Abstract
Intracranial pseudoaneurysms account for about 1% of intracranial aneurysms with a high mortality. The natural history of intracranial pseudoaneurysm is not well-understood, and its management remains controversial. This review provides an overview of the etiology, pathophysiology, clinical presentation, imaging, and management of intracranial pseudoaneurysms. Especially, this article emphasizes the factors that should be considered for the most appropriate management strategy based on the risks and benefits of each treatment option.
Keywords: intracranial pseudoaneurysms, trauma, iatrogenic, management, endovascular treatment
Introduction
Intracranial pseudoaneurysm is a rare entity and represents about 1% of all intracranial aneurysms, with an associated mortality of 20% or higher (1). The most common cause of pseudoaneurysm is trauma (2). Other causes are iatrogenic, infectious disease, radiation exposure, connective tissue disease, and sometimes they occur spontaneously (3–6). A pseudoaneurysm or false aneurysm is the product of damaging vessel wall resulting in an encapsulated hematoma in communication with the ruptured artery. Clinical presentations may vary depending on the rupture status, location, and size of the intracranial pseudoaneurysm (7). If untreated, the mortality rate for patients with intracranial pseudoaneurysm can reach high up to 50% due to delayed rupture and disastrous bleeding (1, 8, 9). Therefore, early diagnosis and efficient treatment are mandatory. In this review, we provide a comprehensive evaluation of the risks and benefits of different treatment options available for pseudoaneurysms, such as observation, microsurgical clipping, and endovascular embolization. Besides, the etiology, pathophysiology, clinical presentation, and imaging of intracranial pseudoaneurysms are also discussed.
Classification
Pseudoaneurysms account for about 1% of aneurysms in adults; however, the incidence rate in the pediatric group is more than 19% (9, 10). Anatomical anomalies, venous sinus thrombosis, multiple surgeries, and prior radiotherapy increase the incidence rate of the pseudoaneurysm. Intracranial pseudoaneurysms can be classified as traumatic, infectious, iatrogenic, and other types.
Traumatic Pseudoaneurysms
Head trauma is the most common cause of intracranial pseudoaneurysms. Closed or penetrating head trauma to cerebral blood vessels, which lead to pseudoaneurysm, could be classified as direct or indirect (1). A penetrating wound resulting from a variety of weapons and cutlery leads to direct vascular injury. Indirect vascular trauma is often encountered in seriously closed brain injuries, such as traffic accidents and bony prominences during major brain shifts.
Infectious Pseudoaneurysms
Infectious pseudoaneurysms can be caused by bacteria, tuberculous bacilli, or fungi (11–13). In comparison with other intracranial aneurysms, infectious aneurysms have a slight preference for younger people. Ruptured aneurysms have a higher rate of mortality. Most of the infectious pseudoaneurysms are located in the anterior circulation, and those aneurysms can be multiple in many cases. The definition of infectious pseudoaneurysms should be based on the angiographic features and demonstration of infection.
Iatrogenic Pseudoaneurysms
In addition to trauma and infection, iatrogenic vascular injury is another important cause of intracranial pseudoaneurysms. They generally involve the internal carotid artery (ICA) due to ICA injury after transsphenoidal or transcranial resection of sellar region tumors (6, 14–19). Iatrogenic pseudoaneurysm are less common in the anterior cerebral artery (12, 20–24), the basilar artery (18, 25–27), and the middle cerebral artery (22, 28–30). Pseudoaneurysms after mechanical thrombectomy or stent angioplasty is one of the potential complications associated with the endovascular procedure (31, 32). Although rare, we should raise the suspicion for this potentially lethal complication.
Other Types of Pseudoaneurysms
Other causes of intracranial pseudoaneurysms include Marfan's syndrome, fibromuscular dysplasia, vasculitis, rupture of true cerebral aneurysm or arteriovenous malformation, associated with moyamoya disease, and radiotherapy (4, 5, 33, 34). Dissecting pseudoaneurysms and blood blister-like aneurysms were out of the scope of the discussion.
Pathophysiology
Compared with the extracranial arteries, the intracranial arteries are thinner and stiffer. They have a thinner media and adventitia, absence of an external elastic lamina, and possess a thicker internal elastic lamina (35, 36). These features make the intracranial arteries more vulnerable to trauma.
Traumatic intracranial aneurysms can be histologically categorized as true or false. True aneurysms usually develop following a partial disruption of the arterial wall. The intima, internal elastic lamina, and media are damaged, whereas the adventitia is intact (1, 3, 8, 36, 37). False aneurysms or pseudoaneurysms result from disruption of the entire arterial wall (Figure 1). A contained hematoma forms outside the vessel, being restricted by perivascular connective tissues. However, it continues to communicate with the injured artery and is more likely to rebleed.
Figure 1.

Brain trauma leads to rupture of the intima, media, and adventitia of the blood vessel (A,B), forming an organized hematoma cavity (C). When the hematoma forms outside the arterial wall, it continues to communicate with the injured vessel, thus predisposing it to re-bleeding (D).
Iatrogenic pseudoaneurysms can be classified as saccular and fusiform (36). Saccular pseudoaneurysms occur secondary to penetration or complete laceration of the arterial wall. They lack a true wall and are only contained by a fragile layer of connective tissue (7, 19). Fusiform pseudoaneurysm may result from thinning of the adventitia during surgical peeling of tumor from the adjacent vessel. In comparison to saccular pseudoaneurysms, they usually do not rupture.
A pseudoaneurysm can also form at the tip of the “true” aneurysm (34, 38). We speculated that it might be the thick hematoma around the aneurysm (38). The temporary disorder of cerebrospinal fluid circulation may also play a significant role in the formation of pseudoaneurysm, since the blood extravasating from the vessel could gather into a hematoma but not diffuse into the cerebrospinal fluid. Thus, a sufficient volume of the subarachnoid pool may result in the occurrence of the pseudoaneurysm (34).
Clinical Presentation
Intracranial pseudoaneurysms may present with intracranial hemorrhage, epistaxis, headaches, seizures, neurological deficits, and associated with other cerebrovascular diseases.
Intracranial hemorrhage is the most common presentation, manifesting as acute hemorrhage associated with the initial injury or in a delayed manner (39). Intraoperative arterial bleeding occurred in the majority of patients with iatrogenic vascular injury. Patients with no evidence of vascular injury during the operation may suffer postoperative or delayed hemorrhage, including intracerebral, intraventricular, and subarachnoid hemorrhage. Pseudoaneurysms of the middle meningeal artery typically are associated with epidural or subdural hematoma (40, 41).
Epistaxis is the common symptom of intracavernous ICA pseudoaneurysms. The intracavernous ICA is close to the sphenoid sinus, mostly bulging into the lateral sinus wall (37). A congenitally thin or even absent bony structure covering the cavernous ICA within the sphenoid sinus may provide less protection against bony erosion (42). Massive epistaxis may be caused by erosion of the lateral wall of the sphenoid sinus. We also cannot exclude the internal maxillary artery as another source of hemorrhage (9). Epistaxis may be delayed in 7 days to 8 months after trauma or iatrogenic intracavernous ICA injury. The initial episodes of epistaxis may be mild and not fatal. However, recurrent bleeding can lead to fatal blood loss. Thus, we should not neglect it, in order to prevent delayed diagnosis and treatment.
Focal neurological deficits are often associated with traumatic pseudoaneurysms of the ICA. Due to its proximity to other cavernous structures, including cranial nerves II, III, IV, V1, V2, and VI. Traumatic ICA pseudoaneurysms may present with cranial nerve deficits, unilateral blindness, or a carotid-cavernous fistula associated to skull base fractures (9, 11, 37, 43). Other symptoms include headache, seizures, neck rigidity, decreased mental state, paralysis, or reduced level of consciousness.
Imaging
Due to the high mortality related to pseudoaneurysm rupture, early diagnosis is mandatory. If intraoperative arterial bleeding occurs, iatrogenic vascular injury should be suspected. Angiography should be used for patients with perioperative hemorrhage or epistaxis. Digital subtraction angiography (DSA) is still the gold standard for the diagnosis of intracranial pseudoaneurysms since CTA and MRA have limited sensitivity for the detection of small aneurysms (44) DSA often demonstrates a globular shaped aneurysmal sac without a neck (5). Delayed filling and stagnation of contrast agents are the features of the pseudoaneurysm. If the initial imaging is negative, angiography shoud be repeated because pseudoaneurysms often develop days to weeks after injury. The optimal time interval between angiographies is still a matter of debate. Some studies reported negative initial angiograms within several hours or days of trauma. Follow-up angiograms showed an aneurysm weeks to months later. Therefore, initial angiography was suggested to be performed 1 or 2 weeks after vascular injury to avoid missed diagnoses (39, 44). However, another study recommends it 6 and 12 months postoperatively (15).
Management
As the causes of pseudoaneurysms are different, treatment options are challenging. Management of intracranial pseudoaneurysms includes microsurgery, embolization, and conservative treatment (Tables 1, 2).
Table 1.
Literature review of intracranial pseudoaneurysms treated with microsurgery*.
| References | Age (ys) /Sex | Artery involved | Etiology | Presentation | Treatment | Immediate aneurysm occlusion | Procedure-related complication | Outcome | Follow-up angiogram |
|---|---|---|---|---|---|---|---|---|---|
| Akamatsu et al. (4) | 75/F | Distal AICA | Radiation | SAH | Trapping and resection | Complete | NA | NA | NA |
| Binning et al. (45) | 16ws/F | ICA C7 | Traumatic | SAH | Surgical suturing /wrapping-clipping | Complete | None | Good | NA |
| Chen et al. (22) | 25/F | MCA branch | Iatrogenic | Delayed ICH | Direct clipping | Complete | None | Good | No recurrence |
| Cikla et al. (36) | 68/M | ICA C6 | Iatrogenic | Intraoperative bleeding | Trapping and bypass | Complete | None | Good | NA |
| Ding et al. (3) | 37/M | ACoA | Unknown | SAH | A fenestrated clip | NA | Intraoperative rupture | Good | NA |
| 50/F | ICA C7 | Unknown | SAH | An encircling clip | NA | Aneurysm avulsion | mRS 3 | NA | |
| Horiuchi et al. (8) | 66/M | Distal MCA | Traumatic | Delayed ICH | Trapping and resection | Complete | None | mRS 2 | NA |
| Imahori et al. (32) | 84/F | MCA M2 | Iatrogenic | Delayed ICH | Surgical suturing | Complete | None | mRS 4 | NA |
| Kumar et al. (39) | 49/M | Frontopolar | Traumatic | ICH | Direct clipping | Complete | None | mRS 4 | NA |
| 20/F | ACA A3/A4 | Traumatic | ICH | Direct clipping | Complete | None | Good | NA | |
| Le et al. (20) | 30/M | ACA A4 | Iatrogenic | Delayed ICH | Resection | Complete | None | NA | NA |
| Raper et al. (46) | 71/F | AChA | Unknown | SAH | Surgical trapping | Complete | None | Good | NA |
| Ravina et al. (23) | 43/M | Proximal A3 | Iatrogenic | SAH/ICH/nfarcts | Trapping and bypass | Complete | None | mRS 6 | / |
| 20/M | Proximal A3 | Traumatic | Recurrent ICH/SAH | Trapping and bypass | Complete | None | mRS 5 | NA | |
| 11/F | A1-A2 | Iatrogenic | Epistaxis/SAH | Trapping and bypass | Complete | None | mRS 4 | NA | |
| Rayes (28) | 22/M | MCA M4 | Iatrogenic | Postoperative ICH | Resection and end-to-end anastomosis | Complete | None | Good | NA |
| Sato et al. (47) | 61/F | Distal LSA | Unknown | IVH | Resection | Complete | None | Good | NA |
| Shirane et al. (48) | 40ws/F | ICA C7 | Iatrogenic | Postoperative IVH | Surgical suturing | Compete | None | Good | No recurrence |
| Sujijantarat et al. (49) | 14/M | BA | Traumatic | Extensive SAH | Staged trapping | Complete | None | mRS 3 | NA |
| Umekawa et al. (50) | 78/M | Distal AICA | Radiation | VII/VIII palsy | Trapping and bypass | Complete | None | mRS 2 | NA |
| Walcott et al. (51) | 26/M | ACA A2 | Traumatic | CTA discovered | Trapping and bypass | Complete | None | Good | No recurrence |
Pseudoaneurysms of the middle meningeal artery were not included.
ACA, anterior cerebral artery; AChA, anterior choroidal artery; ACoA, anterior communicating artery; AICA, anterior inferior cerebellar artery; BA, basilar artery; CTA, computed tomography angiography; ICA, internal carotid artery; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; LSA, lenticulostriate artery; MCA, middle cerebral artery; mRS, modified Rankin Scale; NA, not available; SAH, subarachnoid hemorrhage; ws, weeks.
Table 2.
Literature review of intracranial pseudoaneurysms treated with endovascular embolization from 2010*.
| References | Age (ys) /Sex | Artery involved | Etiology | Presentation | Treatment | Immediate aneurysm occlusion | Complication | Outcome* | Follow-up angiogram |
|---|---|---|---|---|---|---|---|---|---|
| Al-Jehani et al. (52) | 28/M | Cavernous ICA | Traumatic | Epistaxis | Coiling | Near complete | None | Good | No recurrence |
| Aljuboori et al. (53) | 19/M | M2/ICA C6 | Traumatic | SAH | 1st coiling; 2nd flow diversion | Complete | None | Good (mRS 1) | No recurrence |
| Altali et al. (9) | 6/M | Intracavernous ICA | Traumatic | Epistaxis/otorrhagia | Coiling | Complete | None | mRS 2 | Recurrence; retreatment |
| Amenta et al. (7) | 64/F | ICA C5 | Iatrogenic | Intraoperative bleeding | Flow diversion×2 | Decreased filling | None | Good | Complete |
| Chen et al. (22) | 39/M | Distal call. marg. | Iatrogenic | Recurrent ICH | Aneurysm occlusion and PAO (Glubran) | Complete | None | Good | NA |
| Colby et al. (30) | 9ms/F | MCA M1 | Iatrogenic | Delayed ICH | 1st coiling; 2nd flow diversion + coiling | Incomplete | None | Good | Complete |
| Fu et al. (11) | 58/M | Cavernous ICA | Infectious | Epistaxis | 1st coiling; 2nd trapping | Complete | Infarction | mRS 4 | NA |
| Giorgianni et al. (54) | 20/M | Right A1; left A2 | Traumatic | ICH | Flow diversion×2 | Complete; Complete | None | Good | No recurrence |
| Giorgianni et al. (55) | 66/M | Intracavernous ICA | Iatrogenic | Intraoperative bleeding | Flow diversion | Complete | None | Good | No recurrence |
| Griauzde et al. (56) | 7/F | BA | Traumatic | MRI discovered | Stent-assisted coiling | Near complete | None | NA | No recurrence |
| Griauzde et al. (18) | 18/F | BA | Iatrogenic | Intraoperative bleeding | 1st stent-assisted coiling; 2nd flow diversion + coiling | Complete | None | Good (mRS 0) | No recurrence |
| 49/F | Cavernous ICA | Iatrogenic | Intraoperative bleeding | Flow diversion×2 | Complete | None | Good (mRS 0) | No recurrence | |
| 60/M | ICA | Iatrogenic | Intraoperative bleeding | Flow diversion | Complete | None | Good (mRS 1) | No recurrence | |
| Hjortoe et al. (16) | 44/M | Cavernous ICA | Iatrogenic | Intraoperative bleeding | Coiling | Complete | None | Good | Recurrence |
| 63/F | OA | Iatrogenic | Delayed ICH | Coiling | Complete | None | NA | Recurrence | |
| Jadhav et al. (12) | 74 | ACA A3 | Iatrogenic | Postoperative SAH | Trapping (Onyx-34) | Complete | None | NA | NA |
| 61 | Distal MCA | Mycotic | Aortic endocarditis | Trapping (Onyx-34) | Complete | Perforation | Good | NA | |
| 38 | ACA A2 | Iatrogenic | Postoperative SAH | Trapping (Onyx-34) | Complete | None | NA | No recurrence | |
| 56 | ACA A2 | Iatrogenic | Postoperative SAH | Trapping (Onyx-34) | Complete | Thrombosis | mRS 3 | NA | |
| 30 | MCA | Mycotic | Infective endocarditis | Trapping (Onyx-34) | Complete | None | NA | No recurrence | |
| Kim et al. (57) | 13/F | ACA A2 | Traumatic | SDH | Stent-assisted coiling | Complete | None | mRS 2 | No recurrence |
| Kumar et al. (39) | 47/F | ACA A3 | Traumatic | Delayed SAH | Trapping (coils) | Complete | None | Death | / |
| Lee and Luo(24) | 37/M | BA | Iatrogenic | Epistaxis | Coiling | Complete | Rebleeding | Death | / |
| Lim et al. (31) | 60/F | MCA M1 | Iatrogenic | Intraoperative bleeding | Overlapping stents | Near complete | Thrombosis | Good | Complete |
| Lim et al. (58) | 30/M | Supraclinoid ICA | Traumatic | SAH | Stent-assisted coiling + a stent-within-a-stent | Complete | None | Good | No recurrence |
| Liu et al. (59) | 15/F | ICA C6/C7 | Traumatic | Epistaxis | Covered stent×2 | Decreased filling | None | Good | Complete |
| 15/M | ICA C7 | Traumatic | Headache | Covered stent | Decreased filling | None | Good | Complete | |
| Liu et al. (60) | 49/M | ACA A1 | Traumatic | Epistaxis | 1st coiling; 2nd PAO (coils+Onyx-18) | Complete | None | Good | NA |
| Mascitelli et al. (61) | 65/M | Distal AICA | Radiation | SAH | PAO (nBCA) | Complete | Infarction | mRS 2 | No recurrence |
| Matsumura et al. (62) | 64/F | Distal AICA | Radiation | SAH | PAO (coils) | Complete | None | mRS 4 | NA |
| 43/F | Distal ACA/PICA | Radiation | SAH | PAO (coils) | Complete | None | mRS 0 | NA | |
| Morinaga et al. (63) | 68/M | PCoA | Iatrogenic | Recurrent SAH | 1st coiling; 2nd LVIS stent-assisted coiling | Complete | None | Good | No recurrence |
| Munich et al. (24) | 60 | Frontopolar | Iatrogenic | ICH/SAH | PAO (coils+Onyx-34) | Complete | None | Aphasia | NA |
| Murakami et al. (64) | 61/M | AICA (pontine) | Radiation | SAH | PAO (coils) | Complete | Infarction | mRS 2 | No recurrence |
| Nariai et al. (19) | 62/M | Cavernous ICA | Iatrogenic | Epistaxis | Flow diversion | Near complete | None | Good | Complete |
| Ogilvy et al. (65) | 4/M | ICA C6 | Iatrogenic | MRI discovered | Stent-assisted coiling | Near complete | None | Good | Complete |
| OuYang et al. (13) | 49/M | Cavernous ICA | Infectious | Epistaxis | Stent-assisted coiling | Complete | Rebleeding | Death | / |
| Patel et al. (66) | 56/M | Cavernous ICA | Iatrogenic | Intraoperative bleeding | 1st, 2nd: Balloon-assisted (Onyx-500) | 1st: Near complete; 2nd: Complete | None | Good | No recurrence |
| Sami et al. (67) | NA | Cavernous ICA | Iatrogenic | NA | Flow diversion×3 | Decreased filling | None | Good (mRS 0) | Near complete |
| NA | Cavernous ICA | Iatrogenic | NA | Flow diversion | Decreased filling | None | Good (mRS 0) | Complete | |
| NA | ICA C6 | Traumatic | NA | Flow diversion×2 | Decreased filling | None | Good (mRS 1) | Complete | |
| NA | PCA P1 | Traumatic | NA | Flow diversion | Decreased filling | None | Good (mRS 0) | Complete | |
| NA | Cavernous ICA | Traumatic | NA | Flow diversion×2 | Decreased filling | None | mRS 3 | Complete | |
| NA | Cavernous ICA | Traumatic | NA | Flow diversion | Incomplete | Perforation | Death | / | |
| NA | ACA A3 | Traumatic | NA | Flow diversion | Decreased filling | None | Death | / | |
| NA | Cavernous ICA | Traumatic | NA | Flow diversion | Decreased filling | None | Good (mRS 0) | Complete | |
| Sastry et al. (26) | 13/M | BA | Iatrogenic | IVH | 1st coiling; 2nd coiling + flow diversion | Complete | None | Good | No recurrence |
| Shah et al. (29) | 27/M | MCA M4 | Iatrogenic | Delayed ICH | Trapping (nBCA) | Complete | None | mRS 3 | NA |
| Van Rooij and Van Rooij (68) | 28/M | Distal pericall. artery branch | Traumatic | ICH | Trapping (nBCA) | Complete | None | Recovered | NA |
| 22/M | Distal pericall. artery branch | Traumatic | Delayed ICH | Trapping (coils) | Complete | None | Recovered | NA | |
| Wang et al. (2) | 38/M | ICA C4 | Traumatic | Eye blindness | Covered stent | Complete | None | Full recovery | No recurrence |
| 35/M | ICA C5 | Traumatic | Epistaxis | Covered stent | Incomplete | None | Full recovery | Complete | |
| 60/M | ICA C6 | Traumatic | Headache/ptosis | Covered stent×2 | Incomplete | None | Full recovery | Complete | |
| 11/M | ICA C7 | Traumatic | Decreased vision | Covered stent | Complete | None | Full recovery | No recurrence | |
| 36/M | ICA C7 | Traumatic | Decreased vision | Covered stent | Complete | None | Full recovery | No recurrence | |
| 28/M | ICA C6 | Traumatic | Epistaxis | Covered stent | Complete | None | Full recovery | No recurrence | |
| 38/M | ICA C4 | Traumatic | Epistaxis | Covered stent | Complete | None | Full recovery | No recurrence | |
| 40/F | ICA C6 | Traumatic | Decreased vision | Covered stent | Incomplete | None | Full recovery | No recurrence | |
| 16/M | ICA C7 | Traumatic | Decreased vision | Covered stent | Complete | None | Full recovery | No recurrence | |
| 22/M | ICA C4 | Traumatic | Epistaxis | Covered stent | Complete | None | Full recovery | No recurrence | |
| 44/M | ICA C4 | Traumatic | Eye blindness/ptosis | Covered stent | Complete | None | Improvement | No recurrence | |
| 51/M | ICA C4 | Traumatic | Epistaxis | Covered stent | Incomplete | None | Unchanged | Incomplete | |
| Zanaty et al. (69) | 55/M | ICA C7 | Iatrogenic | Intraoperative bleeding | Flow diversion | Complete | None | Good | NA |
Pseudoaneurysms of the middle meningeal artery were not included.
ACA, anterior cerebral artery; AChA, anterior choroidal artery; AICA, anterior inferior cerebellar artery; BA, basilar artery; Call. Marg., callosal marginal; CTA, computed tomography angiography; ICA, internal carotid artery; ICH, intracerebral hemorrhage; MCA, middle cerebral artery; MRI, magnetic resonance imaging; NA, not available; nBCA, n-butylcyanoacrylate; OA, ophthalmic artery; PAO, parent artery occlusion; PCA, posterior cerebral artery; PCoA, posterior communicating artery; Pericall., pericallosum artery; SAH, subarachnoid hemorrhage; SDH, subdural hemorrhage.
Microsurgery
Surgical intervention is typically reserved for the lesions of difficult catheterization or failed endovascular therapy, and presence of significant mass effect in ruptured pseudoaneurysms with acute hematoma, usually followed by clot evaculation and/or decompressive craniectomy. Direct surgery to treat pseudoaneurysms of the cavernous and petrous ICA is difficult. In the distal branch of intracranial arteries, such as the pericallosal artery, surgery still should be considered as an irreplaceable option. Surgical options include direct clipping, suturing, wrapping-clipping, ligation of the parent artery, and trapping with or without bypass (Table 1). However, different experts hold a variety of opinions on surgical strategies for pseudoaneurysms. Direct clipping of the aneurysmal neck may not be feasible because of the lack of a true vessel wall that makes clipping threatening and challenging (9, 45, 53). It often results in aneurysm avulsion and intraoperative bleeding due to the high fragility of the pseudowall (22). The orifice or defect can be repaired with direct microsurgical suturing (32, 45, 48). Subsequent wrapping-clipping supports the fragile wall and maintains the connectivity of the parent artery (45). Ligation of the parent artery can result in distal ischemic complications. Moreover, it may not prevent the rupture of pseudoaneurysms due to collateral retrograde flow into the lesion (37). Trapping, in which clips are placed on the parent vessel, both proximal and distal to the aneurysm, is the definitive treatment modality to eliminate the aneurysm. Trapping with or without bypass revascularization depends on the status of collateral supply. A low-flow bypass is often used to treat distal pseudoaneurysms (50), while a high-flow bypass is recommended for ICA pseudoaneurysms (36, 70). Resection of the aneurysm and end-to-end anastomosis is another possible treatment (28).
Endovascular Embolization
With the advances in techniques and materials, endovascular treatment has been an alternative to surgery for the treatment of intracranial pseudoaneurysms. Endovascular procedures include coiling, stent-assisted coiling, occlusion of the parent artery with or without aneurysm, and flow-diversion. The choice of endovascular technique is based on the location of the pseudoaneurysm, vascular anatomy, and clinical status of the patient.
Packing of the pseudoaneurysm with coils is available for those cases with a narrow neck pseudoaneurysm (52, 71). Because of the fragility of the pseudoaneurysm wall, it has the risk of microcatheter or coil perforation during the procedure. The advantage of selective pseudoaneurysm embolization is the preservation of the parent artery (Figure 2). However, pseudoaneurysm recurrence is still a major issue for patients treated with simple coiling (7, 9, 11, 14, 16, 26, 30, 53, 60, 63). Due to coil impaction into the thrombus (14), flow pulsatility may force into the interstices of the coil mass and lead to recurrent bleeding (27).
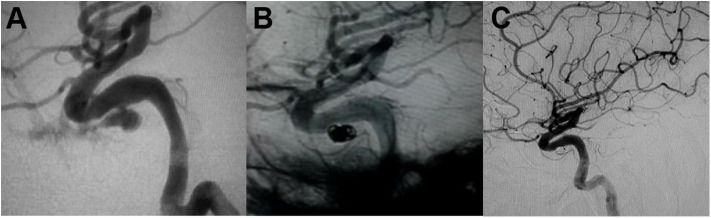
Figure 2.
(A) Angiography demonstrated the intracavernous iatrogenic pseudoaneurysm of the left internal carotid artery (ICA). (B) The pseudoaneurysm was treated by endovascular coiling. (C) Angiogram at 4-month follow-up showed no evidence of aneurysmal filling [adapted from Lin et al. (72)].
Therefore, some studies suggested that occlusion of the parent artery and pseudoaneurysm may be the preferred therapy for distal pseudoaneurysms (12, 73). Because of having good collateral supply or retrograde flow from the distal to the trapped segment, occlusion of the parent artery may be safe in distal ACA (24, 68, 73). Coils (39, 73), or liquid embolization agents including glubran (22), n-butylcyanoacrylate (29, 68), and Onyx (12, 24, 74) can be used in parent artery occlusion. Onyx treatment is especially suitable for pseudoaneurysms with minuscule vessel wall as to avoid coiling. However, occlusion of the parent artery is not recommended for pseudoaneurysms of the ICA. Although having negative balloon occlusion test, 22% of patients develop ischemic complications following parent artery occlusion (Figure 3) (16, 52, 75).
Figure 3.
(A) An axial CT scan showed skull bone fracture and traumatic subarachonoid hemorrhage. (B) Two weeks later, the patient suffered rehemorrhage. A lateral cerebral angiogram of the right ICA demonstrated a large pseudoaneurysm at the C6 segment and a dissection of the C1 segment. Angiogram after balloon occlusion test (C) showed good compensation from the anterior communicating artery (D) and posterior communicating artery (E,F) The pseudoaneurysm and parent artery were trapped with six detachable coils. Postoperative right (G) and left (H) carotid angiograms showed exclusion of the pseudoaneurysm from the circulation [adapted from Lin et al. (72)].
Stent-assisted coiling is a promising treatment option for wide-necked pseudoaneurysms (56–58, 65). It allows for the preservation of the parent artery and avoidance of bypass surgery. However, aneurysm recanalization is not uncommon after single stent-assisted coiling (13, 18, 76). Stent-assisted coil embolization followed by stent-within-a-stent technique has been reported as an effective treatment for pseudoaneurysms (58). Overlapping stents with coils effectively prevent rebleeding and regrowth of the pseudoaneurysm. The overlapping stents may divert the flow away from the pseudoaneurysm, accelerate intraaneurysmal thrombosis, and reconstruct the parent artery by promoting neointima formation along the stent (31, 52, 58, 77).
Another reconstructive endovascular treatment modality is covered stent implantation (2, 59, 78). Endovascular deployment of covered stents can exclude blood flow through the stent as a physical barrier and keep the normal anatomic flow through the parent artery (Figure 4). Compared with the uncovered stents, covered stents decrease the incidence rate of neointimal proliferation and restenosis, at the same time, decrease embolization risk caused by thrombus debris during the process of stent deployment. However, the flexibility of covered stents and the stiffness of the delivery system are the main limitations for its usage in the tortuous ICA, which may result in dissection and vasospasm. Moreover, the occlusion of important perforators by the covered stent may occur when the pseudoaneurysm originates too close to the origin of the ophthalmic artery, the posterior communicating artery, or the anterior choroidal artery.
Figure 4.

(A) Oblique cerebral angiogram showed a pseudoaneurysm in the cavernous segment of righ ICA following endoscopic transsphenoid surgery. (B) A 4*13 mm Willis covered stent was deployed across the pseudoaneurysm. (C) The control angiogram demonstrated complete obliteration of the pseudoaneurysm with preservation of carotid artery patency. (D) A follow-up angiogram showed no recanalization of the aneurysm and patentcy of the parent artery.
Recently, a flow-diverting strategy has been shown to be a promising treatment modality for patients with intracranial pseudoaneurysm. Flow-diverting stent reduces blood flow into the aneurysm, thus promoting thrombosis. It also provides a scaffold for endothelialization and reconstruction of the vessel wall. Previous studies have shown that pseudoaneurysms treated with flow-diverting stents have high complete occlusion rates and low complication rates (6, 7, 18, 19, 26, 30, 53–55, 67, 69, 79). However, the main limitation of flow-diverting stents is delayed aneurysm obliteration due to a lack of immediate thrombosis. It may take weeks for complete aneurysm occlusion, which leaves patients at risk for rebleeding during this time (6, 7, 19, 79). In addition, dural antiplatelet therapy after flow-diverting stent placement may increase the risk of postoperative intracranial hematomas. It should be used judiciously in the setting of ruptured pseudoaneurysms.
Conservative Treatment
Although high mortality rates of pseudoaneurysms were reported, these data are based on a review of the literature and a collection of only case reports. There is no large sample of pseudoaneurysms in single or multiple centers. Therefore, the true natural history of these pseudoaneurysms is unclear. Complete spontaneous resolution of pseudoaneurysms is considered to be an uncommon occurrence. Previous studies have demonstrated existence of spontaneous resolution in peripheral vessels or intracranial vessels, including the middle meningeal artery (80, 81), basilar artery (82–84), posterior cerebral artery (85) and pericallosal artery (39). The mechanism of spontaneous occlusion remains unclear. It may be due to vascular remodeling response to injury as well as spontaneous thrombus formation (84). Those studies suggest that some specific pseudoaneurysms may at least have a potentially benign course with conservative treatment. Observation might be considered in pseudoaneurysms with decreased size and flow in repeated conventional angiography compared with the initial images (84). However, a recent study reported an unusual course of a cerebral pseudoaneurysm (69). The pseudoaneurysm completely disappeared on the second angiogram, but was found to be enlarged on the third angiogram. Therefore, adequate follow-up is mandatory when conservation treatment is considered or even when the lesions have spontaneous obliteration.
An Illustrative Case
We present a case of 45-year-old man harboring an invasive pituitary adenoma, in whom an intracavernous carotid artery tear was caused by aggressive curettage of the left cavernous sinus portion of the lesion. Massive intraoperative bleeding was stopped by surgical packing. Subsequent emergent angiography demonstrated an elliptical shaped pseudoaneurysm located in the intracavernous portion of the left ICA (Figure 2A). The pseudoaneurysm was treated by endovascular coiling (Figure 2B). Complete occlusion of the pseudoaneurysm from the circulation with preservation of the parent artery was achieved with placement of six coils. Angiogram at 4-month follow-up showed no evidence of aneurysmal filling (Figure 2C).
Conclusion
Intracranial pseudoaneurysms are rare pathological entities, representing 1% of all intracranial aneurysms. Rupture of pseudoaneurysm is associated with high rates of morbidity and mortality. Early diagnosis and therapy is critical in those patients with clinical suspicion of a pseudoaneurysm, such as unexplained hemorrhages and epistaxis following a history of head trauma, surgery, or septicemia. Due to spontaneous occlusion of pseudoaneurysms occurred in a few patients, repeated imaging and prompt treatment should be necessary. Endovascular treatments, including coiling with or without stent, covered stent, flow diverting stent, and trapping, should be individualized to aneurysmal location, clinical condition, vascular anatomy, and assessment of the collateral circulation. Microsurgery may be a suitable alternative in cases not amenable to endovascular treatment, yielding favorable outcomes, especially in distal aneurysms or patients with huge hematoma.
Author Contributions
FX and JS: conception and design. YZ and FX drafted the article. ZL: data collection. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by Grant No. (2019D01C093) from the Natural Science Foundation of Xinjiang Province; Grant No. (2018AD32016) from Jiaxing Science and Technology.
References
- 1.Larson PS, Reisner A, Morassutti DJ, Abdulhadi B, Harpring JE. Traumatic intracranial aneurysms. Neurosurg Focus. (2000) 8:e4 10.3171/foc.2000.8.1.1829 [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Li MH, Li YD, Gu BX, Wang J, Zhang PL, et al. Treatment of traumatic internal carotid artery pseudoaneurysms with the willis covered stent: a prospective study. J Trauma. (2011) 70:816–22. 10.1097/TA.0b013e3181f892af [DOI] [PubMed] [Google Scholar]
- 3.Ding H, You C, Yin H. Nontraumatic and noninfectious pseudoaneurysms on the circle of willis: 2 case reports and review of the literature. Surg Neurol. (2008) 69:414–7. 10.1016/j.surneu.2007.02.040 [DOI] [PubMed] [Google Scholar]
- 4.Akamatsu Y, Sugawara T, Mikawa S, Saito A, Ono S, Takayama K, et al. Ruptured pseudoaneurysm following gamma knife surgery for a vestibular schwannoma. J Neurosurg. (2009) 110:543–6. 10.3171/2008.8.JNS08177 [DOI] [PubMed] [Google Scholar]
- 5.Brzozowski K, Frankowska E, Piasecki P, Ziecina P, Zukowski P, Bogusławska-Walecka R. The use of routine imaging data in diagnosis of cerebral pseudoaneurysm prior to angiography. Eur J Radiol. (2011) 80:e401–9. 10.1016/j.ejrad.2010.12.019 [DOI] [PubMed] [Google Scholar]
- 6.Chen SH, McCarthy DJ, Sheinberg D, Hanel R, Sur S, Jabbour P, et al. Pipeline embolization device for the treatment of intracranial pesudoaneurysms. World Neurosurg. (2019) 127:e86–93. 10.1016/j.wneu.2019.02.135 [DOI] [PubMed] [Google Scholar]
- 7.Amenta PS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. Successful treatment of a traumatic carotid pseudoaneurysm with the pipeline stent: case report and review of the literature. Surg Neurol Int. (2012) 3:160. 10.4103/2152-7806.105099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horiuchi T, Nakagawa F, Miyatake M, Iwashita T, Tanaka Y, Hongo K. Traumatic middle cerebral artery aneurysm: case report and review of the literature. Neurosurg Rev. (2007) 30:263–7. 10.1007/s10143-007-0073-9 [DOI] [PubMed] [Google Scholar]
- 9.Altali K, Arruza L, López-Ibor L, Aleo E. Effective coil embolization of intracavernous carotid artery pseudoaneurysm with parental artery preservation following severe head trauma in a pediatric patient. Childs Nerv Syst. (2014) 30:967–70. 10.1007/s00381-013-2312-4 [DOI] [PubMed] [Google Scholar]
- 10.Sakata N, Takebayashi S, Kojima M, Masawa N, Suzuki K, Takatama M. Pathology of a dissecting intracranial aneurysm. Neuropathology. (2000) 20:104–8. 10.1046/j.1440-1789.2000.00275.x [DOI] [PubMed] [Google Scholar]
- 11.Fu M, Patel T, Baehring JM, Bulsara KR. Cavernous carotid pseudoaneurysm following transsphenoidal surgery. J Neuroimaging. (2013) 23:319–25. 10.1111/j.1552-6569.2011.00677.x [DOI] [PubMed] [Google Scholar]
- 12.Jadhav AP, Pryor JC, Nogueira RG. Onyx embolization for the endovascular treatment of infectious and traumatic aneurysms involving the cranial and cerebral vasculature. J Neurointerv Surg. (2012) 5:562–5. 10.1136/neurintsurg-2012-010460 [DOI] [PubMed] [Google Scholar]
- 13.OuYang M, Huang X, Wang Y. Endovascular treatment of infectious pseudoaneurysm of internal carotid artery. World Neurosurg. (2019) 125:42–3. 10.1016/j.wneu.2019.01.147 [DOI] [PubMed] [Google Scholar]
- 14.Kadyrov NA, Friedman JA, Nichols DA, Cohen-Gadol AA, Link MJ, Piepgras DG. Endovascular treatment of an internal carotid artery pseudoaneurysm following transsphenoidal surgery. Case report J Neurosurg. (2002) 96:624–7. 10.3171/jns.2002.96.3.0624 [DOI] [PubMed] [Google Scholar]
- 15.Ciceri EF, Regna-Gladin C, Erbetta A, Chiapparini L, Nappini S, Savoiardo M, et al. Iatrogenic intracranial pseudoaneurysms: neuroradiological and therapeutical considerations, including endovascular options. Neurol Sci. (2006) 27:317–22. 10.1007/s10072-006-0703-y [DOI] [PubMed] [Google Scholar]
- 16.Hjortoe S, Wagner A, Cortsen M. Endovascular embolization of intracranial iatrogenic pseudoaneurysms. A report of two cases and review of the literature. Neuradiol J. (2010) 23:479–83. 10.1177/197140091002300420 [DOI] [PubMed] [Google Scholar]
- 17.Sylvester PT, Moran CJ, Derdeyn CP, Cross DT, Dacey RG, Zipfel GJ, et al. Endovascular management of internal carotid artery injuries secondary to endonasal surgery: case series and review of the literature. J Neurosurg. (2016) 125:1256–76. 10.3171/2015.6.JNS142483 [DOI] [PubMed] [Google Scholar]
- 18.Griauzde J, Ravindra VM, Chaudhary N, Gemmete JJ, Mazur MD, Roark CD, et al. Use of the pipeline embolization device in the treatment of iatrogenic intracranial vascular injuries: a bi-institutional experience. Neurosurg Focus. (2017) 42:E9. 10.3171/2017.3.FOCUS1735 [DOI] [PubMed] [Google Scholar]
- 19.Nariai Y, Kawamura Y, Takigawa T, Hyodo A, Suzuki K. Pipeline embolization for an iatrogenic intracranial internal carotid artery pseudoaneurysm after transspehenoidal pituitary tumor surgery: case report and review of the literature. Interv Neuroradiol. (2020) 26:74–82. 10.1177/1591019919874943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le H, Munshi I, Macdonald RL, Wollmann R, Frank J. Traumatic aneurysm resulting from insertion of an intracranial pressure monitor. Case illustration. J Neurosurg. (2001) 95:720. 10.3171/jns.2001.95.4.0720 [DOI] [PubMed] [Google Scholar]
- 21.Horowitz M, Sharts M, Levy E, Albright AL, Pollack I. Endovascular management of ventricular catheter-induced anterior cerebral artery false aneurysm: technical case report. Neurosurgery. (2005) 57:E374. 10.1227/01.NEU.0000168016.02106.0C [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Zhang J, Miao H, Niu Y, Feng H, Zhu G. Delayed rupture of iatrogenic cerebral pseudoaneurysms after neurosurgical procedures: report of two cases. Clin Neurol Neurosurg. (2013) 115:1552–4. 10.1016/j.clineuro.2012.12.024 [DOI] [PubMed] [Google Scholar]
- 23.Ravina K, Strickland BA, Rennert RC, Chien M, Mack WJ, Amar AP, et al. A3-A3 anastomosis in the management of complex anterior cerebral artery aneurysms: experience with in situ bypass and lessons learned from pseudoaneurysm cases. Oper Neurosurg. (2019) 17:247–60. 10.1093/ons/opy334 [DOI] [PubMed] [Google Scholar]
- 24.Munich SA, Cress MC, Rangel-Castilla L, Krishna C, Siddiqui AH, Snyder KV. Importance of repeat angiography in the diagnosis of iatrogenic anterior cerebral artery territory pseudoaneurysm following endoscopic sinus surgery. J Neurointerv Surg. (2016) 8:e20. 10.1136/neurintsurg-2015-011693.rep [DOI] [PubMed] [Google Scholar]
- 25.Horowitz M, Albright AL, Jungreis C, Levy EI, Stevenson K. Endovascular management of a basilar artery false aneurysm secondary to endoscopic third ventriculostomy: case report. Neurosurgery. (2001) 49:1461–4. 10.1097/00006123-200112000-00031 [DOI] [PubMed] [Google Scholar]
- 26.Sastry RA, Koch MJ, Grannan BL, Stapleton CJ, Butler WE, Patel AB. Flow diversion of a recurrent, iatrogenic basilar tip aneurysm in a pediatric patient: case report. J Neurosurg Pediatr. (2018) 21:90–3. 10.3171/2017.7.PEDS17235 [DOI] [PubMed] [Google Scholar]
- 27.Lee CH, Luo CB. Pseudoaneurysm of the basilar artery presenting with epistaxis. Br J Neurosurg. (2018) 27:1–2. 10.1080/02688697.2018.1445197 [DOI] [PubMed] [Google Scholar]
- 28.Rayes M, Bahgat DA, Kupsky WJ, Mittal S. Middle cerebral artery pseudoaneurysm formation following stereotactic biopsy. Can J Neurol Sci. (2008) 35:664–8. 10.1017/S0317167100009513 [DOI] [PubMed] [Google Scholar]
- 29.Shah KJ, Jones AM, Arnold PM, Ebersole K. Intracranial pseudoaneurysm after intracranial pressure monitor placement. J Neurointerv Surg. (2016) 8:e3. 10.1136/neurintsurg-2014-011410.rep [DOI] [PubMed] [Google Scholar]
- 30.Colby GP, Jiang B, Bender MT, Beaty NB, Westbroek EM, Xu R, et al. Pipeline-assisted coil embolization of a large middle cerebral artery pseudoaneurysm in a 9-month-old infant: experience from the youngest flow diversion case. J Neurosurg Pediatr. (2018) 22:532–40. 10.3171/2018.6.PEDS18165 [DOI] [PubMed] [Google Scholar]
- 31.Lim J, Suh SH, Lee KY, Hong CK, Park SW. Endovascular treatment of iatrogenic intracranial pseudoaneurysm following stent angioplasty. J Neuroimaging. (2012) 22:194–6. 10.1111/j.1552-6569.2011.00591.x [DOI] [PubMed] [Google Scholar]
- 32.Imahori T, Okamura Y, Sakata J, Shose H, Yamanishi S, Kohmura E. Delayed rebleeding from pseudoaneurysm after mechanical thrombectomy using a stent retriever due to small artery avulsion confirmed by open surgery: a case report. World Neurosurg. (2020) 133:150–4. 10.1016/j.wneu.2019.09.141 [DOI] [PubMed] [Google Scholar]
- 33.Nubourgh Y, Bruninx G, Declour C, Vanderkelen B. Unusual occurrence of a pseudo-aneurysm of the middle cerebral artery in a patient with fibromuscular dysplasia. Acta Neurochir. (2000) 142:1311–4. 10.1007/s007010070031 [DOI] [PubMed] [Google Scholar]
- 34.Nomura M, Mori K, Tamase A, Kamide T, Seki S, Iida Y, et al. Pseudoaneurysm formation due to rupture of intracranial aneurysms: case series and literature review. Neuroradiol J. (2017) 30:129–37. 10.1177/1971400916684667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gugliemi G. The interventional neuroradiological treatment of intracranial aneurysms. Adv Tech Stand Neurosurg. (1998) 24:215–60. 10.1007/978-3-7091-6504-1_5 [DOI] [PubMed] [Google Scholar]
- 36.Cikla U, Li Y, Hernandez-Duran S, Kozan A, Baskaya MK. Treatment of supraclinoid internal carotid artery iatrogenic pseudoaneurysm with extracranial-to- intracranial bypass and trapping: demonstration of technique with video presentation. Turk Neurosurg. (2015) 25:305–9. 10.5137/1019-5149.JTN.13039-14.1 [DOI] [PubMed] [Google Scholar]
- 37.Garg K, Gurjar HK, Singh PK, Singh M, Chandra PS, Sharma BS. Internal carotid artery aneurysms presenting with epistaxis-our experience and review of literature. Turk Neurosurg. (2016) 26:357–63. 10.5137/1019-5149.JTN.12598-14.1 [DOI] [PubMed] [Google Scholar]
- 38.D'Urso P, Loumiotis I, Milligan BD, Cloft H, Lanzino G. “Real time” angiographic evidence of “pseudoaneurysm” formation after aneurysm rebleeding. Neurocrit Care. (2011) 14:459–62. 10.1007/s12028-011-9522-y [DOI] [PubMed] [Google Scholar]
- 39.Kumar A, Jakubovic R, Yang V, Dacosta L. Traumatic anterior cerebral artery aneurysms and management options in the endovascular era. J Clin Neurosci. (2016) 25:90–5. 10.1016/j.jocn.2015.05.063 [DOI] [PubMed] [Google Scholar]
- 40.Gerosa A, Fanti A, Del Sette B, Bianco A, Cossandi C, Crobeddu E, et al. Posttraumatic middle meningeal artery pseudoaneurysm: case report and review of the literature. World Neurosurg. (2019) 128:225–9. 10.1016/j.wneu.2019.05.030 [DOI] [PubMed] [Google Scholar]
- 41.Umana GE, Cristaudo C, Scalia G, Passanisi M, Corsale G, Tomarchio L, et al. Chronic epdidural hematoma caused by traumatic intracranial pseudoaneurysm of the middle meningeal artery: review of the literature with a focus on this unique entity. World Neurosurg. (2020) 136:198–204. 10.1016/j.wneu.2019.12.179 [DOI] [PubMed] [Google Scholar]
- 42.Maldonado-Naranjo A, Kshettry VR, Toth G, Bain M. Non-traumatic superior hypophyseal aneurysm with associated pseudoaneurysm presenting with massive epistaxis. Clin Neurol Neurosurg. (2013) 115:2251–3. 10.1016/j.clineuro.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 43.Moon HT, Kim SH, Lee JW, Huh SK. Clinical analysis of traumatic cerebral pseudoaneurysms. Korean J Neurotrauma. (2015) 11:124–30. 10.13004/kjnt.2015.11.2.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wewel J, Mangubat EZ, Muñoz L. Iatrogenic traumatic intracranial aneurysm after endoscopic sinus surgery. J Clin Neurosci. (2014) 21:2072–6. 10.1016/j.jocn.2014.05.017 [DOI] [PubMed] [Google Scholar]
- 45.Binning MJ, Eskandari R, Couldwell WT. Direct surgical repair of carotid pseudoaneurysm in an infant. Childs Nerv Syst. (2010) 26:1151–3. 10.1007/s00381-010-1225-8 [DOI] [PubMed] [Google Scholar]
- 46.Raper DMS, Rutledge WC, Winkler E, Abla AA. Spontaneous perforation of anterior choroidal artery with resultant pseudoaneurysm formation: unusual cause of subarachnoid hemorrhage. World Neurosurg. (2020) 134:141–4. 10.1016/j.wneu.2019.10.174 [DOI] [PubMed] [Google Scholar]
- 47.Sato Y, Ando K, Kawaguchi M, Kakinuma K. Successful resection of a growing distal medial lenticulostriate artery pseudoaneurysm presenting with isolated intraventricular hemorrhage. J Stroke Cerebrovasc Dis. (2017) 26:e206–e209. 10.1016/j.jstrokecerebrovasdis.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 48.Shirane R, Kondo T, Yoshida YK, Furuta S, Yoshimoto T. Ruptured cerebral pseudoaneurysm caused by the removal of a ventricular catheter. J Neurosurg. (1999) 91:1031–3. 10.3171/jns.1999.91.6.1031 [DOI] [PubMed] [Google Scholar]
- 49.Sujijantarat N, Pierson MJ, Kemp J, Coppens JR. Staged trapping of traumatic basilar trunk pseudoaneurysm: a case report and review of literature. World Neurosurg. (2017) 108:e7–12. 10.1016/j.wneu.2017.08.144 [DOI] [PubMed] [Google Scholar]
- 50.Umekawa M, Hasegawa H, Shin M, Kawashima M, Nomura S, Nakatomi H, et al. Radiosurgery-induced anterior inferior cerebellar artery pseudoaneurysm treated with trapping and bypass. World Neurosurg. (2018) 116:209–13. 10.1016/j.wneu.2018.04.161 [DOI] [PubMed] [Google Scholar]
- 51.Walcott BP, Nahed BV, Kahle KT, Sekhar LN, Ferreira MJ. Cerebrovascular bypass and aneurysm trapping for the treatment of an A2-segment anterior cerebral artery pseudoaneurysm and herniation through a skull base defect following trauma. J Clin Neurosci. (2012) 19:149–51. 10.1016/j.jocn.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 52.Al-Jehani HM, Alwadaani HA, Almolani FM. Traumatic intracranial internal carotid artery pseudoaneurysm presenting as epistaxis treated by endovascular coiling. Neurosciences. (2016) 21:60–3. 10.17712/nsj.2016.1.20150514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aljuboori Z, Meyer K, Ding D, James R. Endovascular treatment of a traumatic middle cerebral artery pseudoaneurysm with the pipeline flex embolization device. World Neurosurg. (2020) 133:201–4. 10.1016/j.wneu.2019.10.008 [DOI] [PubMed] [Google Scholar]
- 54.Giorgianni A, Pellegrino C, Minotto R, Mercuri A, Frattini L, Baruzzi F, et al. Flow-diverter stenting of post-traumatic bilateral anterior cerebral artery pseudoaneurysm: a case report. Interv Neuroradiol. (2015) 21:23–8. 10.1177/1591019915575441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giorgianni A, Pozzi F, Pellegrino C, Padovan S, Karligkiotis A, Castelnuovo P, et al. An emergency placement of a flow diverter stent for an iatrogenic internal carotid artery injury during endoscopic pituitary surgery. World Neurosurg. (2019) 122:376–9. 10.1016/j.wneu.2018.10.234 [DOI] [PubMed] [Google Scholar]
- 56.Griauzde J, Gemmete JJ, Chaudhary N, Pandey AS, Garton HJ. Basilar artery pseudoaneurysm presenting at 5-month follow-up after traumatic atlanto-occipital dislocation in a 7-year-old girl treated with intracranial stent placement and coiling. J Neurointerv Surg. (2014) 6:e8 10.1136/neurintsurg-2012-010573.rep [DOI] [PubMed] [Google Scholar]
- 57.Kim SS, Kang DH, Park H, Lee CH, Hwang SH, Jung JM, et al. Short-term clinical and angiographic outcome in child with traumatic pseudoaneurysm in A2 segment of anterior cerebral artery after endovascular treatment: case report. Korean J Neurotrauma. (2014) 10:130–3. 10.13004/kjnt.2014.10.2.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim YC, Kang JK, Chung J. Reconstructive stent-buttressed coil embolization of a traumatic pseudoaneurysm of the supraclinoid internal carotid artery. Acta Neurochir. (2012) 154:477–80. 10.1007/s00701-011-1251-7 [DOI] [PubMed] [Google Scholar]
- 59.Liu P, Yang P, Cai M, Qin J, Pan L. Treatment of pediatric traumatic intracranial pseudoaneurysm using endovascular covered stent: three case reports. World Neurosurg. (2016) 88:693.e1–6. 10.1016/j.wneu.2015.12.037 [DOI] [PubMed] [Google Scholar]
- 60.Liu QL, Xue H, Qi CJ, Zhao P, Wang DH, Li G. Traumatic anterior cerebral artery pseudoaneurysm epistaxis. World Neurosurg. (2017) 100:e9–16. 10.1016/j.wneu.2016.11.138 [DOI] [PubMed] [Google Scholar]
- 61.Mascitelli JR, McNeill IT, Mocco J, Berenstein A, DeMattia J, Fifi JT. Ruptured distal AICA pseudoaneurysm presenting years after vestibular schwannoma resection and radiation. J Neurointerv Surg. (2016) 8:e19. 10.1136/neurintsurg-2015-011736.rep [DOI] [PubMed] [Google Scholar]
- 62.Matsumura H, Kato N, Hosoo H, Fujiwara Y. Subarachonid hemorrhage due to anterior inferior cerebellar artery aneurysms associated with gamma knife surgery for vestibular schwannomas. Acta Neurochir. (2015) 157:1765–7. 10.1007/s00701-015-2552-z [DOI] [PubMed] [Google Scholar]
- 63.Morinaga Y, Nii K, Sakamoto K, Inoue R, Mitsutake T, Hanada H. Stent-assisted coil embolization for a ruptured posterior communicating artery pseudoaneurysm after endoscopic transsphenoidal surgery for pituitary adenoma. World Neurosurg. (2019) 123:301–5. 10.1016/j.wneu.2018.12.047 [DOI] [PubMed] [Google Scholar]
- 64.Murakami M, Kawarabuki K, Inoue Y, Ohta T. Ruptured pseudoaneurysm after gamma knife surgery for vestibular schwannoma. Neurol Med Chir. (2016) 56:38–42. 10.2176/nmc.cr.2015-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogilvy CS, Tawk RG, Mokin M, Yang X, Levy EI, Hopkins LN, et al. Stent-assisted coiling treatment of pediatric traumatic pseudoaneurysm resulting from tumor surgery. Pediatr Neurosurg. (2011) 47:442–8. 10.1159/000339353 [DOI] [PubMed] [Google Scholar]
- 66.Patel AS, Horton TG, Kalapos P, Cockroft KM. Onyx-HD 500 embolization of a traumatic internal carotid artery pseudoaneurysm after transsphenoidal surgery. J Neuroimaging. (2015) 25:656–9. 10.1111/jon.12221 [DOI] [PubMed] [Google Scholar]
- 67.Sami MT, Gattozzi DA, Soliman HM, Reeves AR, Moran CJ, Camarata PJ, et al. Use of PipelineTM embolization device for the treatment of traumatic intracranial pseudoaneurysms: case series and review of cases from literature. Clin Neurol Neurosurg. (2018) 169:154–60. 10.1016/j.clineuro.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 68.Van Rooij WJ, Van Rooij SB. Endovascular treatment of traumatic pericallosal artery aneurysms. A case report. Interv Neuroradiol. (2013) 19:56–9. 10.1177/159101991301900108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zanaty M, Chalouhi N, Jabbour P, Starke RM, Hasan D. The unusual angiographic course of intracranial pseudoaneurysms. Asian J Neurosurg. (2015) 10:327–30. 10.4103/1793-5482.162721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rangel-Castilla L, McDougall CG, Spetzler RF, Nakaji P. Urgent cerebral revascularization bypass surgery for iatrogenic skull base ICA injury. Neurosurgery. (2014) 4:640–8. 10.1227/NEU.0000000000000529 [DOI] [PubMed] [Google Scholar]
- 71.Cappabianca P, Briganti F, Cavallo LM, de Divitiis E. Pseudoaneurysm of the intracavernous carotid artery following endoscopic endonasal transsphenoidal surgery, treated by endovascular approach. Acta Neurochir. (2001) 143:95–6. 10.1007/s007010170144 [DOI] [PubMed] [Google Scholar]
- 72.Lin T, Chen D, Li J, Liao Y, Xu F, Leng B, et al. Diagnosis and treatment differences between iatrogenic and traumatic intracranial pseudoaneurysms. Chin J Neurosurg. (2018) 34:993–8. [Google Scholar]
- 73.Sim SY, Shin YS, Yoon SH. Endovascular internal trapping of traumatic pericallosal pseudoaneurysm with hydrogel-coated self-expandable coil in a child: a case report. Surg Neurol. (2008) 69:418–22. 10.1016/j.surneu.2007.02.042 [DOI] [PubMed] [Google Scholar]
- 74.Medel R, Crowley RW, Hamilton DK, Dumont AS. Endovascular obliteration of an intracranial pseudoaneurysm: the utility of Onyx. J Neurosurg Pediatr. (2009) 4:445–8. 10.3171/2009.6.PEDS09104 [DOI] [PubMed] [Google Scholar]
- 75.Elias AE, Chaudhary N, Pandery AS, Gemmete JJ. Intracranial endovascular balloon test occlusion: indications, methods, and predictive value. Neuroimaging Clin N Am. (2013) 23:695–702. 10.1016/j.nic.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 76.Cohen JE, Gomori JM, Segal R, Spivak A, Margolin E, Sviri G, et al. Results of endovascular treatment of traumatic intracranial aneurysms. Neurosurgery. (2008) 63:476–85. 10.1227/01.NEU.0000324995.57376.79 [DOI] [PubMed] [Google Scholar]
- 77.Ruiz-Juretschke F, Castro E, Mateo Sierra O, Iza B, Manuel Garbizu J, Fortea F, et al. Massive epistaxis resulting from an intracavernous internal carotid artery traumatic pseudoaneurysm: complete resolution with overlapping uncovered stents. Acta Neurochir. (2009) 151:1681–4. 10.1007/s00701-009-0294-5 [DOI] [PubMed] [Google Scholar]
- 78.Saatci I, Cekirge HS, Ozturk MH, Arat A, Ergungor F, Sekerci Z, et al. Treatment of internal carotid artery aneurysms with a covered stent: experience in 24 patients with mid-term follow-up results. Am J Neuroradiol. (2004) 25:1742–9. [PMC free article] [PubMed] [Google Scholar]
- 79.Nerva JD, Morton RP, Levitt MR, Osbun JW, Ferreira MJ, Ghodke BV, et al. Pipeline embolization device as primary treatment for blister aneurysms and iatrogenic pseudoaneurysms of the internal carotid artery. J Neurointerv Surg. (2015) 7:210–6. 10.1136/neurintsurg-2013-011047 [DOI] [PubMed] [Google Scholar]
- 80.Shah Q, Friedman J, Mamourian A. Spontaneous resolution of traumatic pseudoaneurysm of the middle meningeal artery. Am J Neuroradiol. (2005) 26:2530–2. [PMC free article] [PubMed] [Google Scholar]
- 81.Srinivasan A, Lesiuk H, Goyal M. Spontaneous resolution of posttraumatic middle meningeal artery pseudoaneurysm. Am J Neuroradiol. (2006) 27:882–3. [PMC free article] [PubMed] [Google Scholar]
- 82.Adrien C, Pierre-Henri L, Pieere T, Catherine C, Aapolline K, Klau M, et al. Spontaneous resolution of perforator aneurysms of the posterior circulation. J Neurosurg. (2014) 121:1107–11. 10.3171/2014.7.JNS132411 [DOI] [PubMed] [Google Scholar]
- 83.Loevner LA, Ting TY, Hurst RW, Goldberg HI, Schut L. Spontaneous thrombosis of a basilar artery traumatic aneurysm in a child. Am J Neuroradiol. (1998) 19:386–8. [PMC free article] [PubMed] [Google Scholar]
- 84.Turan N, Butler S, Larson TC, 3rd, Marson A. Nontraumatic, posterior circulation pseudoaneurysm of the basilar artery summit with complete spontaneous resolution: case report and literature review. Surg Neurol Int. (2017) 8:50. 10.4103/sni.sni_452_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee CH, Chen SM, Lui TN. Posterior cerebral artery pseudoaneurysm, a rare complication of pituitary tumor transsphenoidal surgery: case report and literature review. World Neurosurg. (2015) 84:e1–3. 10.1016/j.wneu.2015.04.043 [DOI] [PubMed] [Google Scholar]