Abstract
Aim
To assess the intrahepatic and extrahepatic bile duct diameter dilatation after laparoscopic cholecystectomy with magnetic resonance cholangiopancreatography.
Methods
Forty-eight patients (35 women, mean age 54.58 ± 11.83 years) underwent laparoscopic cholecystectomy because of gallstones. The intrahepatic and extrahepatic bile ducts were measured before and three and six months after cholecystectomy. The diameter was measured in the anteroposterior and laterolateral direction at 14 points.
Results
When compared with the preoperative diameter, the common bile duct diameter at the proximal part was significantly wider three months (P = 0.006) and six months (P = 0.0001) after cholecystectomy; the common hepatic duct was significantly wider three months (P = 0.001) and six months (P = 0.003) after cholecystectomy; the right and left hepatic bile ducts were significantly wider six months after cholecystectomy (P < 0.0001, P = 0.01, respectively); and the segmental intrahepatic bile ducts in both hepatic lobes were significantly wider three months (P < 0.0001) and six months after cholecystectomy (P < 0.0001).
Conclusion
This study showed that significant post-cholecystectomy dilatation occurred only at certain points and not along the whole extrahepatic bile duct. We also found a significant dilatation of the main intrahepatic and segmental intrahepatic bile ducts.
The common bile duct (CBD) dilatation occurs due to obstructive changes such as gallbladder tumor or pancreas tumor, choledocholithiasis, previous surgical procedures, and periampular diverticul (1-3). Additional causes may be cholecystectomy (4-9), age (4,10-12), and certain medications (13). However, some ultrasound (US) studies reported no dilatation after cholecystectomy (14-16) and one study reported dilatation after cholecystectomy in patients older than 60 years but not in younger patients (17). An endoscopic retrograde cholangiopancreatography (ERCP) study found CBD to be oval-shaped, meaning that the CBD diameter measured in the anteroposterior direction differed from the diameter measured in the laterolateral direction (18).
CBD diameter changes after cholecystectomy were observed with US, computerized tomography (CT), and ERCP, but most of the studies on this issue were retrospective and performed by US or CT. Due to its noninvasiveness, availability, and lower costs, US is the most frequently used technique for the analysis of bile ducts (19). It is a subjective, observer-dependent method, limited in the cases of colon meteorism or obesity. CT scan is also frequently used, although it can detect the CBD only in 30%-68% of patients (20-22). US and CT scan techniques do not register the juncture of the cystic duct and common hepatic duct, meaning that they do not differentiate between the common hepatic duct and common bile duct (CBD), which is why both ducts together are called the CBD. In patients with suspected cholelithiasis, ERCP has a sensitivity of 0.90 and a specificity of 0.95 (23).
Magnetic resonance cholangiopancreatography (MRCP) is a non-invasive “gold standard” for the evaluation of gallbladder and CBD pathology used to analyze both extrahepatic and intrahepatic bile ducts (24,25). So far, MRCP has been used only in the detection of choledocholithiasis and consequent dilatation, but not in the analysis of post-cholecystectomy dilatation. MRCP is close to the ideal diagnostic modality when it is based on correct clinical suspicion and predictive evaluation (26,27).
Considering the earlier controversial results on the change in the extrahepatic bile ducts diameter after cholecystectomy and the lack of previous research on post-cholecystectomy intrahepatic bile duct changes, this prospective MRCP study aimed to determine the extrahepatic and intrahepatic bile ducts diameter in the anteroposterior and laterolateral direction before and three and six months after laparoscopic cholecystectomy. We also aimed to resolve the controversies around the upper normal diameter limit of the bile ducts in MRCP examination in post-cholecystectomy patients.
PATIENTS AND METHODS
This prospective study involved 50 patients older than 18 years who were referred for elective laparoscopic cholecystectomy because of cholelithiasis or cholesterolosis in the Department of Surgery University Hospital “Sveti Duh” in Zagreb from March 2017 till July 2018. The exclusion criteria were liver diseases (congenital disorders, metabolic disorders, cirrhosis, autoimmune diseases, infections, hepatitis, malignancies), pancreatic diseases (congenital disorders, autoimmune disorders, inflammatory disorders, malignancies), gallbladder and bile ducts disorders (acute inflammation of the gall bladder, acute inflammation of the bile ducts, choledocholithiasis), conversion of laparoscopic surgery to open cholecystectomy, and taking of glucagon, opioids, calcium blockers, atropine, progesterone, histamine2- receptor stimulators, theophylline, octreotide acetate, indomethacin, or erythromycin within three days before surgery. Patients with fat liver infiltration and benign liver tumor changes such as adenoma, hemangioma, and focal nodular hyperplasia were not excluded from the study. Patients were also not excluded from if more than six weeks passed from the end of the treatment of acute gall bladder inflammation until surgery and if they had regular laboratory tests that did not detect inflammation (leukocyte, blood differential test, C-reactive protein). One patient was excluded due to choledocholithiasis three months after surgery and one due to intrahepatic cholelithiasis six months after surgery. The final study group comprised of 48 patients (33 women).
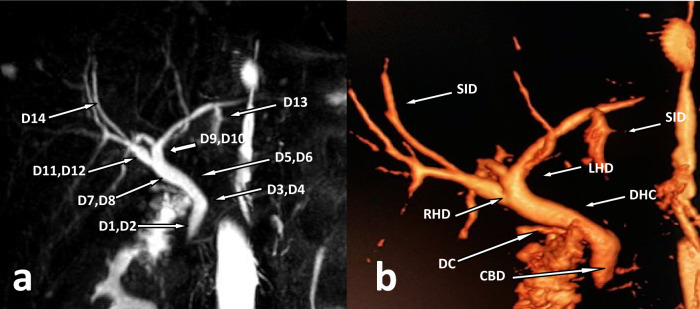
The study was performed in the Department of Radiology of the University hospital “Sveti Duh,” on MR device Phillips Achieva 1.5T (Phillips Medical Systems 2008, Eindhoven, the Netherlands). Patients underwent the examination after at least 8 hours of fasting, within 7 days before cholecystectomy, three months after cholecystectomy, and six months after cholecystectomy. The study was performed with body matrix coil in the MRCP protocol, using coronal three-dimensional high-resolution (3D MRCP HR) sequence with fat saturation: repetition time 1204 ms, echo time 650 ms, slice thickness 1.6 mm, and axial breath-hold balanced turbo field echo sequence: TR 3.7 ms, TE 1.8 ms, SL 5 mm. The examination covered the entire pancreaticobiliary tree. The common bile duct diameter was measured at two points in the distal part above the papilla Vateri and in the proximal part immediately below the connection of the cystic duct and the common hepatic duct. The common hepatic duct diameter was measured at two points in the distal part above the connection of the cystic duct and the common hepatic duct and in the proximal part. The left and right hepatic duct diameters were measured at a distance of 1 cm to their confluence (Figure 1). The diameters were measured in millimeters, rounded to one decimal, in the anteroposterior and laterolateral direction from the inner mucous layer to the inner mucous layer of the wall perpendicular to the longitudinal bile duct axis. In the left and right liver lobe, the widest segmental intrahepatic duct was measured in one dimension. The examination was processed at the same workstation by the same radiologist with eight years of experience.
Figure 1.
Magnetic resonance cholangiopancreatography (MRCP) of the intrahepatic and extrahepatic biliary tree (A) 3D MRCP – measurement points. (B) MRCP volume rendering technique – anatomy; CBD – common bile duct; DHC – common hepatic duct; DC – cystic duct; RHD – right hepatic bile duct; LHD – left hepatic bile duct; SID – segmental intrahepatic bile duct.
In total, the intrahepatic and extrahepatic bile ducts diameters were analyzed at 14 points: D1 – diameter of the common bile duct in the distal part in the anteroposterior direction, D2 – diameter of the common bile duct in the distal part in the laterolateral direction, D3 – diameter of the common bile duct in the proximal part in the anteroposterior direction, D4 – diameter of the common bile duct in the proximal part in the laterolateral direction, D5 – diameter of the common hepatic duct in the distal part in the anteroposterior direction, D6 – diameter of the common hepatic duct in the distal part in the laterolateral direction, D7 – diameter of the common hepatic duct in the proximal part in the anteroposterior direction, D8 – diameter of the common hepatic duct in the proximal part in the laterolateral direction, D9 – diameter of the right hepatic duct in the anteroposterior direction, D10 – diameter of the right hepatic duct in the laterolateral direction, D11 – diameter of the left hepatic duct in the anteroposterior direction, D12 – diameter of the left hepatic duct in the laterolateral direction, D13 – diameter of the widest segmental intrahepatic duct in the right hepatic lobe, D14 – diameter of the widest segmental intrahepatic duct in the left hepatic lobe. The extrahepatic bile ducts were measured at eight points. The intrahepatic bile ducts were measured at six points, the right and left main hepatic bile duct at four points, and the segmental intrahepatic bile duct at one point in each hepatic lobe. The study was approved by the Ethics Committee of the University Hospital “Sveti Duh” (01-580).
Statistical analysis
Categorical data are expressed as absolute and relative frequencies. The normality of distribution was tested with the Kolmogorov-Smirnov test. Numerical data are expressed as mean and standard deviation and median and interquartile range, where applicable. To assess the significance of differences in diameter before and after cholecystectomy, we used the repeated measures ANOVA test and Friedman ANOVA test, where applicable. In the post-hoc analysis, the Wilcoxon test and paired samples t test were used, where applicable. Post-hoc tests P values were Bonferroni corrected, and P < 0.0166 was considered as statistically significant. For the remaining results, the level of statistical significance was set at P < 0.05. The statistical analysis was conducted with MedCalc, version 16.2.0 (MedCalc Software bvba, Ostend, Belgium).
RESULTS
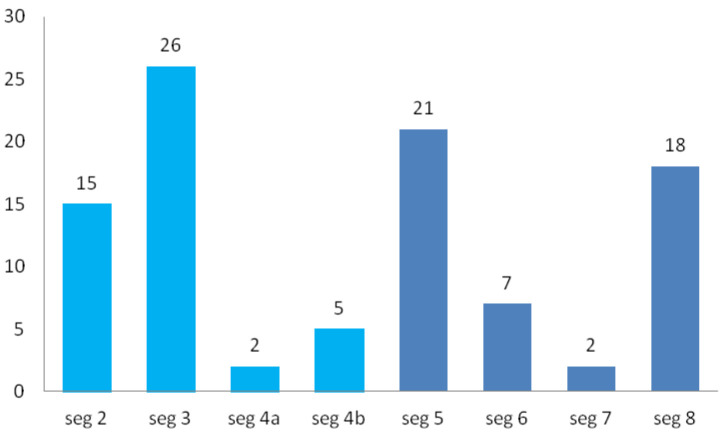
The study comprised of 48 patients who underwent laparoscopic cholecystectomy, with a mean age of 54.58 ± 11.83 years (33 or 69% women). In all patients, gallstones were confirmed after surgery. Men and women did not significantly differ in mean age (men: 58.33 ± 11.36 years; women: 52.87 ± 11.81 years). After MRCP examination, no patients were diagnosed with Mirizzi syndrome. When compared with the preoperative diameter, the extrahepatic bile duct diameter at the points D1 (F = 2.67, df2 = 94, P = 0.07), D2 (F = 1.47, df2 = 94, P = 0.23), D5 (F = 2.35, df2 = 94, P = 0.10), and D6 (F = 2.13, df2 = 94, P = 0.12) did not change significantly after cholecystectomy. The extrahepatic bile duct diameter at the points D3, D4, and D8 was significantly wider three months after cholecystectomy and at the points D4, D7, and D8 was significatly wider six months after cholecystectomy (Table 1). The intrahepatic bile duct diameter at the points D10, D13, and D14 was significantly wider three months after cholecystectomy when compared with the preoperative diameter. The intrahepatic bile duct diameter at the points D11, D13, and D14 six months after cholecystectomy was significantly wider compared with the diameter three months after cholecystectomy. The intrahepatic bile duct diameter at the points D10, D11, D12, D13, and D14 six months after cholecystectomy was significantly wider compared with the preoperative diameter (Table 2). In the left hepatic lobe, the largest number of patients had the widest diameter of the segmental intrahepatic bile duct in the third segment and in the right hepatic lobe, in the fifth segment (Figure 2).
Table 1.
Measurement points of the extrahepatic bile duct with significant diameter changes before and after cholecystectomy
| Bile duct diameter | p¶ |
|||||
|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M2-M1 | M3-M2 | M3-M1 | |
| D3, mm | 4.2 (3.60-5.30) | 4.92 ± 1.16 | 5.0 (4.05-5.55) | 0.006** | 0.67** | 0.03** |
| D4, mm | 4.85 ± 1.21 | 5.50 ± 1.33 | 5.55 ± 1.28 | <0.0001‡ | 0.99‡ | 0.0001‡ |
| D7, mm | 4.02 ± 1.22 | 4.20 (3.20-5.45) | 4.59 ± 1.32 | 0.05** | 0.35** | 0.003** |
| D8, mm | 4.25 (3.50-5.15) | 5.02 ± 1.36 | 5.15 ± 1.49 | 0.001** | 0.39** | <0.0001** |
*M1 – the bile duct diameter before cholecystectomy; M2 – the bile duct diameter three months after cholecystectomy; M3 – the bile duct diameter six months after cholecystectomy; D3 – diameter of the common bile duct in the proximal part in the anteroposterior direction; D4 – diameter of the common bile duct in the proximal part in the laterolateral direction; D7 – diameter of the common hepatic duct in the proximal part in the anteroposterior direction; D8 – diameter of the common hepatic duct in the proximal part in the laterolateral direction.
†Data are expressed as mean ± standard deviation or median with interquartile range.
‡Repeated measures ANOVA test.
§Paired samples t test.
¶Bonferroni corrected.
**Wilcoxon test.
Table 2.
The intrahepatic bile duct diameters measured before cholecystectomy, three months after cholecystectomy, and six months after cholecystectomy on six levels
| Bile duct diameter |
P¶ |
|||||
|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M2-M1 | M3-M2 | M3-M1 | |
| D9, mm | 3.21 ± 0.75 | 3.30 (2.80-4.00) | 3.40 (3.00-3.80) | 0.02** | 0.92** | 0.04** |
| D10, mm | 3.49 ± 0.72 | 4.00 ± 0.96 | 4.00 (3.60-4.50) | 0.0001§ | 0.15** | <0.0001** |
| D11, mm | 3.61 ± 0.62 | 3.66 ± 0.82 | 3.85 (3.25-4.55) | 0.67§ | 0.003** | 0.01** |
| D12, mm | 3.49 ± 0.77 | 3.80 ± 0.88 | 3.86 ± 0.88 | 0.02‡ | 0.99‡ | 0.008‡ |
| D13, mm | 1.00 (0.90-1.20) | 1.34 ± 0.35 | 1.70 (1.20-1.90) | <0.0001** | <0.0001** | <0.0001** |
| D14, mm | 1.00 (0.95-1.15) | 1.41 (1.10-1.65) | 1.68 ± 0.43 | <0.0001** | <0.0001** | <0.0001** |
*M1 – the bile duct diameter before cholecystectomy; M2 – the bile duct diameter three months after cholecystectomy; M3 – the bile duct diameter six months after cholecystectomy; D9 – the diameter of right hepatic duct in the anteroposterior direction; D10 – the diameter of right hepatic duct in the laterolateral direction; D11 – the diameter of left hepatic duct in the anteroposterior direction; D12 – the diameter of left hepatic duct in the laterolateral direction; D13 – the diameter of the widest segmental intrahepatic duct in the right hepatic lobe; D14 – the diameter of the widest segmental intrahepatic duct in the left hepatic lobe.
†Data are expressed a mean ± standard deviation or median with interquartile range.
‡Repeated measures ANOVA test.
§Paired samples t test.
¶Bonferroni corrected.
**Wilcoxon test.
Figure 2.
The most dilated segmental intrahepatic bile ducts in the liver segments (left liver lobe: segments 2, 3, 4a, 4b; right liver lobe: segments 5, 6, 7, 8).
DISCUSSION
This study showed that the intrahepatic bile duct significantly dilatated after cholecystectomy and that significant post-cholecystectomy dilatation occurred only at certain points along the extrahepatic bile ducts.
Different techniques produce different CBD diameter values. ERCP may show a larger CBD diameter due to increased pressures caused by the contrast medium injection in the CBD (28,29). CT measurements may be slightly larger (by 1.7 mm) than the US measurements because CT measurements are performed outer wall-outer wall, while US measurements are performed inner wall-inner wall (30,31). Most of the techniques (such as US and CT) cannot image the cystic duct because it is too small, so special care must be taken not to include the cystic duct in the CBD measurement (32). Our study has several advantages: the exclusion of patients who were taking medicines and had comorbidities that may affect the bile duct diameter; the intrahepatic and extrahepatic bile ducts diameter measurement in the frontal and sagittal planes at multiple points, and the high sensitivity and specificity of the MRCP examination, contributing to data accuracy. Three-dimensional approach used in this study achieves optimal visibility of the intrahepatic and extrahepatic bile ducts compared with 2D sequence (33). The rate of choledocholithiasis in our study was 1%, which is consistent with the study by Valkovic et al (7), who reported 2% of CBD stones after cholecystectomy. Previous studies have only analyzed extrahepatic bile ducts because most of the radiological methods do not detect intrahepatic bile ducts except if they are dilatated. However, MRCP is a non-invasive imaging modality that is able to analyze both the intrahepatic and extrahepatic bile ducts. By measuring the cross-section of the extrahepatic bile ducts, we established that they were oval-shaped, which is consistent with the study by Wachsberg et al (18). The CBD diameter did not significantly increase between the third and the sixth month, so we can conclude that the CBD was maximally dilatated three months after cholecystectomy. Many other studies also found post-cholecystectomy CBD dilatation (4,7-9,34-37). Most of these studies measured the CBD at 1-3 points, whereas our study used 8 measurement points. In addition, the previous studies were US, CT, or EUS-based, while our study used MRCP. The follow-up length in other studies was similar to ours and was mostly three, six, or twelve months, except in the study by Kaim et al (4), who followed their patients for 15 years. The upper limit of the normal extrahepatic bile duct diameter has been reported as 6 mm (9,38). In our study, at no point did the diameter exceed the upper limit of the normal diameter of 6 mm, although half of the measured points of the extrahepatic bile duct showed significantly wider diameter after cholecystectomy, which is consistent with the study by Feng (9).
No study so far has investigated the change of the intrahepatic bile duct diameter after cholecystectomy. We showed that, as opposed to the extrahepatic bile duct diameter, the intrahepatic bile duct diameter continues to dilatate after three months post-cholecystectomy. The upper limit of the normal intrahepatic bile duct diameter has been reported to be 2 mm (39). In our study, all the measured points of the segmental intrahepatic bile ducts showed significantly wider diameter after cholecystectomy but at no point did the diameter exceed the upper limit of 2 mm.
A limitation of our study is a short-term follow-up after cholecystectomy. Future studies should include a follow-up of at least two years or more to determine the long-term impact of cholecystectomy on the bile duct diameter changes. All the measurements were performed on the same machine by one experienced radiologist, so another limitation is the lack of inter-observer variability.
In conclusion, this study showed that post-cholecystectomy dilatation of the extrahepatic bile ducts only occurred at certain points. The common bile duct diameter significantly increased three months after cholecystectomy at the proximal part and remained increased six months after cholecystectomy, and the same happened with the common hepatic duct in the proximal part. We also found significant dilatation of the intrahepatic bile ducts. The upper limit of the normal extrahepatic bile ducts diameter was smaller than 6 mm and that of the intrahepatic segmental bile ducts was smaller than 2 mm, so only the values greater than these require further treatment and correlate with clinical and laboratory findings.
The unexpected dilatation of the extrahepatic bile ducts may present a treatment challenge. Knowing if cholecystectomy patients have a wider bile duct diameter compared with the general population can prevent unnecessary tests, especially costly procedures such as CT or ERCP examinations, which expose patients to radiation and potentially lead to complications.
Acknowledgments
Funding None.
Ethical approval given by the Ethics Committee of the University Hospital “Sveti Duh” (01-580).
Declaration of authorship all authors conceived and designed the study; TP and ST acquired the data; TP and RT analyzed and interpreted the data; all authors drafted the manuscript; all authors critically revised the manuscript for important intellectual content; all authors gave approval of the version to be submitted; all authors agree to be accountable for all aspects of the work.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Campbell WL, Foster RG, Miller WJ, Lecky JW, Zajko AB, Lee KY. Changes in extrahepatic bile duct caliber in liver transplant recipients without evidence of biliary obstruction. AJR Am J Roentgenol. 1992;158:997–1000. doi: 10.2214/ajr.158.5.1566706. [DOI] [PubMed] [Google Scholar]
- 2.Reinus WR, Shady K, Lind M, Scott R. Ultrasound evaluation of the common duct in symptomatic and asymptomatic patients. Am J Gastroenterol. 1992;87:489–92. [PubMed] [Google Scholar]
- 3.Rajnakova A, Goh PM, Ngoi SS, Lim SG. ERCP in patients with periampullary diverticulum. Hepatogastroenterology. 2003;50:625–8. [PubMed] [Google Scholar]
- 4.Kaim A, Steinke K, Frank M, Enriquez R, Kirsch E, Bongartz G, et al. Diameter of the common bile duct in the elderly patient: measurement by ultrasound. Eur Radiol. 1998;8:1413–5. doi: 10.1007/s003300050563. [DOI] [PubMed] [Google Scholar]
- 5.Oddi R. D ʼune disposition sphincter special de l ʼouverture du canal cholodoque. Arch Ital Biol. 1887;8:317–22. [Google Scholar]
- 6.Daradkeh S, Tarawneh E, Al-Hadidy A. Factors affecting common bile duct diameter. Hepatogastroenterology. 2005;52:1659–61. [PubMed] [Google Scholar]
- 7.Valkovic P, Miletic D, Zelic M, Brkljacic B. Dynamic changes in the common bile duct after laparoscopic cholecystectomy: a prospective longitudinal sonographic study. Ultraschall Med. 2011;32:479–84. doi: 10.1055/s-0031-1273224. [DOI] [PubMed] [Google Scholar]
- 8.Park SM, Kim WS, Bae IH, Kim JH, Ryu DH, Jang LC, et al. Common bile duct dilatation after cholecystectomy: a one-year prospective study. J Korean Surg Soc. 2012;83:97–101. doi: 10.4174/jkss.2012.83.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng B, Song Q. Does the common bile duct dilate after cholecystectomy? Sonographic evaluation in 234 patients. AJR Am J Roentgenol. 1995;165:859–61. doi: 10.2214/ajr.165.4.7676981. [DOI] [PubMed] [Google Scholar]
- 10.Wu CC, Ho YH, Chen CY. Effect of aging on common bile duct diameter: a real-time ultrasonographic study. J Clin Ultrasound. 1984;12:473–8. doi: 10.1002/jcu.1870120804. [DOI] [PubMed] [Google Scholar]
- 11.Perret RS, Sloop GD, Borne JA. Common bile duct measurements in an elderly population. J Ultrasound Med. 2000;19:727–30, quiz 31. doi: 10.7863/jum.2000.19.11.727. [DOI] [PubMed] [Google Scholar]
- 12.Bachar GN, Cohen M, Belenky A, Atar E, Gideon S. Effect of aging on the adult extrahepatic bile duct - A sonographic study. J Ultrasound Med. 2003;22:879–82. doi: 10.7863/jum.2003.22.9.879. [DOI] [PubMed] [Google Scholar]
- 13.Economou G, Ward-McQuaid JN. A cross-over comparison of the effect of morphine, pethidine, pentazocine, and phenazocine on biliary pressure. Gut. 1971;12:218–21. doi: 10.1136/gut.12.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puri SK, Gupta P, Panigrahi P, Kumar N, Gupta S, Chaudhary A. Ultrasonographic evaluation of common duct diameter in pre and post cholecystectomy patients. Trop Gastroenterol. 2001;22:23–4. [PubMed] [Google Scholar]
- 15.Hunt DR, Scott AJ. Changes in bile duct diameter after cholecystectomy: a 5-year prospective study. Gastroenterology. 1989;97:1485–8. doi: 10.1016/0016-5085(89)90393-4. [DOI] [PubMed] [Google Scholar]
- 16.Mueller PR, Ferrucci JT, Jr, Simeone JF, Wittenberg J, vanSonnenberg E, Polansky A, et al. Postcholecystectomy bile duct dilatation: myth or reality? AJR Am J Roentgenol. 1981;136:355–8. doi: 10.2214/ajr.136.2.355. [DOI] [PubMed] [Google Scholar]
- 17.Csendes A, Csendes P, Burdiles P, Diaz JC, Maluenda F, Burgos AM. Behavior of the common bile duct diameter before and 12 years after choledochostomy for cholecystolithiasis and choledocholithiasis. A prospective study. J Gastrointest Surg. 2007;11:1294–7. doi: 10.1007/s11605-006-0078-7. [DOI] [PubMed] [Google Scholar]
- 18.Wachsberg RH, Kim KH, Sundaram K. Sonographic versus endoscopic retrograde cholangiographic measurements of the bile duct revisited: Importance of the transverse diameter. AJR Am J Roentgenol. 1998;170:669–74. doi: 10.2214/ajr.170.3.9490950. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal SJ, Cox GG, Wetzel LH, Batnitzky S. Pitfalls and differential diagnosis in biliary sonography. Radiographics. 1990;10:285–311. doi: 10.1148/radiographics.10.2.2183299. [DOI] [PubMed] [Google Scholar]
- 20.Foley WD, Wilson CR, Quiroz FA, Lawson TL. Demonstration of the normal extrahepatic biliary tract with computed tomography. J Comput Assist Tomogr. 1980;4:48–52. doi: 10.1097/00004728-198002000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Co CS, Shea WJ, Goldberg HI. Evaluation of common bile duct diameter using high resolution computed tomography. J Comput Assist Tomogr. 1986;10:424–7. [PubMed] [Google Scholar]
- 22.Hangiandreou NJ. AAPM/RSNA physics tutorial for residents. Topics in US: B-mode US: basic concepts and new technology. Radiographics. 2003;23:1019–33. doi: 10.1148/rg.234035034. [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Mo JJ, Lin L, Li CQ, Zhang JF. Diagnostic value of magnetic resonance cholangiopancreatography in choledocholithiasis. World J Gastroenterol. 2015;21:3351–60. doi: 10.3748/wjg.v21.i11.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shanmugam V, Beattie GC, Yule SR, Reid W, Loudon MA. Is magnetic resonance cholangiopancreatography the new gold standard in biliary imaging? Br J Radiol. 2005;78:888–93. doi: 10.1259/bjr/51075444. [DOI] [PubMed] [Google Scholar]
- 25.Park MS, Kim TK, Kim KW, Park SW, Lee JK, Kim JS, et al. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: findings at MRCP versus ERCP. Radiology. 2004;233:234–40. doi: 10.1148/radiol.2331031446. [DOI] [PubMed] [Google Scholar]
- 26.Menezes N, Marson LP. debeaux AC, Muir IM, Auld CD. Prospective analysis of a scoring system to predict choledocholithiasis. Br J Surg. 2000;87:1176–81. doi: 10.1046/j.1365-2168.2000.01511.x. [DOI] [PubMed] [Google Scholar]
- 27.Topal B, Van de Moortel M, Fieuws S, Vanbeckevoort D, Van Steenbergen W, Aerts R, et al. The value of magnetic resonance cholangiopancreatography in predicting common bile duct stones in patients with gallstone disease. Br J Surg. 2003;90:42–7. doi: 10.1002/bjs.4025. [DOI] [PubMed] [Google Scholar]
- 28.Itoi T, Kamisawa T, Fujii H, Inui K, Maguchi H, Hamada Y, et al. Extrahepatic bile duct measurement by using transabdominal ultrasound in Japanese adults: multi-center prospective study. J Gastroenterol. 2013;48:1045–50. doi: 10.1007/s00535-012-0702-0. [DOI] [PubMed] [Google Scholar]
- 29.Mueller PR, Ferrucci JT, Simeone JF, vanSonnenberg E, Hall DA, Wittenberg J. Observations on the distensibility of the common bile duct. Radiology. 1982;142:467–72. doi: 10.1148/radiology.142.2.7054839. [DOI] [PubMed] [Google Scholar]
- 30.McArthur TA, Planz V, Fineberg NS, Berland LL, Lockhart ME. CT evaluation of common duct dilation after cholecystectomy and with advancing age. Abdom Imaging. 2015;40:1581–6. doi: 10.1007/s00261-014-0308-5. [DOI] [PubMed] [Google Scholar]
- 31.McGillicuddy EA, Schuster KM, Brown E, Maxfield MW, Davis KA, Longo WE. Acute cholecystitis in the elderly: use of computed tomography and correlation with ultrasonography. Am J Surg. 2011;202:524–7. doi: 10.1016/j.amjsurg.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Horrow MM. Ultrasound of the extrahepatic bile duct issues of size. Ultrasound Q. 2010;26:67–74. doi: 10.1097/RUQ.0b013e3181e17516. [DOI] [PubMed] [Google Scholar]
- 33.Chiu NC, Chiou YY. Role of MRCP in the measurement of the CBD diameter. J Chin Med Assoc. 2012;75:423–4. doi: 10.1016/j.jcma.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Chawla S, Trick WE, Gilkey S, Attar BM. Does Cholecystectomy status influence the common bile duct diameter? A matched-pair analysis. Dig Dis Sci. 2010;55:1155–60. doi: 10.1007/s10620-009-0836-y. [DOI] [PubMed] [Google Scholar]
- 35.Senturk S, Miroglu TC, Bilici A, Gumus H, Tekin RC, Ekici F, et al. Diameters of the common bile duct in adults and postcholecystectomy patients: A study with 64-slice CT. Eur J Radiol. 2012;81:39–42. doi: 10.1016/j.ejrad.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Benjaminov F, Leichtman G, Naftali T, Half EE, Konikoff F. Effects of age and cholecystectomy on common bile duct diameter as measured by endoscopic ultrasonography. Surgical Endoscopy and Other Interventional Techniques. 2013;27:303–7. doi: 10.1007/s00464-012-2445-7. [DOI] [PubMed] [Google Scholar]
- 37.McArthur TA, Planz V, Fineberg NS, Berland LL, Lockhart ME. CT evaluation of common duct dilation after cholecystectomy and with advancing age. Abdom Imaging. 2015;40:1581–6. doi: 10.1007/s00261-014-0308-5. [DOI] [PubMed] [Google Scholar]
- 38.Bowie JD. What is the upper limit of normal for the common bile duct on ultrasound: How much do you want it to be? Am J Gastroenterol. 2000;95:897–900. doi: 10.1111/j.1572-0241.2000.01925.x. [DOI] [PubMed] [Google Scholar]
- 39.Parulekar SG. Transabdominal sonography of bile ducts. Ultrasound Q. 2002;18:187–202. doi: 10.1097/00013644-200209000-00004. [DOI] [PubMed] [Google Scholar]