Abstract
INTRODUCTION:
Endoscopic cyclophotocoagulation (ECP) has been effective in the management of a variety of difficult pediatric and adult glaucomas. This study reports long-term efficacy and safety of ECP in pediatric glaucoma following cataract surgery (GFCS).
METHODS:
ECP was performed on 35 eyes of 24 patients under 16 years of age with GFCS. Patients were followed for a minimum of 2 years. Treatment failure was defined as consecutive postoperative intraocular pressures (IOP) of >24 mm Hg, alternative glaucoma procedure following ECP, or occurrence of visually significant complications. Analysis was performed to estimate risk factors for failure.
RESULTS:
Success rate was 54% (19 out of 35 eyes). 27 aphakic and 8 pseudophakic eyes were included in this study. Pretreatment IOP averaged 33.9 ± 7.9 mmHg. Average degrees of treatment per procedure was 218°. Final IOP after a mean follow-up period of 7.2 years was 18.9 ± 8.8 mmHg (p <0.001). Patients with single ECP demonstrated significant improvement in visual acuity from baseline to most recent follow up. 62% of eyes received one treatment only. Failure rate was not increased in pseudophakic patients relative to aphakic patients. Estimated risk factors for treatment failure include increased IOP at first measurement following cataract extraction, increased baseline IOP prior to ECP, and increased time between first IOP measured following cataract extraction and first ECP.
CONCLUSION:
Analysis of longitudinal IOP and visual acuity data demonstrates that ECP remains a successful tool in the treatment of pediatric GFCS and has a low rate of visually significant complications.
Keywords: glaucoma, children, cataract
Introduction
Pediatric glaucoma is a difficult clinical problem and can cause irreversible blindness in children. In particular, glaucoma following cataract surgery (GFCS) is an unfortunate common occurrence. The Infant Aphakia Treatment Study (IATS) revealed the risk of developing glaucoma or being a glaucoma suspect at 4.8 years after surgery to be 17% and 31%, respectively.1 These patients are often inadequately responsive to medical management. Surgical options include trabeculectomy, seton implantation, and cyclodestructive procedures. Success rates related to these procedures in GFCS have been previously reported.2
Endoscopic cyclophotocoagulation (ECP), a ciliary destructive procedure, can be used in the management of a variety of glaucoma cases, though it remains an infrequently used tool in the management of pediatric glaucoma. Its safety and effectiveness have been demonstrated in prior studies of difficult secondary glaucomas in which the etiology of increased intraocular pressure was due to a variety of mechanisms.2,3 ECP has been shown to be effective as a primary intervention for cases of glaucoma following cataract surgery (GFCS) in young children over an average follow up of 3.7 years.2
This study aims to determine the efficacy and safety of ECP over a longer follow up period, increasing minimum follow-up from one year to two years. In this retrospective study, we reviewed the records of aphakic and pseudophakic patients treated with ECP and analyzed the baseline data and treatment outcomes to better understand which patients benefit most from the procedure. The present study expands previous studies by providing longer follow-up, longitudinal analysis of postoperative IOP, and reporting visual acuity as a functional outcome of the procedure.
From initial use of ECP at our institution in 1994, it seemed that of the varied mechanisms of pediatric glaucoma, the aphakic subgroup was suggested to be best suited for this treatment modality. Another aim of this study is to further investigate if pseudophakia is a risk factor for treatment failure.
Methods
The Indiana University Institutional Review Board approved this study, which was performed in accordance with the Health Insurance Portability and Accountability Act. Suitable patients were identified by procedure codes and charts were retrospectively reviewed.
Inclusion Criteria:
Children under 16 years of age with GFCS treated with ECP from 1994 to 2014 at Riley Children’s Hospital in Indianapolis, IN were included. Patients were followed for a minimum of 2 years or until a treatment failure had been declared.
Treatment failure
was defined as the occurrence of any of the following during post-operative follow-up: 1) Any elevated IOP greater than 24 on consecutive visits despite glaucoma medications, 2) any other glaucoma procedure other than ECP, or 3) any sight-threatening complications such as hypotony or retinal detachment.
Exclusion Criteria:
Patients with congenital glaucoma, anterior segment dysgenesis, or other secondary glaucomas were excluded from this study.
The diagnosis of GFCS was based on unacceptable intraocular pressures combined with evidence of optic nerve damage. Medical therapy was instituted in most patients prior to the first ECP treatment. In all patients, ECP was the first glaucoma surgical intervention. Any eye that had a suboptimal IOP and received further ECP was not deemed a treatment failure unless IOP at last follow-up exceeded that required for the definition of success. The decision to proceed with further ECP versus an alternative glaucoma intervention such as tube-shunt placement was made by the primary surgeons (DAP, DEN).
Treatment for all patients was administered with the Microprobe (Endo Optiks Inc., Little Silver, NJ). A schematic illustration of this procedure has been previously published.2 Specific technique as well as preoperative and postoperative care was held consistent to the methods described in detail by Carter, et al.2 Using sterile technique a limbal incision was created with aid of an operating microscope. The 20 gauge endoscopic hand piece with 810 nm diode laser capable of 1.2 W of available power, focused via a 670 nm diode laser aiming-beam was then inserted across the anterior chamber, through the pupil, and posterior to the iris to visualize the ciliary processes. Pulses of 200–800 mW were applied to the individual processes, titrated to achieve blanching and contracture of the process. Treatments ranged from 120° to 300°. A second limbal incision was often fashioned to facilitate visualization of additional ciliary processes. All limbal incisions were closed and a combination antibiotic/steroid eye drop was applied followed by application of a soft patch, which remained in place until removal in the clinic on postoperative day-1 exam. In the postoperative period, antibiotic/steroid drops were used four times daily as well as once daily application of 1% atropine. Prednisolone 1% was added if significant anterior chamber inflammation developed. With rare exception, follow-up examinations took place at postoperative day 1, week 1, and months 1, 3, and 6. Data collected included current medications, visual acuity, and intraocular pressure.
Results
Thirty-five eyes of 24 patients were included in the study. Laterality of disease is shown in Table 1. Patient characteristics and baseline data including gender; corneal diameter; age at dates of lensectomy, diagnosis of GFCS, and first ECP treatments; IOP at baseline and last follow up; and total follow-up time are summarized in Table 2. At the time of treatment, all patients had undergone prior lensectomy and were either aphakic (27 eyes) or pseudophakic (8 eyes).
Table 1.
Laterality of disease
| Laterality | No. of patients (n = 24) |
|---|---|
| Bilateral cataract/bilateral glaucoma | 14 |
| Bilateral subluxed lenses/bilateral glaucoma | 1 |
| Bilateral cataract/unilateral glaucoma | 6 |
| Unilateral cataract/unilateral glaucoma | 4 |
Table 2.
Eye characteristics and outcomes
| No. (%) or Mean (Range) | |
|---|---|
| No. of patients | 24 |
| No. of eyes | 35 |
| Male | 17 (49%) |
| Female | 18 (51%) |
| Right | 17 (49%) |
| Left | 18 (51%) |
| Aphakic | 27 (77%) |
| Pseudophakic | 8 (23%) |
| No. of patients treated bilaterally | 10 (42%) |
| Corneal diameter at lensectomy (mm)* | 9.9±1.9 (5.4–14.0) |
| Age at lensectomy (yr) | 0.7±1.7 (0.1–6.4) |
| Age at diagnosis of GFCS (yr) | 4.0±2.5 (0.3–8.0) |
| Age at first ECP treatment (yr) | 6.0±3.8 (0.4–12.3) |
| Baseline ECP IOP (mm Hg) | 34.1±8.3 (20–50) |
| Final Visit IOP (mm Hg) | 18.9±8.8 (8–57) |
| Follow Up Time (yr) | 7.2±3.6 (2.2–17.1) |
Recorded in 18 out of 35 eyes
General Analysis
As shown in Table 2, baseline preoperative IOP of 34.1±8.3 mm Hg was reduced to 18.9±8.8 mm Hg after an average follow up time of 7.2±3.6 years. This represents an overall decrease in IOP of 45% at most recent visit. On average, the diagnosis of GFCS was made 3.3 years after lensectomy, and ECP was performed 2.0 years after diagnosis of GFCS. All patients were followed for a minimum of 2 years. Seven patients were followed for more than 10 years at the time of the study. Of the eyes included in this study, 80% had single ECP and in this subgroup 14% required further alternative surgery, and 20% of all eyes required multiple ECP with 86% in this subgroup requiring further alternative surgery.
Success Rate
Of the 35 eyes treated with ECP, 19 eyes (54%) were considered treatment successes based on most recent intraocular pressure data. Of the 27 aphakic eyes, 13 (48%) were successfully treated. Of the 8 pseudophakic eyes, 6 (75%) were successfully treated. Table 3 shows success rates based on number of ECP procedures. Of the 7 eyes that received multiple ECP treatments, only 1 eye (14% of this group), which had two ECP procedures, was a treatment success. Neither of the 2 eyes which received 3 ECP treatments were successful. The mean number of ECP treatments per eye was 1.3±0.6 (1–3) times. The average degree of treatment for first ECP procedure was 230±42.5 (120–270). The average degrees of treatment for repeat ECP was 151±54.9 (90–240). Four patients had bilateral ECP success; four patients had bilateral ECP failure. One patient had ECP success in one eye and failure in the other eye.
Table 3.
Success rates by number of ECP treatments for GFCS
| Treatment Number | No. of eyes treated | No. of successfully treated eyes | Success rate (%) |
|---|---|---|---|
| One | 28 | 18 | 64 |
| Two | 5 | 1 | 20 |
| Three | 2 | 0 | 0 |
Longitudinal Analysis
Longitudinal analysis results are compared between single ECP eyes and multiple ECP eyes in Figure 1 and successful and failed Eyes in Figure 2. There is a statistically significant difference in baseline IOP in successful eyes vs. failed eyes with values of 32.1±7.4 (20–48) and 36.1±8.1 (24–48), respectively. At the 6-month postoperative IOP measurement, there is a significant difference (p = 0.03) between successful and failed eyes with values of 18.6±4.0 (10–24) and 28.6±13.3 (12–53), respectively.
Figure 1.
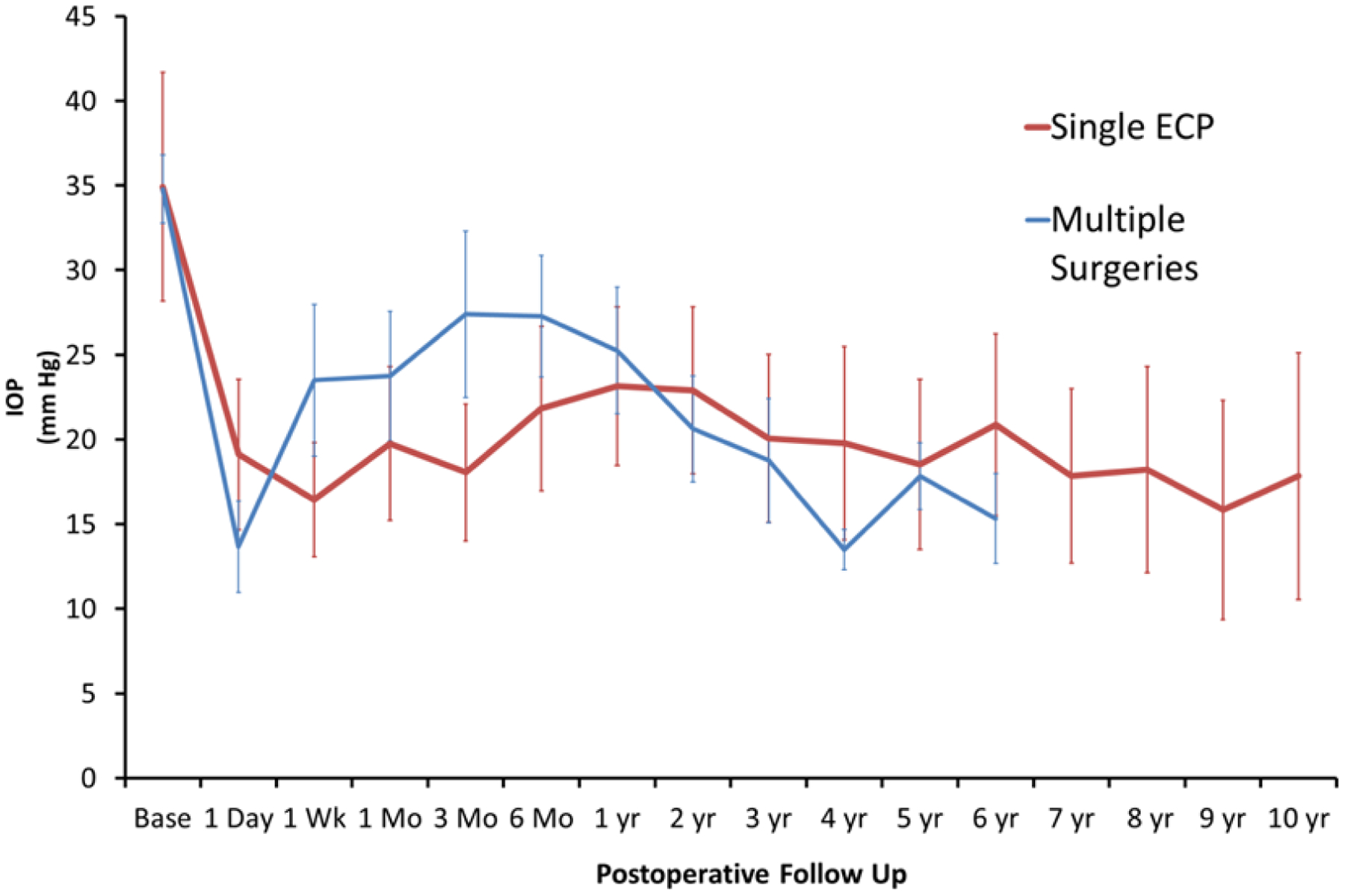
Mean baseline and postoperative IOP (with standard deviation) plotted over time in eyes which received single ECP treatment versus eyes that received multiple surgeries.
Figure 2.
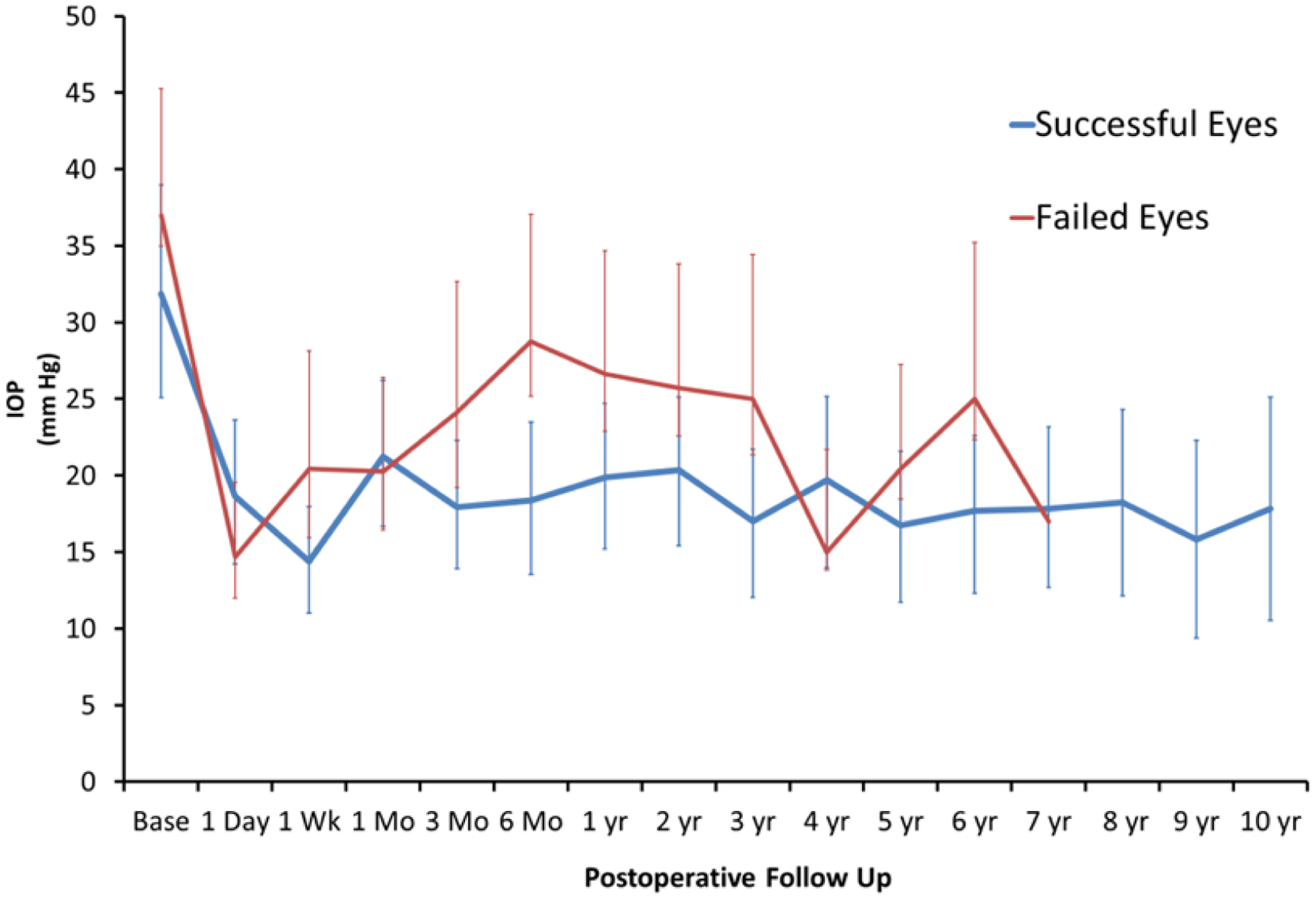
Mean baseline and postoperative IOP (with standard deviation) plotted over time in successful and failed eyes.
Kaplan-Meier survival curves with 95% confidence intervals (CI) were plotted to facilitate visualization of the results and are shown for the single ECP group and the multiple ECP group in Figure 3 and 4, respectively. For the single ECP group, failure time was defined as time from baseline (first ECP procedure) to the first treatment failure. For the multiple ECP group, failure time was defined as time from baseline to the second treatment failure. It is found that, for the single ECP group, the failure probability is 0.23 (CI: 0.06, 0.36) at 6 months, 0.32 (CI: 0.14, 0.47) at 12 months, and 0.39 (CI: 0.20, 0.55) at 24 months. For the multiple ECP group, the (second treatment) failure probability is 0.22 (CI: 0.01, 0.45) at 12 months, 0.35 (CI: 0.01, 0.60) at 24 months, and 0.74 (CI: 0.17, 0.98) at 36 months.
Figure 3.
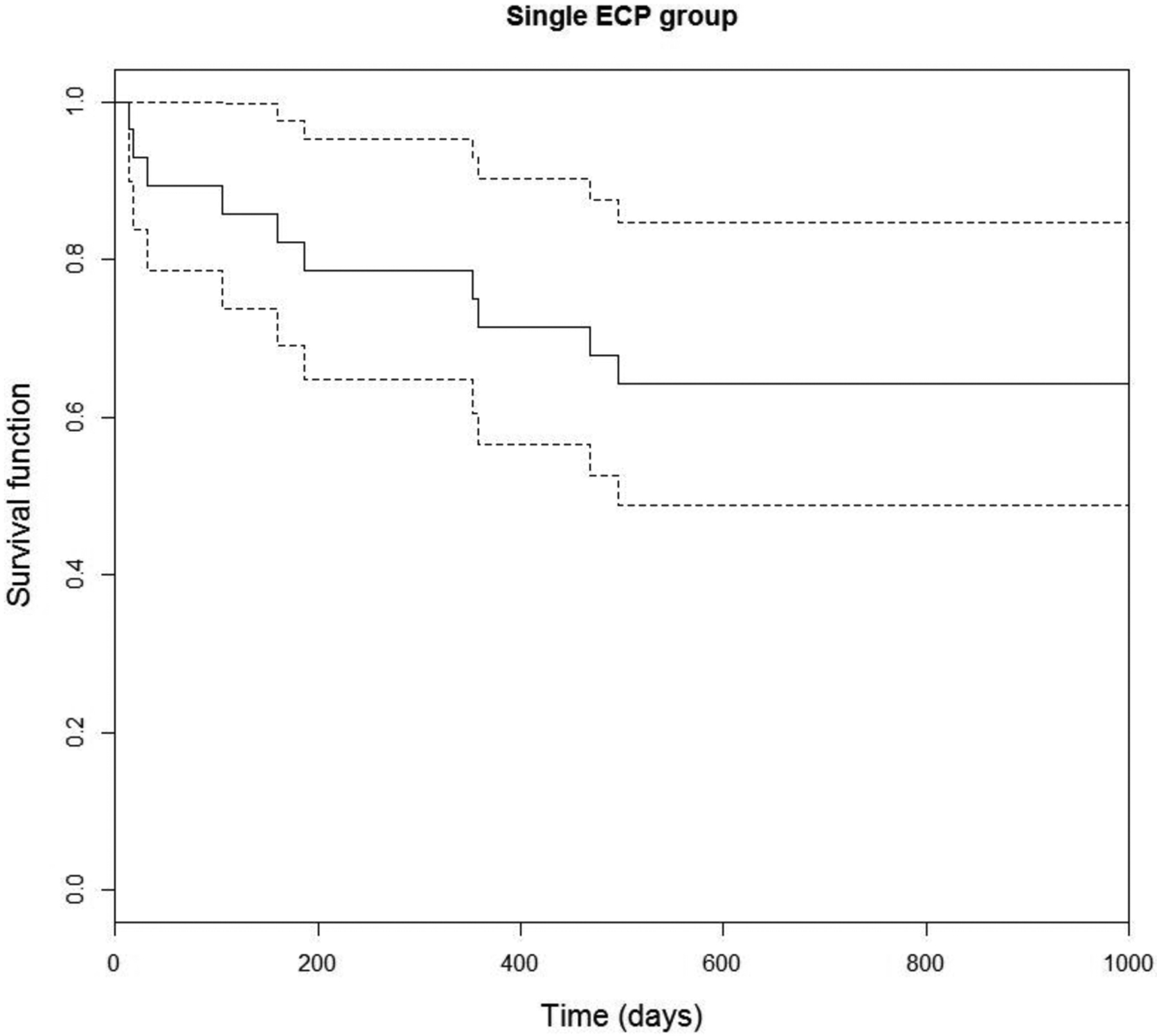
Survival analysis demonstrating time to first treatment failure.
Figure 4.
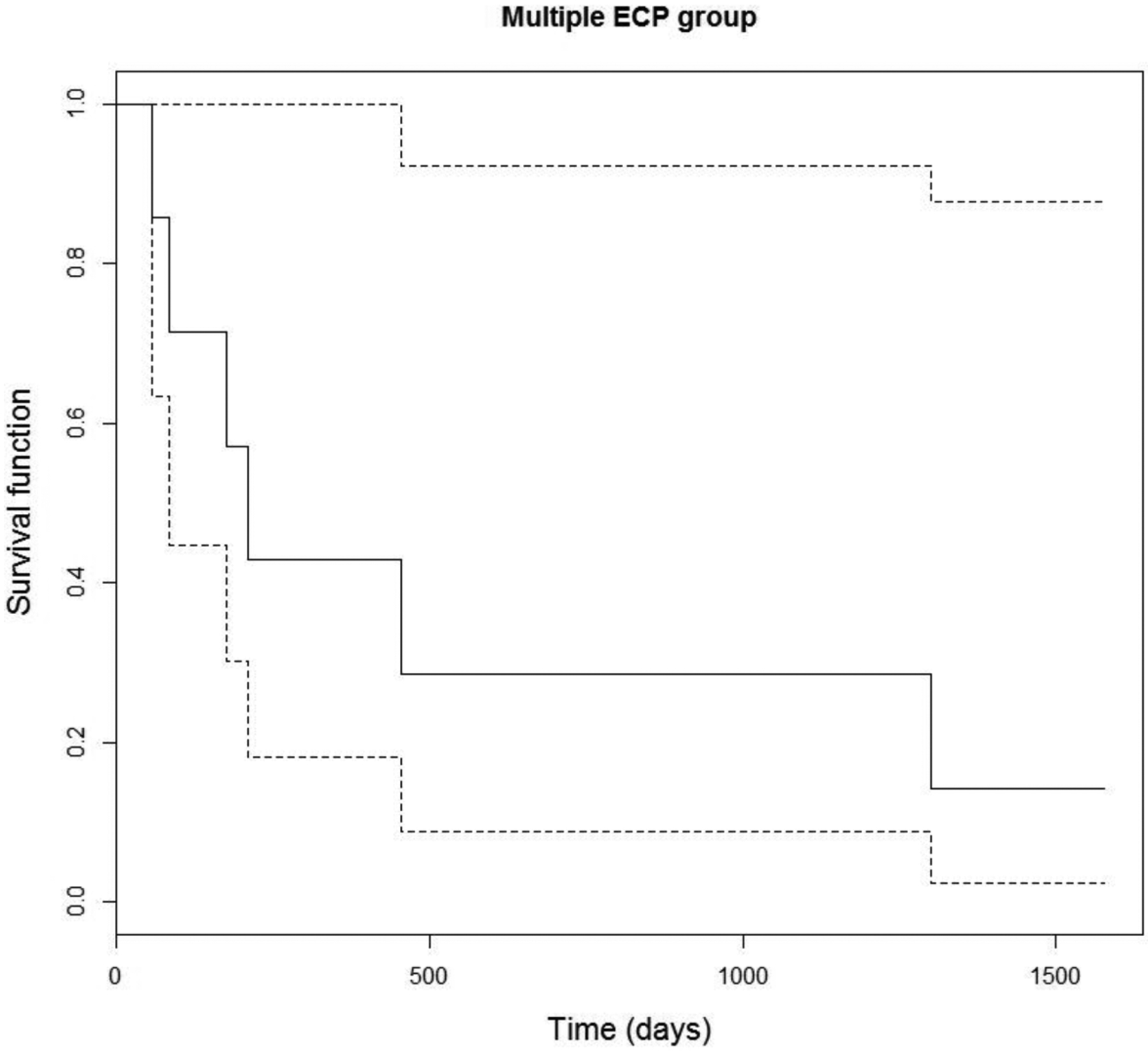
Survival analysis demonstrating time to second treatment failure.
Failure Risk Factors
All analyzed risk factors for ECP failure are shown in Table 4. Significant risk factors include the following factors: 1) increased IOP at first measurement following lensectomy, 2) increased number of ECP procedures performed, 3) decreased time between 1st IOP after lensectomy and ECP, and 4) increased number of glaucoma medications taken after ECP. Other factors which showed a trend for failure which were not statistically significant (0.05 < p < 0.20) include increased IOP at time of glaucoma diagnosis, decreased patient age at time of first ECP, decreased time between lensectomy and ECP, decreased time between glaucoma diagnosis and ECP, and increased baseline IOP at time of first ECP. There were no significant findings for failure rate based on sex or race.
Table 4.
Risk Factors for Failure
| Success | Failure | ||||
|---|---|---|---|---|---|
| Mean±SD | Range | Mean±SD | Range | P | |
| Lensectomy | |||||
| Age (yrs) | 0.8±1.8 | 0.1–6.4 | 0.5±1.5 | 0.0–6.2 | 0.65 |
| Time to first post-op IOP (yrs) | 1.1±1.2 | 0.1–4.5 | 1.8±2.1 | 0.0–5.9 | 0.24 |
| First measured post-op IOP (mm Hg) | 17±7.8 | 5–32 | 25.1±8.8 | 14–42 | 0.01* |
| Glaucoma Diagnosis | |||||
| Time between lensectomy and glaucoma diagnosis (yrs) | 3.8±2.3 | 0.2–7.9 | 2.8±2.5 | 0.2–6.5 | 0.23 |
| IOP at time of glaucoma diagnosis (mm Hg) | 28.6±7.7 | 21–48 | 32±6.4 | 22–42 | 0.16 |
| ECP | |||||
| Number of ECP treatments | 1.1±0.2 | 1–2 | 1.5±0.7 | 1–3 | 0.03* |
| Degrees of treatment of 1st ECP (°) | 224±47 | 120–270 | 238±36 | 180–270 | 0.35 |
| Patient age at time of first ECP (yrs) | 7.1±3.8 | 0.4–12.3 | 4.7±3.5 | 0.4–9.8 | 0.06 |
| Time between lensectomy and ECP (yrs) | 6.3±3.8 | 0.2–12.1 | 4.2±3.6 | 0.3–9.8 | 0.09 |
| Time between 1st IOP after lensectomy and ECP (yrs) | 5.2±3.7 | 0.0–11.4 | 2.3±2.5 | 0.0–8.3 | 0.01* |
| Time between glaucoma diagnosis and ECP (yrs) | 3.3±3.2 | 0.1–9.6 | 1.9±2.0 | 0.1–6.2 | 0.11 |
| Baseline IOP at time of 1st ECP (mm Hg) | 32.1±7.4 | 20–48 | 36.5±8.8 | 24–50 | 0.12 |
| Number of glaucoma medications before ECP | 1.8±1.3 | 0–3 | 1.7±1.0 | 0–3 | 0.95 |
| Number of glaucoma medications after ECP | 0.8±1.3 | 0–3 | 1.8±1.2 | 0–3 | 0.03* |
Visual Acuity
At most recent follow up, 3 out of 35 (9%) of all eyes had visual acuity of hand motion or less. Among 32 remaining eyes, in those which received single ECP, LogMAR visual acuity demonstrated a significant improvement from baseline at 0.80 ± 0.39 (0.10 – 1.60) to 0.60 ± 0.41 (0.00–1.40) (p < 0.01); in those which received multiple ECP, visual acuity did not show significant change from baseline 0.99 ± 0.26 (0.70–1.30) to 0.89 ± 0.46 (0.30–1.48) (p = 0.75).
Complications
Three surgical complications were encountered in this study. 1) One eye received pars plana vitrectomy and capsulotomy at 5 months after ECP and immediately developed endophthalmitis which progressed to phthisis. 2) One eye had G-probe performed 4 months after ECP, and gradually progressed to phthisis. 3) One eye had 3 ECP procedures performed followed by a trabeculotomy, then gradually progressed to phthisis.
Discussion
Compared previous similar studies, this study demonstrated that the procedure of ECP applied on children with glaucoma following cataract surgery can be successful over a longer follow up period up to an average of 7.2 years. One patient in this study was followed for more than 17 years.
Longitudinal analysis demonstrates the progression of patients’ clinical course following ECP. There is an immediate decrease in IOP in the first week postoperatively across all groups. In failed eyes, an upward trend in IOP is noted after 1 week and peaks at 6 months to an average IOP of 29 mm Hg which is statistically significant when compared to 19 mm Hg in successful eyes at that time. Thus, IOP at 6 months may be a good predictor of whether a patient is likely to fail ECP. A similar peak in IOP is seen in patients’ eyes that underwent multiple ECP procedures, corresponding to the fact that most eyes which received multiple ECP were ultimately unsuccessful.
Visual acuity is perhaps the best marker for treatment success as it represents a functional outcome related to quality of life for the patient. Increasing minimum follow-up time in a study results in better estimation of visual acuity especially since ECP was performed at an age where visual acuity cannot be measured on a numerical scale in many children. An improvement in visual acuity from baseline was demonstrated in successful patients. This improvement should be interpreted as successful prevention of vision loss.
In patients with higher IOPs at time of GFCS diagnosis or who fail initial ECP, the chance of success of the procedure is lower. The previous study in this population demonstrated average IOP decrease of 30% from baseline to final postoperative measurement.3 This study shows average IOP decrease of 45%. A success rate of 54% in this study is similar as that in the previous study which had a shorter average follow up (53%). Previously, 38% of eyes received one ECP treatment compared to 83% currently. The previous study experienced more success in multiple ECP eyes. This may be due to the fact that many initial eyes received 180 degrees of treatment, which was considered by the primary surgeons to be an undertreatment and was increased to a more aggressive treatment of 270 degrees in later years. Complications were rare in both studies. No retinal detachments occurred in this study, and while no hypotony was recorded (IOP <5), there were three cases of phthisis which developed after procedures other than ECP.
In this study, failed eyes had higher IOPs at diagnosis of GFCS and higher baseline number of glaucoma medications prior to ECP. This likely represents eyes with a more advanced glaucoma pathology. It is interesting to note that eyes which had ECP performed sooner after their first IOP measurement following lensectomy were significantly at risk to fail. This is counterintuitive to the thought that earlier treatment should avoid glaucoma progression. However, the likely explanation in this study is that these eyes failed multiple medications sooner and thus were more quickly advanced to surgical intervention. This could represent a glaucoma course that develops more quickly in failed eyes. This hypothesis could explain why increased medications preoperatively is a risk factor for failure. Importantly, retreatment with ECP resulted in failure in 6 out of 7 eyes which received multiple treatments. Though a small population sample, this failure risk factor was statistically significant. The data suggests that retreatment with ECP may not improve outcome.
Numerous investigations have allowed ophthalmologists to better determine which infants and children may be most at risk to develop GFCS, although much remains unknown. Overall incidence of the condition ranges from 3 to 41% in various study populations,4–7 and time from cataract extraction to glaucoma diagnosis also appears variable, with reports citing from 4.0 to 6.8 years.6–9 However, this study found an average time from cataract extraction to glaucoma diagnosis to be less at 3.3±2.4 (0.2–7.9) years.
In the early postoperative phase, IOPs were often observed to be mildly elevated in the 21–24 mm Hg range. There is a lack of conclusive data demonstrating this initial elevation is associated with any optic nerve damage. Also, it has been established that most aphakic children have a higher recorded central corneal thickness (CCT)2, resulting in artificially elevated applanation pressure, thus allowing the tolerable pressure cutoff to be slightly higher than in other pediatric subgroups. Small corneal diameter may be most predictive of the disease and has been implicated as a possible causative agent in more than one study.6,8 Chart review in this study failed to identify cataract type consistently, though previous reports have found nuclear and PFV cataracts have been found to be associated with GFCS.6 Likewise, age at cataract extraction may play a role, with children less than 1 year of age carrying the most risk of development of GFCS.5 In contrast to previous findings, there was not increased risk of failure in pseudophakic eyes compared to aphakic eyes.
The importance of corneal diameter and its role as a risk factor in the development of GFCS is also highlighted. 14 of the 35 eyes were diagnosed with GFCS within 12 months of cataract extraction. Documentation of corneal diameter at the time of cataract extraction was found in 18 out of the 35 eyes. The importance of this value in development of GFCS was again apparent, as the average corneal diameter at the time of cataract extraction was 9.9±1.9 (5.4–14.0) mm. This data is comparable to that provided by Parks et al,6 who noted that GFCS developed in 31.9% of eyes with a corneal diameter of less than 10 mm, compared with 2.9% in eyes with a corneal diameter of greater than 10 mm. Aphakic eyes with microcornea have been found to have thicker central cornea thickness and greater measured IOPs than normal eyes, and it has been suggested that this measurement be included in assessing aphakic eyes with microcornea for possible glaucoma.10
Laterality of glaucoma in these patients may yield more insight into the pathophysiology of the disease. Simon et al7 noted no evidence of bilateral glaucoma in the setting of unilateral aphakia, and no unilateral glaucoma when bilateral aphakia was present. Although our data support the former finding, we did encounter 6 eyes with unilateral GFCS in the setting of bilateral aphakia. This finding was also supported by Chen et al,9 who had 30 patients with history of bilateral lensectomy but unilateral GFCS. The absence of bilateral glaucoma in unilateral aphakia suggests the pathology is intrinsic to the eye, and the presence of glaucoma developing in only one eye in a bilaterally aphakic patient suggests there are further variables which may determine the progression of glaucoma.
Limitations to this study include the possibility of selection bias, as is the case for any study of a retrospective nature. Two eyes were from a patient with Turner Syndrome who had bilateral subluxed lenses rather than cataracts, which represents a likely variation in pathological development of glaucoma. Some eyes from this study undoubtedly are the same eyes studied previously at this institution by Carter, et al.2 Thus, these studies should not be considered as having independent data. However, it is beneficial to have continued follow-up data from eyes which were previously studied. In future study, incorporating analysis of postoperative visual fields to IOP will better illustrate patients’ functional outcomes.
Conclusion
ECP is a viable treatment in the setting of GFCS over an average follow-up period of 7.2 years. Results did not show increased failure in pseudophakic eyes, suggesting the presence of an IOL does not affect the surgical outcome. Survival analysis demonstrates retreatment did not improve the overall success rate, which contrasts previous findings in this population. Continued long-term follow-up and comparison to other glaucoma procedures in pediatric populations will allow more definitive conclusions concerning procedure outcomes of preventing progression of glaucoma and preserving vision in children.
Acknowledgements
The authors thank laboratory assistant, Tierra Nolan, for her assistance in collecting data from chart review. The authors also thank staff at the Indiana University Eugene and Marilyn Glick Eye Institute and Riley Hospital for Children at IU Health for assistance in locating charts for patients involved in this study.
Financial support: This study is supported by a Research to Prevent Blindness (RPB) Unrestricted Grant to the Glick Eye Institute.
Footnotes
Commercial relationship: All authors have no commercial relationship.
REFERENCES
- 1.Freedman SF, Lynn MJ, Beck AD. Glaucoma-Related Adverse Events in the First 5 Years After Unilateral Cataract Removal in the Infant Aphakia Treatment Study. JAMA Ophthalmol. 2015; 133: 907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter BC, Plager DA. Endoscopic diode laser cyclophotocoagulation in the management of aphakic and pseudophakic glaucoma in children. J AAPOS 2007;11:34–40. [DOI] [PubMed] [Google Scholar]
- 3.Neely DE, Plager DA. Endocyclophotocoagulation for management of difficult pediatric glaucomas. J AAPOS 2001;5:221–9. [DOI] [PubMed] [Google Scholar]
- 4.Keech RV, Tongue AC, Scott WE. Complications after surgery for congenital and infantile cataracts. Am J Ophthalmol 1989;108:136–41. [DOI] [PubMed] [Google Scholar]
- 5.Mills MD, Robb RM. Glaucoma following childhood cataract surgery. J AAPOS 1994;31:355–60. [DOI] [PubMed] [Google Scholar]
- 6.Parks MM, Johnson DA, Reed GW. Long term visual results and complications in children with aphakia. Ophthalmology 1993;100:826–41. [DOI] [PubMed] [Google Scholar]
- 7.Simon JW, Mehta N, Simmons ST, Catalano RA, Lininger LL. Glaucoma after pediatric lensectomy/vitrectomy. Ophthalmology 1991;98:670–4. [DOI] [PubMed] [Google Scholar]
- 8.Wallace DK, Plager DA. Corneal diameter in childhood aphakic glaucoma. J AAPOS 1996;33:230–4. [DOI] [PubMed] [Google Scholar]
- 9.Chen TC, Walton DS, Bhatia LS. Aphakic glaucoma after congenital cataract surgery. Arch Ophthalmol 2004;122:1819–25. [DOI] [PubMed] [Google Scholar]
- 10.Bayoumi NH, El Shakankiri NM. Central Corneal Thickness in Aphakic Children with Microcornea-Microphthalmia. J Glaucoma. 2016;25(6):497–500. [DOI] [PubMed] [Google Scholar]


