Abstract
Purpose
Retrospective, single-institution analysis of clinical outcomes and treatment-related toxicity in patients treated with salvage I-125 low-dose rate (LDR) brachytherapy (BT) for locally-recurrent prostate cancer after radiotherapy.
Materials and methods
Between 2008 and 2018, 30 patients with biopsy-confirmed prostate cancer recurrence underwent salvage treatment with I-125 LDR-BT. Of these 30 patients, 14 were previously treated with primary external beam radiotherapy (EBRT; median dose, 73 Gy) and 16 with primary I-125 LDR-BT (145 Gy and 160 Gy in 14 and 2 cases, respectively). At seed implantation, the mean age was 75.8 years, with a median Gleason score of 7 and pre-salvage PSA of <10 ng/mL. Six patients received androgen deprivation therapy for six months after relapse diagnosis. The prescribed salvage I-125 BT dose to the gland was 120−130 Gy, with dose restrictions of Dmax <135% (urethra) and <100% (rectum). Toxicity was evaluated according to the CTCAE scale (v4.0).
Results
At a median follow-up of 45 months, the biochemical recurrence-free survival rates at 1, 3 and 5 years were 86.7%, 56.7% and 53.3%, respectively. Overall survival at 5 years was 87%. On the multivariate analysis, two variables were significant predictors of recurrence: PSA at relapse and nadir PSA post-salvage. Grade 3 genitourinary toxicity was observed in 5 patients (radiation-induced cystitis in 3 cases and urethral stenosis in 2) and G3 gastrointestinal toxicity in 3 patients (rectal bleeding).
Conclusion
Salvage therapy with I-125 brachytherapy is a safe and effective treatment option for locally-recurrent prostate cancer in previously-irradiated patients. High pre-salvage PSA and post-salvage nadir PSA values were significantly associated with a worse disease control after salvage I-125 LDR-BT. In well-selected patients, I-125 LDR-BT is comparable to other salvage therapies in terms of disease control and toxicity. However, more research is needed to determine the optimal management of locally-recurrent prostate cancer.
Keywords: Prostate cancer, Salvage brachytherapy, Permanent seed I-125 implant
1. Introduction
In those patients who develop recurrent disease following primary radiation therapy for prostate cancer (PCa), there are three main clinical scenarios: local, regional and/or distance recurrence.1,2 The challenge for the clinicians is to determine whether the PSA-level elevation originates from local disease (recurrence) of cancer or from regional/metastases or both. Of these, only locally-recurrent PCa is suitable for a second local treatment. In these patients, salvage treatment may even be curative, or at least delay the need for systemic therapy (and treatment-related side effects), which may cause additional comorbidities and negatively impact the quality of life.3 Several options are available for salvage therapy in these patients,4 including low-dose rate brachytherapy (LDR-BT), which is considered an effective option for patients with organ-confined disease.5,6
There is evidence—though not level 1—supporting LDR-BT as a safe and potentially curative treatment in well-selected patients.7,8 However, more data are needed to better characterize the role of salvage LDR-BT in locally-recurrent PCa. In this context, the aim of the present study was to describe our single institution experience with salvage LDR-BT to provide further data on the effects of this treatment, in terms of local control and toxicity, in previously-irradiated patients with recurrent organ-confined disease. Also, we identified potential predictors of biochemical failure after salvage BT.
2. Methods and materials
2.1. Patient characteristics
Between 2008 and 2018, 30 patients at our institution underwent salvage LDR-BT with I-125 seeds to treat biochemical recurrence (BCR). The diagnosis of local recurrence was based on the Phoenix criteria9 and histologically-confirmed by transrectal biopsy saturation (confirmed by pathologists with experience in biopsy of previously-irradiated tissue). All patients underwent choline positron-emission computed tomography (PET-CT) and multiparametric MRI (mpMRI) scans to rule out regional and/or distant involvement. Table 1 shows the patients’ clinical characteristics prior to the first treatment and after recurrence. All 30 patients had previously undergone primary radiotherapy: 14 with external beam radiotherapy (EBRT; median dose 73 Gy) and 16 with LDR-BT (145 Gy and 160 Gy in 14 and 2 cases, respectively). Pre-salvage PSA was <10 ng/mL in all cases. None of the patients had genitourinary (GU) or gastrointestinal (GI) toxicity > grade (G)2 after primary radiotherapy. Six patients had received androgen deprivation therapy (ADT).
Table 1.
Clinical characteristics of the patients.
| Initial treatment | Salvage BT | |
|---|---|---|
| Median age (range), years | 79 (62−89) | 80 (75−89) |
| Median PSA at diagnosis | 8.15 ng/mL (6.85−13.95) | <10 ng/mL |
| EBRT | 3.37 (3.06−3.85) | |
| LDR-BT | 3.74 (2.86−5.74) | |
| Clinical stage | ||
| T1c | 11 | |
| T2a | 7 | 8 |
| T2b | 5 | 16 |
| T2c | 3 | 7 |
| T3a | 2 | |
| T3b | 1 | |
| Unknown | 1 | |
| Gleason | ||
| ≤6 | 13 | 4 |
| 7 | 15 | 19 |
| >7 | 1 | 4 |
| Unknown | 1 | 3 |
| D´Amico risk group | ||
| Low risk | 13 | |
| Intermediate risk | 12 | |
| High risk | 6 | |
| Treatment received | ||
| EBRT | 14 | |
| <72 Gy | 8 | |
| >72 Gy | 6 | |
| LDR-BT | 16 | 30 |
| 120 Gy | 21 | |
| 125Gy | 1 | |
| 130Gy | 8 | |
| 145 Gy | 14 | |
| 160Gy | 2 | |
| PSA nadir | ||
| EBRT | 0.27 ng/mL (0−1.8) | 0.14 ng/mL (0.06−0.75) |
| LDR-BT | 0.4 ng/mL (0.1−1.2) | 0.45 ng/mL (0.01−1.31) |
| ADT | 19/30 | 6/30 |
ADT indicates androgen-deprivation therapy; PSA, prostate-specific antigen; EBRT, external beam radiation therapy; BT, brachytherapy.
The decision to offer patients salvage LDR-BT was made after careful deliberation by the Urological Tumor Board at our hospital. The patients were fully informed of all available treatment options, the potential adverse effects of each, and the reported efficacy based on published data before agreeing to undergo this procedure.
2.2. Salvage brachytherapy
I-125 seed implant is performed using a real-time, transrectal ultrasound-guided (TRUS) intraoperative technique. The total prescribed dose to the prostate gland ranges from 120−130 Gy depending on whether patients have previously been treated with BT 145 vs, 160 Gy or EBRT <or > 72 Gy. The patients who had been treated with 145 Gy or <72 Gy were reirradiated with 130 Gy and the patients who had been treated with 160 Gy or >72 Gy were rescued with 120 Gy. In some of the patients that we rescue with 120 Gy, sometimes we administer this dose to the whole gland and a boost of 10 Gy in the volume where the saturation biopsy confirms (we do not perform a fusion with MRI) that there is a relapse. Stranded seeds were implanted and distributed spatially in a peripheral manner, with modifications made based on the lesion localization and the prostate volume. The SPOT treatment planning system (TPS; Elekta/Nucletron) was used, together with the SeedSelectron charging device. Target volumes were defined according to ESTRO/UAE/EORTC recommendations.10 The prescription was done to the prostate gland.
On day 0 (just after implantation), a CT scan was performed to ensure implant quality. At one month after implantation, a thoracoabdominal x-ray was performed to check for possible seed migration. CT and T2 MRI were also performed.
The mean prescribed D90 to the prostate was 100 Gy. Since the sample consisted of previously-irradiated patients, the dosimetric plan was designed to limit the coverage of the prescription dose to the risk volume—defined according to imaging and biopsy data—rather than to the whole gland. For the organs at risk (OAR), the limits were as follows: rectum D2cc <120 Gy, and urethra D30 < 156 Gy and D10 < 180 Gy. The implant dosimetric parameters are shown in Table 2.
Table 2.
Dosimetric parameters for the targets.
| Volumens of interest | Dose-volume parameters objetives | Obtained dosimetric parameters (median value and range) |
|---|---|---|
| Prostate gland | D90% ≥ 100% | 104.9% 79.68-118.51 |
| V100% ≥ 90% | 93.06% 76.12−97.96 | |
| V150% ≤ 60% | 47.07% 30.10−67.04 | |
| V200% | 21.2% 12.90−32.13 |
2.3. Follow up
The initial post-salvage follow-up (clinical interview and PSA determination) was performed at one month post-implant. Afterwards, follow-up was performed every 3 months for the first two years, every 6 months until the fifth year, and annually thereafter. BCR was defined according to the Phoenix nadir +2 ng/mL criterion.9 Treatment-related toxicity was evaluated with the Radiation Therapy Oncology Group (RTOG)11 and the Common Terminology Criteria for Adverse Effects (CTCAE v4.0) scales.12
2.4. Data collection and statistical analysis
Since 2004, our institution has routinely collected data from all prostate cancer patients treated with I-125 seed implants, both for primary treatment of localized cancer and for salvage in cases of local relapse. The data were retrospectively collected from clinical records and reviewed for the current study.
The study variables are presented as means (standard deviation) or medians (1 st and 3rd quartile) for continuous variables, and as absolute and relative frequencies for categorical variables. Survival curves were estimated for each group using the Kaplan-Meier method and compared statistically using the log rank test, also known as Mantel-Haenszel test. The Chi-square test was used to compare differences in proportions of qualitative variables. The Wilcoxon test was used to compare differences in the point estimated of the continuous quantitative variables. Ordinal regression models were used to determine the association between the following variables—previous treatment with EBRT or BT, urethral Dmax, and time between the two treatments—with GI and GU toxicity. Multivariate Cox regression models were used to assess for predictors of recurrence and survival outcomes. The statistical software R (v. 3.6.2) was used for all statistical analyses.13 The cut-off for statistical significance was set at p < 0.05.
3. Results
3.1. Outcomes
Median follow-up after salvage therapy was 48 months (range 25–64). The median (range) time elapsed between primary and salvage therapy was 71 (27–134) months. Median (range) prostate volume at salvage was 24.5 cc (12–62). The median (range) number of seeds implanted was 38.5 (25–77), with a median (range) activity at the time of the implant level of 0.48 mCi (0.319−0.66).
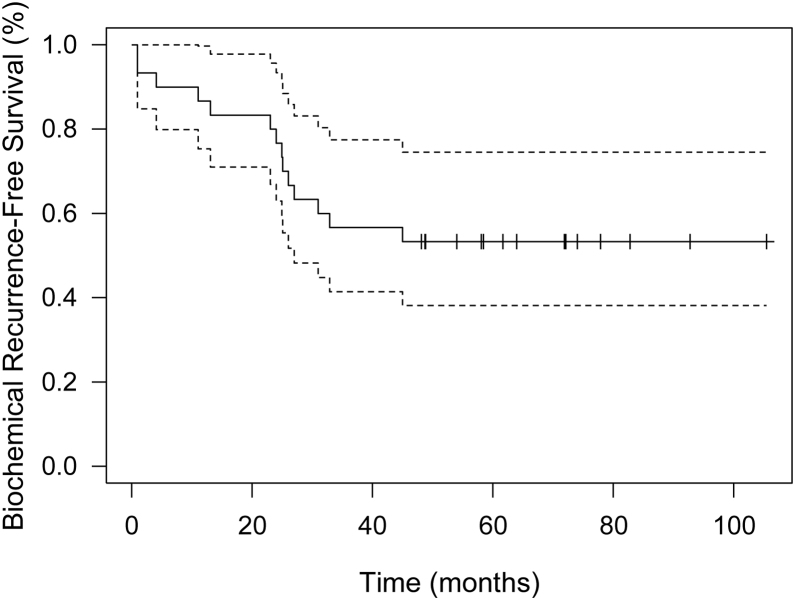
Biochemical recurrence-free survival (BRFS) rates at years 1, 3 and 5 were 86.7%, 56.7% and 53.3%, respectively. Overall survival at five years was 87%. Six patients died due to other causes during the follow-up period. The mean (range) time between salvage I-125 seed implant and a new treatment (ADT, second generation HT or chemotherapy) was 40.4 months (14.7-89), with a median (Q1-Q3) of 28.44 (25.2, 51.12) (Fig. 1).
Fig. 1.
Biochemical recurrence-free survival curve. The dashed lines represent the 95% confidence interval of the Kaplar Meier curve.
Fifteen out of the 30 patients (50%) developed another recurrence after salvage therapy. Of these fifteen, four occurred during the first year, with choline PET-CT confirming regional relapse (multiple lymph node involvement in 3 cases and a single node in the remaining case). Of these four relapses, the time elapsed from the primary treatment until the second relapse was 1.5 years (2 cases) while in the other two patients, the time elapsed was > 4 years. As we observed in our study, the mean (SD) time between salvage I-125 seed implant and a new treatment was 40.4 months (2.29) and in the studies that we had reviewed,25, 26, 27, 28, 29, 30 the FFbF is greater than 70% at 5 years in most cases. Based on this and our own experience we consider that with the rescue with BT we can delay a new treatment for almost 4 years.
At salvage, six of the 30 patients (20%) had received ADT. Although the relapse (and BRFS) rate was higher in the ADT versus non-ADT group (66.67% vs. 41.7%), the differences were not statistically significant. The median PSA nadir was also higher in the ADT group (0.4 vs. 0.17), but not with a significant p value.
On the univariate analysis, two variables were significantly associated with recurrence: PSA at relapse and post-salvage PSA nadir, with higher values associated with a correspondingly greater risk of relapse post-salvage. These variables remained significant on the multivariate analysis.
Other potential predictors of recurrence—risk group, Gleason score, TNM (based on MRI)—showed no significant association on the univariate and multivariate analyses (Table 3, Table 4, Table 5).
Table 3.
Multivariate and univariate analysis using logistic-regression model of factors that may influence recurrence.
| Univariate Cox Regression |
Multivariate Cox Regression |
|||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | |
| PSA relapse | 1.82 | [1.33, 2.49] | < 0.001 | 2.02 | (1.24, 3.29) | 0.005 |
| Time to relapse | 0.88 | [0.72, 1.11] | 0.27 | 1.15 | (0.81, 1.64) | 0.44 |
| Nadir PSA post- salvage | 1.63 | [1.14, 2.35] | 0.008 | 1.84 | (1.09, 3.13) | 0.023 |
| Age | 1.01 | [0.93, 1.11] | 0.76 | 0.95 | (0.84, 1.07) | 0.40 |
Table 4.
Regression model analysis of toxicity.
| Ordinal regression model GI toxicity | OR | 95% CI | p-value |
|---|---|---|---|
| Years from first to second treatment | 0.82 | [0.44, 1.32] | 0.45 |
| Dmax urethra, Gy | 0.99 | [0.94, 1.04] | 0.81 |
| Initial treatment-seeds | 0.28 | [0.01, 2.47] | 0.29 |
| Ordinal regression model GU toxicity | OR | 95% CI | p-value |
| Years from first to second treatment | 0.82 | [0.49, 1.24] | 0.38 |
| Dmax urethra, Gy | 0.99 | [0.96, 1.02] | 0.61 |
| Initial treatment-seeds | 2.23 | [0.36, 18.26] | 0.40 |
| Ordinal regression model toxicity GI | OR | 95% CI | p-value |
| Volume*Seeds | 1.003 | [0.998, 1.009] | 0.27 |
| D90 | 1.05 | [0.92, 1.29] | 0.58 |
| V100 | 1.01 | [0.85, 1.30] | 0.91 |
| V150 | 0.93 | [0.80, 1.07] | 0.33 |
| V200 | 0.92 | [0.68, 1.20] | 0.54 |
| Ordinal regression model GU toxicity | OR | 95% CI | p-value |
| Volume*Seeds | 1.004 | [0.999, 1.011] | 0.12 |
| D90 | 1.01 | [0.91, 1.17] | 0.84 |
| V100 | 0.997 | [0.86, 1.20] | 0.97 |
| V150 | 0.98 | [0.87, 1.10] | 0.77 |
| V200 | 0.88 | [0.67, 1.12] | 0.32 |
CI, confidence interval; OR, odds ratio; GI, gastrointestinal; GU, genitourinary.
*Volume and seeds have been considered at the same time.
Table 5.
Summary of salvage BT series after local failure of radiation therapy.
| Author (year) | Technique and dose | Patients, N | Follow Up (months) | Freedom from biochemical failure (FFbF) |
|---|---|---|---|---|
| Burri et al (2010)25 | LDR I-125 135 Gy Pd-103 110 Gy | 37 | 86 | 65 % 5y 54% 10y |
| Vargas et al (2014)26 | LDR Pd-103 | 69 | 60 | 74% 5y |
| Peters et al (2016)27 | LDR 144 Gy | 20 | 36 | 70% 5y |
| Henríquez et al (2019)28 | LDR 145 Gy | 119 | 52 | 71% 5y |
| HDR 30 Gy | ||||
| Wojcieszek et al (2016)29 | HDR 30 Gy | 83 | 41 | 76% 3y 67% 5y |
| Murgic et al (2018)30 | HDR 27 Gy | 15 | 36 | 61% 3y |
| Kollmeier et al (2017)31 | LDR 125 Gy | 98 | 31 | 60% 3y |
| HDR 32 Gy | ||||
| Current study | LDR 120-130Gy | 30 | 45 | 53.3% 5y |
3.2. Toxicity
In terms of GU toxicity, 23 developed G0-G1 toxicity, two had G2 toxicity (polyuria and hematuria), and five presented G3 toxicity. In the 5 patients with G3 toxicity, two required permanent catheterization and the other 3 developed radiation-induced cystitis, which was treated with hyperbaric oxygen. In terms of GI toxicity, two patients developed G2 proctitis (mucosanguineous secretion) and one patient had G3 toxicity (rectal bleeding).
There were no statistically significant differences in toxicity between patients previously treated with EBRT versus BT. Similarly, no significant association was found between the time elapsed between treatments and toxicity, nor between the urethral Dmax value and toxicity.
To check for other possible treatment-related toxicities, we performed ordinal regression models for the variables D90, V100, V150, V200 and the interaction between prostate volume and seeds, but toxicity rates were too low to detect any statistically significant associations (toxicity for most variables was null; thus, there was no statistical power to detect significant differences).
4. Discussion
Routine assessment of PSA levels is recommended in patients who undergo radical treatment for organ-confined prostate cancer.14 In patients with histologically-confirmed recurrent disease, re-staging is necessary to determine whether the patient is eligible for a second radical therapy or palliative treatment. Local relapse in previously irradiated patients is common, affecting from 21 to 26% of patients at 10 years,15 and up to 24% at 15 years.16 Despite advances in radiotherapy techniques and dose escalation in the last decade, the relapse rate continues to be non-negligible.17
The present study was performed to evaluate patients with locally recurrent disease (within the prostate gland) detectable on imaging scans.18,19 Several treatment options are available for these patients, including surgery, LDR-BT, HDR-BT, cryotherapy and high-intensity focused ultrasound (HIFU), all of which present a non-negligible risk of toxicity.20 Treatment of local recurrences is important because it decreases the risk of metastasis,21 possibly by eliminating tumor cell clones that might otherwise migrate to other parts of the body.16 Until a few years ago, the most common treatment for patients with recurrent disease was ADT,22 but not only is ADT associated with a wide range of adverse effects, it is rarely curative. ADT is more useful in patients with subclinical metastasis, rather than as a treatment to achieve local control of a recurrence.23 In salvage settings, ADT is more important in men with high-risk disease, such as those with stage pT3b/4 disease.23 However, most men will eventually develop hormone-resistance, generally within three years after treatment initiation.24
The BRFS and OS rates in our series are comparable to other published reports of whole gland salvage BT. We show a table with a summary of salvage BT series after local failure of radiation therapy.
Several previous studies25, 26, 27, 28 have reported an association between pre-salvage PSA levels below 10 ng/mL and better post-treatment local control. In our series, all of the patients selected for salvage BT had pre-salvage PSA levels <10 ng/mL, with a median PSA < 4 overall, regardless of the primary treatment (EBRT or LDR-BT). Despite these low PSA values, the risk of relapse increased as a function of higher pre-salvage PSA values. The post-salvage PSA nadir is a significant predictor of the risk of a new relapse. Unfortunately, in our study, the sample size was too small to reliably estimate cut-off points for these two variables (pre-salvage PSA and post-salvage PSA nadir). Although Kollmeier et al.31 did not find any significant associations between these two variables and risk of recurrence, they did find an association with PSA doubling time (PSADT) < 12 months. Wojcieszek et al.29 found that biochemical control rates increased as a function of the time interval to post-salvage PSA nadir, with longer times associated with better biochemical control.
Although we evaluated numerous variables—Gleason score, risk group, T stage, time to relapse and age—none of these was a significant predictor of recurrence in our sample. Of the four patients who experienced a relapse within one year of salvage therapy, two had a high PSA three months after salvage, suggesting that these patients may have not been candidates for local salvage because they probably already had non-organ confined subclinical disease at the time of seed implantation. In this regard, more work is needed to identify other predictors of post-salvage recurrence to better characterize the best candidates for local salvage with LDR-BT.
Various treatment options for local recurrences, including focal/partial treatment of the prostate gland, have been proposed to minimize toxicity; in these scenarios, multiparametric MRI plays a key role.32 Several studies have found that this approach is associated with lower toxicity rates. For example, in the study by Hsu et al.,33 there were no cases of G3 toxicity in the arm that received LDR-BT as focal salvage therapy, even though those authors administered a higher total dose than in our study. Similarly, Sasaky et al.34 reported no G3 toxicity in patients who received a total dose of 145 Gy. Peters et al.27 reported a 5-year BRFS of 60%, with no cases of ≥ G3 toxicity. Likewise, Kunogi and colleagues35 administered 145 Gy to the PTV, also finding no cases of chronic G3 toxicity; moreover, in patients with toxicity ≤ G2, this resolved quickly. The main advantage of focal/partial therapy versus whole gland treatment is to limit the dose to the OARs, thereby reducing the likelihood of inducing severe toxicity.
Baumann et al.36 took a different approach to reducing the risk of severe toxicity. Those authors treated the whole gland but with a relatively low dose (median LDR prescribed dose 100 Gy; median HDR dose 30 Gy in 6 fractions over 4 weeks), administered with neoadjuvant ADT (4–6 months) and adjuvant ADT. Local control rates were acceptable (five and 7-year relapse-free survival were 79% and 67%; and overall survival 94% and 85%, respectively) with a low toxicity profile.
Other local salvage options are also associated with important adverse effects. Salvage prostatectomy, which must be performed in experienced centres, is associated with severe morbidity and, thus, rarely performed nowadays.37,38 The excellent systematic review by Chade et al.39 found that rectal injury occurred in 0%-28% of cases, and anastomotic stricture in 7% to 41% of cases; moreover, in more recent series, most complications are less common (except for anastomotic stricture). Cryotherapy is associated with severe incontinence, ranging from 3% to 19%,40, 41, 42, 43 while serious adverse effects are generally only observed in previously irradiated patients. In patients who undergo HIFU, reported rates of urethral stenosis, urinary incontinence, and rectovesical fistula range from 18 to 38%, 7–40% and 0.4–6%, respectively.44, 45, 46, 47 Rectourethral fistula leading to colostomy is a particularly worrisome complication of HIFU in patients previously treated with EBRT.
In this study, we were unable to identify any significant predictors—not even previous treatment with EBRT or LDR-BT, nor any of the dosimetric variables—of post-treatment toxicity. By contrast, the RTOG-0526 study48 found that V100 was a predictor of GU/GI toxicity.
Overall, the findings of our small, retrospective study are consistent with the available literature regarding local control and toxicity. It is important to emphasize that patients with locally recurrent disease can potentially be "cured" by salvage BT, provided they are carefully selected. Even in patients who cannot be cured, salvage BT can delay initiation of systemic treatment (and the side effects thereof) by postponing the start of this treatment by nearly four years.
In our LDR-BT salvage scheme, we treat the whole gland to a mean dose of 100 Gy, with a total dose to the tumor ranging from 120 to 130 Gy. By applying dose constraints for the rectum (D2cc <120 Gy) and urethra (D30% <156 Gy, and D10% <180 Gy), we were able to achieve disease control and toxicity in line with other studies, with acceptable toxicity. These data support the use of salvage LDR-BT in selected patients with locally recurrent disease.
The main aim of this study was to evaluate the role of salvage LDR-BT in locally recurrent prostate cancer. Our findings—at a median follow-up of 45 months—show that treatment outcomes with salvage LDR-BT were excellent, with BRFS rates of 86.7%, 56.7% and 53.3% at years 1, 3 and 5. Overall survival at 5 years was 87%. Two variables—PSA at relapse and PSA nadir post-salvage—were significant predictors of recurrence on the multivariate analysis. Overall, toxicity was acceptable and consistent with previous reports. These findings support the use of LDR-BT in patients with locally recurrent prostate cancer.
5. Conclusion
BT in locally recurrent PCa is potentially curative and it can delay initiation of palliative hormone therapy.
The current data suggest that it is possible to achieve lasting disease control with acceptable toxicity using the LDR-BT approach and criteria described here, provided that patients are carefully selected and that key prognostic factors—including PSA at relapse and PSA nadir post-salvage—are considered.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
For this type of study formal consent is not required.
Compliance with ethics standards
This study was approved by the Ethics Committee of Biomedical Research of the Hospital Universitari I Politécnic La Fe (Valencia, Spain).
Financial disclosure
None declared.
Conflict of interest
None declared.
References
- 1.Zelefsky M.J., Ben-Porat L., Scher H.I. Outcome predictors for the increasing PSA State after definitive external-beam radiotherapy for prostate cancer. J Clin Oncol [Internet] 2005;23(4):826–831. doi: 10.1200/JCO.2005.02.111. http://www.ncbi.nlm.nih.gov/pubmed/15681527 Feb 1 [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 2.Michalski J., Winter K., Roach M. Clinical outcome of patients treated with 3D conformal radiation therapy (3D-CRT) for prostate cancer on RTOG 9406. Int J Radiat Oncol [Internet] 2012;83(3):e363–370. doi: 10.1016/j.ijrobp.2011.12.070. http://www.ncbi.nlm.nih.gov/pubmed/22633552 Jul 1 [cited 2020 Jan 26] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keating N.L., O’Malley A.J., Smith M.R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol [Internet] 2006;24(27):4448–4456. doi: 10.1200/JCO.2006.06.2497. http://www.ncbi.nlm.nih.gov/pubmed/16983113 Sep 20 [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich A., Bolla M., Joniau S. EAU guidelines on prostate cancer. Update [Internet] 2014;53(Feb):31–45. http://www.uroweb.org/fileadmin/tx_eauguidelines/2005/Pocket/Prostate_Cancer.pdf Available from: [Google Scholar]
- 5.Grimm P., Billiet I., Bostwick D., Dicker A.P., Frank S. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by rmdicaltherapy. Results from the prostate cancer results study group. BJU Int. 2012;109(Supl 1):22–29. doi: 10.1111/j.1464-410X.2011.10827.x. [DOI] [PubMed] [Google Scholar]
- 6.Guinot J.L., Ricós J.V., Tortajada M.I. Comparison of permanent (125)I seeds implants with two different techniques in 500 cases of prostate cancer. J Contemp Brachytherapy [Internet] 2015;7(4):258–264. doi: 10.5114/jcb.2015.53525. http://www.ncbi.nlm.nih.gov/pubmed/26622228 Aug [cited 2020 Jan 26] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada Y., Kollmeier M.A., Pei X. A Phase II study of salvage high-dose-rate brachytherapy for the treatment of locally recurrent prostate cancer after definitive external beam radiotherapy. 2014 doi: 10.1016/j.brachy.2013.11.005. [cited 2020 Jan 26]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C.P., Weinberg V., Shinohara K. Salvage HDR brachytherapy for recurrent prostate cancer after previous definitive radiation therapy: 5-year outcomes. Int J Radiat Oncol [Internet] 2013;86(2):324–329. doi: 10.1016/j.ijrobp.2013.01.027. http://www.ncbi.nlm.nih.gov/pubmed/23474112 Jun 1 [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 9.Abramowitz M.C., Li T., Buyyounouski M.K. The Phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer [Internet] 2008;112(1):55–60. doi: 10.1002/cncr.23139. Jan 1 [cited 2020 Feb 9] Available from: [DOI] [PubMed] [Google Scholar]
- 10.Salembier C., Lavagnini P., Nickers P. Tumour and target volumes in permanent prostate brachytherapy: a supplement to the ESTRO/EAU/EORTC recommendations on prostate brachytherapy. Radiother Oncol. 2007 doi: 10.1016/j.radonc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC) Int J Radiat Oncol Biol Phys [Internet] 1995;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. https://www.redjournal.org/article/0360-3016(95)00060-C/pdf#.W4ZvdUDsxQQ.mendeley Mar 30 [cited 2018 Aug 29] Available from: [DOI] [PubMed] [Google Scholar]
- 12.National Institute of Cancer . NIH Publ.; 2010. . Common terminology criteria for adverse events ( CTCAE ) [Google Scholar]
- 13.The R Project for Statistical Computing. R version 3.2.3 (Wooden Christmas-tree).
- 14.Roach M. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix consensus Conference. Int J RAdiat Oncol Biol Phhys. 2006;62:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Kittel J.A., Reddy C.A., Smith K.L. Long-term efficacy and toxicity of Low-dose-rate (1)(2)(5)I prostate brachytherapy as monotherapy in Low-, intermediate-, and High-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2015 doi: 10.1016/j.ijrobp.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 16.Fuks Z., Leibel S.A., Wallner K.E. The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with 125I implantation. Int J Radiat Oncol Biol Phys [Internet] 1991;21(3):537–547. doi: 10.1016/0360-3016(91)90668-t. http://www.ncbi.nlm.nih.gov/pubmed/1869452 Aug [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 17.Pons-Llanas O., Roldan-Ortega S., Celada-Alvarez F. Permanent seed implant brachytherapy in low-risk prostate cancer: preoperative planning with 145 Gy versus real-time intraoperative planning with 160 Gy. Reports Pract Oncol Radiother [Internet] 2018;23(4):290–297. doi: 10.1016/j.rpor.2018.06.009. https://www.sciencedirect.com/science/article/pii/S1507136718300762 Jul 1 [cited 2018 Aug 15] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pucar D., Hricak H., Shukla-Dave A. {A figure is presented}Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007;69(Sep 1(1)):62–69. doi: 10.1016/j.ijrobp.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 19.Arrayeh E., Westphalen A.C., Kurhanewicz J. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal MRI and MRSI study. Int J Radiat Oncol Biol Phys. 2012;82(Apr 1(5)) doi: 10.1016/j.ijrobp.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alongi F., De Bari B., Campostrini F. Salvage therapy of intraprostatic failure after radical external-beam radiotherapy for prostate cancer: a review. Crit Rev Oncol Hematol [Internet] 2013;88(3):550–563. doi: 10.1016/j.critrevonc.2013.07.009. http://www.ncbi.nlm.nih.gov/pubmed/23953795 Dec [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 21.Ghilezan M., Martinez A., Gustason G. High-dose-rate brachytherapy as monotherapy delivered in two fractions within one day for favorable/intermediate-risk prostate cancer: preliminary toxicity data. Int J Radiat Oncol Biol Phys. 2012;83(Jul 1(3)):927–932. doi: 10.1016/j.ijrobp.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal P.K., Sadetsky N., Konety B.R., Resnick M.I., Carroll P.R. Treatment failure after primary and salvage therapy for prostate cancer. Cancer [Internet]. 2008;112(2):307–314. doi: 10.1002/cncr.23161. Jan 15 [cited 2020 Jan 22] Available from: [DOI] [PubMed] [Google Scholar]
- 23.Gandaglia G., Fossati N., Karnes R.J. Use of concomitant androgen deprivation therapy in patients treated with early salvage radiotherapy for biochemical recurrence after radical prostatectomy: Long-term results from a large, multi-institutional series. Eur Urol [Internet] 2018;73(4):512–518. doi: 10.1016/j.eururo.2017.11.020. http://www.ncbi.nlm.nih.gov/pubmed/29229176 Apr [cited 2020 Jan 22] Available from: [DOI] [PubMed] [Google Scholar]
- 24.Harris W.P., Mostaghel E.A., Nelson P.S., Montgomery B. Vol. 6. Nature Publishing Group; 2009. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion; pp. 76–85. (Nature clinical practice urology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burri R.J., Stone N.N., Unger P., Stock R.G. Long-term outcome and toxicity of salvage brachytherapy for local failure after initial radiotherapy for prostate cancer. Int J Radiat Oncol [Internet] 2010;77(5):1338–1344. doi: 10.1016/j.ijrobp.2009.06.061. https://linkinghub.elsevier.com/retrieve/pii/S0360301609010153 Aug [cited 2019 Aug 24] Available from: [DOI] [PubMed] [Google Scholar]
- 26.Vargas C., Swartz D., Vashi A. Salvage brachytherapy for recurrent prostate cancer. Brachytherapy [Internet] 2014;13(1):53–58. doi: 10.1016/j.brachy.2013.10.012. https://linkinghub.elsevier.com/retrieve/pii/S1538472113003796 Jan [cited 2019 Aug 24] Available from: [DOI] [PubMed] [Google Scholar]
- 27.Peters M., Maenhout M., van der Voort van Zyp J.R.N. Focal salvage iodine-125 brachytherapy for prostate cancer recurrences after primary radiotherapy: a retrospective study regarding toxicity, biochemical outcome and quality of life. Radiother Oncol [Internet] 2014;112(1):77–82. doi: 10.1016/j.radonc.2014.06.013. https://linkinghub.elsevier.com/retrieve/pii/S0167814014002722 Jul [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 28.Henríquez I., Sancho G., Hervás A. Salvage brachytherapy in prostate local recurrence after radiation therapy: predicting factors for control and toxicity. Radiat Oncol [Internet] 2014;9(1):102. doi: 10.1186/1748-717X-9-102. https://ro-journal.biomedcentral.com/articles/10.1186/1748-717X-9-102 Apr 30 [cited 2019 Aug 24] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wojcieszek P., Szlag M., Głowacki G. Salvage high-dose-rate brachytherapy for locally recurrent prostate cancer after primary radiotherapy prostate cancer salvage brachytherapy failure. Radiother Oncol. 2016;119(3) doi: 10.1016/j.radonc.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 30.Murgic J., Morton G., Loblaw A. Focal salvage High dose-rate brachytherapy for locally recurrent prostate cancer after primary radiation therapy failure: results from a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2018;102(3) doi: 10.1016/j.ijrobp.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 31.Kollmeier M.A., McBride S., Taggar A. Salvage brachytherapy for recurrent prostate cancer after definitive radiation therapy: a comparison of low-dose-rate and high-dose-rate brachytherapy and the importance of prostate-specific antigen doubling time. Brachytherapy. 2017;16(6) doi: 10.1016/j.brachy.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Westphalen A.C., Coakley F.V., Roach M., McCulloch C.E., Kurhanewicz J. Locally recurrent prostate cancer after external beam radiation therapy: diagnostic performance of 1.5-T endorectal MR imaging and MR spectroscopic imaging for detection. Radiology [Internet] 2010;256(2):485–492. doi: 10.1148/radiol.10092314. http://www.ncbi.nlm.nih.gov/pubmed/20551184 Aug [cited 2020 Jan 26] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu C.C., Hsu H., Pickett B. Feasibility of MR Imaging/MR spectroscopy-planned focal partial salvage permanent prostate implant (PPI) for localized recurrence after initial PPI for prostate cancer. Int J Radiat Oncol [Internet] 2013;85(2):370–377. doi: 10.1016/j.ijrobp.2012.04.028. https://linkinghub.elsevier.com/retrieve/pii/S0360301612005755 Feb [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 34.Sasaki H., Kido M., Miki K. Salvage partial brachytherapy for prostate cancer recurrence after primary brachytherapy. Int J Urol [Internet] 2014;21(6):572–577. doi: 10.1111/iju.12373. Jun [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 35.Kunogi H., Wakumoto Y., Yamaguchi N., Horie S., Sasai K. Focal partial salvage low-dose-rate brachytherapy for local recurrent prostate cancer after permanent prostate brachytherapy with a review of the literature. Vol. 8. Journal of Contemporary Brachytherapy. 2016 doi: 10.5114/jcb.2016.60452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumann B.C., Baumann J.C., Christodouleas J.P., Soffen E. Salvage of locally recurrent prostate cancer after external beam radiation using reduced-dose brachytherapy with neoadjuvant plus adjuvant androgen deprivation. Brachytherapy. 2017;16(Mar 1(2)):291–298. doi: 10.1016/j.brachy.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Bianco F.J., Scardino P.T., Stephenson A.J., DiBlasio C.J., Fearn P.A., Eastham J.A. Long-term oncologic results of salvage radical prostatectomy for locally recurrent prostate cancer after radiotherapy. Int J Radiat Oncol [Internet] 2005;62(2):448–453. doi: 10.1016/j.ijrobp.2004.09.049. https://linkinghub.elsevier.com/retrieve/pii/S0360301604026999 Jun [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 38.Vaidya A., Soloway M.S. Salvage radical prostatectomy for radiorecurrent prostate cancer: morbidity revisited. J Urol [Internet] 2000;164(6):1998–2001. http://www.ncbi.nlm.nih.gov/pubmed/11061900 Dec [cited 2020 Jan 26] Available from: [PubMed] [Google Scholar]
- 39.Chade D.C., Eastham J., Graefen M. Cancer control and functional outcomes of salvage radical prostatectomy for radiation-recurrent prostate cancer: a systematic review of the literature. Vol. 61. Eur Urol. 2012:961–971. doi: 10.1016/j.eururo.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 40.Elkjær M.C., Borre M. Only carefully selected patients may have a beneficial effect of salvage cryoablation in recurrent prostate cancer after radiotherapy. Dan Med J [Internet] 2013;60(12):A4756. http://www.ncbi.nlm.nih.gov/pubmed/24355453 Dec [cited 2020 Jan 26] Available from: [PubMed] [Google Scholar]
- 41.Spiess P.E., Levy D.A., Pisters L.L., Mouraviev V., Jones J.S. Outcomes of salvage prostate cryotherapy stratified by pre-treatment PSA: update from the COLD registry. World J Urol [Internet] 2013;31(6):1321–1325. doi: 10.1007/s00345-012-0982-2. http://link.springer.com/10.1007/s00345-012-0982-2 Dec 23 [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 42.Williams A.K., Martínez C.H., Lu C., Ng C.K., Pautler S.E., Chin J.L. Disease-Free survival following salvage cryotherapy for biopsy-proven radio-recurrent prostate cancer. Eur Urol [Internet] 2011;60(3):405–410. doi: 10.1016/j.eururo.2010.12.012. https://linkinghub.elsevier.com/retrieve/pii/S0302283810011905 Sep [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 43.Ng C.K., Moussa M., Downey D.B., Chin J.L. Salvage cryoablation of the prostate: followup and analysis of predictive factors for outcome. J Urol [Internet] 2007;178(4):1253–1257. doi: 10.1016/j.juro.2007.05.137. http://www.ncbi.nlm.nih.gov/pubmed/17698104 Oct [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 44.Crouzet S., Murat F.-J., Pommier P. Locally recurrent prostate cancer after initial radiation therapy: early salvage high-intensity focused ultrasound improves oncologic outcomes. Radiother Oncol [Internet] 2012;105(2):198–202. doi: 10.1016/j.radonc.2012.09.014. http://www.ncbi.nlm.nih.gov/pubmed/23068708 Nov [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 45.Gelet A., Chapelon J.Y., Poissonnier L. Local recurrence of prostate cancer after external beam radiotherapy: early experience of salvage therapy using high-intensity focused ultrasonography. Urology [Internet] 2004;63(4):625–629. doi: 10.1016/j.urology.2004.01.002. http://www.ncbi.nlm.nih.gov/pubmed/15072864 Apr [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 46.Uddin Ahmed H., Cathcart P., Chalasani V. Whole-gland salvage high-intensity focused ultrasound therapy for localized prostate cancer recurrence after external beam radiation therapy. Cancer [Internet] 2012;118(12):3071–3078. doi: 10.1002/cncr.26631. http://www.ncbi.nlm.nih.gov/pubmed/22071795 Jun 15 [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 47.Uchida T., Shoji S., Nakano M. High-intensity focused ultrasound as salvage therapy for patients with recurrent prostate cancer after external beam radiation, brachytherapy or proton therapy. BJU Int [Internet] 2011;107(3):378–382. doi: 10.1111/j.1464-410X.2010.09518.x. http://www.ncbi.nlm.nih.gov/pubmed/21265984 Feb [cited 2020 Jan 26] Available from: [DOI] [PubMed] [Google Scholar]
- 48.Crook J.M., Zhang P., Pisansky T.M. A prospective phase 2 trial of transperineal ultrasound-guided brachytherapy for locally recurrent prostate cancer after external beam radiation therapy (NRG Oncology/RTOG-0526) Int J Radiat Oncol Biol Phys. 2019;103(Feb 1(2)):335–343. doi: 10.1016/j.ijrobp.2018.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]