ABSTRACT
The cardiac conduction system (VCS) is essential for normal myocardial excitation and contraction. Heritable and acquired syndromes perturbing conduction system formation or function are responsible for a substantial burden of cardiovascular disease, including heart block, triggered and reentrant arrhythmias, sudden cardiac death, myocardial dyssynchrony, and progression of heart failure. Our laboratory has employed stem cell models, genetically encoded conduction system reporter mice, comparative transcriptional profiling, and a battery of functional assays to elucidate the molecular determinants of conduction system development, physiology, and disease pathogenesis. Through these strategies, we have uncovered a diversity of novel conduction system-enriched genes, including transcription factors, receptors, and signaling molecules that modulate conduction system physiology. Our long-term goals are to leverage these discoveries for therapeutic impact and to diminish the burden of diseases resulting from abnormal cardiac rhythmicity.
INTRODUCTION
The healthcare burden from arrhythmogenic cardiovascular disease is substantial (1-3). An estimated 350,000 sudden cardiac deaths occur in the United States each year, primarily from ventricular tachyarrhythmias that arise in the setting of acute ischemia, acquired heart disease, or inherited syndromes, including channelopathies and cardiomyopathies. Pharmacological anti-arrhythmic therapy for patients at risk of lethal ventricular arrhythmias has been a major disappointment, with an unacceptably high risk of pro-arrhythmia or lack of efficacy (4). Accordingly, the main type of therapy has been the use of electronic devices such as pacemakers and implantable cardioverter-defibrillators. While effective, these devices can lead to significant morbidity and mortality, and they place an enormous strain on the overall healthcare budget.
The specialized cardiac conduction system (CCS) comprises a heterogeneous network of cells that orchestrate the initiation and propagation of a wave of electrical excitation throughout the myocardium (5). Purkinje cells are the most distal component of the CCS, and they deliver the depolarizing wave to the working myocytes of the ventricular myocardium (6). More than 40 years ago, Hoffman and colleagues proposed that enhanced phase 4 depolarization in Purkinje cells might “be a significant factor in human arrhythmias” (7). Since that time, substantial accumulated experimental data support the concept that Purkinje cells play a key mechanistic role in triggering a broad range of life-threatening ventricular arrhythmias. Indeed, primarily through catheter-based mapping and ablation studies, the Purkinje fiber network has been implicated in the initiation of ventricular tachycardia following myocardial infarction, in dilated cardiomyopathies, idiopathic ventricular fibrillation, and even in inherited syndromes including catecholaminergic polymorphic VT (CPVT), Brugada syndrome, and long QT syndrome (LQTS), as recently reviewed by Boyden, Haissaguerre, and others (6,8-12).
A number of laboratories, including our own, have made some progress in identifying transcriptional regulators essential for formation of the VCS and/or regulation of VCS-specific target genes. Murine loss-of-function studies have been especially instructive in this regard and have revealed critical roles for several transcriptional regulators, including NKX2-5 and TBX5, HF-1b, the homeobox transcription factor HOP, and Iroquois family members including Irx3 (reviewed in 5,12). Despite this progress, many of the key factors involved in VCS formation and function are yet to be discovered and characterized. This gap in knowledge has served as the impetus for some of the studies described herein.
MATERIALS AND METHODS
Genetically Engineered Mouse and Cellular Models
CCS-lacZ transgenic mice (13), Cntn2-EGFP BAC transgenic mice (14), and Etv1nlz/+ reporter mice (15) have been described previously. All animal experiments were performed according to protocols approved by the NYU Institutional Animal Care and Use Committee and conformed to the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals.
Stem Cell Models
Murine embryonic stem cells harboring both the CCS-lacZ and Cntn2-EGFP BAC reporter genes (16) and human-induced pluripotent stem cell-derived cardiomyocytes have been described previously (17). High-content screening of murine ESCs was performed using compound libraries from Sigma LOPAC, MicroSource US-Drug, and Preswick Chemical, as previously described (16).
Differential Gene Expression Screen
Purkinje cells and working ventricular cardiomyocytes were isolated from adult hearts of Cntn2-EGFP BAC transgenic mice, and transcriptional profiling was performed using Affymetrix Arrays, as previously described (18).
Cellular Electrophysiology and Electrocardiographic Analysis
Patch-clamp recordings to measure action potentials and individual ionic currents and electrocardiograms (ECGs) to measure in vivo cardiac rhythm were performed, as previously described (15,17).
RESULTS
Identification of Novel Inducers of Purkinje Cells in Stem Cell Model Systems
Purkinje cells comprise less than 1% of ventricular cardiomyocytes. Their rarity is a substantial impediment to molecular, biochemical, and functional studies. Leveraging fluorescent reporter genes that are active only or are primarily in Purkinje cells, but not working ventricular myocytes, we performed a high-throughput chemical screen to identify compounds that pushed differentiating murine embryonic stem cells toward a Purkinje cell fate. We discovered that a small molecule of sodium nitroprusside efficiently promoted the generation of cells with gene expression profiles and cellular electrophysiological properties that were quite similar to bona fide Purkinje cells isolated directly from mouse hearts (16,19), as shown in Figure 1. Further dissection of the signaling pathway suggested that modulation of cyclic AMP signaling may play a mechanistic role in Purkinje cell differentiation. A similar strategy using reporter genes active in human-induced pluripotent stem cells is currently underway, with the goal of translating our early murine studies into more clinically relevant applications.
Fig. 1.
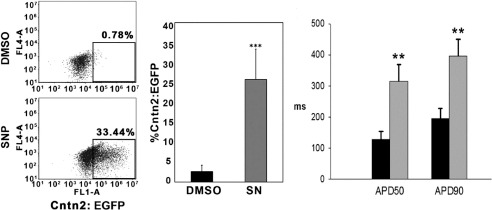
Sodium nitroprusside enhances generation of cardiac conduction system cells. A. Flow cytometry analysis of Cntn2-EGFP expression. Murine ESC-derived cardiomyocytes were treated for five days with either DMSO or sodium nitroprusside (SN) and harvested at Day 25 of differentiation. Quantification of Cntn2-EGFP expression is shown in the right panel. B. Comparison of action potential duration, quantified by APD(50) and APD(90) between GFP− (black bars) presumptive ventricular cardiomyocytes and GFP+ presumptive Purkinje-like myocytes (gray bars). Adapted from (16).
Identification of Novel Transcription Factors
In previous studies, we identified the growth factor neuregulin-1 as being critically important for normal ventricular conduction system development and function (20). Using a combined signal transduction/kinase inhibitor and differential gene expression screen, we asked what transcription factors might link neuregulin signaling with transcriptional modulation. Through this strategy, we identified highly enriched expression of the MAP kinase-dependent transcription factor ETV1 in the proximal and distal VCS, including cardiac Purkinje cells. Importantly, loss of function of ETV1 resulted in reduced expression of key regulators of rapid conduction within these cells, including Nkx2-5, Gja5, and Scn5a—the latter two genes encode the major gap junction channel protein (Cx40) and voltage-gated sodium channel protein (NaV1.5) in the VCS, respectively. Consistent with reduced expression of these two key channel proteins, mutant mice displayed profound conduction slowing within the VCS. In addition, ETV1 mutant mice displayed marked VCS hypoplasia, as shown in Figure 2, suggesting a role for this transcription factor in promoting Purkinje cell identity or survival, as well as cell-type specific gene expression. In terms of relevance to human physiology and disease, a phenome-wide association study identified a link between ETV1 and bundle branch block and heart block in humans (15).
Fig. 2.
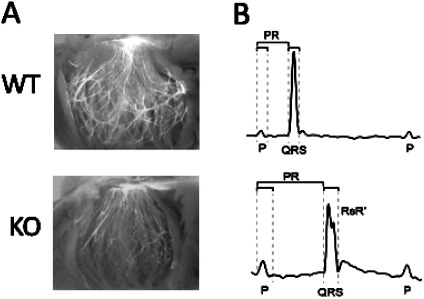
Abnormal conduction system structure and function in Etv1 mutant mice. A. Appearance of the ventricular conduction system as visualized by the Cntn2-EGFP reporter gene in postnatal Day 18 wildtype (WT) and ETV1 knockout (KO) mice. B. Representative electrocardiograms in postnatal Day 18 WT and KO mice, showing prolonged P, PR, and QRS intervals in the KO mice. Adapted from (15).
Induction of Purkinje Cell Gene Expression and Physiology
Yet to be determined is whether unraveling the details of the transcriptional circuitry responsible for Purkinje cell biology can be translated into therapeutic benefit. Toward that end, we have begun to explore the consequences of heterologous expression of the key transcription factor ETV1—both in rodent ventricular myocytes and in human IPSC-derived cardiomyocytes. In both model systems, forced expression of ETV1 resulted in upregulation of key transcripts responsible for rapid conduction, including the aforementioned Nkx2-5, Gja5, and Scn5a, as well as substantial upregulation of cardiac sodium currents (17). These data may be helpful in developing a strategy for the marked conduction slowing associated with a broad range of acquired and heritable cardiomyopathies.
CONCLUSIONS
The ventricular conduction system is an important trigger of life-threatening arrhythmias and a common site of conduction slowing and heart block. Together, these reflections of aberrant cardiac electrophysiology account for substantial morbidity and mortality throughout the United States and worldwide. To date, therapies for conduction system disease have relied heavily upon biomedical engineering and device-based therapies, including implantable defibrillators and pacemakers. Likewise, antiarrhythmic pharmacotherapy has been plagued by off-target effects and an unacceptably high incidence of pro-arrhythmia, as exemplified by the Cardiac Arrhythmia Suppression Trial (4).
In recent years, we and others are beginning to unravel the transcriptional logic regulating conduction system development and the mechanisms governing conduction system physiology in health and disease. We are also actively trying to identify target genes within the ventricular conduction system, specifically those responsible for the unique electrophysiological properties that define this rare population of cardiomyocytes. While we have focused on transcription factors in this brief review, our gene discovery program has identified a promising list of candidate genes that might serve as targets for Purkinje cell-specific therapies: Purkinje cell protein-4 (PCP4), differentially expressed G-protein coupled receptors (GPCRs) including members of the dopamine receptor family, cell adhesion proteins such as Contactin-2, ion channel subunits, and various kinases and phosphatases (18). Many of these differentially expressed genes are logical candidates for targeted Purkinje cell-directed therapies. Moreover, our gene expression screens have also revealed additional transcription factors that are expressed in the developing heart and highly enriched in mature Purkinje cells. As in our findings with ETV1, murine loss-of-function experiments reveal essential roles for these factors in ventricular conduction system development and function. In the coming years, we anticipate a more complete understanding of the transcriptional circuitry responsible for electrophysiological specialization in the heart, thereby providing a roadmap for therapeutic reprogramming.
ACKNOWLEDGMENTS
The findings described in this report represent the work of many former and current members of the Fishman Laboratory, and their contributions are warmly acknowledged. Our studies have been supported by National Institutes of Health Grants HL105983, HL142498, and HL82727; a New York State NYSTEM award; and a grant from Fondation Leducq.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Due to technical problems with the Grand Hotel audiovisual equipment, the questions by Drs. Pinsky, Sacher, and Merajver associated with this paper and the responses by Dr. Fishman could not be transcribed.
REFERENCES
- 1.Wong CX, Brown A, Lau DH, et al. Epidemiology of sudden cardiac death: global and regional perspectives. Heart Lung Circ. 2019;28:6–14. doi: 10.1016/j.hlc.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 2.Isbister J, Semsarian C. Sudden cardiac death: an update. Intern Med J. 2019;49:826–33. doi: 10.1111/imj.14359. [DOI] [PubMed] [Google Scholar]
- 3.Israel CW. Mechanisms of sudden cardiac death. Indian Heart J. 2014;66((Suppl 1)):S10–7. doi: 10.1016/j.ihj.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 5.Park DS, Fishman GI. The cardiac conduction system. Circulation. 2011;123:904–15. doi: 10.1161/CIRCULATIONAHA.110.942284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyden PA, Dun W, Robinson RB. Cardiac Purkinje fibers and arrhythmias; The GK Moe Award Lecture 2015. Heart Rhythm. 2016;13:1172–81. doi: 10.1016/j.hrthm.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer DH, Lazzara R, Hoffman BF. Interrelationship between automaticity and conduction in Purkinje fibers. Circ Res. 1967;21:537–58. doi: 10.1161/01.res.21.4.537. [DOI] [PubMed] [Google Scholar]
- 8.Haissaguerre M, Extramiana F, Hocini M, et al. Mapping and ablation of ventricular fibrillation associated with long QT and Brugada syndromes. Circulation. 2003;108:925–8. doi: 10.1161/01.CIR.0000088781.99943.95. [DOI] [PubMed] [Google Scholar]
- 9.Weerasooriya R, Hsu LF, Scavee C, et al. Catheter ablation of ventricular fibrillation in structurally normal hearts targeting the RVOT and Purkinje ectopy. Herz. 2003;28:598–606. doi: 10.1007/s00059-003-2491-y. [DOI] [PubMed] [Google Scholar]
- 10.Iyer V, Roman-Campos D, Sampson KJ, Kang G, Fishman GI, Kass RS. Purkinje cells as sources of arrhythmias in long QT syndrome Type 3. Sci Rep. 2015;5:13287. doi: 10.1038/srep13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haissaguerre M, Vigmond E, Stuyvers B, Hocini M, Bernus O. Ventricular arrhythmias and the His-Purkinje system. Nat Rev Cardiol. 2016;13:155–66. doi: 10.1038/nrcardio.2015.193. [DOI] [PubMed] [Google Scholar]
- 12.Park DS, Fishman GI. Development and function of the cardiac conduction system in health and disease. J Cardiovasc Dev Dis. 2017:4. doi: 10.3390/jcdd4020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logan C, Khoo WK, Cado D, Joyner AL. Two enhancer regions in the mouse En-2 locus direct expression to the mid/hindbrain region and mandibular myoblasts. Development. 1993;117:905–16. doi: 10.1242/dev.117.3.905. [DOI] [PubMed] [Google Scholar]
- 14.Pallante BA, Giovannone S, Fang-Yu L, et al. Contactin-2 expression in the cardiac Purkinje fiber network. Circ Arrhythm Electrophysiol. 2010;3:186–94. doi: 10.1161/CIRCEP.109.928820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shekhar A, Lin X, Liu FY, et al. Transcription factor ETV1 is essential for rapid conduction in the heart. J Clin Invest. 2016;126((12)):4444–59. doi: 10.1172/JCI87968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai SY, Maass K, Lu J, Fishman GI, Chen S, Evans T. Efficient generation of cardiac Purkinje cells from ESCs by activating cAMP signaling. Stem Cell Reports. 2015;4:1089–102. doi: 10.1016/j.stemcr.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shekhar A, Lin X, Lin B, et al. ETV1 activates a rapid conduction transcriptional program in rodent and human cardiomyocytes. Sci Rep. 2018;8:9944. doi: 10.1038/s41598-018-28239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim EE, Shekhar A, Lu J, et al. PCP4 regulates Purkinje cell excitability and cardiac rhythmicity. J Clin Invest. 2014;124:5027–36. doi: 10.1172/JCI77495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maass K, Shekhar A, Lu J, et al. Isolation and characterization of embryonic stem cell-derived cardiac Purkinje cells. Stem Cells. 2015;33:1102–12. doi: 10.1002/stem.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rentschler S, Zander J, Meyers K, et al. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci U S A. 2002;99:10464–9. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]


