Abstract
Both prenatal substance exposure (PSE, alcohol/drugs) and experiences during the first years of life have powerful effects on brain development. However, only a few studies have investigated the combined effect of PSE and adverse childhood experiences (ACEs) on mental and behavioral disorders among exposed adolescents and adults. This longitudinal register-based cohort study 1) compared the nature and extent of diagnosed mental and behavioral disorders among youth with PSE and matched unexposed controls, and 2) investigated the influence of PSE, health in infancy and ACEs (maternal risk factors and out-of-home care, OHC) on diagnoses of mental and behavioral disorders. The data consisted of 615 exposed youth aged 15–24 years and 1787 matched unexposed controls. Data from hospital medical records and nine registers were merged for the analysis. Descriptive analysis methods and Cox regression were used. The results showed that the prevalence of mental and behavioral disorders was twice as high among exposed compared with controls. The highest levels of mental and behavioral disorders and ACEs were found among exposed with at least one OHC episode. The difference in the risk of mental and behavioral disorders between exposed and controls diminished after controlling for the effect of ACEs. Low birth weight, maternal risk factors, and OHC were the strongest predictors of mental and behavioral disorders. The results suggest that PSE alone does not explain poorer mental health among exposed youth. Risk factors accumulate, and low birth weight and ACEs are strongly associated with increased risk of mental and behavioral disorders.
Keywords: Prenatal substance exposure, FASD, Youth, Adverse childhood experiences, Mental and behavioral disorders
Highlights
-
•
Mental health problems are common in youth with prenatal substance exposure (PSE).
-
•
Adverse childhood experiences (ACEs) accumulate among youth with PSE.
-
•
PSE alone does not predict the high rate of mental and behavioral disorders.
-
•
Youths with PSE and many ACEs had the highest risk of mental health problems.
Introduction
Up to date prevalence rates of substance use (alcohol/drugs) during pregnancy from Finland are lacking. According to previous estimations, 6% of Finnish women use substances during pregnancy (Pajulo et al., 2001) but heavy drinking is rarer and decreases with gestational age (Halmesmäki et al., 1987; Halmesmäki , 2000). In light of the recent studies, these figures may be underestimations (Mårdby et al., 2017; Popova et al., 2017). The global prevalence of consuming any amount of alcohol during pregnancy is estimated to be 9.8%, 25.2% in Europe (Popova et al., 2017), and 14% in Finland (Mårdby et al., 2017). The usage of illicit drugs in Finland has been continuously increasing since 1992 (THL, 2019).
Alcohol and drugs easily cross the placenta and may affect the normal development of the brain and other organs of the fetus (Behnke et al., 2013; Lambert & Bauer, 2012). Studies consistently show that prenatal alcohol exposure (PAE) can cause cognitive and behavioral dysfunction (Riley et al., 2011), congenital anomalies and overall morbidity short- and long-term (Popova et al., 2016). The detrimental effects of PAE manifest in many developmental domains including learning and memory, executive function, attention, and language development (Autti-Rämö, 2000; Korkman et al., 2003, 1998; Mattson et al., 2011; Riley et al., 2011). Fetal Alcohol Spectrum Disorders (FASD) is an umbrella term for all structural anomalies, growth retardation and neurobehavioral disabilities caused by PAE (Hoyme et al., 2016). PAE is a leading known cause of intellectual disability in the Western world (Popova et al., 2017). Prenatal drug exposure (PDE) has not been reported to cause as serious consequences for neurocognitive and somatic development, as are caused by PAE (Behnke et al., 2013; Lambert & Bauer, 2012; Messinger et al., 2004; Minnes et al., 2011) but also PDE can increase the risk of deficiencies in language abilities (Bandstra et al., 2011; Irner, 2012; Lambert & Bauer, 2012), executive functioning (Lambert & Bauer, 2012), and problem-solving and abstract reasoning (Fried et al., 2003; Richardson et al., 2015). Opiate exposure is associated with Neonatal Abstinence Syndrome (NAS) including a combination of physiologic and neurobehavioral signs (Behnke et al., 2013; Wachman et al., 2018).
The prevalence of mental and behavioral disorders is very high both among individuals with PAE (Easey et al., 2019; Popova et al., 2016; Streissguth et al., 2004) and PDE (Ackerman et al., 2010; Irner, 2012). Brain damage caused by PAE results in impaired mental function and behavioral problems and makes affected children prone to additional mental and behavioral problems caused by adverse childhood experiences (Riley et al., 2011; Streissguth et al., 1996). Even low to moderate levels of maternal alcohol use during pregnancy are associated with offspring mental health (Easey et al., 2019). PDE may have similar effects on mental function as PAE (Lambert & Bauer, 2012). Impairments in sustained attention and behavioral self-regulation are frequently reported among children with PAE and PDE and many are diagnosed with attention deficit hyperactivity disorder (ADHD), (Ackerman et al., 2010; Khoury et al., 2018; Lambert & Bauer, 2012; Pei et al., 2011; Weyrauch et al., 2017). Also internalizing problems (e.g. depression and anxiety disorders), have been found (Gray et al., 2005; Lambert & Bauer, 2012; Pei et al., 2011).
A great challenge in studies on prenatal substance exposure (PSE, alcohol and drugs) is the fact that polydrug use is common among pregnant women with substance use problems (Bada et al., 2012; Pajulo et al., 2001; Sarkola, Kahila, Gissler, & Halmesmäki, 2007)), and it is difficult to separate the effects of PAE and PDE. Developmental outcomes may reflect the combined impact of multiple exposures (Ackerman et al., 2010; Irner, 2012; Minnes et al., 2011). Also other associated risks, for example, low birth weight, prematurity, poverty, and poor caregiving environment that are typically associated with substance misuse during pregnancy, contribute to less optimal intellectual and emotional development (Buckingham-Howes et al., 2013; Lambert & Bauer, 2012).
Studies on PAE have been criticized for not investigating the dual effect of prenatal alcohol exposure and postnatal traumatic experiences (Fisher et al., 2011; Koponen, Kalland, & Autti-Rämö, 2009; Koponen, Kalland, Autti-Rämö, Laamanen, & Suominen, 2013; ; Price et al., 2017). The few existing studies showed that those with dual exposure had more deficits in language, attention, memory, and intelligence, and they exhibited more severe behavioral problems compared with children with PAE only (Price et al., 2017). Behavioral problems have also been found to be more common among children in foster or adoptive care (Khoury et al., 2018; Sarkola, Gissler, Kahila, Autti-Rämö, & Halmesmäki, 2011) and stable and nurturing home care seems to prevent these problems (Streissguth et al., 2004). According to Pei et al. (2011), research on FASD is gradually moving toward an integrated multifactorial approach incorporating genetics, PAE, and postnatal environmental factors.
PDE research has focused more systematically on environmental risk factors than PAE research. A shared view is that environmental factors play a key role in moderating and explaining the effects of PDE on children's and adolescents' cognitive and emotional development and may even overshadow the effects of PDE (Ackerman et al., 2010; Buckingham-Howes et al., 2013; Lambert & Bauer, 2012; Minnes et al., 2011; Schempf, 2007). Environmental risk factors may increase, and protective factors attenuate the detrimental effects of PDE (Bada et al., 2012; Lambert & Bauer, 2012; Richardson et al., 2015).
Already during the first weeks of life, the neonate starts to adapt to his/her caregiving environment (Shonkoff et al., 2009). Recurrent traumatic experiences, such as family violence, physical/emotional abuse, neglect, maternal depression and frequent losses of attachment figures (e.g. due to multiple OHCs), cause severe toxic stress and can generate similar structural damage in the brain as PAE (Glaser, 2000; Hart & Rubia, 2012; Miguel et al., 2019). Chronic, overwhelming stress hinders neural integration and the mind's capacity to function as a well-integrated system (Lambert & Bauer, 2012; Schore, 2003; Siegel, 2001). It is possible that environmental adversities, especially during the first years of life, increase the neurocognitive abnormalities already present at birth in individuals with PSE.
More longitudinal studies extending into adulthood with large sample sizes and multiple variables describing adverse childhood experiences (ACEs) are needed (Irner, 2012; Minnes et al., 2011; Pei et al., 2011). The present study was designed to account for this and 1) compares the nature and extent of diagnosed mental and behavioral disorders among youth aged 15–24 years with PSE (exposed) and matched controls without PSE (controls), and 2) investigates the influence of PSE, health in infancy and ACEs (maternal risk factors and OHC) on these disorders. We hypothesize that 1) diagnosed mental and behavioral disorders are more common among youth with PSE, and 2) childhood adversities significantly contribute to these disorders in both groups and decrease the differences between them.
Methods
Data collection
This study is the second follow-up of a cohort of children born 1992–2001 to mothers with alcohol/drug misuse problems during pregnancy and unexposed controls in the Helsinki metropolitan area. Public health maternity clinic nurses referred pregnant women with a significant substance abuse problem (Alcohol Use Disorders Identification Test (AUDIT) ≥ 8 or illegal drug misuse or nonmedical use of central nervous system medication or opioid maintenance treatment) to tertiary care special antenatal HAL-clinics (an abbreviation of illicit drugs, alcohol, medicines) at HUS (Helsinki University Hospital) for intensified counseling and pregnancy follow-up scheduled every 2–4 weeks according to the individual need. All pregnant women fill the AUDIT-test and the referral decision is made on the basis of the result and the general evaluation of the mother's life situation. During the pregnancy follow-up, women were offered easy access to addiction treatment and/or psychiatric care and intensified support by an experienced multi-disciplinary addiction treatment team consisting of an obstetrician, nurse, midwife, psychiatric nurse and social worker (Kahila, Gissler, Sarkola, Autti-Rämö, & Halmesmäki, 2010; Kahila, Saisto, Kivitie-Kallio, Haukkamaa, & Halmesmäki, 2007; Sarkola et al., 2007).
Unexposed controls without any evidence of maternal substance misuse in any of the national health and social welfare registers at delivery were collected from the Medical Birth Register. The mother-child control dyads were matched with exposed dyads for maternal age, parity, number of fetuses, a month of birth and a delivery hospital of the index child. Mothers' mean age at the time of the child's birth was 27 years (SD 6.5, range 15–45 years). Matching was done for maternal characteristics and therefore children were not matched by sex. The proportion of boys among newborns in Finland is 51.1% (Statistics Finland). In the present data, the proportion of boys is 49.3% among exposed and 51.7% among unexposed.
Hospital medical records of substance misusing mothers and their children were reviewed for the first follow-up of the study cohort (Kahila et al., 2007; Sarkola et al., 2007). Register data from multiple mandatory national health and social welfare registers were collected for each mother-child dyad and linked with data from medical records. Similar register data from the national registers were gathered for the control mother-child dyads. In the present second follow-up, the data collected for the first follow-up were used and new data up to the age of 15–24 years were collected. Thus, from the oldest in the cohort born in 1992, we have data from birth until the age of 24 years and from the youngest born in 2001 from birth until the age of 15 years.
All data linkages were made by using the unique identification number assigned to each Finnish citizen at birth or immigration. Identification numbers were concealed and replaced with study numbers after data linkages. The first follow-up of the cohort included 638 exposed and 1914 unexposed mother-child dyads. The present second follow-up included 615 exposed and 1787 unexposed mother-child dyads after exclusions (missing/secured ID information, died in infancy, incorrect birth year). The data collection process and registers are described in detail in Koponen, Nissinen, Gissler, Sarkola, Autti-Rämö, and Kahila (2020).
Ethics
The study was approved by the local ethical committee of HUS (Dnro 333/E8/02). All register organizations: Digital and Population Data Services Agency (DVV), Statistics Finland, Social Insurance Institution (Kela), Legal Register Center (LRC), Finnish Center for Pensions (ETK), and Finnish Institute for Health and Welfare (THL) approved the use of their register data. No study subjects are contacted. All register linkages were performed by a statistical authority (THL) and the data were analyzed anonymously by researchers with research permission provided by the register keepers. According to Finnish law, informed consent is not required in register studies when study subjects are not contacted.
Variables and register sources
Variables used in the present study are listed below. These include variables on childhood adversities considered important for children's neurocognitive and emotional development (e.g. Miguel et al., 2019; Shonkoff et al., 2009).
Outcome variable - mental or behavioral disorder
Primary diagnosis for mental or behavioral disorder. At least one inpatient episode (public and private hospitals, 1992–2016) or outpatient hospital visit (public hospitals, 1998–2016) with ICD-9 codes 290–319 (1992–1995), and ICD-10 codes F00–F99 (1996–2016) based on the Hospital Discharge Register (1992–1993) and Care Register for Health Care (1994–2016).
Children's demographic background factors
Sex (male, female): Medical Birth Register.
Mortality: (no, yes; date of death): Cause of Death Register.
Native language (Finnish, Swedish, other): Population Information System.
Marital status (married, unmarried (single/divorced/widow)): Population Information System.
Completed secondary education (no, yes): Population Census.
Prenatal substance exposure (alcohol/illicit drugs)
Alcohol/drug exposure (alcohol only, illicit drugs only, alcohol and drugs, other multiple substance combination including legal drugs): Medical records from the HAL-clinics.
FASD (no, yes): Register of Congenital Malformations and Hospital Discharge Register: at least one inpatient episode or outpatient hospital visit with ICD-9 code 76071 or ICD-10 code Q86.0.
Neonatal Abstinence Syndrome (NAS), (no, yes): Medical records from HAL-clinics, Medical Birth Register and Hospital Discharge Register including inpatient episodes and outpatient hospital visits with ICD-9 code 7795 or ICD-10 code P96.1.
Maternal smoking during pregnancy
Exposure to mother's daily smoking (no exposure or mother stopped smoking after the first trimester, mother smoked throughout pregnancy): Medical Birth Register.
Newborn health
Gestational weeks at delivery (<37 weeks, ≥37 weeks): Medical Birth Register.
Apgar score at 1 min (0–6, 7–10): Medical Birth Register.
Intensive care by the age of 7 days of life (no, yes): Medical Birth Register.
Birth weight (<2500 g, ≥2500 g; median): Medical Birth Register.
Birth height (median): Medical Birth Register.
Adverse childhood experiences
Out-of-home care (OHC):
Out-of-home care (no OHC, at least one episode; age at first episode; the number of OHC episodes; the proportion of OHC during lifetime). Information on OHC during 1992–2016 is based on the Child Welfare Register. In the Finnish child welfare system, preventive measures and support are always offered first and OHC is only indicated if preventive measures have failed (Child Welfare Act). OHC ends at the age of 18 years, but extended aftercare services were available for OHC youth until the age of 24 years at the time of the study.
Mother's life-situation at delivery:
Age: Medical Birth Register.
Marital status (married, unmarried (single/divorced/widow)): Medical Birth Register.
Socioeconomic status (0 = high (self-employed/lower-level employee/upper-level employee), 1 = low (manual worker/student/pensioner/other)): Medical Birth Register.
Maternal risk factors
Diagnosed mental or behavioral disorder: At least one outpatient visit or inpatient episode with a primary diagnosis for mental or behavioral disorder: ICD-9 codes (1987–1995) 290 and 293–319, and ICD-10 codes (1996–2016) F00–F09 and F20–F99 based on the Hospital Discharge Register or the Care Register for Health Care.
Diagnosed substance misuse: At least one outpatient visit or inpatient episode with a primary or a secondary diagnosis or external cause for alcohol and/or drug-related misuse: ICD-9 (1987–1995) codes: 291–292, 303–305, 3570, 4255, 5353, 5710, 5711–5713, 6483, 6555, 9650, and 9696–9697 and ICD-10 (1996–2016) codes E24.4, F10–F16, F18–F19, G31.2, G40.5, G40.51, G40.52, G62.1, G72.1, I42.6, K29.2, K70, K85.2, K86, O35.4-O35.5, P04.4, R78.0-R78.5, T40, T43.6, T50.2-T50.3, T51, Z71.4, Z72.1-Z72.2, X45, and X69 based on the Hospital Discharge Register or the Care Register for Health Care.
Mortality (no, yes; date of death): Cause of Death Register.
Criminal record (0 = no sentence, 1 = at least one sentence during 1985–2018): Criminal Records Register.
Social assistance (0 = no long-term social assistance, 1 = long-term social assistance (10–12 months during a one year period)) in 2002–2016: Register of Social Assistance, covered the years 2002–2016. Social assistance is the last-resort financial assistance for individuals and families in order to guarantee a minimum standard of living.
Statistical analysis
This is a longitudinal population- and register-based matched cohort study. Data from hospital medical records and nine registers were merged for the analysis. In descriptive analyses, baseline comparisons between cohorts were made with Pearson chi2-tests, Mann-Whitney U test or Kruskal-Wallis-test, as appropriate. Cox regression modeling was used for univariate and multivariate analyses. The follow-up of the first diagnosed mental or behavioral disorder started from birth. Time of the first incidence of maternal risk factors (e.g. child's age at the mother's first mental or behavioral disorder diagnosis) was taken into account in the analyses. Crude and adjusted Hazard Ratios (HRs) with 95% confidence intervals (CIs) were calculated. The level of statistical significance was set at p < 0.05.
Variables in the multivariate Cox regression models were chosen on a theoretical and statistical basis. Spearman correlations between study variables were explored prior to multivariate analyses to check multicollinearity. Due to moderate or relatively strong correlations between exposure status and maternal risk factors (0.24–0.68, p < 0.001), a sum score of maternal risk factors was created in order to avoid the multicollinearity problem. Child and maternal age were not included in the multivariate Cox regression models as groups were matched by these factors. SPSS version 25 was used.
Results
Sample description
About half in the exposed and control groups were male and 60% were 18–24 years old in 2016. Almost all (96%) of the exposed and 86% of the controls had Finnish as their native language. In both groups, 1% had died during the follow-up, and under 2% were married. Eighteen percent of the exposed and 23% of the controls had completed secondary education. Almost 8% of the exposed had a FASD continuum diagnosis and 8% had a NAS-diagnosis (Table 1.). Based on data from the HAL-clinics, 29% (n = 175) were exposed to alcohol only, 10% (n = 63) to illicit drugs only, 10% (n = 63) to alcohol and illicit drugs and 51% (n = 314) had a multiple substance exposure including legal drugs. However, the presented figures are only estimates as precise data on substance misuse is challenging to obtain even prospectively.
Table 1.
Childhood adversities by exposure status and out-of-home care (OHC) status.
| Exposed N = 615 % (n) |
Controls N = 1787 % (n) |
p-value | Exposed, no OHC N = 222 % (n) |
Exposed, OHC N = 393 % (n) |
Controls, no OHC N = 1640 % (n) |
Controls, OHC N = 147 % (n) |
p-value | |
|---|---|---|---|---|---|---|---|---|
| Gender and health in infancy | ||||||||
| Male | 49.3 (303) | 51.7 (923) | 0.308 | 47.3 (105) | 50.4 (198) | 51.4 (843) | 54.4 (80) | 0.558 |
| Exposed to daily smoking | 75.3 (463) | 18.9 (337) | <0.001 | 62.6 (139) | 82.4 (324) | 16.8 (275) | 42.2 (62) | <0.001 |
| Gestational age <37 weeks |
9.1 (55) | 9.1 (162) | 0.985 | 8.7 (19) | 9.3 (36) | 9.0 (147) | 10.3 (15) | 0.956 |
| Birth weight <2500 g | 12.5 (77) | 6.7 (120) | <0.001 | 10.8 (24) | 13.5 (53) | 6.7 (109) | 7.5 (11) | <0.001 |
| Birth weight < median (3450g) | 65.0 (400) | 44.5 (793) | <0.001 | 59.5 (132) | 68.2 (268) | 43.7 (716) | 52.4 (77) | <0.001 |
| Birth height < median (50 cm) | 60.0 (361) | 39.4 (694) | <0.001 | 54.6 (118) | 63.0 (243) | 38.7 (627) | 46.5 (67) | <0.001 |
| Intensive care by the age of 7 days of life | 20.7 (127) | 9.8 (176) | <0.001 | 16.7 (37) | 22.9 (90) | 9.8 (161) | 10.2 (15) | <0.001 |
| FASD | 7.5 (46) | 2.7 (6) | 10.2 (40) | 0.001 | ||||
| Any congenital anomaly including FASD | 15.6 (96) | 9.2 (164) | <0.001 | 12.2 (27) | 17.6 (69) | 9.0 (148) | 10.9 (16) | <0.001 |
| NAS | 8.1 (50) | 5.4 (12) | 9.7 (38) | 0.063 | ||||
| Mother's life-situation at delivery | ||||||||
| Mother's age <25 years |
37.7 (232) | 37.0 (661) | 0.745 | 43.2 (96) | 34.6 (136) | 34.9 (572) | 60.5 (89) | <0.001 |
| Unmarried | 79.5 (485) | 39.9 (703) | <0.001 | 80.7 (176) | 78.8 (309) | 37.4 (605) | 67.6 (98) | <0.001 |
| Lower socioeconomic status (manual worker, student, pensioner, other) | 65.7 (364) | 42.7 (719) | <0.001 | 63.1 (128) | 67.2 (236) | 40.8 (635) | 65.1 (84) | <0.001 |
| Maternal risk factors | ||||||||
| Mother died | ||||||||
| No | 88.5 (544) | 99.3 (1774) | <0.001 | 95.5 (212) | 84.5 (332) | 99.3 (1629) | 98.6 (145) | <0.001 |
| Yes | 11.5 (71) | 0.7 (13) | 4.5 (10) | 15.5 (61) | 0.7 (11) | 1.4 (2) | ||
| Child's age at mother's death | ||||||||
| <7 years | 31.0 (22) | 0.0 (0) | 0.019 | 40.0 (4) | 29.5 (18) | 0.0 (0) | 0.0 (0) | 0.114 |
| ≥7 years | 69.0 (49) | 100.0 (13) | 60.0 (6) | 70.5 (43) | 100.0 (11) | 100.0 (2) | ||
| Median | 8.0 | 13.0 | 7.5 | 8.0 | 11.0 | 18.0 | ||
| Mother's diagnosed mental or behavioral disorder (substance misuse exl.) | <0.001 | <0.001 | ||||||
| No | 48.3 (297) | 81.8 (1462) | 55.4 (123) | 44.3 (174) | 84.7 (1389) | 49.7 (73) | ||
| Yes | 51.7 (318) | 18.2 (325) | 44.6 (99) | 55.7 (219) | 15.3 (251) | 50.3 (74) | ||
| Mother's first diagnosed mental or behavioral disorder (substance misuse exl.) | <0.001 | <0.001 | ||||||
| Before delivery | 45.9 (146) | 12.9 (42) | 43.4 (43) | 47.0 (103) | 10.8 (27) | 20.3 (15) | ||
| After delivery | 54.1 (172) | 87.1 (283) | 56.6 (56) | 53.0 (116) | 89.2 (224) | 79.7 (59) | ||
| Median | 0.0 | 7.0 | 0.0 | 0.0 | 7.0 | 5.0 | ||
| Child's age at mother's first diagnosed mental or behavioral disorder (substance misuse exl.) | <0.001 | <0.001 | ||||||
| <7 years | 77.0 (245) | 48.0 (156) | 69.7 (69) | 80.4 (176) | 45.4 (114) | 56.8 (42) | ||
| ≥7 years | 23.0 (73) | 52.0 (169) | 30.3 (30) | 19.6 (43) | 54.6 (137) | 43.2 (32) | ||
| Diagnosed substance misuse problem (all substance misuse related diagnoses) | <0.001 | <0.001 | ||||||
| No | 31.2 (192) | 94.7 (1693) | 47.3 (105) | 22.1 (87) | 96.8 (1588) | 71.4 (105) | ||
| Yes | 68.8 (423) | 5.3 (94) | 52.7 (117) | 77.9 (306) | 3.2 (52) | 28.6 (42) | ||
| Child's age at mother's first diagnosed substance misuse disorder (as a primary diagnosis) | <0.001 | <0.001 | ||||||
| <7 years | 79.5 (260) | 40.3 (25) | 77.1 (54) | 80.2 (206) | 27.3 (9) | 55.2 (16) | ||
| ≥7 years | 20.5 (67) | 59.7 (37) | 22.9 (16) | 19.8 (51) | 72.7 (24) | 44.8 (13) | ||
| Median | −0.61 | 9.0 | −0.83 | −0.37 | 13.3 | 4.04 | ||
| Mother's criminal record | <0.001 | <0.001 | ||||||
| No | 88.9 (547) | 99.2 (1772) | 95.5 (212) | 85.2 (335) | 99.4 (1630) | 96.6 (142) | ||
| Yes | 11.1 (68) | 0.8 (15) | 4.5 (10) | 14.8 (58) | 0.6 (10) | 3.4 (5) | ||
| Mother's first criminal record | 0.143 | 0.383 | ||||||
| Before the delivery | 76.5 (52) | 93.3 (14) | 80.0 (8) | 75.9 (44) | 100.0 (10) | 80.0 (4) | ||
| After the delivery | 23.5 (16) | 6.7 (1) | 20.0 (2) | 24.1 (14) | 0.0 (0) | 20.0 (1) | ||
| Median | −8.0 | −11.0 | −7.0 | −8.0 | −11.0 | −13.0 | ||
| Received social assistance Long-term social assistance |
72.8 (448) | 15.0 (268) | <0.001 | 50.0 (111) | 85.8 (337) | 11.2 (183) | 57.8 (85) | <0.001 |
| Sum of maternal risk factors | <0.001 | <0.001 | ||||||
| 0 | 8.1 (50) | 72.2 (1291) | 18.9 (42) | 2.0 (8) | 76.6 (1256) | 23.8 (35) | ||
| 1 | 20.5 (126) | 18.7 (334) | 30.2 (67) | 15.0 (59) | 17.7 (290) | 29.9 (44) | ||
| 2 | 28.5 (175) | 6.1 (109) | 27.9 (62) | 28.8 (113) | 4.1 (68) | 27.9 (41) | ||
| 3-5 risks | 42.9 (264) | 3.0 (53) | 23.0 (51) | 54.2 (213) | 1.6 (26) | 18.4 (27) | ||
Childhood adversities
Sixty-four percent (n = 393) of the exposed and 8% (n = 147) of the controls had been at least once in OHC. Among the exposed, the median age at the first OHC episode was two years, 76% were placed before school-age (<7 years), and the median number of placements was three. Among controls, the median age at first OHC placement was 10 years, 34% were placed before school age and the median number of placements was two. The median proportion of lifetime in OHC was 51% among exposed and 6% among controls.
Table 1 shows childhood adversities related to health in infancy, mother's life-situation at delivery and maternal risk factors among exposed and controls, and separately in different OHC groups. Among exposed, mothers' smoking during pregnancy was more common (75% vs. 19%), birth weight and height were lower, and neonatal intensive care was more common than among controls. Exposed with OHC had the poorest health outcomes. Compared with exposed without OHC, they had a higher prevalence of FASD (p = 0.001), lower median birth weight (p = 0.029), lower median height (p = 0.046), and maternal smoking was more common (p < 0.001). Differences in the prevalence of NAS (p = 0.063), any congenital anomalies (p = 0.077) and neonatal intensive care (p = 0.067) were close to statistical significance. In the control group, those with OHC were more often exposed to maternal smoking (p < 0.001) and had lower median birth weight (p = 0.043). No differences were found regarding the gestational age or 1-min Apgar-scores (data not shown) in the different exposure or OHC groups.
Mothers of the exposed were more often unmarried and had lower socioeconomic status compared with control mothers. In addition, exposed had more maternal risk factors in terms of diagnosed mental and behavioral disorders, criminal records and need for social assistance. Among controls, 72% had no recorded maternal risk factors. Among the exposed, 8% had no risk factor, while 43% had three to five risks (Table 1.).
The prevalence of maternal adversities was highest in the exposed OHC group in which also the death rate of mothers was extremely high (15.5%). The prevalence of maternal adversities was also high among controls with OHC regarding sociodemographic factors, mental health and substance misuse problems, and the cumulative number of risk factors. All these differences between controls with and without OHC were statistically significant (p < 0.001). (Table 1.).
The timing of maternal adversities varied somewhat between exposed and controls. In the exposed group, the mother's first mental or behavioral disorder diagnosis was typically received before the child's birth or before school age (77%). In the control group, the corresponding proportion was 48%. If the mother had one or more criminal records, the first one was typically received before the child's birth in both groups. Child age at maternal death was usually more than 7 years. In the control group, maternal death was uncommon and no mother had died before the child's school age (Table 1).
Diagnosed mental and behavioral disorders among exposed and control youths
The prevalence of diagnosed mental or behavioral disorders was twice as high among exposed (55%) as among controls (26%). The median age at the first mental or behavioral disorder (later F-diagnosis) was 10 years in both groups and 27% of the exposed and 31% of the controls had received F-diagnosis before the school age. Only a few had received F-diagnosis after the age of 20 years. Exposed had more outpatient episodes with F-diagnosis but no more inpatient episodes or days in inpatient care. Exposed with OHC and controls with OHC had the highest prevalence of F-diagnoses and the largest number of outpatient visits with these diagnoses. (Table 2).
Table 2.
Mental and behavioral disorders (F-diagnoses) among exposed and controls a.
| Exposed, N = 615 |
Controls, N = 1787 |
p-value | Exposed, No OHC N = 222 |
Exposed, OHC N = 393 |
Controls, No OHC N = 1640 |
Controls, OHC N = 147 |
p-value | |
|---|---|---|---|---|---|---|---|---|
| F-diagnosis % (n) | <0.001 | <0.001 | ||||||
| No | 45.4 (279) | 73.6 (1316) | 65.3 (145) | 34.1 (134) | 77.7 (1275) | Please, check the lines27.9 (41) | ||
| Yes | 54.6 (336) | 26.4 (471) | 34.7 (77) | 65.9 (259) | 22.3 (365) | 72.1 (106) | ||
| Age at first F-diagnosis | 0.174 | 0.397 | ||||||
| % (n) These numbers should be in the next line. | 26.8 (90) | 31.2 (147) | 24.7 (19) | 27.4 (71) | 30.1 (110) | 34.9 (37) | ||
| <7 years | 73.2 (246) | 68.8 (324) | 75.3 (58) | 72.6 (188) | 69.9 (255) | 65.1 (69) | ||
| ≥7 years | 10.2 | 10.1 | 12.5 | 9.8 | 10.4 | 8.6 | ||
| Median Here medians from the line above. | ||||||||
| Number of outpatient visits with F-diagnosis | <0.001 | <0.001 | ||||||
| Mean (SD) | 43.7 (70.5) | 28.7 (41.6) | 39.0 (62.5) | 45.0 (72.7) | 24.4 (37.3) | 43.8 (51.5) | ||
| Range | 1–839 | 1–313 | 1–328 | 1–839 | 1–207 | 1–313 | ||
| Median | 20.0 | 10.0 | 15.0 | 22.5 | 8.0 | 28.0 | ||
| Number of inpatient episodes with F-diagnosis | 0.086 | 0.173 | ||||||
| Mean (SD) | 2.9 (3.2) | 2.1 (1.7) | 2.3 (2.4) | 3.0 (3.4) | 2.1 (1.7) | 2.2 (1.8) | ||
| Range | 1–24 | 1–7 | 1–12 | 1–24 | 1–7 | 1–6 | ||
| Median | 2.0 | 1.0 | 1.0 | 2.0 | 1.0 | 1.0 | ||
| Number of days in inpatient care with F-diagnosis | 0.141 | 0.386 | ||||||
| Mean (SD) | 63.0 (111.0) | 48.8 (96.6) | 66.4 (121.5) | 62.1 (108.8) | 48.6 (93.6) | 49.3 (103.3) | ||
| Range | 1–618 | 1–565 | 1–500 | 1–618 | 1–565 | 1–493 | ||
| Median | 21.0 | 15.0 | 19.5 | 24.0 | 16.00 | 13.0 | ||
Comparison of categorical variables based on Chi-Square test. Comparison of means based on Mann-Whitney U test and Kruskal-Wallis test.
The largest diagnosis groups both among exposed and controls were ‘Behavioral and emotional disorders with onset usually occurring in childhood and adolescence’ (F90–F98), ‘Disorders of psychological development’ (F80–F89), ‘Neurotic, stress-related and somatoform disorders’ (F40–F49), and ‘Mood (affective) disorders’ (F30–F39). When the effect of OHC status was taken into account, the results showed that the prevalence of diagnosed mental or behavioral disorders was no different among exposed with OHC than among controls with OHC regarding all F-diagnoses (p = 0.170). When the F-subgroups were analyzed separately, the results revealed only small differences between exposed and controls with OHC. Controls with OHC had more often received diagnoses ‘Schizophrenia, schizotypal and delusional disorders’ (F20–F29, p = 0.014) and ‘Neurotic, stress-related and somatoform disorders’ (F40–F49, p = 0.002) compared with exposed with OHC. No other statistically significant differences were found between the two OHC groups (Table 2, Fig. 1).
Fig. 1.
Prevalence of diagnosed mental and behavioral disorders by the exposure status and out-of-home care status.
*F10–F19 Mental and behavioral disorders due to psychoactive substance use. F20–F29 Schizophrenia, schizotypal and delusional disorders. F30–F39 Mood [affective] disorders. F40–F48 Neurotic, stress-related and somatoform disorders. F50–F59 Behavioral syndromes associated with physiological disturbances and physical factors. F60–F69 Disorders of adult personality and behavior. F70–F79 Intellectual disability. F80–F89 Disorders of psychological development. F90–F98 Behavioral and emotional disorders with onset usually occurring in childhood and adolescence.
Among exposed, type of substance exposure and NAS-diagnosis were not associated with F-diagnosis (data not shown) but those with FASD had more often received F-diagnosis (HR = 1.66, 95% CI 1.16–2.37, p = 0.006). In addition, low birth weight, mother's diagnosed substance misuse problem, mother's criminal record, mother's long-standing social assistance, the sum of maternal adversities, and child's OHC were independently associated with the child's F-diagnosis. Among controls, all analyzed maternal adversities, except for the mother's death, were associated with a child's F-diagnosis. Moreover, the child's OHC was strongly associated with an increased risk of F-diagnosis. (Table 3).
Table 3.
Associations between childhood adversities and diagnosed mental and behavioral disorders from birth to early adulthood among exposed and unexposed controls. Cox regression hazard model, n = 2402. a
| Exposed, n = 615 HR (95% CI) | p-value | Controls, n = 1787 HR (95% CI) | p-value | |
|---|---|---|---|---|
| Gender and health in infancy | ||||
| Gender | 0.543 | 0.061 | ||
| Female | 1 | 1 | ||
| Male | 1.07 (0.86–1.32) | 1.19 (0.99–1.43) | ||
| Birth weight | 0.039 | 0.009 | ||
| ≥2500 g | 1 | 1 | ||
| <2500 g | 1.38 (1.02–1.87)) | 1.54 (1.11–2.13) | ||
| Intensive care by the age of 7 days of life | 0.063 | 0.004 | ||
| No | 1 | 1 | ||
| Yes | 1.28 (0.99–1.65) | 1.51 (1.14–2.0) | ||
| Mother smoked | 0.600 | 0.001 | ||
| No | 1 | 1 | ||
| Yes | 0.94 (0.73–1.20) | 1.45 (1.17–1.79) | ||
| Mother's sociodemographic characteristics at delivery | ||||
| Mother's age | 0.460 | <0.001 | ||
| ≥25 years | 1 | 1 | ||
| <25 years | 1.09 (0.87–1.35) | 1.55 (1.29–1.86) | ||
| Mother's marital status | 0.403 | <0.001 | ||
| Married | 1 | 1 | ||
| Not married | 1.12 (0.86–1.47) | 1.59 (1.33–1.91) | ||
| Socioeconomic status | 0.550 | <0.001 | ||
| Higher | 1 | 1 | ||
| Lower | 1.08 (0.85–1.37) | 1.46 (1.21–1.76) | ||
| Maternal risk factors | ||||
| Mother's mental or behavioral disorder | ||||
| No | 1 | 1 | ||
| Yes | 1.09 (0.88–1.35) | 0.441 | 2.25 (1.84–2.74) | <0.001 |
| Child's age at mother's first diagnosed mental or behavioral disorder (substance misuse exl.) | 1 | 1 | ||
| No diagnosis | ||||
| <7 years | 1.07 (0.85–1.34) | 0.583 | 2.44 (1.89–3.17) | <0.001 |
| ≥7 years | 1.16 (0.83–1.62) | 0.393 | 2.08 (1.61–2.69) | <0.001 |
| Mother's first mental or behavioral disorder | ||||
| No | 1 | 1 | ||
| Before birth | 1.13 (0.87–1.48) | 0.368 | 2.75 (1.75–4.33) | <0.001 |
| After birth | 1.05 (0.82–1.36) | 0.690 | 2.18 (1.77–2.69) | <0.001 |
| Mother's diagnosed substance misuse problem | 0.026 | <0.001 | ||
| No | 1 | 1 | ||
| Yes | 1.31 (1.03–1.66) | 2.58 (1.92–3.46) | ||
| Child's age at mother's first diagnosed substance misuse problem | ||||
| No diagnosis | 1 | 1 | ||
| <7 years | 1.23 (0.98–1.55) | 0.069 | 2.72 (1.60–4.63) | <0.001 |
| ≥7 years | 1.25 (0.88.1.79) | 0.220 | 2.03 (1.25–3.30) | 0.004 |
| Mother's criminal record | 0.039 | 0.028 | ||
| No | 1 | 1 | ||
| Yes | 1.40 (1.02–1.92) | 2.31 (1.09–4.87) | ||
| Mother's first criminal record | ||||
| No criminal record | 1 | Move to next line 0.528 | 1 | |
| Before child's birth | 1.13 (0.78–1.64) | <0.001 | 2.04 (0.91–4.56) | 0.083 |
| After child's birth | 2.85 (1.67–4.89) | 11.5 (1.61–82.2) | 0.015 | |
| Mother died | 0.248 | 0.461 | ||
| No | 1 | 1 | ||
| Yes | 0.81 (0.57–1.16) | 1.39 (0.58–3.36) | ||
| Mother's long-term social assistance | 0.001 | <0.001 | ||
| No | 1 | 1 | <0.001 | |
| Yes | 1.57 (1.21–2.04) | 2.39 (1.94–2.94) | ||
| Sum of maternal adversities | ||||
| 0 | 1 | 1 | ||
| 1 | 1.58 (0.92–2.71) | 0.095 | 2.01 (1.63–2.50) | <0.001 |
| 2 | 2.39 (1.43–4.00) | 0.001 | 3.16 (2.36–4.23) | <0.001 |
| 3–5 | 2.15 (1.30–3.54) | 0.003 | 3.66 (2.51–5.35) | <0.001 |
| Out-of home care | ||||
| No OHC | 1 | <0.001 | 1 | <0.001 |
| At least once in OHC | 2.41 (1.87–3.11) | 4.84 (3.89–6.03) | ||
| Age at first OHC | ||||
| No placement | 1 | 1 | ||
| <7 years | 2.38 (1.82–3.10) | <0.001 | 5.66 (3.99–8.03) | <0.001 |
| ≥7 years | 2.51 (1.80–3.49) | <0.001 | 4.52 (3.50–5.84) | <0.001 |
All associations are bivariate.
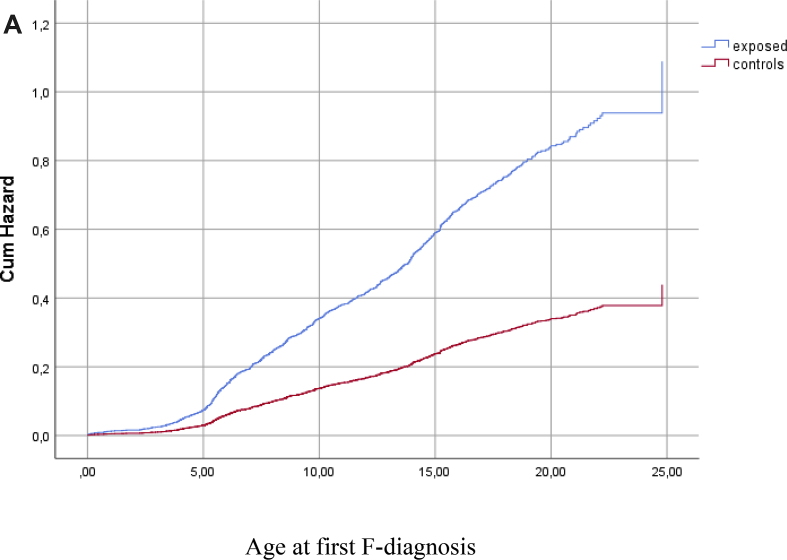
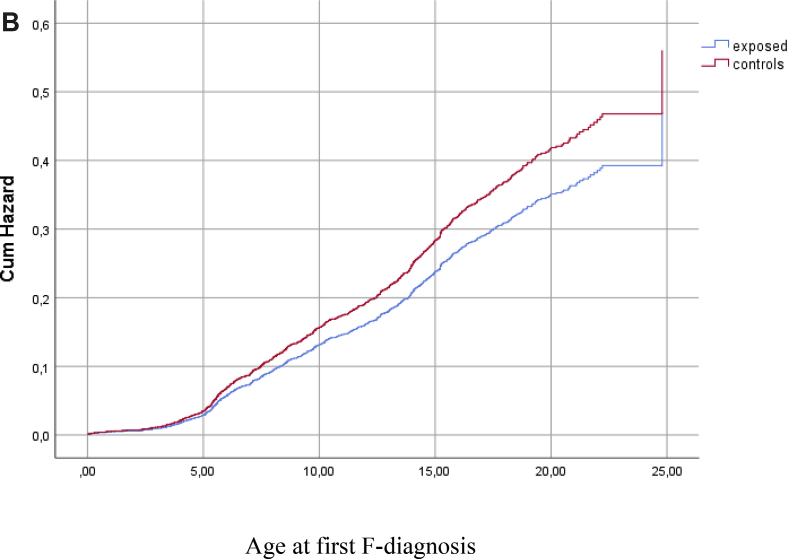
In the adjusted models, the difference between exposed and controls in the risk of F-diagnosis attenuated after controlling for the effects of maternal risk factors and child's OHC status. The final model showed that the strongest risk factors associated with a child's F-diagnosis were low birth weight, the mother being unmarried at the time of the delivery, a high number of maternal risk factors and OHC (Table 4, Fig. 2a, Fig. 2b).
Table 4.
Association between childhood adversities and diagnosed mental and behavioral disorders from birth to early adulthood. Cox regression hazard models, n = 2402.
| Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | Model 4 HR (95% CI) | Model 5 HR (95% CI) | |
|---|---|---|---|---|---|
| Exposure Controls Exposed |
1 2.48 (2.16–2.85) *** |
1 2.19 (1.84–2.60) *** |
1 1.91 (1.59–2.30) *** |
1 1.13 (0.91–1.39) |
1 0.84 (0.67–1.05) |
| Sex Female Male |
1 1.14 (1.00–1.31) |
1 1.11 (0.96–1.28) |
1 1.11 (0.96–1.29 |
1 1.11 (0.96–1.29) |
|
| Birth weight ≥2500 g <2500 g |
1 1.44 (1.15–1.80) ** |
1 1.55 (1.23–1.95) *** |
1 1.56 (1.24–1.97) *** |
1 1.45 (1.15–1.83) ** |
|
| Exposure to smoking No Yes |
1 1.20 (1.01–1.42) * |
1 1.11 (0.92–1.32) |
1 0.96 (0.81–1.15) |
1 0.84 (0.70–1.01) |
|
| Mother's socioeconomic status at delivery High Low |
1 1.27 (1.10–1.48) ** |
1 1.16 (0.99–1.35) |
1 1.11 (0.95–1.29) |
||
| Mother's marital status at delivery Married Not married |
1 1.33 (1.13–1.57) *** |
1 1.26 (1.07–1.49) ** |
1 1.24 (1.06–1.46) ** |
||
| Number of maternal risk factors 0 1 2–5 |
1 1.92 (1.56–2.35) *** 2.92 (2.33–3.66) *** |
1 1.71 (1.38–2.10) *** 1.97 (1.54–2.52) *** |
|||
| Out-of-home care No Yes |
1 2.90 (2.35–3.57) *** |
*p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 2a.
Hazard function for diagnosed mental or behavioral disorder (F-diagnosis) among exposed and controls before adjustment for covariates, p < 0.001.
Fig. 2b.
Hazard function for diagnosed mental or behavioral disorder (F-diagnosis) among exposed and controls after adjustment for covariates (sex, birth weight, exposure to smoking, mother's socioeconomic status, mother's marital status, sum of maternal risk factors and out-of-home care), p > 0.05.
Discussion
This register-based cohort study focused on the nature, extent, and predictors of diagnosed mental and behavioral disorders (F-diagnoses) among adolescents and young adults with PSE and matched unexposed controls. In line with the first hypothesis, exposed had received F-diagnoses twice as often as controls. The largest diagnostic categories were similar in both groups. The largest category was ‘Behavioral and emotional disorders with onset usually occurring in childhood and adolescence’ (F90–F98), which includes ADHD and conduct disorders. The result is consistent with many previous studies that report high levels of attention and behavioral dysregulation problems among individuals with PAE and PDE (e.g. Irner, 2012; Weyrauch et al., 2017). However, the F90–F98 category includes also less often reported emotional disorders such as separation anxiety, phobic anxiety, and social anxiety.
The risk of F-diagnoses was highest among exposed with OHC episodes. Also, the accumulation of ACEs was highest among them and they were more often diagnosed with FASD and had poorer health in infancy compared with exposed without OHC. Youths exposed to PSE with OHC had all prenatal and postnatal adversities that according to Miguel et al. (2019) cause modifications in brain structure and function (amygdala and hippocampus) leading to behavioral dysfunctions and risk for mental health problems. A minority of mothers with substance misuse problems during pregnancy had been able to raise their children without any OHC episode. The results suggest that among these mothers substance misuse and mental health problems were less severe than among mothers whose children had been taken into OHC. The result is consistent with the findings by Sood et al. (2001) and Raitasalo et al. (2019). These studies found that women with severe alcohol misuse had an increased risk of not living with their children.
ACEs and F-diagnoses were common also among controls with OHC. As predicted, ACEs increased the risk of F-diagnoses both among exposed and controls. However, the associations were weaker among the exposed. The close relationship between the mother's drinking during pregnancy and other maternal risk factors seems to decrease the explanatory power of the single risk variables. In line with the second hypothesis, the difference between exposed and controls was attenuated after adjusting for the effect of ACEs. In the final model, low birth weight, maternal risk factors, and OHC were the strongest predictors of F-diagnoses.
The results of this study are in line with previous findings showing a strong association between traumatic experiences (Henry et al., 2007; Kambeitz et al., 2019; Koponen, 2006; Koponen et al, 2009; 2013; Lambert & Bauer, 2012; Price et al., 2017), residential care (Sarkola et al., 2011; Fagerlund et al., 2011) and an increased risk of mental health problems among children and adolescents with PAE and PDE. Numerous studies carried out among the general population have also revealed the relationship between socioeconomic inequalities, multiple ACEs and high levels of mental health problems (Hughes et al., 2017; Paananen et al., 2013; Reiss, 2013; Ristikari et al., 2018). In contrast, Mukherjee et al. (2019) did not find any additional increase in the prevalence of ADHD, social and communication disorders and autistic spectrum disorders caused by postnatal neglect among children and young people with FASD.
The results of the present study suggest that traumatic experiences may have a greater effect on the mental and behavioral disorders of children and youth with PAE than previously reported. Previous studies have typically compared exposed children with unexposed children matched with age, sex, ethnic origin, and parents’ socioeconomic status (e.g. Fryer et al., 2007; Mattson & Roebuck, 2002), but have not considered the overall quality of the caregiving environment. However, traumatic experiences may not be associated with all mental and behavioral disorders or the strength of the association may vary depending on the disorder. Environmental factors are seen to affect secondary mental health problems but neurocognitive disabilities are seen as primary being present at birth and caused by PAE (Streissguth et al., 1996). Thus, the effect of environmental factors may be weak on functions that are strongly related with biological maturation of the central nervous system (Disorders of psychological development, F80–F89) and activities that require cognitive involvement (Hyperkinetic disorders, F90), for example. The present study used the complete F-diagnosis category as an outcome and did not differentiate between primary and secondary disabilities.
Further more detailed longitudinal studies investigating the relationship between PSE, ACEs, and different categories of cognitive and mental health problems are needed. Because of central nervous system disruptions caused by PAE and PDE (Lambert & Bauer, 2012; Riley et al., 2011), exposed infants have an impaired ability to habituate to environmental stimuli (Fried et al., 1987; Streissguth et al., 1993) and thus may be very vulnerable to environmental influences. ACEs may increase cognitive and behavioral dysfunction caused by PSE and not only secondary mental health problems. Also, the effect of OHC needs to be investigated more thoroughly. The effects may vary depending on the age of the first placement, the number of placements, time in care, and the type of placement.
Limitations
The exposed cohort represents 0.4% of all children born in the Helsinki metropolitan area in 1992–2001 (Kahila et al., 2007) indicating that only women with the most severe misuse problem were identified for the study. The results do not reveal the effects of slight and/or occasional substance use. Based on medical records from the HAL-clinics, we estimated that a majority in the exposed group were exposed to alcohol or alcohol and drugs. However, detailed information on the type, timing and amount of exposure is limited as in all studies on prenatal substance exposure (Behnke et al., 2013). Further, despite careful checking of the health and social welfare registers, we cannot be absolutely certain that mothers in the control group had not used any substances during pregnancy.
F-diagnoses were received from specialized health care. Diagnoses from primary care are not available for the complete study period and therefore the prevalence of F-diagnoses may be higher than found in the study and less severe disabilities were not identified. Moreover, we were not able to get direct evidence of child abuse and neglect from the registers. However, a majority of exposed had been in OHC, which suggests that many of them may have faced these adversities as previously reported by Kalland and Sinkkonen (2001). An additional limitation is the lack of information on fathers and the quality of life in foster/adoptive care.
Strengths
This is one of the first longitudinal studies investigating the combined effect of PSE and ACEs on mental and behavioral disorders in exposed adolescents and young adults. In comparison to previous studies, we were able to use a unique combination of medical records and register data with a large sample size, a matched control group, long-term follow-up, and multiple variables describing ACEs. In Finland, health care is publicly funded and all Finnish citizens have access to it. Almost all (99.7%) pregnant women attend antenatal services provided by maternity clinics of health care centers (Kahila et al., 2010), which made it possible to identify those with a substance misuse problem.
The reporting to the national health and social welfare registers used in this study is mandatory, and their good coverage, quality, and suitability for epidemiological research have been verified (Aro et al., 1990; Gissler & Haukka, 2004; Sund, 2012). Diagnoses are made by medical professionals and registered in national databases. Most children and adolescents use free-of-charge child and school health services provided by the municipalities. Thus, everyone has access to health services and they are referred to special care when needed.
In sum, the results revealed a high accumulation of postnatal ACEs and a strong relationship between ACEs and an increased risk of mental and behavioral disorders both among exposed and controls. This study emphasizes the need for preventive actions comprising primary education on the detrimental effects of prenatal alcohol/drug exposure, pregnancy follow-up of women with substance misuse problem, continued treatment for misuse after child’ birth, and support for the family and the child from the prenatal period up to adulthood.
Conclusion
The results of the study indicate that PSE alone does not explain the high prevalence of mental and behavioral disorders among exposed youth. PSE is associated with a high accumulation of ACEs and ACEs independently increase the risk of mental and behavioral disorders. The risk was highest among youths with PSE, OHC, and a high rate of maternal risk factors.
Credit roles
Anne M. Koponen: Acquired funding, designed the data collection, planned the study, formulated hypotheses, conducted statistical analyses, interpreted results, and wrote the first draft of the manuscript and all the later versions, data curation.
Niina-Maria Nissinen: Data merging and curation, manuscript reviewing and revising.
Mika Gissler: Participated in data collection design, anonymized the original data, reviewed and revised the manuscript, statistical expert and adviser.
Ilona Autti-Rämö: Participated in data collection design, reviewed and revised the manuscript.
Taisto Sarkola: Participated in data collection design, and reviewed and revised the manuscript.
Hanna Kahila: Participated in data collection design, and reviewed and revised the manuscript.
All the authors approved the final manuscript for submission to the journal.
Ethical statement
The study was approved by the local ethical committee of HUS (Dnro 333/E8/02). All register organizations: Digital and Population Data Services Agency (DVV), Statistics Finland, Social Insurance Institution (Kela), Legal Register Center (LRC), Finnish Center for Pensions (ETK), and Finnish Institute for Health and Welfare (THL) approved the use of their register data. No study subjects are contacted. All register linkages were performed by a statistical authority (THL) and the data were analyzed anonymously by researchers with research permission provided by the register keepers. According to Finnish law, informed consent is not required in register studies when study subjects are not contacted.
Declaration of competing interest
None.
Acknowledgments
This work was supported by Samfundet Folkhälsan i svenska Finland, Juho Vainio Foundation, Signe and Ane Gyllenberg Foundation, and Medicinska Understödsföreningen Liv och Hälsa.
Footnotes
The study was approved by the local ethical committee of Helsinki University Hospital (Dnro 333/E8/02).
References
- Ackerman J.P., Riggins T., Black M.M. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125(3):554–565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro S., Koskinen R., Keskimäki I. Reliability of hospital discharge data concerning diagnosis, treatments and accidents. Duodecim; Lääketieteellinen Aikakauskirja. 1990;106(21):1443–1450. [PubMed] [Google Scholar]
- Autti-Rämö I. Twelve-year follow-up of children exposed to alcohol in utero. Developmental Medicine and Child Neurology. 2000;42:406–411. doi: 10.1017/s0012162200000748. [DOI] [PubMed] [Google Scholar]
- Bada H.S., Bann C.M., Whitaker T.M., Bauer C.R., Shankaran S., LaGasse L.…Higgins R. Protective factors can mitigate behavior problems after prenatal cocaine and other drug exposures. Pediatrics. 2012;130(6):e1479–e1488. doi: 10.1542/peds.2011-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandstra E.S., Morrow C.E., Accornero V.H., Mansoor E., Xue L., Anthony J.C. Estimated effects of in utero cocaine exposure on language development through early adolescence. Neurotoxicology and Teratology. 2011;33(1):25–35. doi: 10.1016/j.ntt.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Behnke M., Smith V.C., Committee on Substance Abuse Prenatal substance abuse: Short-and long-term effects on the exposed fetus. Pediatrics. 2013;131(3):e1009–e1024. doi: 10.1542/peds.2012-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham-Howes S., Berger S.S., Scaletti L.A., Black M.M. Systematic review of prenatal cocaine exposure and adolescent development. Pediatrics. 2013;131(6):e1917–e1936. doi: 10.1542/peds.2012-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child Welfare Act https://www.finlex.fi/en/laki/kaannokset/2007/en20070417_20131292.pdf
- Easey K.E., Dyer M.L., Timpson N.J., Munafò M.R. Prenatal alcohol exposure and offspring mental health: A systematic review. Drug and Alcohol Dependence. 2019;197:344–353. doi: 10.1016/j.drugalcdep.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlund Å., Autti‐Rämö I., Hoyme H.E., Mattson S.N., Korkman M. Risk factors for behavioural problems in foetal alcohol spectrum disorders. Acta Paediatrica. 2011;100(11):1481–1488. doi: 10.1111/j.1651-2227.2011.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P.A., Lester B.M., DeGarmo D.S., Lagasse L.L., Lin H., Shankaran S.…Whitaker T. The combined effects of prenatal drug exposure and early adversity on neurobehavioral disinhibition in childhood and adolescence. Development and Psychopathology. 2011;23(3):777–788. doi: 10.1017/S0954579411000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried P.A., Watkinson B., Dillon R.F. Neonatal neurological status in a low-risk population after prenatal exposure to cigarettes, marijuana, and alcohol. Developmental and Behavioral Pediatrics. 1987;8(6):318–326. [PubMed] [Google Scholar]
- Fried P.A., Watkinson B., Gray R. Differential effects on cognitive functioning in 13-to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicology and Teratology. 2003;25(4):427–436. doi: 10.1016/s0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Fryer S.L., McGee C.L., Matt G.E., Riley E.P., Mattson S.N. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007;119(3):e733–e741. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- Gissler M., Haukka J. Finnish health and social welfare registers in epidemiological research. Norsk Epidemiologi. 2004;14:113–120. [Google Scholar]
- Glaser D. Child abuse and neglect and the brain—a review. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2000;41(1):97–116. [PubMed] [Google Scholar]
- Gray K.A., Day N.L., Leech S., Richardson G.A. Prenatal marijuana exposure: Effect on child depressive symptoms at ten years of age. Neurotoxicology and Teratology. 2005;27(3):439–448. doi: 10.1016/j.ntt.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Halmesmäki E. Päihteiden käyttäjien raskaus [Pregnancy among substance abusing women] Duodecim. 2000;116:1513–1519. [Google Scholar]
- Halmesmäki E., Raivio K.O., Ylikorkala O. Patterns of alcohol consumption during pregnancy. Obstetrics & Gynecology. 1987;69(4):594–597. [PubMed] [Google Scholar]
- Hart H., Rubia K. Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J., Sloane M., Black-Pond C. Neurobiology and neurodevelopmental impact of childhood traumatic stress and prenatal alcohol exposure. Language, Speech, and Hearing Services in Schools. 2007;38:99–108. doi: 10.1044/0161-1461(2007/010). [DOI] [PubMed] [Google Scholar]
- Hoyme H.E., Kalberg W.O., Elliott A.J., Blankenship J., Buckley D., Marais A.…Abdul-Rahman O. Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics. 2016;138(2):e2015–4256. doi: 10.1542/peds.2015-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K., Bellis M.A., Hardcastle K.A., Sethi D., Butchart A., Mikton C.…Dunne M.P. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. The Lancet Public Health. 2017;2(8):e356–e366. doi: 10.1016/S2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- Irner T.B. Substance exposure in utero and developmental consequences in adolescence: A systematic review. Child Neuropsychology. 2012;18(6):521–549. doi: 10.1080/09297049.2011.628309. [DOI] [PubMed] [Google Scholar]
- Kahila H., Gissler M., Sarkola T., Autti-Rämö I., Halmesmäki E. Maternal welfare, morbidity and mortality 6–15 years after a pregnancy complicated by alcohol and substance abuse: A register-based case-control follow-up study of 524 women. Drug and Alcohol Dependence. 2010;111(3):215–221. doi: 10.1016/j.drugalcdep.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Kahila H., Saisto T., Kivitie-Kallio S., Haukkamaa M., Halmesmäki E. A prospective study on buprenorphine use during pregnancy: Effects on maternal and neonatal outcome. Acta Obstetricia Et Gynecologica Scandinavica. 2007;86(2):185–190. doi: 10.1080/00016340601110770. [DOI] [PubMed] [Google Scholar]
- Kalland M., Sinkkonen J. Finnish children in foster care: Evaluating the breakdown of long-term placements. Child Welfare. 2001;80(5):513–527. [PubMed] [Google Scholar]
- Kambeitz C., Klug M.G., Greenmyer J., Popova S., Burd L. Association of adverse childhood experiences and neurodevelopmental disorders in people with fetal alcohol spectrum disorders (FASD) and non-FASD controls. BMC Pediatrics. 2019;19(1):1–9. doi: 10.1186/s12887-019-1878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury J.E., Jamieson B., Milligan K. Risk for childhood internalizing and externalizing behavior problems in the context of prenatal alcohol exposure: A meta‐analysis and comprehensive examination of moderators. Alcoholism: Clinical and Experimental Research. 2018;42(8):1358–1377. doi: 10.1111/acer.13805. [DOI] [PubMed] [Google Scholar]
- Koponen A.M., Kalland M., Autti-Rämö I., Laamanen R., Suominen S. Socio-emotional development of children with foetal alcohol spectrum disorders in long-term foster family care: A qualitative study. Nordic Social Work Research. 2013;3(1):38–58. [Google Scholar]
- Koponen A.M., Nissinen N-M., Gissler M., Sarkola T., Autti-Rämö I., Kahila H. Cohort profile: ADEF Helsinki - a longitudinal register-based study on exposure to alcohol and drugs during foetal life. Nordic Studies on Alcohol and Drugs. 2020;37(1):32–42. doi: 10.1177/1455072519885719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen A. Sikiöaikana päihteille altistuneiden lasten kasvuympäristö ja kehitys [Life of children exposed to alcohol or drugs in utero]. The Kotu Research Publications 5. Finnish Association on Mental Retardation; 2006. http://ethesis.helsinki.fi [Google Scholar]
- Koponen A.M., Kalland M., Autti-Rämö I. Caregiving environment and socio-emotional development of foster-placed FASD-children. Children and Youth Services Review. 2009;31(9):1049–1056. [Google Scholar]
- Korkman M., Autti-Rämö I., Koivulehto H., Granström M. Neuropsychological effects at early school age of fetal alcohol exposure of varying duration. Child Neuropsychology. 1998;4(3):199–212. [Google Scholar]
- Korkman M., Kettunen S., Autti-Rämö I. Neurocognitive impairment in early adolescence following prenatal alcohol exposure of varying duration. Child Neuropsychology. 2003;9(2):117–128. doi: 10.1076/chin.9.2.117.14503. [DOI] [PubMed] [Google Scholar]
- Lambert B.L., Bauer C.R. Developmental and behavioral consequences of prenatal cocaine exposure: A review. Journal of Perinatology. 2012;32(11):819. doi: 10.1038/jp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdby A., Lupattelli A., Hensing G., Nordeng H. Consumption of alcohol during pregnancy - a multinational European study. Women and Birth. 2017;30(4):e207–e213. doi: 10.1016/j.wombi.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Mattson S.N., Crocker N., Nguyen T.T. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychology Review. 2011;21(2):81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson S.N., Roebuck T.M. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2002;26(6):875–882. [PubMed] [Google Scholar]
- Messinger D.S., Bauer C.R., Das A., Seifer R., Lester B.M., Lagasse L.L.…Smeriglio V.L. The maternal lifestyle study: Cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics. 2004;113(6):1677–1685. doi: 10.1542/peds.113.6.1677. [DOI] [PubMed] [Google Scholar]
- Miguel P.M., Pereira L.O., Silveira P.P., Meaney M.J. Early environmental influences on the development of children's brain structure and function. Developmental Medicine and Child Neurology. 2019;61(10):1127–1133. doi: 10.1111/dmcn.14182. [DOI] [PubMed] [Google Scholar]
- Minnes S., Lang A., Singer L. Prenatal tobacco, marijuana, stimulant, and opiate exposure: Outcomes and practice implications. Addiction Science & Clinical Practice. 2011;6(1):57–70. [PMC free article] [PubMed] [Google Scholar]
- Mukherjee R.A., Cook P.A., Norgate S.H., Price A.D. Neurodevelopmental outcomes in individuals with fetal alcohol spectrum disorder (FASD) with and without exposure to neglect: Clinical cohort data from a national FASD diagnostic clinic. Alcohol. 2019;76:23–28. doi: 10.1016/j.alcohol.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Paananen R., Ristikari T., Merikukka M., Gissler M. Social determinants of mental health: A Finnish nationwide follow-up study on mental disorders. Journal of Epidemiology & Community Health. 2013;67(12):1025–1031. doi: 10.1136/jech-2013-202768. [DOI] [PubMed] [Google Scholar]
- Pajulo M., Savonlahti E., Sourander A., Helenius H., Piha J. Antenatal depression, substance dependency and social support. Journal of Affective Disorders. 2001;65(1):9–17. doi: 10.1016/s0165-0327(00)00265-2. [DOI] [PubMed] [Google Scholar]
- Pei J., Denys K., Hughes J., Rasmussen C. Mental health issues in fetal alcohol spectrum disorder. Journal of Mental Health. 2011;20(5):473–483. doi: 10.3109/09638237.2011.577113. [DOI] [PubMed] [Google Scholar]
- Popova S., Lange S., Probst C., Gmel G., Rehm J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. The Lancet Global Health. 2017;5(3):e290–e299. doi: 10.1016/S2214-109X(17)30021-9. [DOI] [PubMed] [Google Scholar]
- Popova S., Lange S., Shield K., Mihic A., Chudley A.E., Mukherjee R.A., Rehm J. Comorbidity of fetal alcohol spectrum disorder: A systematic review and meta-analysis. The Lancet. 2016;387(10022):978–987. doi: 10.1016/S0140-6736(15)01345-8. [DOI] [PubMed] [Google Scholar]
- Price A., Cook P.A., Norgate S., Mukherjee R. Prenatal alcohol exposure and traumatic childhood experiences: A systematic review. Neuroscience & Biobehavioral Reviews. 2017;80:89–98. doi: 10.1016/j.neubiorev.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Raitasalo K., Holmila M., Jääskeläinen M., Santalahti P. The effect of the severity of parental alcohol abuse on mental and behavioural disorders in children. European Child & Adolescent Psychiatry. 2019;28(7):913–922. doi: 10.1007/s00787-018-1253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss F. Socioeconomic inequalities and mental health problems in children and adolescents: A systematic review. Social Science & Medicine. 2013;90:24–31. doi: 10.1016/j.socscimed.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Richardson G.A., Goldschmidt L., Larkby C., Day N.L. Effects of prenatal cocaine exposure on adolescent development. Neurotoxicology and Teratology. 2015;49:41–48. doi: 10.1016/j.ntt.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley E.P., Infante M.A., Warren K.R. Fetal alcohol spectrum disorders: An overview. Neuropsychology Review. 2011;21(2):73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristikari T., Merikukka M., Hakovirta M.K. Timing and duration of social assistance receipt during childhood on early adult outcomes. Longitudinal and Life Course Studies. 2018;9(3):312–326. [Google Scholar]
- Sarkola T., Gissler M., Kahila H., Autti-Rämö I., Halmesmäki E. Early healthcare utilization and welfare interventions among children of mothers with alcohol and substance abuse: A retrospective cohort study. Acta Paediatrica. 2011;100(10):1379–1385. doi: 10.1111/j.1651-2227.2011.02317.x. [DOI] [PubMed] [Google Scholar]
- Sarkola T., Kahila H., Gissler M., Halmesmäki E. Risk factors for out‐of‐home custody child care among families with alcohol and substance abuse problems. Acta Paediatrica. 2007;96(11):1571–1576. doi: 10.1111/j.1651-2227.2007.00474.x. [DOI] [PubMed] [Google Scholar]
- Schempf A.H. Illicit drug use and neonatal outcomes: A critical review. Obstetrical and Gynecological Survey. 2007;62(11):749–757. doi: 10.1097/01.ogx.0000286562.31774.76. [DOI] [PubMed] [Google Scholar]
- Schore A.N. WW Norton & Company; 2003. Affect dysregulation and disorders of the self. Norton series on interpersonal neurobiology. [Google Scholar]
- Shonkoff J.P., Boyce W.T., McEwen B.S. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Siegel D.J. Toward an interpersonal neurobiology of the developing mind: Attachment relationships, “mindsight”, and neural integration. Infant Mental Health Journal. 2001;22(1-2):67–94. [Google Scholar]
- Sood B., Delaney-Black V., Covington C., Nordstrom-Klee B., Ager J., Templin T.…Sokol R.J. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. Dose-response effect. Pediatrics. 2001;108(2):e34. doi: 10.1542/peds.108.2.e34. [DOI] [PubMed] [Google Scholar]
- Statistics Finland . 2019. Väestötieteen perusteet.https://tilastokoulu.stat.fi/verkkokoulu_v2.xql?course_id=tkoulu_vaesto&lesson_id=5&subject_id=3&page_type=sisalto [Introduction to population demographics] [Google Scholar]
- Streissguth A.P., Barr H.M., Kogan J., Bookstein F.L. Final report. University of Washington School of Medicine; Washington: 1996. Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE) [Google Scholar]
- Streissguth A.P., Bookstein F.L., Barr H.M., Sampson P.D., O'Malley K., Young J.K. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental and Behavioral Pediatrics. 2004;25(4):228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Streissguth A.P., Bookstein F.L., Sampson P.D., Barr H.M. The University of Michigan Press; Ann Arbor: 1993. The enduring effects of prenatal alcohol exposure on child development: Birth through seven years, a partial least squares solution. [Google Scholar]
- Sund R. Quality of the Finnish hospital discharge register: A systematic review. Scandinavian Journal of Public Health. 2012;40(6):505–515. doi: 10.1177/1403494812456637. [DOI] [PubMed] [Google Scholar]
- THL . THL; 2019. Suomalaisten huumeiden käyttö ja huumeasenteet 2018 [Drugs use and attitudes towards drugs in Finland]. Statistical Report 2/2019, 25.2.2019.https://thl.fi/fi/tilastot-ja-data/tilastot-aiheittain/paihteet/huumeet/suomalaisten-huumeiden-kaytto-ja-huumeasenteet [Google Scholar]
- Wachman E.M., Schiff D.M., Silverstein M. Neonatal abstinence syndrome: Advances in diagnosis and treatment. Journal of the American Medical Association. 2018;319(13):1362–1374. doi: 10.1001/jama.2018.2640. [DOI] [PubMed] [Google Scholar]
- Weyrauch D., Schwartz M., Hart B., Klug M.G., Burd L. Comorbid mental disorders in fetal alcohol spectrum disorders: A systematic review. Journal of Developmental and Behavioral Pediatrics. 2017;38(4):283–291. doi: 10.1097/DBP.0000000000000440. [DOI] [PubMed] [Google Scholar]