Abstract
Bacterial chorioamnionitis is an intrauterine infection that occurs during pregnancy and involves the membranes that extend from the placenta to form the sac encasing the amniotic fluid and developing fetus. Chorioamnionitis is most commonly caused by bacteria that likely ascend from the vagina into the gravid uterus and can result in devastating complications such as preterm labor, membrane rupture, fetal stillbirth, or severe infection of the newborn. Surviving babies exposed to chorioamnionitis in utero have an increased risk of lifelong disability. Unfortunately, most chorioamnionitis is clinically silent unless a bad outcome occurs, which compels the need for better diagnostic, therapeutic, and preventive approaches. Our lab has a primary interest in defining the early steps in disease pathogenesis of bacterial chorioamnionitis, when microbes first make contact with the fetal membranes. Through team science, we are using organ-on-chip models of human fetal membranes to define host-microbe interactions critical to the development of chorioamnionitis and its complications.
INTRODUCTION
In studying acute bacterial chorioamnionitis, certain principles motivate our team science approach to preventing adverse pregnancy outcomes. First, all groups of people experience pregnancy and childbirth. Second, a healthy birth is important to living a long, healthy life. Third, the in utero environment influences fetal development and the short- and long-term health of offspring. Last, improving maternal-child health is a significant factor in advancing population health.
THE CHALLENGE
Maternal-child health in the United States faces significant challenges. A 2018 study by Papanicolas and colleagues compared health care spending and quality metrics in the United States with those in 10 other high-income countries (1). The United States ranked the worst of all 11 countries in life expectancy for women aged ≥40 years, maternal mortality, neonatal mortality, and infant mortality (1). Preterm birth, which impacts one in every 10 deliveries in the United States (or nearly 380,000 preterm births per year) (2), is a major driver of both neonatal and infant mortality (3). Conservative estimates suggest that 25% of all preterm deliveries are associated with intrauterine infection (4), though the earlier in gestation a pregnancy progresses to labor the more likely this reflects intrauterine infection (5).
BACTERIAL CHORIOAMNIONITIS
Chorioamnionitis refers to inflammation involving the fetal membranes (also known as the extraplacental or gestational membranes) that extend from the placenta to create the sac of amniotic fluid that contains the developing fetus (5). Acute chorioamnionitis, which is commonly caused by bacterial infection, is histologically characterized by neutrophilic infiltrates of the fetal membranes. It is more often identified microscopically postpartum than it is during pregnancy due to clinical signs or symptoms (5). Bacteria are thought to infect the membranes after ascending from the vagina, across the cervix, and into the gravid uterus. Three potentially devastating, immediate adverse outcomes of chorioamnionitis include preterm labor, membrane rupture (“water breaking”), and infection of the fetus, the latter of which can result in stillbirth or early-onset neonatal sepsis (5). However, babies who survive intrauterine exposure to chorioamnionitis can experience lifelong disabilities that involve almost any major organ system (6)
Since chorioamnionitis is frequently clinically silent but can present with a severe, irreversible complication, the early steps in disease pathogenesis must be better understood and actionable targets for prevention, treatment, or risk stratification must be identified.
FETAL MEMBRANE STRUCTURE
The normal fetal membranes have an elegant but simple structure (Figure 1). This somewhat translucent membrane is relatively avascular and represents an important anatomical aspect of the maternal-fetal interface. The innermost (fetal) side of the membranes is the amnion layer, which has a single layer of amniotic epithelial cells resting atop a dense bed of extracellular matrix (largely collagen) laid down by mesenchymal fibroblasts (7). The amnion provides mechanical strength to the fetal membrane and acts largely as a structural barrier, though it also appears to play a role in host defense and inflammation (8). Beneath the amnion is the chorion—a layer defined by the presence of trophoblasts, which are specialized cells that are the defining cell type of the placenta (the organ from which the fetal membranes extend) (7). Both the chorion and the amnion are genetically fetal and are derived from the implanted blastocyst (9). Thus, these anatomical regions of the membranes can be genetically male or female, for example. The outermost layer of the membranes is not fetal in origin but maternal. It is the decidua, a region largely populated by decidualized stromal cells (10). The cells of the decidua and chorion are highly intermingled and present a true maternal-fetal interface.
Fig. 1.

Simplified diagram of the fetal membranes infected with bacteria. Shown are distinct layers of the membranes including those that are genetically fetal and those that are maternally derived. Both maternal and fetal immune cells are found in this maternal-fetal interface. Shown are macrophages, the primary innate phagocyte of the membranes. Lymphocytes and NK cells, while present in this tissue, are not shown, for simplicity. Bacteria are thought to infect the membranes primarily via ascension from the vagina through the cervix.
Throughout all layers of the fetal membranes are immune cells, including lymphocytes, NK cells, and macrophages (11). Neutrophils are not a normal population found in uninflamed membranes. The extent to which bacteria that cause chorioamnionitis are recognized by both immune and nonimmune cells within the fetal membranes is a focus of our research (12). We want to better define the signals cells send to each other following microbial threat and determine how such signals sometimes lead to resolution of infection and other times result in adverse consequences.
EXISTING MODELS OF THE HUMAN FETAL MEMBRANE
Current studies of human fetal membrane immunology employ traditional cell and tissue culture models (13,14). Cell culture is limited by the loss of the biological context provided by neighboring cells and matrix, while tissue culture is crippled by an inability to maintain viability ex vivo for prolonged periods of time (without contamination). Tissue culture also lacks the capability to dissect the roles of individual cell types within the context of whole tissue. Typically, independent cell and tissue culture experiments are conducted and terminated at discrete points throughout the course of infection. This disjointed approach creates challenges for understanding the dynamics of host-microbial relationships. In addition, these culture systems suffer from dilutional effects imposed by the relatively large culture media volumes employed. Animal models are also limited and present endocrine, anatomical, and immunological differences from humans. Thus, there is an urgent need to develop better human chorioamnionitis models, which would eliminate species-specific differences and accelerate research in immunology and microbiology.
The instrumented fetal membrane on a chip (IFMOC), an innovative model of fetal membrane immunology, bridges in vitro with in vivo human studies (13,14) (Figure 2). IFMOC advantages include the following:
A highly defined, living model of human fetal membrane that can be maintained for days to weeks.
The ability to define the contribution(s) of individual cell types to the immunology of intact membranes, facilitating high-resolution mapping of autocrine and paracrine signaling networks within this compartment.
The potential to incorporate transgenic and gene-deficient cell types within the membranes and to define the contribution of particular genes and gene-networks to human reproductive immunology (and physiology).
The capacity to better model covariates such as fetal sex or race/ethnicity at the tissue level.
The ability to incorporate the IFMOC into novel imaging tools and downstream analytics while preserving the capacity to perform longitudinal studies throughout the course of infection: from colonization to invasion.
Fig. 2.
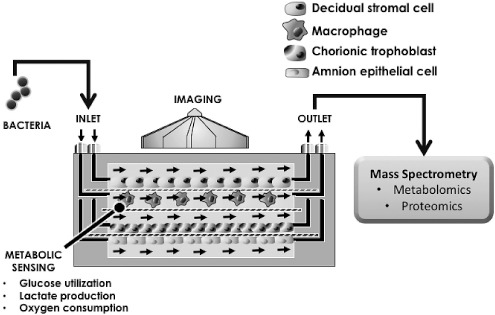
Simplified diagram of the instrumented fetal membrane on chip (IFMOC). Diagrammed here is a four-chamber IFMOC with inlet ports for microfluidic culture media flow, where stimuli such as bacterial pathogens can be introduced. Each layer of the IFMOC can be populated with individual or mixed cell populations, with or without an extracellular matrix. Shown here are four cell types separated by permeable, porous membranes. The device is translucent and can be imaged. Effluent can be collected for analysis by any number of technologies including mass spectrometry, as illustrated here.
EARLY USE OF THE IFMOC
We initially used the IFMOC to examine host-microbe interactions in the decidua, the outermost aspect of the fetal membranes. We used a single-chamber IFMOC to model the human decidua using decidualized telomerase-immortalized endometrial stromal cells cultured alone or in combination with the macrophage-like THP-1 cell (15). Cells were challenged with the Gram-negative bacterial outer membrane component lipopolysaccharide (LPS) from Escherichia coli, which is a common cause of chorioamnionitis and neonatal sepsis. We found that the decidual stromal cells inhibited macrophage production of the proinflammatory cytokine tumor necrosis factor (TNF)-α through a paracrine mechanism involving the lipid mediator prostaglandin E2 (15). We have since collaborated with investigators to demonstrate the utility of a two-chamber IFMOC to examine decidual-amnion interactions in response to oxidative stress (16). We have been able image cells on the IFMOC using light and fluorescent microscopy.
FUTURE APPLICATIONS OF THE IFMOC
Preliminary data suggest we can conduct experiments on chip and then subject the devices to scanning electron microscopy as well. We are also working with collaborators to equip the IFMOC with metabolic sensors that would allow us to better objectively measure tissue-level responses to perturbations such as infection. An exciting possibility is leveraging organ-on-chip technologies to recreate the maternal-fetal interface for mother-offspring pairs to better understand the contribution of the in utero environment to adverse health outcomes, aligning with the developmental origins of health and disease (DOHaD) paradigm. For example, maternal comorbidities, such as infections or metabolic stressors (e.g., diabetes, obesity), are known to increase the risk of adverse health outcomes in offspring, but exact mechanisms of disease risk transmission are not well defined. We envision a time when mother-offspring pairs affected by an illness or syndrome of interest (and unaffected controls matched for relevant covariates) could be enrolled long after birth into studies where noninvasive samples (such as buccal swabs or skin biopsies) are used to derive induced pluripotent stem cells that can then be differentiated on chip into critical cellular populations of the maternal-fetal interface: a newly, retrospectively designed placenta (or fetal membrane) that can then be interrogated to test hypotheses about transgenerational risk transmission. Team science that brings together biomedical engineers, chemists, reproductive biologists, and physician scientists will surely benefit maternal-child health.
Acknowledgments and Financial Support
I would like to thank my wife, Kelly Hill, for her outstanding support. Special thanks also goes to Lisa Rogers, my laboratory manager. In addition, my collaborators and lab members (current and past) are all sincerely appreciated!
This work has been funded by grants from the U.S. Environmental Protection Agency (EPA, Grant No. 83573601), the National Institutes of Health (NIH, R01 AI134036), and the March of Dimes. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the funding agencies, the U.S. federal government, the NIH, or the EPA. Furthermore, the EPA does not endorse the purchase of any commercial products or services mentioned in this publication.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Due to technical problems with the Grand Hotel audiovisual equipment, the questions by Drs. Hochberg, Merajver, and Palmer associated with this paper and the responses by Dr. Aronoff could not be transcribed.
REFERENCES
- 1.Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319((10)):1024–39. doi: 10.1001/jama.2018.1150. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton BE, Martin JA, Osterman MJK, Rossen LM. Births: provisional data for 2018. Vital Statistics Rapid Release; no 7. Hyattsville, MD: National Center for Health Statistics; May 2019. [Google Scholar]
- 3.Heron M. Deaths: leading causes for 2017. Hyattsville, MD: National Center for Health Statistics; 2019. National Vital Statistics Reports 2018;68(6) [PubMed] [Google Scholar]
- 4.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345((6198)):760–5. doi: 10.1126/science.1251816. PMCID: PMC4191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henríquez GMG, Rodrigo FG-M. Chorioamnionitis and neonatal morbidity: current perspectives. Research and Reports in Neonatology. 2017;7:41–52. [Google Scholar]
- 6.Galinsky R, Polglase GR, Hooper SB, Black MJ, Moss TJ. J Pregnancy. 2013;2013:412831. doi: 10.1155/2013/412831. PMCID: 3606792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verbruggen SW, Oyen ML, Phillips AT, Nowlan NC. Function and failure of the fetal membrane: modeling the mechanics of the chorion and amnion. PLoS One. 2017;12((3)):e0171588. doi: 10.1371/journal.pone.0171588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boldenow E, Hogan KA, Chames MC, Aronoff DM, Xi C, Loch-Caruso R. Role of cytokine signaling in group B Streptococcus-stimulated expression of human beta defensin-2 in human extraplacental membranes. Am J Reprod Immunol. 2015;73((3)):263–72. doi: 10.1111/aji.12325. PMCID: PMC4323989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Sousa Barros JJ. Embryology and Anatomy of Placental Membranes. Amniotic Membrane: Springer; 2015. pp. 3–18. [Google Scholar]
- 10.Areia AL, Moura P. Amniotic Membrane in Health and Disease: An Obstetrical Perspective. Amniotic Membrane: Springer; 2015. pp. 77–101. [Google Scholar]
- 11.Castillo-Castrejon M, Meraz-Cruz N, Gomez-Lopez N, Flores-Pliego A, Beltrán-Montoya J, Viveros-Alcaráz M, et al. Choriodecidual cells from term human pregnancies show distinctive functional properties related to the induction of labor. Am J Reprod Immunol. 2014;71((1)):86–93. doi: 10.1111/aji.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anders AP, Gaddy JA, Doster RS, Aronoff DM. Current concepts in maternal-fetal immunology: recognition and response to microbial pathogens by decidual stromal cells. Am J Reprod Immunol. 2017;77((3)) doi: 10.1111/aji.12623. PMCID: PMC5321847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnecco JS, Anders AP, Cliffel D, Pensabene V, Rogers LM, Osteen K, et al. Instrumenting a fetal membrane on a chip as emerging technology for preterm birth research. Current Pharmaceutical Design. 2017;23((40)):6115–24. doi: 10.2174/1381612823666170825142649. [DOI] [PubMed] [Google Scholar]
- 14.Hutson MS, Alexander PG, Allwardt V, Aronoff DM, Bruner-Tran KL, Cliffel DE, et al. Organs-on-chips as bridges for predictive toxicology. Applied In Vitro Toxicology. 2016;2((2)):97–102. [Google Scholar]
- 15.Rogers LM, Anders AP, Doster RS, Gill EA, Gnecco JS, Holley JM, et al. Decidual stromal cell-derived PGE2 regulates macrophage responses to microbial threat. Am J Reprod Immunol. 2018;80((4)):e13032. doi: 10.1111/aji.13032. PMCID: PMC6461368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson LS, Osteen K, Saade GR, Aronoff D, Menon R. Fetal membrane organ on chip: an innovative approach to study feto-maternal cellular interactions. Am J Obstetrics and Gynecology. 2019;220((1)):S512. [Google Scholar]


