Abstract
Aim
We aim to evaluate the variables affecting the frequency of adaptive radiotherapy (ART) in vulvar cancer.
Background
ART may be needed throughout a definitive RT course for vulvar carcinoma due to changes in patient’s anatomy and tumor response.
Materials and methods
Charts of patients charts who had been treated with definitive concurrent chemo-radiotherapy for vulvar carcinoma, between January 2015 and December 2019 were inquired. Radiation therapy was delivered using intensity modulated radiotherapy (IMRT) with daily image-guided radiotherapy (IGRT). ART was defined as re-simulation and re-planning based on deformation in the irradiated volume by more than 1 cm. Univariate analysis was conducted to study the impact of patient’s demographics as well as tumor characteristics on the frequency of ART.
Results
22 patients were eligible for analysis. Median age at diagnosis was 55 years (range 43–82). Radiotherapy dose was 60−66 Gy over 30–35 fractions (fx). Median primary tumor volume was 30cc (9–140). Median Body Mass Index (BMI) was 32 (range 21–40). Thirteen out of 22 patients (59%) required ART, with median timing at 25 fx (19–31). On univariate analysis, larger primary tumor volume (> = 30cc) was associated significantly with increased frequency of ART (p value = 0.0005). There was no significant impact of ART on the frequency with respect to patient’s age, BMI, tumor stage, grade and location.
Conclusion
Changes in radiation target volume are common among vulvar carcinoma patients who are treated with definitive radiotherapy, especially large primary tumors. This review highlights the importance of ART for patients with vulvar carcinoma treated with definitive radiotherapy.
Keywords: Adaptive, Radiotherapy, Vulva, Factors
1. Background
Vulvar carcinoma is a rare disease, ranking as the 4th gynecological malignancy as per GLOBOCAN 2018.1 However, a recent report has shown an increasing trend in vulvar carcinoma incidence in women <50 years possibly due to change in sexual behavior.2
Radiation therapy is considered one of the main modalities in the management of vulvar carcinoma. It is delivered with or without chemotherapy as adjuvant treatment for resectable tumors, or concurrently with chemotherapy as definitive treatment for unresectable disease or medically inoperable patients.3 Due to its rarity, literature lacks randomized evidence for the management of inoperable primary disease.4 However, phase II studies have shown good local control with complete clinical response reaching up to 66.8%.5,6
Computerized tomography (CT) simulation is required for radiation therapy planning prior to starting treatment. However; re-simulation and re-planning due to changes in patient’s anatomy and tumor response might be needed throughout the treatment course. This has been defined as adaptive radiation therapy (ART) as first quoted by yan et al., indicating individualized re-planning to accommodate anatomy change and tumor response by radiation therapy.7
As per Thornqvist et al., ART has been categorized into 3 main approaches: 1) on- or offline re-planning, 2) online plan selection and 3) offline MLC/field adjustment.8
2. Aim
Although several reports have addressed the use of ART for pelvic and gynecological malignancies in radiation therapy, to the best of our knowledge, this is the first report in the literature evaluating the frequency of ART in vulvar carcinoma.
3. Methods
After obtaining the Institutional Review Board (IRB) approval (IRB No. 19 KHCC 89), a retrospective chart review was performed for patients who had received radiation therapy for vulvar carcinoma as definitive treatment at King Hussein Cancer Center (KHCC). Pathological review for patients operated outside our center was undertaken. After obtaining history and physical examination, staging via pelvic magnetic resonance imaging (MRI) and CT scan for the chest and abdomen was performed. A positron emission tomography (PET) scan was not routinely requested for this group of patients, unless borderline regional and distant in which metastasis is suspected. Cases then were discussed at the gynecological multidisciplinary clinic (MDC) for a management plan. Locally advanced vulvar carcinoma patients with unresectable primary disease or those unfit for surgery were referred for definitive chemoradiation. Patients who had unresectable primary disease and enlarged inguinal lymph nodes underwent bilateral inguinal lymph node dissection followed by definitive chemoradiation. Sentinel lymph node biopsy was not routinely performed.
3.1. Simulation
CT scan simulation was conducted using Philips Brilliance Big Bore CT (85-cm bore). Patients were simulated in a frog leg position using Orfit knee rest and grip ring at the ankle level, 3 mm axial cuts were obtained and sent to the treatment planning system.
3.2. Contouring and planning
Radiation therapy planning was performed via Pinnacle3 16.2 (Philips Medical System) using Intensity modulated Radiation Therapy (IMRT) or Volumetric Modulated Arc Therapy (VMAT) technique. Primary gross target volume (GTV-P) was defined as tumor seen in the clinical examination, CT simulation scan and fused MR and PET scans (if performed). Primary clinical target volume (CTV-P) included the whole vulva area for phase I and GTV with a 1–1.5 cm margin for the subsequent phases. Nodal gross target volume included suspicious lymph nodes on scans if nodal dissection was not performed. Nodal clinical target volume included GTV-N with a 1 cm margin. Elective nodal clinical target volume (CTV- E) included the pelvic vessels with a 1 cm margin and inguinal vessels with 1.5 cm margin. All CTVs were manually modified with respect to anatomical adjacent structures. Planned target volume (PTV) was taken as 1 cm expansion in all directions from CTV (with avoidance of 5 mm skin strip for non-involved skin).
Radiation therapy was delivered in sequential phases. In phase I the vulva, pelvic lymph nodes and inguinal lymph nodes were included, unless the patient had undergone bilateral lymph node dissection with no evidence of microscopic involvement on microscopic examination where phase I radiation volume was confined to the vulva, while phase II involved further boost to the involved lymph nodes and primary tumor. The dose range for the initial phase was 45–50 Gray (Gy) over 25 fractions (fx) followed by a boost delivered to the primary and involved lymph nodes up to 60−66 Gy using 1.8−2 Gy per fx.
3.3. Radiation therapy
Based on KHCC’s policy, all radiation therapy plans were reviewed during the departmental peer review meeting.9 After plan approval, physics quality assurance was run prior to treatment initiation. Afterwards, patient proceeded with the radiation treatment as planned using the daily cone beam CT (CBCT) to ensure optimal plan delivery. These are checked via radiotherapy technician on daily basis who reported to the radiation oncologist on any marked positioning shift or anatomical change. Otherwise, all CBCT were checked by the radiation oncologist on weekly basis throughout the weekly chart round. Any shift at radiating region (beam or arc entrance) by more than 1 cm (PTV margin) would require re-simulation and re-planning using offline ART strategy. New plans were completed within the same day to avoid treatment interruption.10,11 Fig. 1 illustrates the CBCT during treatment showing a significant difference in the patient outline due to tumor response, requiring re-simulation to optimize the radiation therapy plan.
Fig. 1.
Matching CT simulation scan (in purple) to the daily CBCT (in green) in axial, sagittal and coronal views, with significant difference at fraction 25 (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Chemotherapy was given as a radiosensitizer during the radiation therapy using weekly Cisplatin IV 70 mg/m2.5
3.4. Statistics
Patients’ characteristics and disease information were presented as counts and percentages. Comparisons between patients who required ART and those who did not, according to demographics and disease information, were held using Fisher’s exact test. Patient’s age at diagnoses, body mass index (BMI), tumor stage, location, volume and grade were correlated with the frequency of ART using univariate analysis.
A significance criterion of p ≤ 0.05 was used in the analysis. All analysis was performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
4. Results
Between Jan 2015 and Dec 2019, 22 patients treated with definitive radiation therapy to primary vulvar cancer were eligible for analysis. Patient and tumor characteristics are shown in Table 1. Histopathology was Squamous Cell Carcinoma (SCC) for all patients, tumor grading included: grade 1 for 1 patient (5%), grade 2 for 18 patients (82%) and grade 3 for 3 patients (13%). The median patient’s age was 57 years (range 43–82). The mean tumor volume at presentation was 30cc range (9–140), the body mass index (BMI) mean was 32 range (21–40). Ten (45%) patients were simulated and treated in the straight leg position, and 12 (55%) in the frog leg position. Sixteen (73%) patients had centrally located tumor (clitoral and periurethral), while 6 (27%) had lateral origin (labia minora and labia majora).
Table 1.
Patient demographics and tumor characteristics.
| Number (%) | |
|---|---|
| Age | |
| 40−49 | 6 (27%) |
| 50−59 | 8 (36%) |
| 60−69 | 4 (18%) |
| 70−79 | 3 (14%) |
| 80−89 | 1 (5%) |
| Site | |
| Labia Minora | 5 (23%) |
| Labia Majora | 1 (5%) |
| Clitoral | 8 (36%) |
| Periurethral | 8 (36%) |
| Stage | |
| II | 4 (18%) |
| IIIA | 4 (18%) |
| IIIB | 9 (41%) |
| IIIC | 2 (9%) |
| IVA | 3 (14%) |
| Body mass index | |
| Normal | 4 (18%) |
| Overweight | 7 (32%) |
| Obese | 11 (50%) |
| Radiation technique | |
| IMRT | 4 (18%) |
| VMAT | 18 (82%) |
| Tumor grade | |
| I | 1 (5%) |
| II | 18 (82%) |
| III | 3 (13%) |
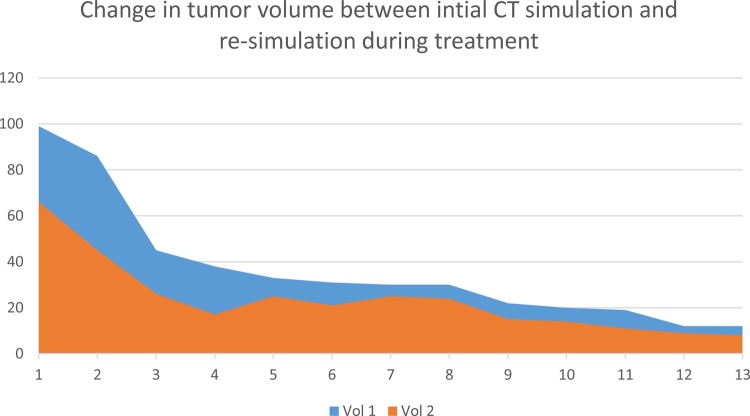
Thirteen out of 22 patients (59%) in the definitive treatment required ART, the average time for ART was 25 days (range; 19–31). The change in tumor volume mean was 12 cc (range 3−41cc) between the initial simulation CT and re-simulation CT are shown in Fig. 2 for the 13 patients who underwent ART, indicating a higher difference for larger tumors.
Fig. 2.
Change in tumor volume in cc (on the left) between initial CT simulation and re-simulation during treatment.
Acute radiation toxicity was seen during radiation therapy course and reported based on Radiation Therapy Oncology Group (RTOG) criteria12; 2 patients had grade 1 RTOG skin toxicity (10%), 17 patients had grade 2 RTOG skin toxicity (77%), while 3 patients had grade 3 RTOG skin toxicity (13%). No acute gastrointestinal toxicity was noted, while 4 patients had grade 2 genitourinary RTOG toxicity. Although most of acute toxicity was managed with symptomatic treatment and local creams; radiation toxicity resulted in treatment hold in 2 patients for 3 days. With regard to late skin toxicity, 1 (5%) patient developed lymphangitis, and no patients had vulvar necrosis or nonhealing ulceration.
Tumor response after completion of treatment was assessed by a clinical exam and pelvic MRI. Out of 22 patients; 15 (68%) had complete response while 7 had partial response (32).
The comparison of the use of ART between patients in correlation with patient demographics and tumor characteristics are shown in Table 2. Patient’s age at diagnoses, prior lymph node dissection, higher BMI, higher tumor grade, peripheral location, and advanced T-stage were not significantly associated with a higher frequency for ART, while large tumor volume (more than 30cc) was correlated with higher frequency for ART (23.1% vs. 76.9%; p-value 0.0005).
Table 2.
Variables affecting the use of ART.
| Re-simulation |
|||||
|---|---|---|---|---|---|
| Value | Total | No | Yes | P-value | |
| Location | Clitoral/Periurethral | 16(72.7%) | 7(77.8%) | 9(69.2%) | 0.999 |
| Labia Minora/Majora | 6(27.3%) | 2(22.2%) | 4(30.8%) | ||
| T stage | TIb | 6(27.3%) | 3(33.3%) | 3(23.1%) | 0.658 |
| TII | 14(63.6%) | 6(66.7%) | 8(61.5%) | ||
| TIII | 2(91%) | 2(15.4%) | |||
| Lymph node dissection | No | 13(59.1%) | 4(44.4%) | 9(69.2%) | 0.384 |
| Yes | 9(40.9%) | 5(55.6%) | 4(30.8%) | ||
| BMI category | Normal | 4(18.2%) | 2(22.2%) | 2(15.4%) | 0.271 |
| Obese | 11(50.0%) | 6(66.7%) | 5(38.5%) | ||
| Overweight | 7(31.8%) | 1(11.1%) | 6(46.2%) | ||
| Grade | 1 | 1(4.5%) | 1(7.7%) | 0.999 | |
| 2 | 18(81.8) | 8(88.9%) | 10(76.9%) | ||
| 3 | 3(13.6%) | 1(11.1%) | 2(15.4%) | ||
| Age | < = 55.5 | 11(50.0%) | 4(44.4%) | 7(53.8%) | 1.000 |
| >55.5 | 11(50.0%) | 5(55.6%) | 6(46.2%) | ||
| Tumor volume | < = 30 cc | 12(54.5%) | 9(100%) | 3(23.1%) | 0.0005 |
| >30 cc | 10(45.5%) | 10(76.9%) | |||
5. Discussion
This report discusses the factors affecting the use of ART in treating primary vulvar carcinoma. In this cohort larger tumor volume at the time of treatment was a significant predictor for undergoing re-simulation and re-planning through the course of definitive chemo-radiation. ART, thus, does not only ensure tumor coverage but also spares the normal tissues, which might also result in a less acute and less chronic side effects.13
The target definition in the primary vulvar disease can be guided by discrimination of primary tumor and nearby soft tissue densities, but may still be challenging at re-simulation scans due to the edema caused by the radiation therapy. This will raise the question of sparing the soft tissue previously involved by the tumor from the full prescribed radiation dose. That is why, we included soft tissue initially involved by tumor in the CTV but avoided pretreatment tumor bulk outside skin at re-simulation. Another remaining controversy is the variation between the radiation oncologist’s contouring to target volumes, as previous reported,14 which is also reported in other gynecological malignancies.15,16
Contouring for GTV-P over the irradiated area might not be accurate in re-simulation settings, so the initial CTV and PTV volumes are still used with respect to the anatomical boundaries and changes related to vulvar edema and perineal soft tissue swelling.17
The decision to proceed with ART is based on the discretion of treating radiation oncologist, which leads to interpersonal variation similar to the case of target contouring. However, following the contouring guidelines and consensus agreement for re-simulation criteria minimizes such variations. In addition, all cases are being reviewed at our departmental quality assurance round to ensure adherence to contouring, dose fractionation and dosimetry in radiation therapy plans.
Weight change is not uncommon for patients receiving pelvic chemoradiation, an issue that should be addressed carefully when prescribing the chemotherapy dose and monitoring by the radiation oncologist for the patient’s anatomy change through IGRT. Nutritional support is crucial to ensure minimal BMI alteration and change in the patient’s body fat content that might lead to changes in dosimetry.18
Recently, the use of functional imaging has been utilized to help target delineation in vulvar carcinoma. In addition to its significant role in tumor staging, it also aids tumor and nodal target definition for radiation therapy planning.19
To obtain a proper target dose coverage, tissue equivalent bolus is frequently added via radiation therapy plan and used over the surface area during treatment. This is applicable for vulvar carcinoma mainly to improve the coverage for tumors involving the skin. Dramatic tumor shrinkage during the treatment phase may lead to changes in the volume and surface integrity beneath the bolus leading to air gap between the bolus and skin, which could alter the planned dose distribution.20
The dose fractionation used for patients in this report was 1.8−2 Gy/fx, in sequential settings, simultaneous higher dose integrated boost was avoided to offer more room for ART intervention.21
Despite IMRT having been proved to cause fewer side effects and a lesser need for treatment interruption which might affect local control,22 ART may provide better outcomes through the avoidance of unnecessary dose to the adjacent normal tissue resulting in less radiation toxicity.
In this report grade 2 RTOG acute radiation toxicity was seen in majority of patients (77%) and less grade 3 (13%), while late toxicity was noted in 1 patient (5%). This could be related to the use of IMRT and the addition of ART. These findings are close to previous reports in literature regarding the use of IMRT in vulvar carcinoma.23
While ART is a promising methodology in radiation therapy resulting in personalizing the delivered plan to each patient, while ensuring proper target coverage and avoiding unnecessary dose to adjacent organs and soft tissue; it still increases the burden on the departmental machines and requires more personnel effort and time and possible additional cost which can be an issue, especially in developing countries. In addition, complex re-planning could result in treatment interruption; which is an unfavorable event in vulvar carcinoma.24 Extra time used to obtain the daily CBCT and contact the treating radiation oncologist might be another challenge prolonging the daily treatment list which can be an issue in busy departments with long treatment lists.
This report is considered the first in literature that discusses the use of ART in vulvar carcinoma. However, it is limited by its retrospective nature and the small sample size. Nonetheless, we suggest the application of scheduled re-simulation between fractions 22–25 for patients with vulvar carcinomas larger than 30cc who had received definitive chemoradiation to ensure adequate target coverage as well as minimize toxicity.
6. Conclusion
Change in the radiation target volume during definitive radiation therapy in vulvar carcinoma is common. IGRT is the elementary tool for ART implementation. ART is crucial to ensure proper radiation therapy delivery especially with large primary tumors more than 30cc. Future prospective larger cohorts are needed to confirm these findings.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Kang Y.J., Smith M., Barlow E., Coffey K., Hacker N., Canfell K. Vulvar cancer in high-income countries: increasing burden of disease. Int J Cancer. 2017;141(11):2174–2186. doi: 10.1002/ijc.30900. [DOI] [PubMed] [Google Scholar]
- 3.Gill B.S., Bernard M.E., Lin J.F. Impact of adjuvant chemotherapy with radiation for node-positive vulvar cancer: a National Cancer data Base (NCDB) analysis. Gynecol Oncol. 2015;137(3):365–372. doi: 10.1016/j.ygyno.2015.03.056. [DOI] [PubMed] [Google Scholar]
- 4.Mitra S., Sharma M.K., Kaur I. Vulvar carcinoma: dilemma, debates, and decisions. Cancer Manag Res. 2018;10:61–68. doi: 10.2147/CMAR.S143316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore D.H., Ali S., Koh W.J. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: a gynecologic oncology group study. Gynecol Oncol. 2012;124(3):529–533. doi: 10.1016/j.ygyno.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Tans L., Ansink A.C., van Rooij P.H., Kleijnen C., Mens J.W. The role of chemo-radiotherapy in the management of locally advanced carcinoma of the vulva: single institutional experience and review of literature. Am J Clin Oncol. 2011;34(1):22–26. doi: 10.1097/COC.0b013e3181cae6a1. [DOI] [PubMed] [Google Scholar]
- 7.Yan D., Vicini F., Wong J., Martinez A. Adaptive radiation therapy. Phys Med Biol. 1997;42(1):123–132. doi: 10.1088/0031-9155/42/1/008. [DOI] [PubMed] [Google Scholar]
- 8.Thornqvist S., Hysing L.B., Tuomikoski L. Adaptive radiotherapy strategies for pelvic tumors - a systematic review of clinical implementations. Acta Oncol. 2016;55(8):943–958. doi: 10.3109/0284186X.2016.1156738. [DOI] [PubMed] [Google Scholar]
- 9.Khader J.K., Al-mousa A.M., Mohamad I.A. Enhancing value of quality assurance rounds in improving radiotherapy management: a retrospective analysis from King Hussein Cancer center in Jordan. Radiat Oncol J. 2019;37(1):60–65. doi: 10.3857/roj.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holsti L.R., Taskinen P.J. Effect of unplanned interruption of radiation therapy. A retrospective survey. Acta Radiol Ther Phys Biol. 1964;2:365–376. doi: 10.3109/02841866409134069. [DOI] [PubMed] [Google Scholar]
- 11.Ohri N., Rapkin B.D., Guha C., Kalnicki S., Garg M. Radiation therapy noncompliance and clinical outcomes in an urban academic cancer center. Int J Radiat Oncol Biol Phys. 2016;95(2):563–570. doi: 10.1016/j.ijrobp.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 12.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the radiation therapy oncology group (RTOG) and the european organization for research and treatment of cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 13.Sonke J.J., Aznar M., Rasch C. Adaptive radiotherapy for anatomical changes. Semin Radiat Oncol. 2019;29(3):245–257. doi: 10.1016/j.semradonc.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Vinod S.K., Jameson M.G., Min M., Holloway L.C. Uncertainties in volume delineation in radiation oncology: a systematic review and recommendations for future studies. Radiother Oncol. 2016;121(2):169–179. doi: 10.1016/j.radonc.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Weiss E., Richter S., Krauss, T. Conformal radiotherapy planning of cervix carcinoma: Differences in the delineation of the clinical target volume. A comparison between gynaecologic and radiation oncologists. Radiother Oncol. 2003;67(1):87–95. doi: 10.1016/s0167-8140(02)00373-0. [DOI] [PubMed] [Google Scholar]
- 16.Wu D.H., Mayr N.A., Karatas Y. Interobserver variation in cervical cancer tumor delineation for image-based radiotherapy planning among and within different specialties. J Appl Clin Med Phys. 2005;6(4):106–110. doi: 10.1120/jacmp.v6i4.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaffney D.K., King B., Viswanathan A.N. Consensus recommendations for radiation therapy contouring and treatment of vulvar carcinoma. Int J Radiat Oncol Biol Phys. 2016;95(4):1191–1200. doi: 10.1016/j.ijrobp.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGough C., Baldwin C., Frost G., Andreyevet H.J. Role of nutritional intervention in patients treated with radiotherapy for pelvic malignancy. Br J Cancer. 2004;90(12):2278–2287. doi: 10.1038/sj.bjc.6601868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viswanathan C., Kirschner K., Truong M., Balachandran A., Devine C., Bhosale P. Multimodality imaging of vulvar cancer: Staging, therapeutic response, and complications. AJR Am J Roentgenol. 2013;200(6):1387–1400. doi: 10.2214/AJR.12.9714. [DOI] [PubMed] [Google Scholar]
- 20.Sroka M., Regula J., Lobodziec W. The influence of the bolus-surface distance on the dose distribution in the build-up region. Rep Pract Oncol Radiother. 2010;15(6):161–164. doi: 10.1016/j.rpor.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler J.F. Biological factors influencing optimum fractionation in radiation therapy. Acta Oncol. 2001;40(6):712–717. doi: 10.1080/02841860152619124. [DOI] [PubMed] [Google Scholar]
- 22.Hymel R., Jones G.C., Simone C.B., 2nd Whole pelvic intensity-modulated radiotherapy for gynecological malignancies: a review of the literature. Crit Rev Oncol Hematol. 2015;94(3):371–379. doi: 10.1016/j.critrevonc.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao Y.J., Chundrury A., Schwarz J.K. Intensity modulated radiation therapy for squamous cell carcinoma of the vulva: treatment technique and outcomes. Adv Radiat Oncol. 2017;2(2):148–158. doi: 10.1016/j.adro.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorino C., Cozzarini C., Passoni P. The promise of adaptive radiotherapy for pelvic tumors: "too high cost for too little result" or "a low cost for a significant result"? Acta Oncol. 2016;55(8):939–942. doi: 10.1080/0284186X.2016.1203460. [DOI] [PubMed] [Google Scholar]