PubMed] [Google Scholar]
- 39.Munn Z., Tufanaru C., Aromataris E. JBI's systematic reviews: data extraction and synthesis. AJN Am J Nursing. 2014;114(7):49–54. doi: 10.1097/01.NAJ.0000451683.66447.89. [DOI] [PubMed] [Google Scholar]
- 40.Modesti P.A., Reboldi G., Cappuccio F.P., Agyemang C., Remuzzi G., Rapi S. Settings EWGoCRiLR: Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS ONE. 2016;11(1) doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 42.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 43.Huedo-Medina T.B., Sánchez-Meca J., Marín-Martínez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 44.L. StataCorp Stata statistical software (version release 14) 2015 Author College Station, TX.
- 45.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olarinoye J., Kuranga S., Katibi I., Adediran O., Jimoh A., Sanya E. Prevalence and determinants of erectile dysfunction among people with type 2 diabetes in Ilorin, Nigeria. Nigerian Postgraduate Med J. 2006;13(4):291–296. [PubMed] [Google Scholar]
- 47.Ugwumba F.O., Okafor C.I., Nnabugwu I.I., Udeh E.I., Echetabu K.N., Okoh A.D. Prevalence of, and risk factors for erectile dysfunction in male type 2 diabetic outpatient attendees in Enugu, South East Nigeria. Ann African Med. 2018;17(4):215. doi: 10.4103/aam.aam_3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ugwu T., Ezeani I., Onung S., Kolawole B., Ikem R. Predictors of erectile dysfunction in men with type 2 diabetes mellitus referred to a tertiary healthcare centre. Adv Endocrinol. 2016;2016 [Google Scholar]
- 49.Obi P., Anyanwu A., Nwatu C., Ekwueme N., Mbaike A., Onyegbule O. Pattern of serum testosterone and glycated haemoglobin among adult males with type 2 diabetes mellitus and erectile dysfunction attending a Tertiary Hospital in South Eastern Nigeria. J Adv Med Med Res. 2016:1–8. [Google Scholar]
- 50.BELLO KS pattern and determinants of sexual dysfunction among adults with type 2 diabetes attending the general out patient clinic of ahmadu bello university teaching hospital zaria, Nigeria. Faculty of Family Medicine 2017.
- 51.Seid A., Gerensea H., Tarko S., Zenebe Y., Mezemir R. Prevalence and determinants of erectile dysfunction among diabetic patients attending in hospitals of central and northwestern zone of Tigray, northern Ethiopia: a cross-sectional study. BMC Endocr Disorders. 2017;17(1):16. doi: 10.1186/s12902-017-0167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walle B., Lebeta K.R., Fita Y.D., Abdissa H.G. Prevalence of erectile dysfunction and associated factors among diabetic men attending the diabetic clinic at Felege Hiwot Referral Hospital, Bahir Dar, North West Ethiopia, 2016. BMC Res Notes. 2018;11(1):130. doi: 10.1186/s13104-018-3211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Worku D, Hamza L, Woldemichael K: Patterns of diabetic complications at jimma university specialized hospital, southwest ethiopia. Ethiopian J Health Sci 2010;20(1). [DOI] [PMC free article] [PubMed]
- 54.Asefa A., Nigussie T., Henok A., Mamo Y. Prevalence of sexual dysfunction and related factors among diabetes mellitus patients in Southwest Ethiopia. BMC Endocr Disorders. 2019;19(1):1–8. doi: 10.1186/s12902-019-0473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amidu N., Owiredu W.K., Alidu H., Sarpong C., Gyasi-Sarpong C.K., Quaye L. Association between metabolic syndrome and sexual dysfunction among men with clinically diagnosed diabetes. Diabetol Metab Syndrome. 2013;5(1):42. doi: 10.1186/1758-5996-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Annani-Akollor M.E., Addai-Mensah O., Fondjo L.A., Sallah L., Owiredu E.-W., Acheampong E. Predominant complications of type 2 diabetes in kumasi: a 4-year retrospective cross-sectional study at a teaching hospital in Ghana. Medicina. 2019;55(5):125. doi: 10.3390/medicina55050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kemp T., Rheeder P. The prevalence and associations of erectile dysfunction in a South African male diabetic urban population. J Endocr Metab Diabetes South Africa. 2015;20(3):134–139. [Google Scholar]
- 58.Likata G.M.U., Kuria M.W., Olando Y., Owiti F.R. Sexual dysfunction among patients with diabetes mellitus. Green J Med Sci. 2012;2:138–145. [Google Scholar]
- 59.Pasipanodya Ian Machingura V.C. Erectile Dysfunction among Diabetic Patients at Parirenyatwa Group of Hospitals in Zimbabwe Texila. Int J Public Health. 2018;6:2. [Google Scholar]
- 60.Mutagaywa R.K., Lutale J., Aboud M., Kamala B.A. Prevalence of erectile dysfunction and associated factors among diabetic men attending diabetic clinic at Muhimbili National Hospital in Dar-es-Salaam, Tanzania. Pan African Med J. 2014:17. doi: 10.11604/pamj.2014.17.227.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalter-Leibovici O., Wainstein J., Ziv A., Harman-Bohem I., Murad H., Raz I. Clinical, socioeconomic, and lifestyle parameters associated with erectile dysfunction among diabetic men. Diabetes Care. 2005;28(7):1739–1744. doi: 10.2337/diacare.28.7.1739. [DOI] [PubMed] [Google Scholar]
- 62.Nisahan B., Kumanan T., Rajeshkannan N., Peranantharajah T., Aravinthan M. Erectile dysfunction and associated factors among men with diabetes mellitus from a tertiary diabetic center in Northern Sri Lanka. BMC Res Notes. 2019;12(1):210. doi: 10.1186/s13104-019-4244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kiskac M., Zorlu M., Cakirca M., Buyukaydin B., Karatoprak C., Yavuz E. Frequency and determinants of erectile dysfunction in Turkish diabetic men. Nigerian J Clin Practice. 2015;18(2):209–212. doi: 10.4103/1119-3077.151043. [DOI] [PubMed] [Google Scholar]
- 64.Lo W.H., Fu S.N., Wong C.K.H., San Chen E. Prevalence, correlates, attitude and treatment seeking of erectile dysfunction among type 2 diabetic Chinese men attending primary care outpatient clinics. Asian J Androl. 2014;16(5):755. doi: 10.4103/1008-682X.127823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Djordjevic D., Vukovic I., Milenkovic Petronic D., Radovanovic G., Seferovic J., Micic S. Erectile dysfunction as a predictor of advanced vascular age. Andrology. 2015;3(6):1125–1131. doi: 10.1111/andr.12105. [DOI] [PubMed] [Google Scholar]
- 66.Kamenov Z. A comprehensive review of erectile dysfunction in men with diabetes. Exp Clin Endocrinol Diabetes. 2015;123(03):141–158. doi: 10.1055/s-0034-1394383. [DOI] [PubMed] [Google Scholar]
- 67.Selvin E., Feinleib M., Zhang L., Rohrmann S., Rifai N., Nelson W.G. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30(2):234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 68.Hull E.M., Du J., Lorrain D.S., Matuszewich L. Testosterone, preoptic dopamine, and copulation in male rats. Brain Res Bull. 1997;44(4):327–333. doi: 10.1016/s0361-9230(97)00211-6. [DOI] [PubMed] [Google Scholar]
- 69.Algeffari M., Jayasena C., MacKeith P., Thapar A., Dhillo W., Oliver N. Testosterone therapy for sexual dysfunction in men with Type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabet Med. 2018;35(2):195–202. doi: 10.1111/dme.13553. [DOI] [PubMed] [Google Scholar]
- 70.Buvat J., Maggi M., Gooren L., Guay A.T., Kaufman J., Morgentaler A. Endocrine aspects of male sexual dysfunctions. J Sex Med. 2010;7(4):1627–1656. doi: 10.1111/j.1743-6109.2010.01780.x. [DOI] [PubMed] [Google Scholar]
- 71.Cheng J., Ng E., Ko J., Chen R. Physical activity and erectile dysfunction: meta-analysis of population-based studies. Int J Impot Res. 2007;19(3):245–252. doi: 10.1038/sj.ijir.3901521. [DOI] [PubMed] [Google Scholar]
- 72.Hecht M.J., Neundörfer B., Kiesewetter F., Hilz M.J. Neuropathy is a major contributing factor to diabetic erectile dysfunction. Neurol Res. 2001;23(6):651–654. doi: 10.1179/016164101101198965. [DOI] [PubMed] [Google Scholar]
- 73.Kouidrat Y., Pizzol D., Cosco T., Thompson T., Carnaghi M., Bertoldo A. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med. 2017;34(9):1185–1192. doi: 10.1111/dme.13403. [DOI] [PubMed] [Google Scholar]
- 74.Giuliano F.A., Leriche A., Jaudinot E.O., de Gendre A.S. Prevalence of erectile dysfunction among 7689 patients with diabetes or hypertension, or both. Urology. 2004;64(6):1196–1201. doi: 10.1016/j.urology.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 75.Rosen R.C., Wing R.R., Schneider S., Wadden T.A., Foster G.D., West D.S. Erectile dysfunction in type 2 diabetic men: relationship to exercise fitness and cardiovascular risk factors in the Look AHEAD trial. J Sex Med. 2009;6(5):1414–1422. doi: 10.1111/j.1743-6109.2008.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moreira Júnior E.D., Bestane W.J., Bartolo E.B., Fittipaldi J.A.S. Prevalence and determinants of erectile dysfunction in Santos, southeastern Brazil. Sao Paulo Med J. 2002;120(2):49–54. doi: 10.1590/S1516-31802002000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andersson DP, Ekström U, Lehtihet M: Rigiscan evaluation of men with diabetes mellitus and erectile dysfunction and correlation with diabetes duration, age, BMI, lipids and HbA1c. PLoS One 2015, 10(7). [DOI] [PMC free article] [PubMed]
- 78.Ahmed I., Au A., Anwar E., Ali S.S., Ali A., Ali A. Erectile dysfunction and type 2 diabetes mellitus in northern Pakistan. J Pak Med Assoc. 2013;63(12):1486–1490. [PubMed] [Google Scholar]
- 79.Jamieson F., Chalmers J., Duncan C., Prescott R.J., Campbell I.W. Erectile dysfunction in type 1 diabetic males. Br J Diab Vasc Dis. 2008;8(5):232–234. [Google Scholar]
- 80.Binmoammar TA, Hassounah S, Alsaad S, Rawaf S, Majeed A: The impact of poor glycaemic control on the prevalence of erectile dysfunction in men with type 2 diabetes mellitus: a systematic review. JRSM Open 2016, 7(3):2054270415622602. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.