Abstract
Background
Cystinosis is a metabolic disease caused by intracellular accumulation of cystine within lysosomes. Development of symptoms can be delayed significantly by a life-long therapy with cysteamine, a drug that enters the lysosome and reacts with cystine thereby enabling its export from the organelle.
Methods
During a period of 16 years, blood samples of 330 cystinosis patients were analyzed to investigate therapeutic adherence and metabolic control in patients treated with immediate-release cysteamine. The accepted therapeutic goal is to measure intracellular cystine levels in white blood cells every 3 months and to keep them below 0.5 nmol cystine/mg protein (= 1 nmol hemicystine/mg protein).
Results
42% of measurements were within the desired 3-month interval, 38% were done every 3–5 months, 11% every 6–8 months, 5% every 9–12 months and 4% after a 12-month interval only. 64.4% of the measurements were higher than the therapeutic target value. Median cystine levels increased with longer control intervals.
Conclusions
The majority of the cystinosis patients showed insufficient metabolic adjustment. Intracellular cystine levels were not done as often as recommended and were not within therapeutic range. Poor therapy adherence is likely to be caused by gastrointestinal side effects of immediate-release cysteamine. Incorrect intervals between drug intake and blood sampling could contribute to the results.
Keywords: Adherence, Cysteamine, Cystine level, Cystinosis, Metabolic monitoring
1. Introduction
Cystinosis is an autosomal recessive metabolic disease characterized by reduced cystine export from lysosomes due to absent or abnormal cystinosin, the integral lysosomal membrane cystine transporter [1]. This results in storage of lysosomal cystine, which is released as cysteine by intralysosomal protein degradation and rapidly forms the disulfide, cystine [2]. Cystine is poorly soluble und forms crystals that lead to cell damage, especially in the kidney. Untreated cystinosis patients have cystine levels in leukocytes that are on average 80-fold higher than those observed in healthy individuals [3]. Cystine accumulation leads to toxic reactions caused by an inhibition of oxidative enzyme activities with secondary effects on cell functions, presumably leading to apoptotic or necrotic cell death [4]. Prevalence of the disease is approximately 1:100.000–1:200.000 [5].
A cornerstone of therapy is the oral administration of cysteamine, which is rapidly absorbed from the gut and reacts inside the lysosomes with cystine to form the mixed disulfide cysteine-cysteamine. This product can be exported out of the lysosomes by the cationic amino acid transporter PQLC2, thereby decreasing the cellular cystine level [6].
Cystagon© (Mylan Pharmaceuticals, Canonsburg, PA) is an immediate-release form of cysteamine and contains 50 or 150 mg of cysteamine per capsule as mercaptamine bitartrate. It received the FDA approval in the USA based on clinical data after various clinical tests in 1994 and is taken orally 4 times per day in regular intervals of 6 h immediately after or together with a meal. After 2 h, the cellular cystine level drops to a minimum, and rises again to its original high level after 6 h [7]. Until the age of 12 years, standard daily dose is 1.30 g/m2 body surface area. In elder patients over 50 kg, the recommended daily dose is 2 g up to a maximum of 1.95 g/m2. Monitoring of the cystine concentration in leukocytes at specific intervals is required for correct adjustment of the dose. The drug must be taken life-long. The therapeutic target level in white blood cells (WBCs) is ≤0.5 nmol cystine/mg protein (< 1,0 mmol hemicystine). Cysteamine therapy can delay disease progression very efficiently [8].
Many patients experience considerable side effects upon cysteamine treatment: mouth and body odour occurs as well as gastrointestinal problems like frequent nausea and vomiting, abdominal discomfort and diarrhoea as well as increased production of gastric acid with subsequent ulceration, side effects which greatly limit therapy adherence [[8], [9], [10]]. Moreover, strictly scheduled dosing every 6 h means getting up during the night to ingest capsules, which can further reduce therapy adherence.
Due to these limitations some patients have poor therapy adherence that might accelerate disease progress, like chronic renal failure, hypothyroidism, photophobia up to retinal blindness, diabetes mellitus, hypogonadism, pulmonary dysfunction, muscle weakness, epilepsy, dementia or cerebral atrophy [5,11]. Proximal tubular damage leads to renal Fanconi syndrome ending in most cases in kidney failure, which often requires kidney transplantation [12].
2. Materials and methods
In this study, data on cystine concentrations in leukocytes from 330 patients was collected between July 1999 and June 2015. Only patients that had at least 3 blood tests during this period and had received immediate-release cysteamine (total: 162 patients) were included in this retrospective study. The first measurement was excluded to avoid the inclusion of pre-therapeutic samples. Data analysis was consented by the local ethics board (number 2019–199-f-S).
For analysis of WBCs cystine level, blood should be drawn exactly 6 h following the last cysteamine dose. The therapeutic target value should then be below 0.5 nmol cystine/mg protein.
2.1. Cystine measurements
The cystine measurement of the leucocytes was carried out by the classical method by Spackman, Stein and Moore [13], a method with ion chromatographical separation of amino acids by a cation exchange column and a step gradient. N-Ethylmaleimide (NEM) was added to capture free sulfhydryl groups. Two internal standards were measured in addition (norvaline and cystine).
3. Results
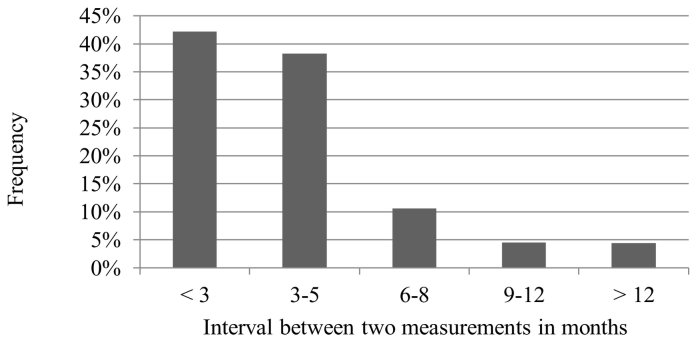
A total of 42% of the controls were within the desired 3-month interval, 38% were done every 3 to 5 months, 11% every 6 to 8 months, 5% every 9 to 12 months, and 4% were done on after more than 12 months (Fig. 1).
Fig. 1.
Time intervals between two blood samplings of cystinosis patients for WBC cystine content measurements (3278 blood samples of 162 patients). The appointments were considered individually and independent of the corresponding patient.
Fig. 2 shows intracellular cystine levels within the respective time intervals in the total group. The dotted line indicates the target value of 0.5 nmol cystine/mg protein. While the results of 3-month follow-ups and follow-ups from 3 to 5 months show barely any differences (the median was 0,6 nmol cystine/mg protein each), an increase in cystine levels was observed with longer intervals (e.g. > 12 month with a median of 0,8 nmol cystine/mg protein). Longer monitoring intervals go along with higher mean intracellular cystine levels. The many outliers found in the first two interval groups are remarkable, especially since some of them are equal to untreated cystinosis.
Fig. 2.

WBC cystine levels measured in the respective time intervals, independent of individual patients to illustrate a correlation between appointment adherence and cystine level (All together: 3349 blood samples). The dotted line indicates the target value of 0,5 nmol cysteine/mg protein. The grey coloured boxes represent the middle 50% of the data, the band inside the boxes shows the median. Stars represent the extreme outliers and circles the milder ones.
However it must be considered that longer intervals between the measurements may result in less frequent adjustment of the medication dose. Even patients who were following the strict medication schedule could suffer from a underdosing represented by the raised median of their higher interval group.
The intra-individual fluctuations of the 10 patients with the most frequent examinations during the mentioned period are illustrated in Fig. 3 with 63 blood measurements the first patient and 45 samples in the last patient. The medians were between 0.40 and 0.79 nmol cystine/mg protein. The best result could be found in patient 1 with 68% of blood samples below 0,5 nmol cystine/mg protein. Despite of two outliers (max. 1,22 nmol cystine/mg protein), 50% of the other 61 blood samples were located between 0,3 and 0,53 nmol cystine/mg protein. Patient 2 had many mild and extreme outliers. Levels of up to 3.6 nmol cystine/mg protein were achieved at times. Patient 4 (47 blood samples) is represented by the 4th box which is the widest one and shows a high degree of fluctuation and the highest median (almost 0,8 nmol cystine/mg protein). Recommended cystine levels could not be achieved permanently in any of the patients.
Fig. 3.
Intracelluar WBC cystine levels of the ten patients with the best adherence to the 3-months time intervals. The different extents of the boxes reflects the considerable fluctuations of the individual cystine levels. The stars and circles represent the extreme and mild outliers, the dotted line the target value of 0,5 nmol cystine/mg protein.
The median of all measurements was 0.63 nmol cystine/mg protein, and therefore above the therapeutic target value (Fig. 4). Including the middle 50% of the data, the box is characterized by an interquartile range of 0,43 till 0,9 nmol cystine/mg protein. The lowest cystine level was 0,04 nmol cystine/mg protein, the highest could be found at 8,9 nmol cystine/mg protein. All together 64,4% of the measurements were over the target value of 0,5 nmol cysteine/mg protein, only 35,6% reached the desired range. It shows the extreme outliers, which were found both in patients who seemed therapy-adherent as well as in patients who were less compliant with follow-up measurements.
Fig. 4.
Illustration of the levels of every analysis of individual patients in nmol cystine/mg protein (A total of: 3349 blood samples).
Fig. 5 demonstrates the relative distribution of cystine levels measured. It shows that 35.6% of the levels were below 0.5 nmol cystine/mg protein, which means that the metabolic monitoring was insufficient in 64.4% of the samples. 28,2% of the levels were > 0,5 and ≤ 0,75, 16,8% >0,75 and ≤ 1, 6,7% >1 and ≤ 1,25. The last three categories show barely any differences: levels >1,25 and ≤ 1,5 could be found in 4,1% and levels >1,5 and ≤ 2 and > 2 were reached by 4,3% each.
Fig. 5.
Relative proportion of measurements per category. The categorical classification of cystine levels in relation to the frequency offers valuable clues to the deficient success at the therapy of cystinosis.
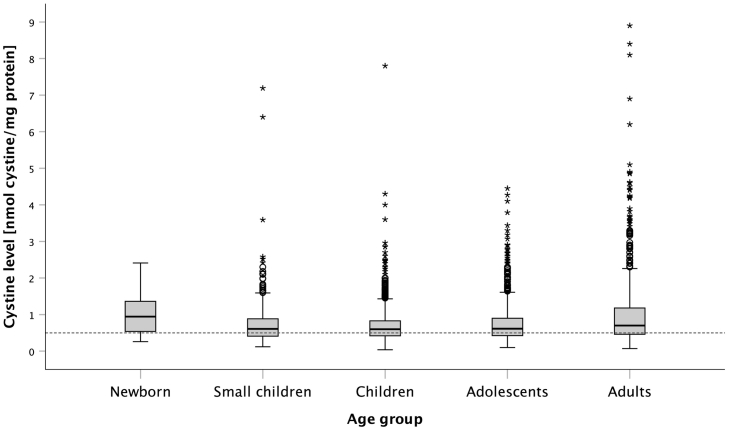
Fig. 6 presents the subdivision of the measurements in the different age groups. Values obtained within the first 12 months of age were assigned to the category Newborn/Infant, from the start of the 2nd year to the end of the child's 3rd year, the category assigned is Small Child, from the start of the child's 4th year until the end of the 12th year, the category is Child, from the start of the 13th year to the end of the 18 year – Youth, and thereafter the category Adult. It can be seen that the median values of Small Children, Children and Youths were almost identical (0.61 to 0.60 to 0.62 nmol cystine/mg protein). The median value was highest for infants at 0.95 nmol cystine/mg protein, followed by the value for adults at 0,7 nmol cystine/mg protein. No statistical outliers are found in the newborn category; the total number of included values is, however, at n = 24 the lowest, whereas the children group accounts for most measurement data (n = 1374). The adult category showed the highest outliers, and the interquartile range was also larger, similarly for the newborn group
Fig. 6.
Measurements in the different age groups. The age of the patient is determined at the follow-up appointment and categorized accordingly.
4. Discussion
White blood cell cystine levels have to be kept all-day-long below a threshold level in order avoid cystine crystal formation and subsequent cellular damage. Several studies monitoring cystinosis patients receiving cysteamine therapy showed that adherence is often insufficient to achieve a stable cystine level below the recommended therapeutic target value. This may be due to the strict medication schedule requiring getting up at night to adhere the proper 6 h dosing interval and to the side effects of the medication, such as bad smell and gastrointestinal problems [7,9,10].
Approximately 14% of the affected patients do not tolerate cysteamine therapy due to strong nausea and vomiting, which worsens compliance [5]. If the drug is tolerated, only 23% of patients comply with the strict medication schedule, 17% medicate only during the day. However, the cystine concentration will greatly increase if the medication is taken with a small delay of 2–3 h [7].
The current study demonstrates that metabolic control in a large cohort of cystinosis patients taking immediate-release cysteamine seems to be poor. Intra-individual fluctuations in different blood samples were high and were not rarely above 1 nmol cystine/mg protein in WBCs. Fluctuations may be due to the deviation from prescribed intake times of 6 h prior to drawing blood or an inaccurate or missed dosage. Due to the pharmacokinetics of immediate-release cysteamine, strict adherence of the blood sampling to the 6-h interval is mandatory.
As an alternative to Cystagon©, which is released from the capsule in the stomach, a drug called Procysbi© (Raptor Pharmaceutical Corporation, USA) was launched in the market, which is released and absorbed in the small intestine due to its enteric coating and shows improved cysteamine pharmacokinetics. It allows reducing the dose required with Cystagon© to 82% and only needs to be taken twice daily [10]. The simplified intake of that drug and fewer gastrointestinal side effects could improve adherence.
Cystinosis patients require life-long care and monitoring of their intracellular cystine levels, which generally requires time and the willingness of having blood drawn. Therefore, it is not surprising that patients, primarily in those examined for many years, in part over the entire period of 16 years, get tired of keeping follow-up appointments. But results show also that strong fluctuations in WBC cystine levels occur even in dedicated patients, who are under regular care.
No information was provided about the prescribed dosage with each patient, the actual dose interval or the time the medication was administered last before the blood sample was taken. If the predefined interval was exceeded the result would be a rise in cystine measurements. Another way how cystine levels could be falsely low would be if the requested 6-h interval between the last cysteamine administration and the blood sampling was shorter.
Whereas this retrospective analysis cannot correlate metabolic control with clinical outcome, it clearly shows insufficient metabolic control or wrong intervals between drug intake and blood sampling with immediate-release cysteamine treatment. In order to delay disease progression ever further, greater care has to be taken with respect to adequate dosing and correct blood sampling.
Declaration of Competing Interest
None declared.
Acknowledgements
T.M. received consulting fees and research grants from Orphan Europe (France; No. 141217) and from Horizon Pharmaceuticals (Ireland; No. 20150904) and acknowledges support from the Open Access Publication Fund of the University of Muenster (Germany).
Contributor Information
Simone Linden, Email: s.linden@my.com.
Sabrina Klank, Email: s_klan04@uni-muenster.de.
Erik Harms, Email: harms@uni-muenster.de.
Marianne Grüneberg, Email: Marianne.Grueneberg@ukmuenster.de.
Julien H. Park, Email: Julien.Park@ukmuenster.de.
Thorsten Marquardt, Email: marquat@uni-muenster.de.
References
- 1.Harms E. Angeborene Stoffwechselkrankheiten bei Erwachsenen. Dahl SV. Springer; Berlin: 2014. Cystinose; pp. 167–175. [Google Scholar]
- 2.Thoene J.G., Oshima R.G., Ritchie D.G., Schneider J.A. Cystinotic fibroblasts accumulate cystine from intracellular protein degradation. Proc. Natl. Acad. Sci. U. S. A. 1977;74(10):4505–4507. doi: 10.1073/pnas.74.10.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider J.A., Bradley K., Seegmiller J.E. Increased cystine in leukocytes from individuals homozygous and heterozygous for cystinosis. Science. 1967;157(3794):1321–1322. doi: 10.1126/science.157.3794.1321. [DOI] [PubMed] [Google Scholar]
- 4.Park M., Helip-Wooley A., Thoene J.G. Lysosomal cystine storage augments apoptosis in cultured human fibroblasts and renal tubular epithelial cells. J. Am. Soc. Nephrol. 2002;13(12):2878–2887. doi: 10.1097/01.asn.0000036867.49866.59. [DOI] [PubMed] [Google Scholar]
- 5.Gahl W.A., Thoene J.G., Schneider J.A. Cystinosis. N. Engl. J. Med. 2002;347(2):111–121. doi: 10.1056/NEJMra020552. [DOI] [PubMed] [Google Scholar]
- 6.Besouw M., Masereeuw R., van den Heuvel L., Levtchenko E. Cysteamine: an old drug with new potential. Drug Discov. Today. 2013;18(15):785–792. doi: 10.1016/j.drudis.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Levtchenko E., van Dael C.M., de Graaf-Hess A.C. Strict cysteamine dose regimen is required to prevent nocturnal cystine accumulation in cystinosis. Pediatr. Nephrol. 2006;21(1):110–113. doi: 10.1007/s00467-005-2052-0. [DOI] [PubMed] [Google Scholar]
- 8.Elmonem M.A., Veys K.R., Soliman N.A., van Dyck M., van den Heuvel L.P., Levtchenko E. Cystinosis: a review. Orphanet J. Rare Dis. 2016;11:47. doi: 10.1186/s13023-016-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ariceta G., Lara E., Camacho J.A. Cysteamine (Cystagon®) adherence in patients with cystinosis in Spain: successful in children and a challenge in adolescents and adults. Nephrol. Dial. Transplant. 2015;30(3):475–480. doi: 10.1093/ndt/gfu329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besouw M., Tangerman A., Cornelissen E., Rioux P., Levtchenko E. Halitosis in cystinosis patients after administration of immediate-release cysteamine bitartrate compared to delayed-release cysteamine bitartrate. Mol. Genet. Metab. 2012;107.1–2:234–236. doi: 10.1016/j.ymgme.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Wilmer M.J., Schoeber J.P., van den Heuvel L.P., Levtchenko E. Cystinosis: practical tools for diagnosis and treatment. Pediatr. Nephrol. 2011;26(2):205–215. doi: 10.1007/s00467-010-1627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heil S.G., Levtchenko E., Monnens L.A., Trijbels F.J., Put N.M.V.D., Blom H.J. The molecular basis of Dutch infantile nephropathic cystinosis. Nephron. 2001;89(1):50–55. doi: 10.1159/000046043. [DOI] [PubMed] [Google Scholar]
- 13.Spackman D.H., Stein W.H., Moore S. Anal.Chem. 1958;30(7):1190–1206. [Google Scholar]