Abstract
Objective
The objective was to evaluate the contraceptive effectiveness, safety, and acceptability of a novel vaginal pH regulator over seven cycles of use.
Study design
A single-arm, open-label, phase 3 study was conducted across 112 sites in the United States in sexually active 18–35-year-old women at risk of pregnancy. Women administered the study treatment ≤ 1 h before each episode of intercourse. Women recorded use of study drug, coital information, and any symptoms experienced in electronic diaries. The primary outcome was the seven-cycle cumulative pregnancy rate as calculated using the Kaplan–Meier methodology; secondary outcomes included safety. Overall satisfaction was assessed via written questionnaires.
Results
A total of 1384 women were enrolled in the study from July 2017 to November 2018. Mean age was 27.7 ± 4.4 years; most women were white (69.0%). The seven-cycle cumulative pregnancy percentage was 13.7% [95% confidence interval (CI): 10.0%–17.5%], meeting the prespecified primary endpoint of having the upper bound 95% CI ≤ 21%. Most common adverse events (AEs) occurring in ≥ 2% of women were vulvovaginal burning sensation, vulvovaginal pruritus, urinary tract infection, vulvovaginal pain, mycotic infection, bacterial vaginosis, and nasopharyngitis. Of 1330 women who used the study drug at least once, fewer than 2% of women discontinued due to any AEs, and < 1% of women discontinued due to genitourinary symptoms. Overall, > 80% of women reported being “very satisfied” or “satisfied” with study treatment.
Conclusions
In this phase 3 study, the novel vaginal pH regulator demonstrated 86.3% contraceptive effectiveness, was safe and well tolerated, and was highly acceptable.
Implications
This novel vaginal pH regulator is a safe, nonhormonal, woman-controlled method of contraception that expands women's options.
Keywords: Contraception, Nonhormonal, Vaginal gel, Phase 3 trial
1. Introduction
The rate of unintended pregnancy in the United States remains high, estimated at 45% in 2011 [1]. In part, the incidence of unintended pregnancy suggests that despite the availability of effective contraceptives, additional contraceptive options are needed in order to meet the multitude of needs and preferences of women as their family planning goals shift over time. Research suggests some women have concerns about hormone-related side effects and would prefer contraceptives that provide control and flexibility, such as those that enable short-term and/or nondaily use [2].
The novel vaginal pH regulator Phexxi™ (previously Amphora and ACIDFORM) is a nonhormonal, woman-controlled, contraceptive vaginal gel under investigation for prevention of pregnancy. It contains three active ingredients (L-lactic acid, citric acid and potassium bitartrate) and is designed to maintain the acidic vaginal environment even in the presence of alkaline semen [[3], [4], [5]]. Preclinical testing of the gel demonstrated bioadhesive and viscosity-retaining properties enabling the gel to stay in the vagina for up to 8–10 h [6,7]. Results from phase 1 clinical studies show use of vaginal pH regulator did not result in intravaginal toxicity, although mild/moderate burning/itching/irritation were reported [8,9]. In early clinical studies, few male partners reported side effects, with most resolving spontaneously and with rates similar to those seen with an over-the-counter lubricant [10].
The objectives of this phase 3 AMPOWER trial were to evaluate the contraceptive effectiveness, safety, and women's satisfaction with the novel vaginal pH regulator over seven cycles of use.
2. Methods
In consultation with the US Food and Drug Administration (FDA) and to support a new drug application, AMPOWER was designed as a multicenter, single-arm, open-label, phase 3 study (ClinicalTrials.gov identifier number: NCT03243305) to evaluate the contraceptive effectiveness of vaginal pH regulator over seven cycles of use. The primary endpoint was the seven-cycle cumulative pregnancy rate with typical use as measured by the Kaplan–Meier statistical method. Safety was measured by adverse event (AE) reporting, clinical labs, and physical exams. All AEs were coded by MedDRA (version 21.0), and the number of women experiencing ≥ 1 AE was summarized by frequency and percentage. Women's overall satisfaction was an exploratory outcome.
2.1. Participants, inclusion and exclusion criteria
The study was conducted at 112 sites in the United States; the protocol was approved by The Advarra Institutional Review Board. Clinical site assessments were conducted to ensure sites had the necessary resources and training/experience for participation. Sites were monitored periodically to review study progress, verify protocol adherence, review maintenance of study records, and ensure International Council for Harmonisation (ICH) and Good Clinical Practice (GCP) guidelines were followed. At the screening visit (visit 1), all participating women provided informed consent. Eligible women were aged 18–35 years, at risk for pregnancy and report normal 21–35 day cyclic menses. Complete inclusion and exclusion criteria are described in Appendices A.1 and A.2, respectively.
Each woman was asked to engage in at least three acts of heterosexual vaginal intercourse per cycle and keep an electronic diary (eDiary) to record coital information, study drug information, use of concomitant medications and other contraceptives, menses, and any symptoms that may indicate an AE.
2.2. Product assignment and sample size
Enrolled women received the study product as prefilled applicators each containing 5 g of study drug (stored at ambient temperature [3]) to use as their on-study contraceptive method while engaging in vaginal intercourse. Women were instructed to administer the study drug intravaginally immediately before or up to 1 h before each episode of intercourse. Women received daily reminders to record data via the eDiary app, which had a 48-h lockout period so data were captured in “real time.” As agreed upon with the FDA, to achieve 90% power to ensure the upper limit of the 95% confidence interval (CI) of the cumulative seven-cycle pregnancy rate was ≤ 21%, accounting for dropout and loss to follow-up, an estimated total sample size of 1349 women enrolled would be required. There were 550 women in screening when the enrollment target was met; therefore, an additional 35 women were enrolled, bringing the total to 1384.
2.3. Follow-up visits
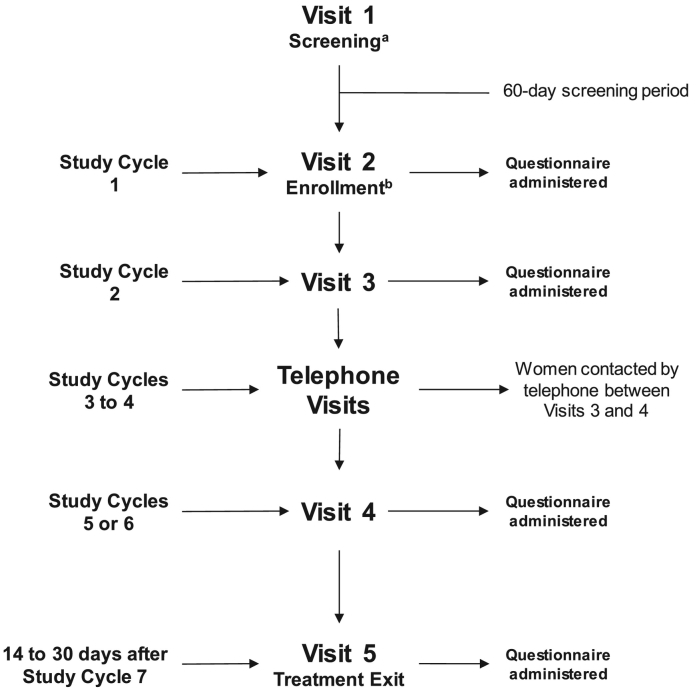
Following enrollment (visit 2), women returned for visits 3 (second study cycle) and 4 (either fifth or sixth study cycle) (Fig. 1). Visit 5/exit visit took place 14–30 days after the seventh study cycle or from the last use of study product for early termination. At each visit, urine pregnancy test and gynecologic examination were performed, vital signs were assessed, and AEs were recorded. Any unused study drug and applicators were collected, and each woman's eDiary was reviewed. Additional study product was distributed at each visit as needed. The total estimated time for a woman's participation in the study was ≤ 10 months. Questionnaires were administered for women to rate their overall satisfaction with their previous contraceptive method at enrollment and to report their satisfaction with the study drug at each study visit.
Fig. 1.
AMPOWER study design.
aWithin 60 days of providing consent, eligible women were assessed for overall and gynecologic health prior to being scheduled for enrollment/visit 2 to begin receiving study treatment.
bThe cycle during which enrollment occurred was considered cycle 0. The woman's seven study cycles were cycles 0 to 6 if the time from enrollment to the woman's next menstrual period was ≥ 21 days. If the time from enrollment to the woman's next menstrual period was < 21 days, the woman's seven study cycles were cycles 1 to 7.
2.4. Statistical analysis and outcome measures
All enrolled women were included in the intent-to-treat (ITT) population. Women in the ITT population who administered the study drug at least once were considered the Safety evaluable population; all safety data were summarized descriptively. The modified intent-to-treat (mITT) population included women from the ITT population who had at least one qualifying cycle or who became pregnant with a conception date between enrollment and < 8 days after final study drug use. The primary effectiveness outcome was the seven-cycle cumulative pregnancy rate in the mITT population. To comply with FDA recommendations, qualifying cycles had to meet rigorous criteria, including: 21–35-day cycle length, no backup or emergency contraception, and at least one recorded act of vaginal intercourse in that cycle. Women's satisfaction was assessed in the Safety population and summarized for each visit using descriptive statistics. Version 9.4 of the SAS® statistical software package was used for all summaries, listings, statistical analyses, and graphical presentations. The statistical analysis plan was submitted to the FDA prior to database lock.
3. Results
3.1. Enrollment, disposition, and participant characteristics
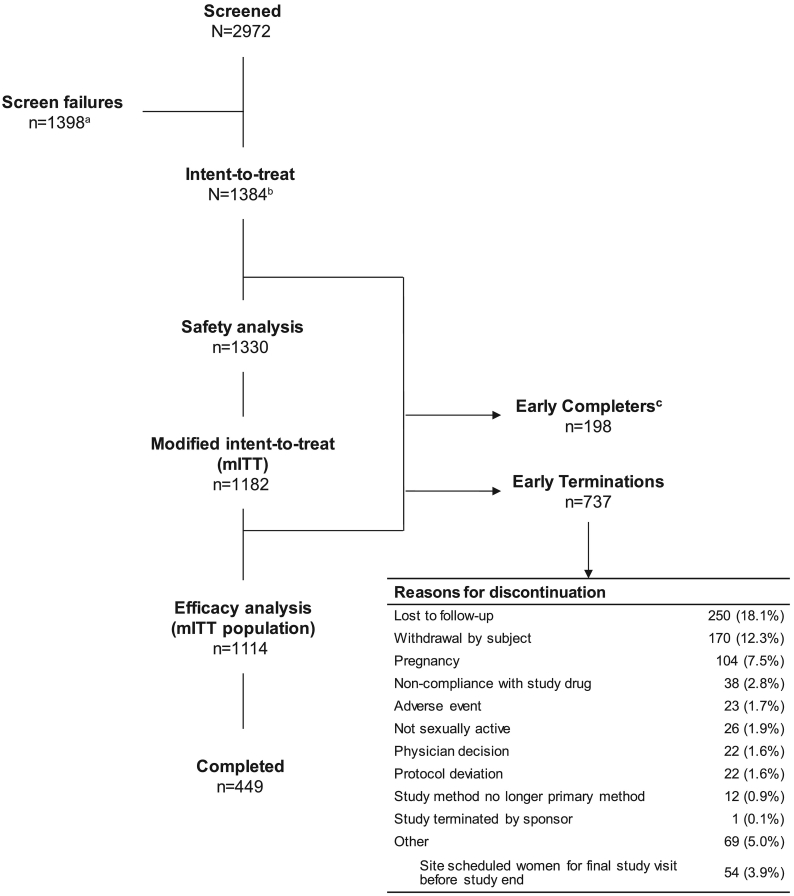
From July 2017 through November 2018, a total of 2972 women were screened and 1384 women were enrolled at 112 study sites (Fig. 2). The average age was 27.7 ± 4.5 years, with mean body mass index of 28.8 ± 8.1 kg/m2. Of the 1384, there were 519 (37.5%) nulligravida, 313 (22.6%) primigravida, and 552 (39.9%) multigravida women prior to enrollment; among 865 primi- and multigravida, the mean number of prior pregnancies was 2.5 ± 1.8 (Table 1). The majority of participating women were white (69.0%) and were of non-Hispanic or non-Latina origin (58.2%). At time of screening, 70% of women (969/1384) reported contraception use, with some women reporting use of at least one contraceptive method. The most frequently reported methods of contraception used at time of screening were male condom (56.9%, 787/1384), withdrawal method (14.2%, 196/1384), and rhythm method (5.1%, 70/1384); approximately 30% of women (415/1384) did not report using any contraceptive method.
Fig. 2.
Participant flow diagram of the AMPOWER study.
All enrolled women were included in the ITT population. Safety population included women in the ITT population who used the study drug at least once throughout the study. The mITT population included women from the ITT population who had at least one qualifying cycle or who became pregnant with a conception date between enrollment and < 8 days after final study drug use.
aScreen failures include > 550 women in screening at the time enrollment target was met.
bThere were 10 women who enrolled in the study more than once; only their first enrollment was counted. Excludes women who had a pregnancy detected after being enrolled but the pregnancy was determined to have started before enrollment date. Women with cycles with backup emergency contraception are excluded unless they became pregnant while in the study.
cThere were 198 “early completers” who had completed at least six study cycles but were among 252 women inadvertently scheduled for their final study visit before study end.
Table 1.
Demographic and baseline characteristics of women enrolled in a phase 3 contraceptive trial for vaginal pH regulator
| Characteristic | Enrolled women (N = 1384) |
|---|---|
| Age in years, mean ± S.D. | 27.7 ± 4.5 |
| Race, n (%) | |
| Asian | 35 (2.5) |
| Black or African American | 348 (25.1) |
| American Indian or Alaska Native | 6 (0.4) |
| Native Hawaiian or Pacific Islander | 2 (0.1) |
| White | 955 (69.0) |
| Other | 38 (2.8) |
| Ethnicity, n (%) | |
| Hispanic or Latina | 571 (41.3) |
| Not Hispanic or Latina | 805 (58.2) |
| Not reported | 8 (0.6) |
| Body mass index at screening (kg/m2, mean ± S.D.) | 28.8 ± 8.1 |
| Gynecological history, n (%) | |
| Bacterial vaginosis | 245 (17.7) |
| Urinary tract infection | 550 (39.7) |
| Yeast infection | 467 (33.7) |
| Contraception use in the past 12 months, n (%) | |
| Any use | 1233 (89.1) |
| Current use | 969 (70.0) |
| Never user | 11 (0.8) |
| Pregnancy history | |
| Number of women reporting previous pregnancies | 865 (62.5) |
| How many times woman has been pregnant, n (%) | |
| 0 | 519 (37.5) |
| 1 | 313 (22.6) |
| 2 | 244 (17.6) |
| 3 | 131 (9.5) |
| > 4 | 177 (12.8) |
| Mean number of pregnancies ± S.D. | 2.5 ± 1.8 |
| Mean number of full-term deliveries ± S.D. | 1.5 ± 1.3 |
| Mean number of elective abortions ± S.D. | 0.4 ± 1.0 |
| Mean number of spontaneous abortions ± S.D. | 0.3 ± 0.7 |
All enrolled women were included in the ITT population. S.D., standard deviation.
No unexpected changes in serum chemistry, hematology, vital signs, or physical exam results were reported. Of the 1330 women included in the safety analysis, the mean time on study was 144.6 ± 75.0 days (median, 175) and the mean total number of cycles per woman was 5.7 ± 2.7 (median, 7). Data from women's eDiaries (n = 1265) showed that the study drug was used a mean of 25.8 ± 21.2 times per woman (median, 22) and was used a mean of 4.2 ± 3.2 (median, 3.6) times per woman per cycle. The completion rate was 46.7 (647/1384), which included 449 women (32.4%) completing seven cycles and 198 “early completers” (14.3%) who completed at least six cycles but were inadvertently scheduled for their treatment exit visit. The most frequent reason for study discontinuation was loss to follow-up (18.1%, 250/1384) and withdrawal by subject (12.3%, 170/1384).
3.2. Contraceptive effectiveness
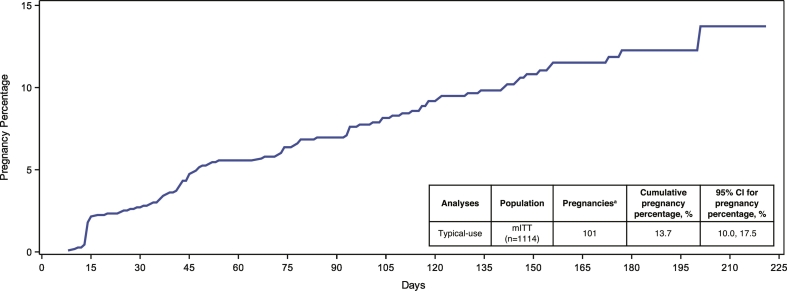
Of 1384 enrolled women, 1182 were included in the mITT population. Sixty-eight women were excluded from the mITT effectiveness analysis (n = 1114) due to (1) having a pregnancy determined to have started before enrollment date or (2) having no qualifying cycles. In total, there were 32,680 reported acts of intercourse, of which 24,289 acts occurred in cycles that met the criteria for inclusion in the primary effectiveness analysis. Of the 1114 women included in the primary effectiveness analysis, 101 on-treatment pregnancies were reported out of over 24,000 qualifying cycles, resulting in a seven-cycle cumulative pregnancy percentage of 13.7% (95% CI: 10.0%–17.5%) with typical use, meeting the prespecified primary endpoint of having the upper bound 95% CI ≤ 21% (Fig. 3). Given that cycles up to 42 days are generally considered ovulatory [11,12], additional effectiveness analyses including 21–42-day cycles reduced the seven-cycle cumulative pregnancy percentage to 12.5% (95% CI: 9.6%–15.3%).
Fig. 3.
Contraceptive effectiveness outcomes in the phase 3 AMPOWER study.
The seven-cycle cumulative pregnancy probability curve with typical use for women in the mITT efficacy analysis population.
aExcludes women who had a pregnancy detected after being enrolled but the pregnancy was determined to have started before enrollment date. Women with cycles with backup emergency contraception are excluded unless they became pregnant while in the study. mITT, modified intent-to-treat.
3.3. Safety and acceptability
Of 1384 women enrolled, 96.1% (1330/1384) of women used the study drug at least once throughout the study (Safety population), providing a total of 7561 cycles evaluable for safety. Throughout the study, 45.2% (601/1330) of women experienced at least one AE (Table 2). The most frequent AEs reported by the largest proportion of women (occurring in ≥ 2.0% of women) were vulvovaginal burning sensation (20.0%), vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), vulvovaginal pain (3.8%), vulvovaginal mycotic infection (2.9%), bacterial vaginosis (2.8%), and nasopharyngitis (2.6%) (Table 2). Most women reported their highest-severity AE to be mild (23.9%, 318/1330) or moderate (18.7%, 249/1330) in severity. There were 108 (8.1%) women who reported experiencing at least one AE that was “definitely” related to treatment. Burning (5.8%) and itching (2.3%) were the most frequently cited reasons for AEs “definitely” related to treatment. Overall, rates of burning and itching generally decreased over time. The rates of burning and itching by acts of intercourse were lower when the study drug was used once per day (2.1% and 0.7%, respectively) compared with two or more times per day (4.6% and 1.0%, respectively).
Table 2.
Summary of women experiencing adverse events on study in a phase 3 contraceptive trial evaluating vaginal pH regulator over seven cycles of use
| Safety population (n = 1330) | |
|---|---|
| Adverse event (preferred term)a | |
| Women with ≥ 1 AE,n (%) | 601 (45.2) |
| Vulvovaginal burning sensation | 266 (20.0) |
| Vulvovaginal pruritus | 149 (11.2) |
| Urinary tract infection | 76 (5.7) |
| Vulvovaginal pain | 51 (3.8) |
| Vulvovaginal mycotic infection | 38 (2.9) |
| Bacterial vaginosis | 37 (2.8) |
| Nasopharyngitis | 35 (2.6) |
| AEs leading to early discontinuation,n (%) | 25 (1.9) |
| Relationship of AEs,bn (%) | |
| Unlikely to be related | 186 (14.0) |
| Possibly related | 166 (12.5) |
| Probably related | 139 (10.5) |
| Definitely related | 108 (8.1) |
| Intensity of AEs | |
| Mild | 318 (23.9) |
| Moderate | 249 (18.7) |
| Severe | 31 (2.3) |
Enrolled women who used the study drug at least once were included in the Safety population. Women could report more than one AE at each study visit.
AEs were coded by MedDRA (version 21.0) system organ class and per MedDRA preferred terms.
Assessed by the investigator.
Fewer than 2% of women discontinued due to any adverse event, and < 1.0% of women (13/1330) discontinued specifically due to genitourinary symptoms. The incidence of serious AEs (SAEs) was low, occurring in 1.3% of women (Table 3), and none were considered to be “definitely” related to study treatment; only a single SAE (cystitis, 0.1%) was considered “probably” to be treatment related.
Table 3.
Incidence of serious adverse events in a phase 3 contraceptive trial evaluating vaginal pH regulator over seven cycles of use
| Parameters, n (%) | Safety population (n = 1330) |
|---|---|
| Total number of SAEs | 17 (1.3) |
| Gastrointestinal disorders | 2 (0.2) |
| Hepatobiliary disorders | 1 (0.1) |
| Infections and infestations | 3 (0.2) |
| Injury, poisoning, and procedural complications | 2 (0.2) |
| Metabolism and nutrition disorders | 1 (0.1) |
| Neoplasms benign, malignant, and unspecified | 1 (0.1) |
| Nervous system disorders | 1 (0.1) |
| Pregnancy, puerperium, and perinatal conditions | 1 (0.1) |
| Psychiatric disorders | 3 (0.2) |
| Reproductive system and breast disorders | 1 (0.1) |
| SAEs leading to early discontinuation | 2 (0.2) |
Enrolled women who used the study drug at least once were included in the Safety population.
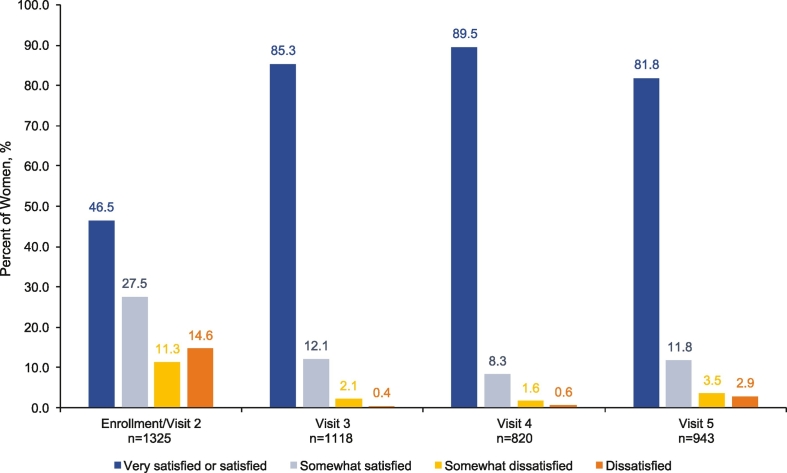
Women's satisfaction was evaluated in 1330 women (ITT population); almost half (46.5% 616/1325) of respondents indicated that they were “very satisfied” or “satisfied” with their contraceptive method prior to the study at enrollment/visit 2. Compared with women's satisfaction with their contraceptive method prior to enrollment, satisfaction with the study treatment nearly doubled; at visits 3, 4, and 5, 85%, 90%, and 82% of women surveyed, respectively, reported being “very satisfied” or “satisfied” (Fig. 4).
Fig. 4.
Women's satisfaction with their most recent contraceptive method. SAE, serious adverse event.
At visit 2, women reported satisfaction with their most recent contraceptive method used prior to enrollment. Women reported their satisfaction with the study treatment at every subsequent study visit; visit 3 corresponded to study cycle 2, visit 4 corresponded to study cycles 5 or 6, and visit 5 occurred 14–30 days after study cycle 7. Visit 5 included responses from women who returned 14–30 days after their seventh cycle or from their last use of study product for early termination.
4. Discussion
This study assessed the contraceptive effectiveness, safety, and acceptability of a novel vaginal pH regulator. In consultation with the FDA, this study design employed rigorous criteria for defining qualifying cycles consistent with their current recommendations for contraceptive trials, including (1) 21–35-day cycle length, (2) no backup/emergency contraception used, and (3) at least one act of intercourse in each cycle. As agreed upon with the FDA, the study was seven cycles in length, consistent with other nonhormonal, on-demand contraceptive trials [13,14]. The primary effectiveness endpoint was met; the typical-use seven-cycle cumulative pregnancy percentage was 13.7%, with an upper limit of the 95% CI being ≤ 21%. Cycle lengths between 21 and 42 days are also considered ovulatory [11,12], and a sensitivity analysis including 21–42-day cycles reduced the seven-cycle cumulative pregnancy percentage to 12.5%. These analyses do not represent efficacy of the study drug with correct and consistent use, nor are they what we would expect with “real-world” use (i.e., where women may use a second method, may not have intercourse every cycle, and/or may have varying cycle lengths when not on hormonal contraception). Therefore, additional ad hoc analyses will be reported separately.
The study drug was safe and well tolerated. The most commonly reported AEs included burning and pruritus at rates similar to those reported in a randomized crossover trial evaluating female and male condom acceptability: 30% and 17% of women using the female and male condom, respectively, experienced burning/itching/irritation [15]. In this study, most women reported their highest-severity AE to be mild or moderate. Fewer than 2% of women discontinued due to AEs, with even fewer discontinuing due to genitourinary AEs. This is much lower than the observed discontinuation rates due to AEs in hormonal contraceptive studies [[16], [17], [18]]. The incidence of serious AEs was low; only one event (cystitis) was considered “probably” related to treatment.
Nearly half (46.8%) of enrolled women completed at least six study cycles. The discontinuation rate (53.3%) was similar to reports from other contraceptive clinical trials where nearly half of enrolled women discontinued [14,[16], [17], [18]]. For women seeking on-demand contraception, these discontinuation rates were not unexpected [19].
This study has several strengths: the large study population, geographically diverse study sites, the range of demographic backgrounds of women included, frequent pregnancy testing, and real-time assessments of sexual intercourse and side effects. The study also included women at the highest risk of pregnancy (i.e., sexually active women with regular 21–35-day cycles), and women with proven fertility (2.5 mean pregnancies; 1.5 mean full-term deliveries). Study limitations include its open-label design, which may have introduced a possibility for bias among women and study personnel. The relatively short study duration might limit the ability to understand long-term effectiveness of the study drug. In contraception studies, the likelihood of pregnancy decreases over time due to attrition, as women at highest risk become pregnant early and exit from the study [[20], [21], [22]]. Furthermore, the likelihood of pregnancy declines over time as women become more proficient with correct use of the contraceptive method [20,22]. These results are also not necessarily indicative of “real-world” failure rates due to the rigorous nature of the clinical trial design. The study design followed key recommendations from the FDA for establishing efficacy in phase 3 hormonal contraceptive trials including the following: US-based, no body mass index restrictions, regular urine pregnancy tests at study visits, and excluding cycles where no sexual intercourse occurred and where backup/emergency contraception was used [23]; however, there has not been any guidance specific to nonhormonal or on-demand contraceptive clinical trial design. As more contraceptive studies are developed with the FDA's recommendations for establishing efficacy, cumulative pregnancy rates observed may be higher than what has been reported historically, similar to the phenomenon observed of reduced efficacy in newer oral combined hormonal contraceptives [22,23].
An international survey found that 49% of women prefer nondaily forms of contraception and 73% prefer a method that allows them to stop using at the moment they choose [2]. Besides contraceptive effectiveness, noncontraceptive benefits are also important to women [24,25]. Its unique viscosity-retaining properties offer women seeking a contraceptive option the noncontraceptive benefit of lubrication, and it can be used with other contraceptives, consistent with research suggesting that some women prefer to use multiple methods simultaneously [26]. This new vaginal pH regulator offers women a contraceptive option that may be used “in the moment” and allows for flexibility and control.
Acknowledgments
The authors would like to acknowledge the contributions of the investigators and staff of the 112 sites in the United States. Medical writing assistance was provided by Ying Hou, Ph.D., of PharmaWrite, LLC, and was funded by Evofem Biosciences Inc. Evofem Biosciences, Inc., provided a full review of the article. The study was sponsored by Evofem Biosciences, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals' “Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3.”
Footnotes
Clinical Trial Registration: This clinical trial was registered at www.ClinicalTrials.gov as: NCT03243305; AMP002 Phase III Contraceptive Study. The URL can be found at: https://clinicaltrials.gov/ct2/show/NCT03243305.
Funding: This study was sponsored by Evofem Biosciences, Inc. Medical writing assistance was provided by PharmaWrite, LLC, and was funded by Evofem Biosciences Inc. Evofem Biosciences, Inc., provided a full review of the article and had a role in the design, execution, data collection and analysis, reporting, and funding of the study. Each clinical site received sufficient supplies for enrolled women.
Disclosure:
M.A.T.: Research, Evofem Biosciences, Inc.
B.T.C.: Research, Evofem Biosciences, Inc.
B.M.: Research and consultant, Evofem Biosciences, Inc.
K.R.C.: Employee, Evofem Biosciences, Inc.
C.D.: Employee, Health Decisions, which received funding from Evofem Biosciences, Inc. to help conduct this study.
B.H.: Employee, Evofem Biosciences, Inc.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conx.2020.100031.
Appendix A. Supplementary data
Supplementary tables
References
- 1.Finer L.B., Zolna M.R. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843–852. doi: 10.1056/NEJMsa1506575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansour D. International survey to assess women’s attitudes regarding choice of daily versus nondaily female hormonal contraception. Int J Womens Health. 2014;6:367–375. doi: 10.2147/IJWH.S59059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg S., Anderson R.A., Chany C.J., 2nd, Waller D.P., Diao X.H., Vermani K. Properties of a new acid-buffering bioadhesive vaginal formulation (ACIDFORM) Contraception. 2001;64:67–75. doi: 10.1016/s0010-7824(01)00217-7. [DOI] [PubMed] [Google Scholar]
- 4.Bayer L.L., Jensen J.T. ACIDFORM: a review of the evidence. Contraception. 2014;90:11–18. doi: 10.1016/j.contraception.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Garg S., Tambwekar K.R., Vermani K., Garg A., Kaul C.L., Zaneveld L.J.D. Compendium of pharmaceuticals for vaginal formulations. Pharm Technol Drug Del. 2001;25:14–24. [Google Scholar]
- 6.Amaral E., Faundes A., Zaneveld L., Waller D., Garg S. Study of the vaginal tolerance to Acidform, an acid-buffering, bioadhesive gel. Contraception. 1999;60:361–366. doi: 10.1016/s0010-7824(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 7.Amaral E., Perdigao A., Souza M.H., Mauck C., Waller D., Zaneveld L. Postcoital testing after the use of a bio-adhesive acid buffering gel (ACIDFORM) and a 2% nonoxynol-9 product. Contraception. 2004;70:492–497. doi: 10.1016/j.contraception.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Amaral E., Perdigao A., Souza M.H., Mauck C., Waller D., Zaneveld L. Vaginal safety after use of a bioadhesive, acid-buffering, microbicidal contraceptive gel (ACIDFORM) and a 2% nonoxynol-9 product. Contraception. 2006;73:542–547. doi: 10.1016/j.contraception.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Keller M.J., Carpenter C.A., Lo Y., Einstein M.H., Liu C., Fredricks D.N. Phase I randomized safety study of twice daily dosing of acidform vaginal gel: candidate antimicrobial contraceptive. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz J.L., Poindexter A., Schmitz S.W., Mauck C., Callahan M.M. Male tolerance of ACIDFORM gel. Contraception. 2005;71:443–446. doi: 10.1016/j.contraception.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Chiazze L., Jr., Brayer F.T., Macisco J.J., Jr., Parker M.P., Duffy B.J. The length and variability of the human menstrual cycle. JAMA. 1968;203:377–380. [PubMed] [Google Scholar]
- 12.Berglund Scherwitzl E., Lindén Hirschberg A., Scherwitzl R. Identification and prediction of the fertile window using NaturalCycles. Eur J Contracept Reprod Health Care. 2015;20:403–408. doi: 10.3109/13625187.2014.988210. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz J.L., Weiner D.H., Lai J.J., Frezieres R.G., Creinin M.D., Archer D.F. Contraceptive efficacy, safety, fit, and acceptability of a single-size diaphragm developed with end-user input. Obstet Gynecol. 2015;125:895–903. doi: 10.1097/AOG.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 14.Chen B.A., Blithe D.L., Muraguri G.R., Lance A.A., Carr B.R., Jensen J.T. Acceptability of the woman’s condom in a phase III multicenter open-label study. Contraception. 2019;99:357–362. doi: 10.1016/j.contraception.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulczycki A., Kim D.-J., Duerr A., Jamieson D.J., Macaluso M. The acceptability of the female and male condom: a randomized crossover trial. Perspect Sex Reprod Health. 2004;36:114–119. doi: 10.1363/psrh.36.114.04. [DOI] [PubMed] [Google Scholar]
- 16.Portman D.J., Kaunitz A.M., Howard B., Weiss H., Hsieh J., Ricciotti N. Efficacy and safety of an ascending-dose, extended-regimen levonorgestrel/ethinyl estradiol combined oral contraceptive. Contraception. 2014;89:299–306. doi: 10.1016/j.contraception.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Kroll R., Ackerman R., Feldman R., Howard B., Weiss H., Hsieh J. Efficacy and safety of a 21/7-active combined oral contraceptive with continuous low-dose ethinyl estradiol. Contraception. 2016;93:249–256. doi: 10.1016/j.contraception.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Archer DF, Merkatz RB, Bahamondes L, Westhoff CL, Darney P, Apter D, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health 2019;7:e1054-e64. [DOI] [PMC free article] [PubMed]
- 19.Trussell J., Vaughan B. Contraceptive failure, method-related discontinuation and resumption of use: results from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999;31:64–72. [93] [PubMed] [Google Scholar]
- 20.Trussell J. Methodological pitfalls in the analysis of contraceptive failure. Stat Med. 1991;10:201–220. doi: 10.1002/sim.4780100206. [DOI] [PubMed] [Google Scholar]
- 21.Trussell J. Understanding contraceptive failure. Best Pract Res Clin Obstet Gynaecol. 2009;23:199–209. doi: 10.1016/j.bpobgyn.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trussell J., Portman D. The creeping pearl: why has the rate of contraceptive failure increased in clinical trials of combined hormonal contraceptive pills? Contraception. 2013;88:604–610. doi: 10.1016/j.contraception.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food and Drug Administration Establishing effectiveness and safety for hormonal drug products intended to prevent pregnancy: guidance for industry. 2019. https://www.fda.gov/media/128792/download Available at:
- 24.Nappi R.E., Albani F., Tonani S., Santamaria V., Pisani C., Terreno E. Psychosexual well-being in women using oral contraceptives containing drospirenone. Funct Neurol. 2009;24:71–75. [PubMed] [Google Scholar]
- 25.Bahamondes L., Valeria Bahamondes M., Shulman L.P. Non-contraceptive benefits of hormonal and intrauterine reversible contraceptive methods. Hum Reprod Update. 2015;21:640–651. doi: 10.1093/humupd/dmv023. [DOI] [PubMed] [Google Scholar]
- 26.Frohwirth L., Blades N., Moore A.M., Wurtz H. The complexity of multiple contraceptive method use and the anxiety that informs it: implications for theory and practice. Arch Sex Behav. 2016;45:2123–2135. doi: 10.1007/s10508-016-0706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables