Abstract
WNK [with-no-lysine (K)] kinases are a family of four members of serine and threonine kinases that regulate renal Na+ and K+ transport. Mutations of WNK1 and WNK4 cause a hereditary hypertensive and hyperkalemic disease known as pseudohypoaldosteronism type II (PHA2). Unlike other WNK isoforms, WNK1 is ubiquitously expressed and regulates many other cellular processes outside the kidney. Oxidative stress response kinase (OSR1) and related STE 20/SPS1-related proline alanine-rich kinase (SPAK) are downstream kinases of WNK kinases. To examine the role of WNK kinase cascade in vivo, we generated global Wnk1-deleted mice and found that Wnk1-ablated mice die in utero from embryonic angiogenesis and cardiac developmental defects. Endothelial-specific Wnk1 deletion reveals that angiogenesis defect is due to WNK1 requirement in endothelium. We further showed that global and endothelial-deletion of Osr1 phenocopies Wnk1 deletion. Furthermore, expression of a catalytic constitutively active Osr1 transgene rescues angiogenesis defects and embryonic lethality of Wnk1-ablated mice. In zebrafish, Wnk1 knockdown causes similar angiogenesis defects to Vegf2 (Flk1) knockdown and that expression of WNK1 partially rescues Flk1 angiogenesis defects. The results indicate that WNK1 is downstream of VEGF signaling cascade. T-lymphocytes isolated from Wnk1-null mice exhibit migration defects. Inhibition of WNK1-OSR1 downstream target Na-K-2Cl cotransporter NKCC1 mimics migration defect of WNK1-deficient T-lymphocytes. Thus, WNK1-OSR1/SPAK cascade is important for angiogenesis. Regulation of ion homeostasis and cell volume may underlie the mechanism for WNK1 regulation of endothelial cell migration and angiogenesis.
Introduction
WNK kinases are a family of four serine-threonine protein kinases, WNK1-4, with an atypical placement of the catalytic lysine (1). Mutations of two members, WNK1 and WNK4, cause pseudohypoaldosteronism type II (PHA2), an autosomal-dominant disease characterized by hypertension and hyperkalemia (2). Mutations in the WNK1 gene are large deletions of the first intron leading to increased expression. Mutations in the WNK4 gene are missense mutations in the coding sequence outside the protein kinase domain. WNK1-4 are products of different genes that contain a conserved kinase domain in the amino terminus (1-3) (Figure 1). WNK1 is widely expressed in multiple spliced forms (1). The full-length WNK1 is produced from the entire 28 exons (referred to as “long WNK1”; L-WNK1) and has a ubiquitous distribution (1). A shorter WNK1 transcript encoding a polypeptide lacking the amino terminal 1-437 amino acids of the long WNK1 is highly expressed in the kidney (referred to as KS-WNK1 for kidney-specific) (4,5). The KS-WNK1 is produced by replacing the first four exons with an alternative exon 4A, which encodes 30 amino acids unique to KS-WNK1. The remaining exons 5 through 28 are the same as the long transcript.
Fig. 1.

Domain structure of WNKs. WNK1 through 4 share conserved kinase domain (“KD”), autoinhibitory domain (“I”), coiled-coil (“C”) domains, and multiple proline-rich (“P”) domains. Only WNK1 and WNK4 are shown here.
In vitro and cell-based studies have shown that WNK1 can activate the furosemide-sensitive Na+-K+-2Cl- cotransporters NNKCC1 and NKCC2 as well as Na+-Cl- cotransporter NCC through activating SPAK (STE20/SPS1-related, proline alanine-rich kinase) and the related OSR1 (oxidative stress-responsive kinase 1) (6-8). SPAK and OSR1 are members of the STE20 kinase subfamily that share a homologous kinase domain, a regulatory S-motif, and a conserved carboxyl-terminus (CCT). Both OSR1 and SPAK bind to the RFXV (arginine-phenylalanine-any amino acid-valine) motif of WNK kinases through the CCT domain (Figure 2) (9). The physical interaction is believed to be important for the activation of OSR1/SPAK by WNKs. The two NKCC isoforms, NKCC1 and NKCC2, and NCC also contain RFXV motif allowing them to bind to and be phosphorylated and activated by SPAK and OSR1. These effects on NKCCs and NCC are important for WNK1-OSR/SPAK kinase cascade regulation of renal Na+ and K+ transport and hypertension and hyperkalemia phenotypes of PHA2 (6).
Fig. 2.
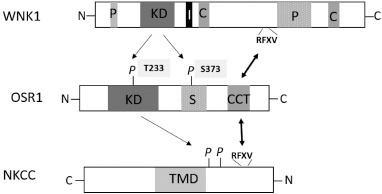
Cascade for regulation of NKCC transporter by WNK1-OSR1/SPAK. The conserved carboxyl-terminus (CCT) of OSR1/SPAK (SPAK not shown, but similar) interacts with WNKs (upstream target) or NKCC (downstream targets) via RFXV (arginine-phenylalanine-any residue-valine) motif. WNK kinase activates OSR1/SPAK by phosphorylating on threonine-233 (T233) in the kinase domain (“KD”) and serine-373 (S373) in the S-motif (“S”). Activated OSR1/SPAK phosphorylates and activates NKCC transporter. Phosphorylation is shown as “P”. Note that the amino (N)-terminus is oriented to the right, in contrast to that for WNK1 and OSR1. “TMD” in NKCC stands for transmembrane domain.
Mice With Deletion of wnk1 Exhibit Angiogenesis Defects
WNK4 is abundantly expressed in epithelial tissues, while WNK3 is abundant in the brain. The expression of WNK2 is less well known. In contrast, WNK1 is ubiquitously expressed and regulates many other cellular processes outside the kidney (6). We found that mice with homozygous deletion of Wnk1 are embryonic lethal (10). Analysis of embryos at various stages of development reveals that homozygous Wnk1 deletion causes death at embryonic (E) day 10.5–12.5. The timing of death indicates cardiovascular developmental defects. Indeed, Wnk1-null E10.5 embryos are much smaller than wild-type counterparts and exhibit pericardial edema and hemorrhage. Staining of endothelial cells by antibody against platelet endothelial cell adhesion molecule (PECAM) reveals that in Wnk1-deleted mice the dorsal aorta and cardinal vein are present but the secondary and tertial branches of intersomitic vessels are absent. Yolk sacs of Wnk1-deleted mice have primitive vascular plexuses but no well-developed large and small vessels. These finding indicate that Wnk1 deletion impairs embryonic angiogenesis; the initial step of blood vessel formation, vasculogenesis, is not affected.
WNK1 regulation of angiogenesis is kinase activity-dependent and mediated by OSR1
Regulation of an ion transporter by WNK1 may be kinase-dependent or -independent. Kinase-dependent activity may be mediated through the downstream OSR1 or SPAK kinase (Figure 2) (6,9). We demonstrated that OSR1 functions downstream of WNK1 in regulating angiogenesis. Mice with global or endothelial ablation of Osr1 also die in utero with essentially identical phenotypes and at the same timing compared to Wnk1-ablated mice. To further demonstrate the cascade WNK1-OSR1 in regulating angiogenesis, we showed that expression of a catalytic constitutively active Osr1 transgene rescues angiogenesis defects and embryonic lethality of Wnk1-ablated mice (11).
WNK1 works downstream of VEGF signaling cascade
We used a zebrafish model system to explore the relationship between vascular endothelial growth factor (VEGF) and WNK1 cascades in the regulation of angiogenesis. We used fli-GFP transgenic fish that mark endothelial cells with green fluorescence. Zebrafish have two Wnk1 genes, Wnk1a and Wnk1b. Morpholino knockdown of Wnk1a or Wnk1b induces angiogenesis defects as evidenced by failure of intersomitic vessels to reach the dorsal longitudinal vessels by 33 hours post-fertilization (12). The pattern of angiogenesis defects resembles that of Flk1 (VegfR2) knockdown. Phosphatidylinositol-3-kinase (PI3K)-mediated Akt kinase causes phosphorylation of WNK1 at threonine-60 (human WNK1, threonine-58 for rodent WNK1) (13,14). We showed that overexpression of wild-type rat WNK1, but not T58A mutant, rescues Flk1 angiogenesis defects (12). The mRNA expression of Wnk1a or Wnk1b is reduced in Flk1 knockdown fish. The results indicate that WNK1 signaling is downstream of VEGF and VEGF signaling cascade regulates WNK1 phosphorylation as well as Wnk1 gene expression.
Role of regulation of ion homeostasis by WNK1-OSR1 cascade in angiogenesis
WNK1-SPAK/OSR1 cascade plays an important role in regulating Na+, K+, and Cl- ion transport (6,9). Increasing evidence supports the relationship between ion homeostasis, angiogenesis, and tumor vascularization (15,16). For example, Cl- and K+ channel inhibitors inhibit new vessel formation in vitro and in vivo (17). The activity of WNK1-OSR1 downstream target NKCC1 positively correlates with hepatocellular carcinoma growth and metastasis (18,19). T-lymphocytes isolated from Wnk1-deleted mice exhibit impaired migration probably due to reduced NKCC1 activity (20).
Summary and future perspectives
WNK1 plays an essential role in early embryonic angiogenesis. WNK1 regulation of angiogenesis is through OSR1 kinase. Emerging evidence supports that ion homeostasis is important for cell migration in many cell types. Regulation of ion homeostasis in migrating endothelial cells by WNK1-OSR1 cascade likely underlies the mechanism by which WNK1-OSR1 cascade controls angiogenesis (Figure 3). WNK1 is downstream of VEGF signaling cascade. VEGF controls the expression of Wnk1 and causes WNK1 protein phosphorylation via VEGF receptor-mediated PI3K-Akt kinase. Akt phosphorylation of WNK1 does not affect its kinase activity (13,14,21). How WNK1 phosphorylation contributes to WNK1-OSR1 regulation of ion homeostasis remains unknown. We speculate that Akt phosphorylation of WNK1 may affect its targeting and/or interaction with downstream targets. In conclusion, WNK1-OSR1 is a novel angiogenesis pathway that acts downstream of the VEGF signaling cascade. It may be a target for antitumor therapy.
Fig. 3.

Working model for WNK1-OSR1 regulation of endothelial cell migration. Arrow indicates the direction of cell movement.
Acknowledgments
Work in Chou-Long Huang's lab is supported by a grant from the National Institutes of Health USA (DK111542). Chiou-Hwa Yuh is supported by a grant from the Taiwan Ministry of Science and Technology (MOST 108-2320-B-400 -005 -MY3).
Footnotes
Potential Conflicts of Interest: None disclosed.
References
- Xu B, English JM, Wilsbacher JL, et al. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem. 2000;275:16795–801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- Wilson FH, Disse-Nicodeme S, Choate KA, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–12. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- Verissimo F, Jordan P. WNK kinases, a novel protein kinase subfamily in multicellular organisms. Oncogene. 2001;20:5562–9. doi: 10.1038/sj.onc.1204726. [DOI] [PubMed] [Google Scholar]
- Delaloy C, Lu J, Houot AM, Disse-Nicodeme S, et al. Multiple promoters in the WNK1 gene: one controls expression of a kidney-specific kinase-defective isoform. Mol Cell Biol. 2003;23:9208–21. doi: 10.1128/MCB.23.24.9208-9221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly M, Marshall E, Speirs HJ, et al. WNK1, a gene within a novel blood pressure control pathway, tissue specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol. 2003;14:2447–56. doi: 10.1097/01.asn.0000089830.97681.3b. [DOI] [PubMed] [Google Scholar]
- McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev. 2011;91:177–219. doi: 10.1152/physrev.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Urushiyama S, Hisamoto N, et al. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280:42685–93. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- Vitari AC, Deak M, Morrice NA, et al. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005;391:17–24. [Google Scholar]
- Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signaling pathway. J Cell Sci. 2008;121:3293–304. doi: 10.1242/jcs.029223. [DOI] [PubMed] [Google Scholar]
- Xie J, Wu T, Xu K, et al. Endothelial-specific expression of WNK1 kinase is essential for angiogenesis and heart development in mice. Am J Pathol. 2009;175((3)):1315–27. doi: 10.2353/ajpath.2009.090094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Yoon J, Yang SS, et al. WNK1 protein kinase regulates embryonic cardiovascular development through the OSR1 signaling cascade. J Biol Chem. 2013;288((12)):8566–74. doi: 10.1074/jbc.M113.451575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JG, Tsai SM, Tu HC, et al. Zebrafish WNK lysine deficient protein kinase 1 (WNK1) affects angiogenesis associated with VEGF signaling. PLoS One. 2014;9((8)):e106129. doi: 10.1371/journal.pone.0106129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu BE, Stippec S, Chu PY, et al. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proceedings of the National Academy of Sciences of the United States of America. 2005;102((29)):10315–20. doi: 10.1073/pnas.0504422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu BE, Stippec S, Lazrak A, et al. WNK1 activates SGK1 by a phosphatidylinositol 3-kinase-dependent and non-catalytic mechanism. J Biol Chem. 2005;280((40)):34218–23. doi: 10.1074/jbc.M505735200. [DOI] [PubMed] [Google Scholar]
- Martial S. Involvement of ion channels and transporters in carcinoma angiogenesis and metastasis. Am J Physiol Cell Physiol. 2016;310((9)):C710–27. doi: 10.1152/ajpcell.00218.2015. [DOI] [PubMed] [Google Scholar]
- Fiorio Pla A, Munaron L. Functional properties of ion channels and transporters in tumour vascularization. Philos Trans R Soc Lond B Biol Sci. 2014;369((1638)):20130103. doi: 10.1098/rstb.2013.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamili C, Kakataparthy RS, Vattikutti UM, et al. Anti-proliferative and anti-angiogenic activities of ion-channel modulators: In-ovo, in-vitro and in-vivo study. Asian Pacific J Trop Biomed. 2017;7((6)):555–62. [Google Scholar]
- Thastrup JO, Rafiqi FH, Vitari AC, et al. SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem J. 2012;441((1)):325–37. doi: 10.1042/BJ20111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Sun W, Chen N, et al. Discovery of NKCC1 as a potential therapeutic target to inhibit hepatocellular carcinoma cell growth and metastasis.pdf. Oncotarget. 2017;8((39)):66328–42. doi: 10.18632/oncotarget.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köchl R, Thelen F, Vanes L, et al. WNK1 kinase balances T cell adhesion versus migration in vivo. Nat Immunol. 2016;17:1075–83. doi: 10.1038/ni.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallolu Kankanamalage S, Karra AS, Cobb MH. WNK pathways in cancer signaling networks. Cell Commun Signal. 2018;16((1)):72. doi: 10.1186/s12964-018-0287-1. Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]


