Abstract
Percutaneous transabdominal lymphangiography and embolization have been reported as useful approaches for intractable chylothorax or chylous ascites. However, they are often difficult to perform after extensive lymph node dissection because disruption of the antegrade lymphatic flow makes leaks identification difficult. When the leakage point cannot be identified or percutaneous transabdominal lymphangiography and embolization fail, a retrograde transvenous approach to the thoracic duct can be used instead. We report 3 cases of refractory chylous ascites after retroperitoneal operation or extensive lymph node dissection that was addressed by retrograde transvenous lymphatic embolization. In one case, a combination of retrograde transvenous lymphatic embolization, transcatheter sclerotherapy, and transcatheter embolization was used. These findings suggest that retrograde transvenous lymphatic embolization appears to be feasible and efficient for postoperative chylous ascites.
Keywords: Chylous ascites, Lymphangiography, Retrograde transvenous lymphatic embolization, Lymphopseudoaneurysm sclerotherapy, Lymphopseudoaneurysm embolization
Introduction
Chylous ascites is a rare, but potentially life-threatening complication after abdominal and pelvic operations, with a reported frequency of 0.3%-11% [1]. It has also been reported to occur in 2.4% of cases involving extensive lymph node dissection [2,3]. This complication may cause significant morbidity, including malnutrition, dehydration, and immunosuppression, with high mortality rates [4]. While chylothorax is primarily caused by damage to the thoracic duct, chylous ascites can be caused by damage to the thoracic duct, cisterna chyli, intestinal lymphatics, or tributaries [5]. A combination of percutaneous transabdominal lymphangiography and embolization has been recently reported as a treatment method for intractable chylothorax, with a response rate of 80%-90% [6,7]. However, a smaller number of studies and reports have also used interventional techniques for the treatment of chylous ascites [2,4,8,9]. The majority of these studies used an antegrade approach, which was usually used following intranodal lymphangiography and involved percutaneous transabdominal embolization similar to that used for chylothorax, upstream lymph node embolization, lymphatic vessel embolization performed by direct puncture, and direct lymphatic fluid cavity embolization [2,4,8,9]. However, these approaches are often difficult to apply after extensive lymph node dissection because disruption of the antegrade lymphatic flow upstream from the leakage site makes leaks identification difficult by conventional intranodal lymphangiography. In fact, according to previous reports [4,9], about half of the leakage lesions cannot be visualized with intranodal lymphangiography or even with catheter-inserted thoracic ductography. When the leakage point cannot be identified, a retrograde transvenous approach via the basilic or cephalic vein through the ostial valve of the thoracic duct [5,10–14] can be used as an alternative therapeutic method. Furthermore, it may be effective for lumbar lymphatic trunk injury located upstream of the cisterna chyli, which is difficult to reach by the percutaneous transabdominal approach.
We report 3 cases of refractory chylous ascites after retroperitoneal operation with or without extensive lymph node dissection that were addressed by retrograde transvenous lymphatic embolization. In one of these cases, the patient was treated with a combination of retrograde transvenous lymphatic embolization, transcatheter sclerotherapy, and transcatheter embolization.
Case reports
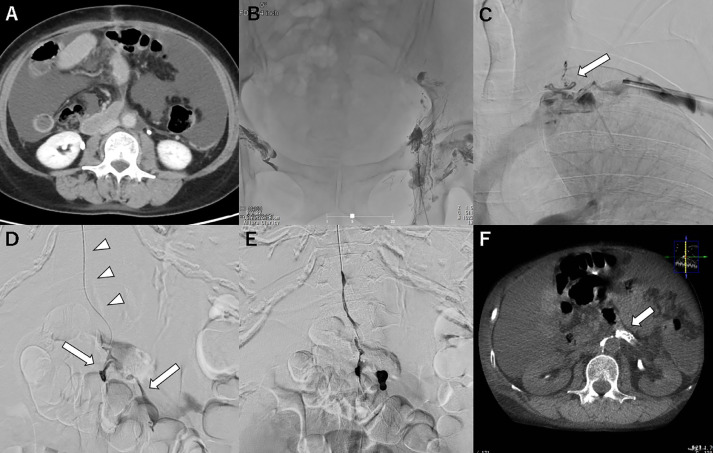
Case 1: A woman in her 50s with endometrial cancer (pT2N0M0) received curative operation with pelvic and para-aortic lymph node dissection. Chylous ascites with a triglyceride level of 848 mg/dL was observed from the drain 3 days after the operation (Fig. 1A). The ascites did not improve after conservative treatment with parenteral nutrition and somatostatin therapy for 2 weeks. Lymphatic angiography with iodized oil from the bilateral inguinal intranodal lymphangiography showed no lymphatic path downstream from the pelvis because of disruption of the antegrade lymphatic drainage after lymph node dissection (Fig. 1B). Therefore, we abandoned the percutaneous transabdominal approach and switched to a retrograde transvenous approach. The anatomical pathway and the junction with the left jugulo-subclavian angle were identified on magnetic resonance imaging (MRI) in advance. A 5-F 25-cm sheath (Terumo, Tokyo, Japan) was placed into the left basilic vein. The thoracic duct was visualized by subclavian venography (Fig. 1C), which is a very rare finding, because the presence of an ostial valve usually hinders its visualization by subclavian venography. A 4-F RIM catheter (Seiya; Medikit, Tokyo, Japan) was successfully inserted into the lymphovenous junction, after which a 1.9-F microcatheter (Progreat λ19; Terumo, Tokyo, Japan) and 0.016-inch guidewire (Meister; Asahi Intec, Nagoya, Japan) were advanced into the lower thoracic duct and reached the cisterna chyli. Retrograde thoracic ductography demonstrated the leakage point and extravasation at the lumbar trunk slightly upstream from the cisterna chyli (Fig. 1D). It was embolized with a 2:1 mixture of iodized oil and N-butyl cyanoacrylate (NBCA) (Histoacryl; B. Braun, Melsungen, Germany) (Fig. 1E). The procedure time was 68 minutes. Chylous ascites improved immediately from the day after treatment.
Fig. 1.
Case 1: retrograde lymphangiography and embolization.
A: Axial computed tomography (CT) image through the mid-abdomen before the embolization procedure showing ascites [this is the same slice as the postoperative CT image shown in (F)]; B: After groin intranodal lymphangiography, no lymphatic path downstream from the pelvis, including the thoracic duct and the cisterna chyli, can be visualized; C: The thoracic duct visualized by subclavian venography (arrows); D: A microcatheter advanced into the thoracic duct (arrowheads) through the jugulovenous angle; lymphangiography at the cisterna chyli showing contrast extravasation at the level of the lumbar trunk slightly upstream from the cisterna chyli (arrow); E: Embolization with 33% N-butyl cyanoacrylate; F: CT examination the day after embolization showing accumulation of the glue mixture filling the lymphatic tributaries and covering the leakage site (arrow).
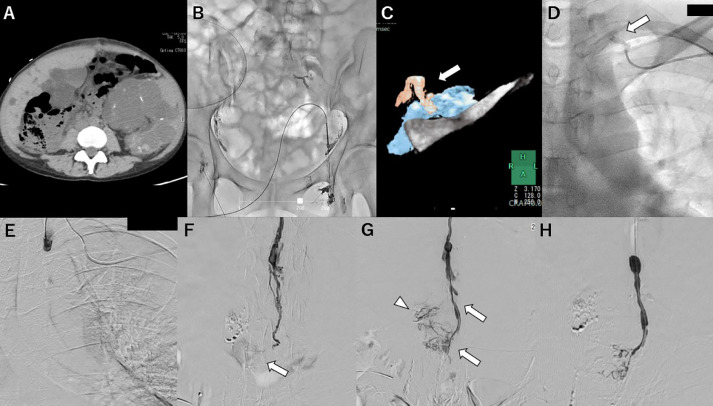
Case 2: A woman in her 40s was treated by right nephrectomy due to uncontrolled infection of a polycystic kidney. Chylous ascites with a triglyceride level of 183 mg/dL was observed 2 days after the operation (Fig. 2A). The patient did not respond to conservative treatment with parenteral nutrition and somatostatin therapy for 2 weeks. Bilateral inguinal intranodal lymphangiography showed no lymphatic path downstream from the pelvis (Fig. 2B). A retrograde transvenous approach using the same procedure as in case 1 was attempted, and a 4-F RIM catheter (Seiya) was successfully inserted into the lymphovenous junction after patiently seeking the left jugulo-subclavian angle with reference to MRI findings (Fig. 2C, D). Then, a 1.8-F microcatheter (Breakthrough AI 18; Boston Scientific, Natick, MA, USA) and 0.016-inch guidewire (Meister) were advanced into the thoracic duct; however, it was difficult to advance the microcatheter beyond the thoracic duct valve in the cervical part (Fig. 2E). By changing the guidewire to another stiff 0.018-inch guidewire (V-18 Control Wire; Boston Scientific, Natick, MA, USA), it was possible to advance the catheter to the cisterna chyli. Retrograde thoracic ductography demonstrated the leakage point, and the extravasation was located upstream of the cisterna chyli (Fig. 2F, G). It was embolized with a 2:1 mixture of iodized oil and NBCA (Fig. 2H). The procedure time was 105 minutes. Chylous ascites improved gradually from the day after treatment.
Fig. 2.
Case 2: retrograde lymphangiography and embolization.
A: Axial computed tomography (CT) image showing a small amount of retroperitoneal lymphatic fluid located close to the surgical site; B: After groin intranodal lymphangiography, a lymphatic path downstream from the pelvis located adjacently to the surgical site can be slightly visualized, but the cisterna chyli, thoracic duct, and extravasation cannot be visualized; C: The thoracic duct visualized on a magnetic resonance (MR) image of a highly fluid-sensitive sequence; 3D-fusion image of the MR image of the thoracic duct (arrow) and contrast-enhanced CT image of the left subclavian vein and clavicle; D: A captured fluoroscopic image of selected angiography at the jugulovenous angle showing the cervical part of the thoracic duct (arrow) leading to the subclavian vein; E: Retrograde lymphangiography at the cervical part of the thoracic duct does not show the entire thoracic duct, suggesting the existence of valves (arrow); F: Lymphangiography at the level of the lumbar trunk showing contrast extravasation; G: Lymphangiography at a later phase showing extravasation spreading around the leakage point and the thoracic duct (arrow) and lymphatic tributaries leading to the lumbar trunk (arrowhead); H: Embolization with 33% N-butyl cyanoacrylate.
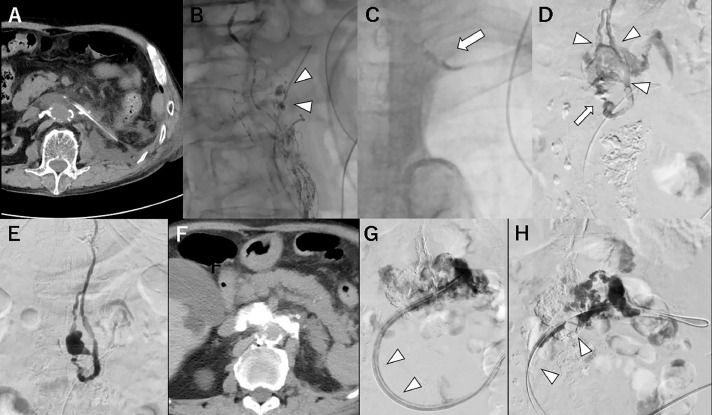
Case 3: A man in his 70s with left renal cell carcinoma (pT3aN0M0) was treated by left nephrectomy and left renal vein resection. Chylous ascites was observed from the drain on the day after the operation (chylous leakage of 200–1200 mL a day) (Fig. 3A). Patient's condition did not improve after conservative treatment with parenteral nutrition and somatostatin therapy for a week. Left inguinal intranodal lymphangiography showed a lymphatic path from the pelvis to the thoracic duct and extravasated iodized oil around the tip of the drainage catheter. The cisterna chyli was not visualized (Fig. 3B). A 4-F RIM catheter (Seiya) was successfully inserted into the thoracic duct with reference to the remaining iodized oil at the left jugulo-subclavian angle (Fig. 3C). A 1.9-F microcatheter (Prograde λ19) was advanced into the lower thoracic duct. Retrograde thoracic ductography identified a duplication of the thoracic duct, and extravasation was revealed upstream of the duplication (position of the cisterna chyli was unclear) (Fig. 3D). It was embolized with a 2:1 mixture of iodized oil and NBCA (Fig. 3E). The procedure time was 208 minutes. However, the chylous ascites persisted. The damage to the thoracic duct was greater than expected, and it was suspected that extravasation from upstream was still continuing. A radiograph obtained with contrast injection into the extravasation cavity via a remaining drainage catheter showed stagnation of the contrast medium around the leakage point (Fig. 3G); subsequently, sclerotherapy was attempted. The existing drainage catheter was exchanged with a 12-F multiport drainage catheter, and the presence of the contrast agent close to the leakage point (the so-called lymphopseudoaneurysm contained by surrounding tissue) was confirmed. Although a total of 10 mL of 95% ethanol was injected, chylous ascites of about half the initial volume persisted after the sclerotherapy. As an alternative therapy, embolization with a 2:1 mixture of iodized oil and NBCA via a 5.2-F balloon catheter (Selecon MP catheter II; Terumo, Tokyo, Japan), which was exchanged with a drainage catheter, was performed (Fig. 3H). A balloon catheter was used to reduce backflow into the catheter insertion pathway. Chylous ascites improved immediately the day after this procedure.
Fig. 3.
Case 3: retrograde lymphangiography, embolization, and direct lymphopseudoaneurysm embolization.
A: Axial computed tomography (CT) image showing a small amount of retroperitoneal lymphatic fluid located adjacently to a drainage catheter; B: After left groin intranodal lymphangiography, the lymphatic path from the pelvis to the thoracic duct and extravasated iodized oil (arrowheads) can be visualized around the tip of the drainage catheter; note that the cisterna chyli is not visualized; C: The lymphovenous junction at the left jugulo-subclavian angle identified by the remnant iodized oil (arrow); D: Duplication of the thoracic duct (arrowheads) identified by retrograde thoracic ductography a and extravasation (arrow) can be visualized upstream from the duplication with the balloon inflation (position of the cisterna chyli was unclear); E: The leakage point embolized with 33% N-butyl cyanoacrylate (NBCA); F: CT image obtained the day after embolization showing accumulation of the glue mixture consistent with fluid collection; G: Radiograph obtained with contrast injection into the extravasation cavity via a remaining drainage catheter (arrowheads) showing stagnation of the contrast medium around the leakage point, suggesting remnant lymphatic extravasation; H: Embolization with 33% NBCA via a 5.2-F balloon catheter (arrowheads); the digital subtraction image during injection of NBCA showing sufficient filling of the lymphopseudoaneurysm.
Discussion[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]
Abdominal lymphorrhea is divided into lymph without chyle and chyle that depends on the location of the leakage. Chylous ascites can occur due to injury to the intestinal lymphatics, thoracic duct, or cisterna chyli [5]. Therefore, if the rupture area is located close to the cisterna chyli, chylous ascites may occur due to reflux, as it was in our cases. Among the available treatment strategies for high-output chylothorax or chylous ascites caused by an injury located from the cisterna chyli to the thoracic duct, percutaneous transabdominal approach under fluoroscopic guidance is the first choice [5–7]. If this approach fails due to technical reasons, inability to identify the leakage point [4], anatomic anomalies, such as a completely left-sided thoracic duct or plexiform variation [5], or lack of access from the abdomen and pelvis as a result of disruption of the lymphatic pathway, as it was in our cases, a percutaneous transvenous retrograde approach via the basilic or cephalic vein through the ostial valve of the thoracic duct is an appropriate alternative method.
The greatest advantage of retrograde embolization is that it is less invasive than the percutaneous transabdominal approach. However, this approach has some issues. One report suggested a success rate for retrograde lymphangiography of only 61.5%, and a much lower one for embolization [14]. Retrograde transvenous catheter cannulation and lymphangiography are challenging procedures due to the following 4 problems. First, the point of leakage may be unknown before the retrograde transverse procedure because it may not be identified with inguinal intranodal lymphangiography or lymphatic scintigraphy when the upstream region is interrupted due to lymph node dissection. Second, the junction of the venous angle and the thoracic duct are usually not visualized with subclavian venography from the subclavian or internal jugular vein due to the presence of an ostial valve at the termination of the thoracic duct [5, [10], [11], [12], [13], [14]. In addition, the termination point of the thoracic duct can have various locations, such as the internal jugular vein, jugulo-subclavian angle, subclavian vein, or another vein, and is generally placed within 2 cm of the jugulo-subclavian angle [5,15], making it necessary to grope for the lymphovenous junction. Moreover, in 1 of the 3 presented cases, the cervical part of the thoracic duct was visualized with venography from the subclavian vein, but no similar findings have been reported in the past. Therefore, intranodal lymphangiography and MR lymphangiography are useful for visualization of the lymphovenous junction and facilitate catheter insertion [5,14]. Technically, on the basis of previous reports [5,10–12], a 4-F or 5-F preshaped catheter, such as RIM catheter or SOS Omni Selective catheter (AngioDynamics, Queensbury, NY, USA) was often used for cannulation of the lymphatic venous junction when it was approached from the left brachial vein. Third, because of the presence of many branches that join from the cervical region and arms or plexiform configuration of the cervical part of the thoracic duct, the contrast agent may not reach the thoracic part even with pressure injection after cannulation of the cervical part [14,16]. Fourth, the upstream thoracic duct and the cisterna chyli may be difficult to reach due to the presence of valves and anatomic anomalies of the thoracic duct, such as duplication or plexiform variation [5,14]. The cervical part of the thoracic duct has been reported to be of the plexiform type in 23%-26% of cases [14,17]. When the plexiform type is present, microcatheter advancement beyond the cervical part to the thoracic part and embolization may fail because of the presence of valves and complex branching [5,14]. In fact, Kariya et al. [14] reported that retrograde microcatheter advancement beyond the cervical part was not possible because of the presence of many duct junctions in 3 out of 12 patients (25%). In our second case, it was difficult to advance the catheter—it could be smoothly advanced to the target site only by replacing the guidewire with a stiffer one. Furthermore, due to the countering antegrade flow, the entire picture of the leakage on the upstream side may not be easy to identify even by lymphangiography from the cisterna chyli. Although this may not be an issue as in previous reports [10,11] and in 3 of our cases, if it does occur, or even if the catheter cannot reach the cisterna chyli, visualizing the site of leakage may be possible using a power injector or balloon catheter [12–14].
If preoperative preparation is sufficient and enough attention is paid to the above issues to facilitate the retrograde transvenous lymphatic embolization, the success rate of the procedure would improve.
Our search of the English literature yielded 7 reports that described retrograde transvenous lymphangiography and embolization [5,9–14]. In these previous reports, thoracic duct embolization was performed for chylothorax in 4 cases [5,11,14] and for chylous ascites in 5 cases [9,10,12,13]. Lymphangiography of the thoracic duct or cisterna chyli showed leakage in 7 of 9 cases (78%). Clinical success of embolization was achieved in 8 of 9 cases (89%). Of these 8 successfully embolized cases, 4 (50%) were addressed by a balloon-occlusion catheter [9,12–14]. Several materials were used for embolization, such as a mixture of iodized oil and NBCA [5,11,14], a mixture of 3% sodium tetradecyl sulfate, iodized oil, and air (3:2:1 ratio) [9,12,13], and/or coils [10,13] and/or microvascular plug [13]. NBCA is also often used for percutaneous transabdominal embolization.
If the percutaneous transabdominal and retrograde transvenous approaches fail, alternative methods can be applied. Baek et al. [2] and Hur et al. [8] reported 3 alternative embolization techniques with NBCA for abdominal or retroperitoneal lymphorrhea under fluoroscopy and/or C-arm CT: closest upstream lymph node embolization, direct upstream lymphatic vessel embolization, and lymphopseudoaneurysm embolization. Closest upstream lymph node embolization and direct upstream lymphatic vessel embolization are performed by direct puncture of the iodized oil-stained lymph node and lymphatic vessel, respectively, with a fine needle such as a 26-gauge needle, followed by injection of the glue mixture with iodized oil (ratio range, 1:1.5–1:9). Lymphopseudoaneurysm (defined as a small extravasated lymphatic fluid collection contained by the surrounding tissue) embolization is performed by direct puncture with a 21-gauge needle and followed by injection of the glue mixture with iodized oil (ratio range, 1:1–1:2). Lymphopseudoaneurysm embolization with NBCA via drainage catheter and sclerotherapy performed by direct puncture or percutaneous catheter drainage with a sclerosant, such as ethanol [18] or OK-432 [5], as well as lymphocele sclerotherapy, are alternative therapeutic options in such cases. Inoue et al. [5] reported that the key to success is to place a drainage tube as close to the source of the leak as possible. The response rate has been reported to reach 70%-85% [4,8,18].
Conclusion
Retrograde transvenous lymphatic embolization is a less invasive technique than percutaneous transabdominal thoracic duct embolization. When the leakage point is not identified by groin intranodal lymphangiography or percutaneous transabdominal lymphangiography fails, retrograde transvenous lymphatic embolization is an alternative option. If the percutaneous transabdominal approach and retrograde transvenous approach fail, lymphopseudoaneurysm embolization with NBCA and sclerotherapy via drainage catheter can cure chylous leaks.
Contributor Information
Kazuhiko Morikawa, Email: k.morikawa@jikei.ac.jp.
Shinsuke Takenaga, Email: takenaga@jikei.ac.jp.
Jun Hasumi, Email: h21ms-hasumi@jikei.ac.jp.
Asami Kano, Email: asamik@jikei.ac.jp.
Tomomi Terayama, Email: yamagishi@jikei.ac.jp.
References
- 1.Weniger M, D'Haese JG, Angele MK, Kleespies A, Werner J, Hartwig W. Treatment options for chylous ascites after major abdominal surgery: A systematic review. Am J Surg. 2016;211:206–213. doi: 10.1016/j.amjsurg.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Baek Y, Won JH, Kong TW, Paek J, Chang SJ, Ryu HS, et al Lymphatic leak occurring after surgical lymph node dissection: a preliminary study assessing the feasibility and outcome of lymphatic embolization. Cardiovasc Intervent Radiol. 2016;39:1728–1735. doi: 10.1007/s00270-016-1435-x. [DOI] [PubMed] [Google Scholar]
- 3.Kong TW, Chang SJ, Kim J, Paek J, Kim SH, Won JH, et al Risk factor analysis for massive lymphatic ascites after laparoscopic retroperitonal lymphadenectomy in gynecologic cancers and treatment using intranodal lymphangiography with glue embolization. J Gynecol Oncol. 2016;27:e44. doi: 10.3802/jgo.2016.27.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadolski GJ, Chauhan NR, Itkin M. Lymphangiography and lymphatic embolization for the treatment of refractory chylous ascites. Cardiovasc Intervent Radiol. 2018;41:415–423. doi: 10.1007/s00270-017-1856-1. [DOI] [PubMed] [Google Scholar]
- 5.Inoue M, Nakatsuka S, Yashiro H, Tanuma M, Suyama Y, Tsukada J. Lymphatic intervention for various types of lymphorrhea: Access and treatment. Radiographics. 2016;36:2199–2211. doi: 10.1148/rg.2016160053. [DOI] [PubMed] [Google Scholar]
- 6.Itkin M, Kucharczuk JC, Kwak A, Trerotola SO, Kaiser LR. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: Experience in 109 patients. J Thorac Cardiovasc Surg. 2010;139:584–590. doi: 10.1016/j.jtcvs.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Pamarthi V, Stecker MS, Schenker MP, Baum RA, killoran TP, Han AS. Thoracic duct embolization and disruption for treatment of chylous effusions: Experience with 105 patients. J Vasc Interv Radiol. 2014;25:1398–1404. doi: 10.1016/j.jvir.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Hur S, Shin JH, Lee IJ, Min SK, Min SI, Ahn S. Early experience in the management of postoperative lymphatic leakage using lipiodol lymphangiography and adjunctive glue embolization. J Vasc Interv Radiol. 2016;27:1177–1186. doi: 10.1016/j.jvir.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Majdalany BS, Khayat M, Downing T, Killoran TP, El-Haddad G, Khaja MS, et al Lymphatic interventions for isolated, iatrogenic chylous ascites: A multi-institution experience. Eur J Radiol. 2018;109:41–47. doi: 10.1016/j.ejrad.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Mittleider D, Dykes TA, Cicuto KP, Amberson SM, Leusner CR. Retrograde cannulation of the thoracic duct and embolization of the cisterna chyli in the treatment of chylous ascites. J Vasc Interv Radiol. 2008;19:285–290. doi: 10.1016/j.jvir.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Koike Y, Hirai C, Nishimura J, Moriya N, Katsumata Y. Percutaneous transvenous embolization of the thoracic duct in the treatment of chylothorax in two patients. J Vasc Interv Radiol. 2013;24:135–137. doi: 10.1016/j.jvir.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasa RN, Gemmete JJ, Osher ML, Hage AN, Chick JF. Endolymphatic balloon-occluded retrograde abdominal lymphangiography (BORAL) and embolization (BORALE) for the diagnosis and treatment of chylous ascites: Approach, technical success, and clinical outcomes. Ann Vasc Surg. 2018;49:49–56. doi: 10.1016/j.avsg.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Hussain JS, Srinivasa RN, Srinivasa RN, Patel A, Gemmete JJ, Chick JF. Balloon-occluded retrograde lymphangiography and embolization of a posttraumatic lymphoenteric fistula. J Vasc Interv Radiol. 2018;29:1032–1033. doi: 10.1016/j.jvir.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 14.Kariya S, Nakatani M, Ueno Y, Yoshida A, Ono Y, Maruyama T. Transvenous retrograde thoracic ductography: Initial experience with 13 consecutive cases. Cardiovasc Intervent Radiol. 2018;41:406–414. doi: 10.1007/s00270-017-1814-y. [DOI] [PubMed] [Google Scholar]
- 15.Phang K, Bowman M, Phillips A, Windsor J. Review of thoracic duct anatomical variations and clinical implications. Clin Anat. 2014;27:637–644. doi: 10.1002/ca.22337. [DOI] [PubMed] [Google Scholar]
- 16.Hsu MC, Itkin M. Lymphatic anatomy. Tech Vasc Interv Radiol. 2016;19:247–254. doi: 10.1053/j.tvir.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsson SI. Almqvist & Wiksell; Stockholm: 1972. Clinical anatomy and pathology of the thoracic duct: an investigation of 122 cases. [Google Scholar]
- 18.Kortes N, Radeleff B, Sommer CM, Bellemann N, Ott K, Richter GM. Therapeutic Lymphangiography and CT-guided Sclerotherapy for the Treatment of Refractory Lymphatic Leakage. J Vasc Interv Radiol. 2014;25:127–132. doi: 10.1016/j.jvir.2013.10.011. [DOI] [PubMed] [Google Scholar]