Summary
Recently, the environmental impacts of microplastics have received extensive attention owing to their accumulation in the environment. However, developing efficient technology for the control and purification of microplastics is still a big challenge. Herein, we investigated the photocatalytic degradation of typical microplastics such as polystyrene (PS) microspheres and polyethylene (PE) over TiO2 nanoparticle films under UV light irradiation. TiO2 nanoparticle film made with Triton X-100 showed complete mineralization (98.40%) of 400-nm PS in 12 h, while degradation for varying sizes of PS was also studied. PE degradation experiment presented a high photodegradation rate after 36 h. CO2 was found as the main end product. The degradation mechanism and intermediates were studied by in situ DRIFTS and HPPI-TOFMS, showing the generation of hydroxyl, carbonyl, and carbon-hydrogen groups during the photodegradation of PS. This study provides a green and cost-efficient strategy for the control of microplastics contamination in the environment.
Subject Areas: Catalysis, Environmental Chemistry, Nanomaterials
Graphical Abstract

Highlights
-
•
Efficient degradation of microplastics under UV light by TiO2 film
-
•
Triton-based TiO2 film showed higher photocatalytic performance
-
•
The role of radical species during microplastics degradation was elucidated
-
•
Degradation mechanism and reaction intermediates were explored
Catalysis; Environmental Chemistry; Nanomaterials
Introduction
Plastics are an ideal choice owing to its light weight, low cost, and high durability; nevertheless, their use still leads to the ever-widening problem of disposal. More than 8 × 109 metric tons of plastic reaches the waterbodies every single year, and pollution is expected to surpass oceanic fish in the next coming years (MacArthur, 2017). Generally, the discharged plastics debased into small particles via combined effects of solar irradiation, physical degradation, and biodegradation (van Weert et al., 2019). The plastic decomposition leads to the formation of the micro (size less than 5 μm) and nano-plastics (size less than 1 μm) (Koelmans et al., 2015). Although recycling is progressively predominant, one-third of all plastics are still small or complex to recuperate economically (Garcia and Robertson, 2017). As a conventional plastic material, polystyrene (PS) and polyethylene (PE) are used in building materials (insulation and molding), foam packaging, food containers, and compact disks (Zan et al., 2004). However, their release in the water environment occurs through poor handling, wastewater treatment plants (McCormick et al., 2014), and microbial decomposition (Andrady, 2011). PS and PE show adverse effects on human health and aquatic organisms owing to their recalcitrant nature (Li et al., 2018). Its contamination within the aquatic environment poses a severe threat not only to aquatic biota but also to terrestrial organisms via food webs (Barboza et al., 2018; Besseling et al., 2012; Fossi et al., 2014; Rochman et al., 2017; Watts et al., 2014). The seriousness and complication of plastics waste problems indicate that a new approach is desperately required for the decomposition of polymers.
The conventional wastewater purification technologies present difficulty in control and elimination of microplastics (Moore, 2008). Several techniques have been used for the removal of microplastics, including biodegradation (Rochman et al., 2017; Yang et al., 2015) and photodegradation (Feng et al., 2010). The biodegradation process is highly dependent on the microbial species and soil environment and requires more stringent storage conditions (Inkinen et al., 2011; Jakubowicz, 2003). Feng and his coworkers first time reported the photo-assisted Fenton degradation of polystyrene microspheres (Feng et al., 2010), whereas other studies on polystyrene degradation mainly dealt with plastic film for the development of photodegradable plastics (Kemp and McIntyre, 2006; Shang et al., 2003; Zan et al., 2006). The main limitation of Fenton-based reaction is the oxidant and iron ions loss due to the radical scavenging effect of hydrogen peroxide (H2O2) (Zhou et al., 2016). Recently, Kang et al. studied the degradation of cosmetic microplastics by catalytic activation of peroxymonosulfate to generate reactive radicals using carbon hybrids (Kang et al., 2019). However, the complete mineralization of microplastics was not achieved.
Photocatalysis is considered as an advanced oxidation process for pollutants removal (Klavarioti et al., 2009). It is a well-established green technique with a primary feature that it exploits free and infinite solar energy, implying its great potential as a low-cost and environmentally friendly treatment technology (Li et al., 2019). TiO2 is widely used as a model photocatalyst owing to its efficient oxidizing ability of organic pollutants (Yuan et al., 2017). TiO2 has performed exceptionally well in various studies for the removal of organic pollutants under UV light, and there is a need to further study its use for microplastic degradation, although few studies have used TiO2/plastic composite materials for the development of photodegradable plastic (Kemp and McIntyre, 2006; Shang et al., 2003; Zan et al., 2006), such as polypropylene (PP) (Kamrannejad et al., 2014), polystyrene (PS) (Shang et al., 2003), and high- (Wang et al., 2015) and low-density polyethylene (PE) films (Thomas et al., 2013; Thomas and Sandhyarani, 2013). TiO2 nanotubes showed enhanced removal of low-density PE under visible light (Ali et al., 2016), whereas the activity of TiO2 was improved using copper phthalocyanine (Zhao et al., 2008), polypyrrole (Li et al., 2010), multiwalled carbon nanotubes (An et al., 2014) for PE removal, whereas iron phthalocyanine (Fa et al., 2008) and ferric stearate (Fa et al., 2013) were applied for photodegradable PS. The photocatalytic degradation for dealing with microplastics has not been explored so far.
In this study, we have developed a novel and generalized approach for solid-phase photodegradation of microplastics (PS and PE) and also introduced the effect of different preparation methods on the photocatalytic performance of TiO2. The degradation mechanism, reaction intermediates, and feasibility of mineralization were investigated. The in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), high-pressure photon ionization (HPPI)-TOFMS, and gas chromatography (GC) spectroscopy results showed that the larger fragments of PS were broken into smaller ones and finally changed into CO2. Considering the natural availability of TiO2 and the global environmental impact of microplastics, TiO2-enhanced photodegradation system may be established into a promising approach to take advantage of natural material for the removal of microplastics. For real application, TiO2-based films can be fabricated on microplastic filters, which can be directly used for the decomposition of microplastics under UV light irradiation, and it can also avoid the release of toxic intermediates into water in the technologies of liquid phase elimination of microplastics.
Results and Discussion
Photocatalytic Degradation of PS and PE
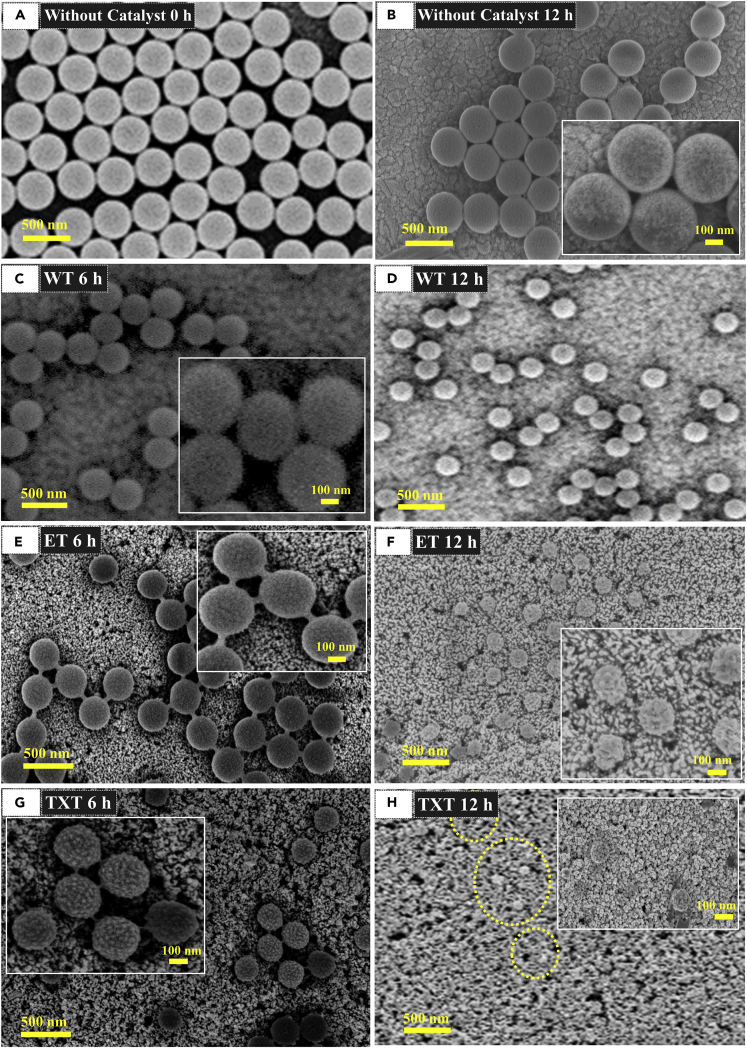
The morphological changes in 400-nm PS on TiO2 nanoparticle films fabricated with water (WT), ethanol (ET), Triton X-100 (TXT), and without catalyst (FTO) before and after UV light irradiation are shown in Figures 1 and S1. It can be seen that PS nanospheres were well dispersed on all films. Figure 1A displays the exact size and morphology of 400-nm PS on FTO (without photocatalyst) at 0 h irradiation. Almost a negligible change in PS size was observed on FTO during the whole photoirradiation reaction (Figure 1B). However, PS spheres exhibited a change in morphology and size on TiO2-based catalysts at the initial 3 h of photoreaction (Figure S1). The light-induced coalescence of PS during degradation is because the photocatalytic degradation (hydroxyl radical oxidation) at the contact point of packed spheres is slower, which suggests that the degradation takes place on the PS-air interface. A more significant change in PS morphology and size was observed after another 3 and 6 h of irradiation (Figures S1, 1D, 1F, and 1H). TXT film exhibited an obvious change in PS morphology (Figure 1H) and size as compared with ET and WT films. Moreover, we further investigated 700 nm, 1 μm, and 5 μm sized PS spheres on TXT film, which showed significant changes in PS morphology and reduction in sphere size during the photoreaction (Figure S2). The change in PS morphology and size was further analyzed by determining the average diameter, degradation efficiency, and volume change. Based on the above observations, it is reasonable to conclude that TXT is very efficient in the photocatalytic degradation of PS.
Figure 1.
FE-SEM Images of PS Spheres after Different Irradiation Time under 365 nm UV Light
(A) PS spheres on FTO (without catalyst) before irradiation.
(B) Without catalyst after 12 h irradiation.
(C) Water-based TiO2 (WT) after 6 h irradiation.
(D) WT after 12 h irradiation.
(E) Ethanol-based TiO2 (ET) after 6 h irradiation.
(F) ET after 12 h irradiation.
(G) Triton X-100 based TiO2 (TXT) after 6 h irradiation.
(H) TXT after 12 h irradiation.
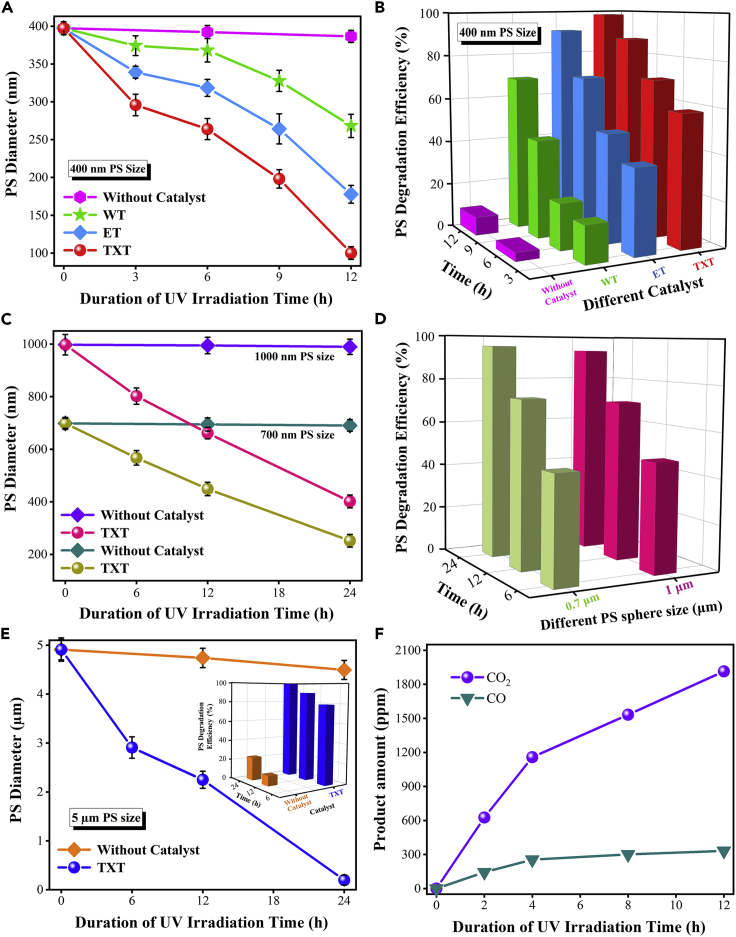
Figure 2 displays the PS diameter, degradation efficiency, and inorganic carbon content during the photodegradation reaction. The diameter change of 400-nm PS was almost negligible on FTO, whereas this diameter became 268.18 nm on WT film after 12 h illumination. Simultaneously, the ET and TXT film shows a significant decrease in PS diameter, which was 177.78 and 100.07 nm, respectively (Figure 2A). The difference in photocatalytic performance of films can be ascribed to band energy, surface area, morphology, charge separation, light absorption, and texture (Vahl et al., 2019). Comparing the photocatalytic activity of fabricated films, TXT showed higher performance, which was attributed to its low band energy and good charge separation, which was studied in detail later. Therefore, TXT produces more electron-hole pairs under light irradiation and also extends the charge separation resulting in significant photoactivity for PS removal, whereas the mediocre performance of WT film is presumably due to its large particle size, low surface area, and a poor charge separation ability. The degradation efficiency of PS was calculated by (Equation 1):
| (Equation 1) |
Figure 2.
Photocatalytic Degradation of Polystyrene
(A) Diameter change of 400-nm PS on TXT, ET, WT films, and without catalyst under different irradiation time intervals.
(B) PS degradation efficiency (%) with (TXT, ET, WT) and without catalyst.
(C) Diameter change of 1-μm and 700-nm PS on TXT film, and without catalyst.
(D) Degradation efficiency (%) of 1-μm and 700-nm PS on TXT film under 365-nm UV light.
(E) Diameter change of 5-μm PS on TXT film, and without catalyst (inserted degradation efficiency [%] of 5 μm) under 254-nm UV light.
(F) Concentrations of carbon dioxide (CO2) and carbon monoxide at different stages of photodegradation reaction.
It was observed that the photodegradation of PS increased with increasing irradiation time. After 12 h of UV irradiation, the degradation efficiency of 400-nm PS was 98.40%, 91.04%, and 69.25% for TXT, ET, and WT films, respectively (Figure 2B). Since the PS degradation efficiency was negligible on FTO (8.01%), indicating the direct photolysis of PS was insignificant. Figures 2C and 2D present the diameter change and the degradation efficiency of 700-nm and 1-μm PS, which was decreased to 251.96 and 401.28 nm, respectively, after 24 h irradiation. Meanwhile, the degradation efficiency for 1-μm and 700-nm PS was 93.49% and 95.30%, respectively, after 24 h irradiation under 365 nm UV light. However, the degradation efficiency of 5-μm PS was 99.99% after 24 h illumination under 254 nm UV and the faster degradation is attributed to the higher energy of photon and more charge carriers generated (Figure 2E).
Figures S3 and S4 present volume and surface area changes in varying sizes of PS, and effectual changes were observed with the increase in irradiation time. Figures 2F and S3D show the concentration of CO2 with TiO2 and PS with TiO2/PS. At the initial phase, the concentration of CO2 increases linearly owing to the fast photodegradation of PS (direct contact of PS with TiO2 nanoparticles) and then slowly decelerates in the subsequent phase because of slow desorption process. CO2 plot of TiO2 with PS was 10 times higher than the control experiment signifying that CO2 was produced from the PS decomposition, whereas in control experiment, a slight quantity of CO2 might be due to the presence of organic impurities. Moreover, a small amount of carbon monoxide (CO) appeared owing to the stretching of C-H bond in the TiO2/PS system (Cho and Choi, 2001).
Furthermore, we conducted an experiment to check PS decomposition in the liquid phase to evaluate if it would make the same contribution as the solid phase. The comparison results are illustrated in Figure S5. Interestingly, the mineralization efficiency of microplastic that occurred on the solid (microplastic)-solid (TiO2) interface was much higher than that in the liquid phase. This is because t there is less chance of hydroxyl radicals and holes to react with microplastic particles in the liquid phase (suspended, close contact between TiO2 and microplastic particle becomes difficult), whereas on the solid-solid interface the key structural feature is that it facilitates charge separation to hinder recombination and enhances the photocatalytic degradation efficiency (Li and Gray, 2007). Moreover, the exposed surface of PS in the solid phase is higher owing to the direct contact with TiO2 film, which leads to the direct attack of reactive species without any hindrance, whereas in the liquid phase the slower degradation is due to the hydrophilicity of PS particles in water solution, which makes the attack of reactive species slower.
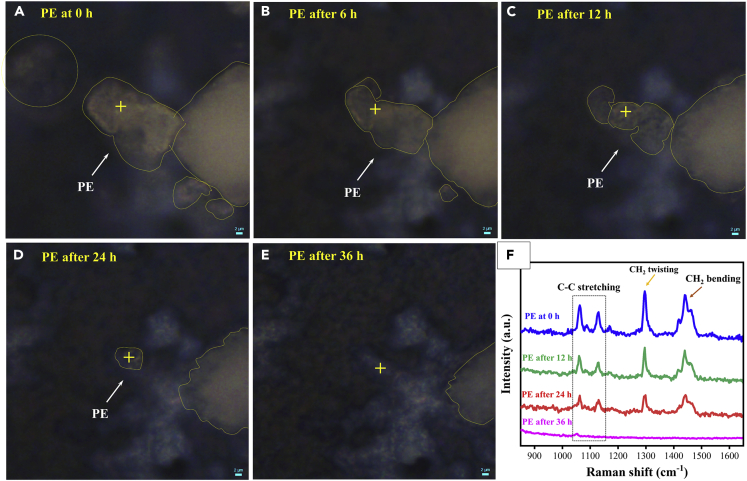
We further investigated the activity of TXT film for the degradation of PE, and analysis was carried out through Raman spectroscopy. Figure 3A presents the actual PE morphology before UV light irradiation, and the plus (+) symbol presents the selected point for Raman detection and spectrum. Interestingly, PE shows changes in morphology and size with the increase in irradiation time (Figures 3B–3D) and finally, tens of micrometers-sized PE particles totally disappeared after 36 h of UV irradiation, signifying the complete degradation of PE (Figure 3E), although an insignificant degradation was observed on FTO (Figure S6). Figure 3F shows the Raman band of PE at 1,064, 1,133, 1,296, and 1,442 cm−1, which corresponds to C-C stretching, CH2 twisting, and CH2 bending (Wright et al., 2019). Raman spectra of PE exhibited a decline in PE peak with irradiation time, but no peak of PE was detectable after 36 h of photoreaction, which was consistent with Raman imaging. In summary, TXT film also showed efficient photocatalytic activity for PE degradation under UV light. The oxidation state of TiO2 film remained the same before and after the degradation reaction, and results are shown in Figure S7. Figure S8 shows the actual morphology of the TXT film before and after the degradation reaction. It can be seen that there was no prominent change in catalyst morphology after 12 h of PS degradation reaction, which suggests that the prepared film is highly stable. We investigated the effect of light energy, wavelength, and degradation rate under different light sources (254 nm, 365 nm, and visible light), and the results are shown in Figure S9.
Figure 3.
Photocatalytic Degradation of PE on TXT Film under 254-nm UV Light
(A) PE at 0 h; (B) PE after 6 h; (C) PE after 12 h; (D) PE after 24 h; (E) PE after 36 h; (F) Raman spectrum of PE with different time profiles. (Highlighted parts present PE, and the same point was used for Raman imaging and detection throughout the experiment.)
Structure, Morphology, and Optical Properties of Prepared Catalysts
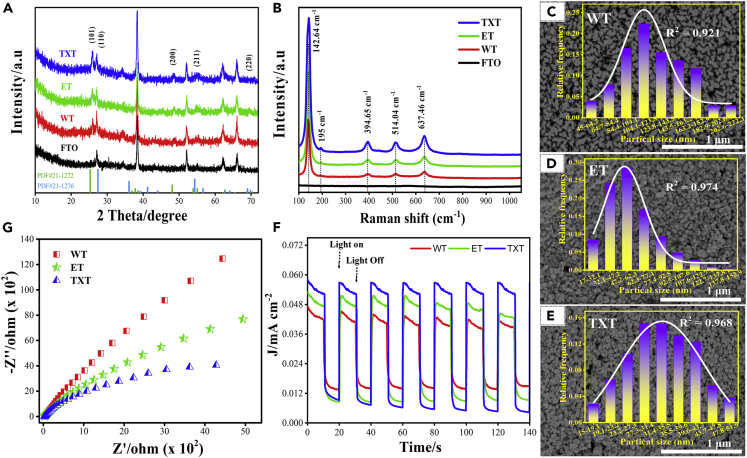
The crystalline structure of the prepared TiO2 films on FTO substrate was examined by X-ray diffraction (XRD) as shown in Figure 4A. All diffraction peaks of the synthesized films correspond well to a reference pattern of tetragonal anatase and rutile phases with the joint committee on powder diffraction standards (JCPDS) file Nos. 21-1,272 and 21-1,276. The diffractogram consists of peaks at (2 Theta) 25.28°, 48.04°, and 55.06° along with Miller indices values (101), (200), and (211) of anatase TiO2, respectively. The peaks at (2 Theta) 27.45° and 36.08° match very well with the diffraction (110) and (101) crystal planes of rutile phase TiO2. It is notable that the width of (101) planes in TXT was stronger at (2 Theta) 25.28° as compared with ET and WT representing the higher nano crystallinity of film. The diffraction peaks from FTO glass was also observed, which can be attributed to the thinness and high porosity of TiO2 films.
Figure 4.
Structure, Morphology, and Optical Properties
(A) XRD patterns of TXT, ET, and WT photocatalysts (XRD pattern of FTO was used as a baseline for comparison with catalyst films).
(B) Raman spectrum of fabricated photocatalyst films.
(C), (D), and (E) FE-SEM images of the nanoporous WT, ET, and TXT photocatalysts with their particle size distribution (inserted).
(F) Amperometric i-t curves of TXT, ET, and WT photocatalysts under chopped illumination.
(G) Electrochemical impedance spectroscopy (EIS) Nyquist plot of TXT, ET, and WT catalyst films.
Raman spectroscopy is beneficial for local structure analysis of the catalysts. Raman spectrum was recorded at wavenumber 100 to 1,100 cm−1, and the FTO glass spectrum was used as a control experiment. The Raman spectrum of the TiO2 show peaks at 142.64 (Eg), 195 (Eg), 394.65 (B1g), 514.04 (A1g), and 637.46 cm−1 (Eg), which are assigned to the anatase phase of TiO2, whereas a minor peak that appears at 445 (Eg) is attributed to the rutile phase of TiO2 (Figure 4B) (Wang et al., 2016). All samples show analogs peak, and no other peak was observed.
The morphology of the prepared TiO2 films was characterized by using field-emission scanning electron microscopy (FE-SEM) (Figures 4C–4E). Figure 4E revealed that the presence of Triton X-100 significantly affected the morphology of TiO2 (Suwanchawalit et al., 2017), such as the formation of nanoparticles, induced a particle size reduction, and fine pore formation (Godinez and Darnault, 2011). Particle size can influence the surface area and the recombination rate of photogenerated electron-hole pairs. Organic solvents act as a morphology-directing agent and effect on the texture of film during the annealing process, which leads to change in its optical and electrical properties and pore content. The particle size of the prepared films was calculated by using the nano measurer 1.2 software (inserted in Figures 4C–4E). The average particle size in TXT could be varied between 27.3 and 31.1 nm. However, the average particle size in ET and WT was ranging from 47.2 to 62.3 nm and 104.1 to 123.8 nm, respectively. It could be seen that the distribution curves fit well the Gaussian function with R2 values of 0.921, 0.974, and 0.968 for WT, ET, and TXT, respectively, indicating that the size distribution of the samples was consistent with a normal distribution. Water contact angle and optical properties measurements of the nanoporous TiO2 films are shown in Figures S10 and S11, respectively. TEM images of the synthesized film showed that TXT film exhibited cuboid and spherical-shaped particles, whereas ET and WT have only a spherical-shaped particle and a dark color in TEM images was observed owing to a high electron density of Ti element (Figure S12). The cross-sectional SEM images show that TiO2 film has a thickness of about 1.21 (Figure S13). TXT film has a high surface area as compared with ET and WT films, as shown in Figure S14 and Table S1.
The charge carrier generation and separation in the synthesized photocatalysts were investigated by using the amperometric i-t technique and electrochemical impedance spectroscopy (EIS) (Figures 4F and 4G). The transient photocurrent response of the films (WT, ET, and TXT) was studied at an applied potential of 0.8 V under simulated solar light illumination. The rise and drop of the photocurrent respond well to chopped light. The photocurrent drops when the light was off and increases instantly when the light was on, specifying that the current was completely due to the light response of the catalyst, and the rate of charge transfer was very speedy. Figure 4F illustrates that the TXT produces the transient photocurrent density of 0.058 mA cm−2, which was higher than the ET (0.053 mA cm−2) and WT (0.046 mA cm−2). This i-t result indicates that the TXT has a better efficiency in the generation, separation, and transport of electron-hole pairs, which was in good agreement with the following EIS result. The smaller arc radius suggests a more effective separation of photogenerated electron-hole pairs and faster interfacial charge transfer. As can be seen from Figure 4G, the arc radius of TXT catalyst was smaller than the ET and WT, signifying that TXT could facilitate the effective separation and transfer of photogenerated electron-hole pairs that occur on the TXT surface. These electric characteristics showed that Triton X-100 played an effective role in improving the photocatalytic efficiency of the film.
Mass Spectrum and Degradation Mechanism
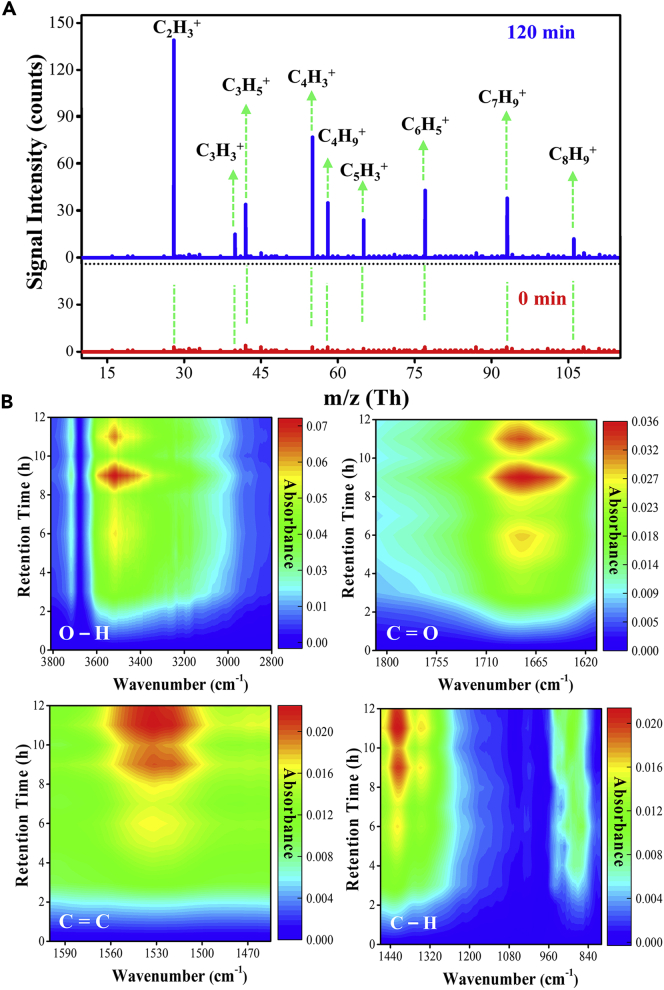
Nature of the chemical substances formed during the degradation process of PS was characterized through high-pressure photon ionization (HPPI)-TOFMS. Mass spectra were obtained before and after 120 min of UV irradiation, and no dominant peak was observed at 0 min. Numerous characteristic ion fragments were formed during the photodegradation of PS such as m/z = 27 (C2H3+), 39 (C3H3+), 41 (C3H5+), 51 (C4H3+), 63 (C5H3+), 77 (C6H5+), 91 (C7H9+), and 105 (C8H9+) (Ng et al., 2018). These fragments were originated from the cleavage of PS spheres. The O-containing intermediates were observed with peak m/z values 43.01 (C2H3O+), 44.94 (C2H5O+), 57.03 (C3H5O+), 59.04 (C3H7O+), and 87.04 (C4H7O2+), but the intensities of these peaks are very weak. Therefore, these peaks were not considered in reaction intermediates. Figure 5A shows the m/z spectra of these commonly observed ions. Among the various degradation intermediates, the m/z 105 belongs to the styrene (Bruns et al., 2017), whereas the m/z 77 and 91 corresponds to the benzene and toluene. The other small fragments with m/z 27, 39, 41, and 51 were derived due to the breakdown of bigger m/z fragments. As the photodegradation reaction proceeds, the PS fragments were further broken and finally changed into CO2 according to the GC result.
Figure 5.
Mass Spectrum and Degradation Mechanism
(A) Mass spectra obtained by high-pressure photon ionization (HPPI)-TOFMS during the photodegradation of PS.
(B) DRIFTS study of PS at different time intervals.
For further consolidation, an in situ DRIFTS study was done to understand the chemical functionality of organic species during the photodegradation reaction of PS (Figures 5B and S15). The IR spectra of TiO2/PS at about 3,600–3,200 cm−1 were attributed to the hydroxyl group formation (Kaczmarek et al., 2008), and the band that appears at 1,650–1700 cm−1 was due to a carbonyl group (C=O) (Subramani and Sepperumal, 2016; Zan et al., 2004). These two bands were assigned to the formation of aromatic and aliphatic ketones of the acetophenone and OH group in the PS chain. The reason for these two bands formation may be the chain scission or a competitive cross-linking or the oxidation of the benzene ring. A minor peak at 2,314 cm−1 was observed, which was due to CO2 formation during the reaction. The band at 1,527 cm−1 was associated with stretching of the C=C bonds (Gipson et al., 2015) from the aromatic ring, and the intensity of these bands increases continuously with increasing the illumination time of TiO2/PS composite. The band that appears at 1,425 cm−1 was due to C-H bend (Truc et al., 2017), whereas the band at 873 cm−1 corresponds to C-H stretching vibration of the aromatic ring and deformation vibration in cyclic carbonyl compounds (Al-Kadhemy et al., 2016). The bond smashing provides evidence that the TiO2 and UV irradiation accelerate the bond cleavage of PS. Figure S16 presents the thermogravimetric analysis (TGA) curve of PS, which signifies the complete mineralization after 24 h irradiation.
In order to understand the photodegradation mechanism of PS, including the role, and possible pathway for the formation of active species, corresponding effective scavengers were added in the reaction system. Figure S17 shows the change in PS diameter over TXT film after 6 h irradiation under different reaction conditions. TBA was employed to scavenge the ⋅OH (hydroxyl radical), EDTA to trap h+ (holes), and the anaerobic experiment was conducted to check the role of oxygen. The addition of EDTA completely inhibited the PS degradation, whereas TBA and anaerobic experiments induced the depression effect. A slight degradation in anaerobic condition was observed owing to the attack of electron on PS particles yielding the PS cation radical, which further leads to its degradation (Lu et al., 2011; Szabó-Bárdos et al., 2011). Along this, holes and hydroxyl radicals were also formed by TiO2 and became dominant oxidants to attack plastic particles that cause degradation (Lee et al., 2014). Therefore, we conclude that h+, ⋅OH, and oxygen play an important role in the photodegradation of PS, whereas the h+ was the dominant active species in our reaction. The possible reaction of h+ with other species produces more active species causing the highly favorable generation of iOH. Moreover, the degradation performance of microplastics as compared with the literature and details are presented in Table S2.
The photodegradation reaction of PS on a solid state was initiated by holes and reactive oxidative species such as. UV light stimulates the TiO2 to produce an electron in the conduction (e−CB) and formation of a positive hole in the valence band (h+VB) (Equation 2). There are two possibilities for reaction initiation. In the first pathway, the oxidation reaction takes place at the valence band. h+VB is a powerful oxidizing agent and capable of oxidizing organic compounds resulting in CO2 and H2O (Equation 3), as well as it can also oxidize the compounds by generating hydroxyl radicals (Equation 4) (Kesselman et al., 1997).radicals have an electrophilic nature; they can non-selectively oxidize all electron-rich organic compounds and eventually result in their mineralization (Equation 5) (Shang et al., 2003).
| (Equation 2) |
| (Equation 3) |
| (Equation 4) |
| (Equation 5) |
n the second pathway, the degradation reaction takes place at the conduction band. The e−CB reacts with oxygen, forming a superoxide radical (Equation 6). This superoxide radical reaction with water produces (Equation 7) that generates hydrogen peroxide (Equation 8). The hydrogen peroxide finally changed into (Equation 9), which reacts with microplastic and leads to its degradation (Cho and Choi, 2001).
| (Equation 6) |
| (Equation 7) |
| (Equation 8) |
| (Equation 9) |
Conclusion
This work proposed the efficient degradation and complete mineralization of microplastics by TiO2 nanoparticles film under UV light irradiation. Among synthesized films, TXT exhibits high performance as compared with ET and WT. A significant photocatalytic degradation and mineralization for varying sizes of PS such as 5 μm, 1 μm, and 700 nm and polyethylene (PE) were observed by TXT. The superior activity of TXT film is due to surface hydrophilicity and film texture. Surface hydrophilicity can enhance the interaction between semiconductors and plastic, particles that improved the film texture leading to charge transfer and separation that results in the fast degradation of microplastics. The degradation mechanism was studied by in situ DRIFTS and mass spectrometer, showing the generation of hydroxyl, carbonyl, and carbon-hydrogen groups, which is evidence for the photodegradation of PS. The film fabrication process does not include any costly, toxic, and hazardous chemicals, which makes this method highly efficient, beneficial, and economically important. Moreover, the solid phase photodegradation of microplastics also avoids the release of possible toxic intermediates into water in the liquid phase technology. Overall, the present study put forward a green and sustainable method for microplastic waste degradation from the environment.
Limitations of the Study
The main limitation of this study is that, initially, microplastics should be separated from water bodies, which can be done by using microplastic filters. Second, the complete mineralization of microplastics was observed under UV light. So, there is need for promising applications for microplastic mineralization under visible light.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Zhang Liwu (zhanglw@fudan.edu.cn).
Materials Availability
This study did not generate new materials. Detailed information on experiments can be found in the accompanying Transparent Methods.
Data and Code Availability
This study did not generate a new code. All relevant data are available from the Lead Contact upon reasonable request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors gratefully acknowledge financial support from National Natural Science Foundation of China (Nos. 21677037, 21976030, 21507011and 21607027), the Ministry of Science and Technology of the People's Republic of China (2016YFE0112200), and the Natural Science Foundation of Shanghai (Nos. 19ZR1471200 and 17ZR1440200).
Author Contributions
L.Z. was the originator of the concept and planned and supervised the experiments. I.N. synthesized the photocatalysts, analyzed data, carried out photodegradation experiments, and wrote the manuscript. A.-U.-R.B., K.L., and H.C. performed the PEC experiments. T.W. and Y.L. did the in situ DRIFTS and HPPI-TOFMS measurements. S.A. and Y.Y. carried out the GC analysis. Y.F. assisted in Raman experiments. All authors reviewed the manuscript.
Declaration of Interests
The authors declare no competing financial interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101326.
Supplemental Information
References
- Al-Kadhemy M.F.H., Rasheed Z.S., Salim S.R. Fourier transform infrared spectroscopy for irradiation coumarin doped polystyrene polymer films by alpha ray. J. Radiat. Res. Appl. Sci. 2016;9:321–331. [Google Scholar]
- Ali S.S., Qazi I.A., Arshad M., Khan Z., Voice T.C., Mehmood C.T. Photocatalytic degradation of low density polyethylene (LDPE) films using titania nanotubes. Environ. Nanotechnol. Monit. Manag. 2016;5:44–53. [Google Scholar]
- An Y., Hou J., Liu Z., Peng B. Enhanced solid-phase photocatalytic degradation of polyethylene by TiO2–MWCNTs nanocomposites. Mater. Chem. Phys. 2014;148:387–394. [Google Scholar]
- Andrady A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011;62:1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Barboza L.G.A., Vethaak A.D., Lavorante B.R., Lundebye A.-K., Guilhermino L. Marine microplastic debris: an emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018;133:336–348. doi: 10.1016/j.marpolbul.2018.05.047. [DOI] [PubMed] [Google Scholar]
- Besseling E., Wegner A., Foekema E.M., Van Den Heuvel-Greve M.J., Koelmans A.A. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.) Environ. Sci. Technol. 2012;47:593–600. doi: 10.1021/es302763x. [DOI] [PubMed] [Google Scholar]
- Bruns E.A., Slowik J.G., Haddad I.E., Kilic D., Klein F., Dommen J., Temime-Roussel B., Marchand N., Baltensperger U., Prévôt A.S. Characterization of gas-phase organics using proton transfer reaction time-of-flight mass spectrometry: fresh and aged residential wood combustion emissions. Atmos. Chem. Phys. 2017;17:705–720. [Google Scholar]
- Cho S., Choi W. Solid-phase photocatalytic degradation of PVC–TiO2 polymer composites. J. Photoch. Photobio. A Chem. 2001;143:221–228. [Google Scholar]
- Fa W., Guo L., Wang J., Guo R., Zheng Z., Yang F. Solid-phase photocatalytic degradation of polystyrene with TiO2/Fe (St)3 as catalyst. J. Appl. Polym. Sci. 2013;128:2618–2622. [Google Scholar]
- Fa W., Zan L., Gong C., Zhong J., Deng K. Solid-phase photocatalytic degradation of polystyrene with TiO2 modified by iron (II) phthalocyanine. Appl. Catal. B Environ. 2008;79:216–223. [Google Scholar]
- Feng H.-M., Zheng J.-C., Lei N.-Y., Yu L., Kong K.H.-K., Yu H.-Q., Lau T.-C., Lam M.H. Photoassisted Fenton degradation of polystyrene. Environ. Sci. Technol. 2010;45:744–750. doi: 10.1021/es102182g. [DOI] [PubMed] [Google Scholar]
- Fossi M.C., Coppola D., Baini M., Giannetti M., Guerranti C., Marsili L., Panti C., de Sabata E., Clò S. Large filter feeding marine organisms as indicators of microplastic in the pelagic environment: the case studies of the Mediterranean basking shark (Cetorhinus maximus) and fin whale (Balaenoptera physalus) Mar. Environ. Res. 2014;100:17–24. doi: 10.1016/j.marenvres.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Garcia J.M., Robertson M.L. The future of plastics recycling. Science. 2017;358:870–872. doi: 10.1126/science.aaq0324. [DOI] [PubMed] [Google Scholar]
- Gipson K., Stevens K., Brown P., Ballato J. Infrared spectroscopic characterization of Photoluminescent polymer nanocomposites. J. Spectrosc. 2015;2015:1–9. [Google Scholar]
- Godinez I.G., Darnault C.J. Aggregation and transport of nano-TiO2 in saturated porous media: effects of pH, surfactants and flow velocity. Water Res. 2011;45:839–851. doi: 10.1016/j.watres.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Inkinen S., Hakkarainen M., Albertsson A.-C., Södergård A. From lactic acid to poly (lactic acid) (PLA): characterization and analysis of PLA and its precursors. Biomacromolecules. 2011;12:523–532. doi: 10.1021/bm101302t. [DOI] [PubMed] [Google Scholar]
- Jakubowicz I. Evaluation of degradability of biodegradable polyethylene (PE) Polym. Degrad. Stab. 2003;80:39–43. [Google Scholar]
- Kaczmarek H., Felczak A., Szalla A. Studies of photochemical transformations in polystyrene and styrene-maleic anhydride copolymer. Polym. Degrad. Stab. 2008;93:1259–1266. [Google Scholar]
- Kamrannejad M.M., Hasanzadeh A., Nosoudi N., Mai L., Babaluo A.A. Photocatalytic degradation of polypropylene/TiO2 nano-composites. Mater. Res. 2014;17:1039–1046. [Google Scholar]
- Kang J., Zhou L., Duan X., Sun H., Ao Z., Wang S. Degradation of cosmetic microplastics via functionalized carbon nanosprings. Matter. 2019;1:745–758. [Google Scholar]
- Kemp T.J., McIntyre R.A. Influence of transition metal-doped titanium (IV) dioxide on the photodegradation of polystyrene. Polym. Degrad. Stab. 2006;91:3010–3019. [Google Scholar]
- Kesselman J.M., Weres O., Lewis N.S., Hoffmann M.R. Electrochemical production of hydroxyl radical at polycrystalline Nb-doped TiO2 electrodes and estimation of the partitioning between hydroxyl radical and direct hole oxidation pathways. J. Phys. Chem. B. 1997;101:2637–2643. [Google Scholar]
- Klavarioti M., Mantzavinos D., Kassinos D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009;35:402–417. doi: 10.1016/j.envint.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Koelmans A.A., Besseling E., Shim W.J. Springer; 2015. Nanoplastics in the Aquatic Environment. Critical Review, Marine Anthropogenic Litter; pp. 325–340. [Google Scholar]
- Lee W.-L.W., Lu C.-S., Lin H.-P., Chen J.-Y., Chen C.-C. Photocatalytic degradation of ethyl violet dye mediated by TiO2 under an anaerobic condition. J. Taiwan Inst. Chem. Eng. 2014;45:2469–2479. [Google Scholar]
- Li A., Zhu W., Li C., Wang T., Gong J. Rational design of yolk–shell nanostructures for photocatalysis. Chem. Soc. Rev. 2019;48:1874–1907. doi: 10.1039/c8cs00711j. [DOI] [PubMed] [Google Scholar]
- Li G., Gray K.A. The solid-solid interface: explaining the high and unique photocatalytic reactivity of TiO2-based nanocomposite materials. Chem. Phys. 2007;339:173–187. [Google Scholar]
- Li J., Liu H., Chen J.P. Microplastics in freshwater systems: a review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018;137:362–374. doi: 10.1016/j.watres.2017.12.056. [DOI] [PubMed] [Google Scholar]
- Li S., Xu S., He L., Xu F., Wang Y., Zhang L. Photocatalytic degradation of polyethylene plastic with polypyrrole/TiO2 nanocomposite as photocatalyst. Polym. Plast. Technol. Eng. 2010;49:400–406. [Google Scholar]
- Lu S.-y., Wu D., Wang Q.-l., Yan J., Buekens A.G., Cen K.-F.J.C. Photocatalytic decomposition on nano-TiO2: destruction of chloroaromatic compounds. Chemosphere. 2011;82:1215–1224. doi: 10.1016/j.chemosphere.2010.12.034. [DOI] [PubMed] [Google Scholar]
- MacArthur E. Beyond plastic waste. Science. 2017;358:843. doi: 10.1126/science.aao6749. [DOI] [PubMed] [Google Scholar]
- McCormick A., Hoellein T.J., Mason S.A., Schluep J., Kelly J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014;48:11863–11871. doi: 10.1021/es503610r. [DOI] [PubMed] [Google Scholar]
- Moore C.J. Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ. Res. 2008;108:131–139. doi: 10.1016/j.envres.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Ng K.M., Lau Y.T.R., Weng L.T., Yeung K.L., Chan C.M. ToF-SIMS and computation analysis: fragmentation mechanisms of polystyrene, polystyrene-d5, and polypentafluorostyrene. Surf. Interface Anal. 2018;50:220–233. [Google Scholar]
- Rochman C.M., Regan F., Thompson R.C. On the harmonization of methods for measuring the occurrence, fate and effects of microplastics. Anal. Methods. 2017;9:1324–1325. [Google Scholar]
- Shang J., Chai M., Zhu Y. Photocatalytic degradation of polystyrene plastic under fluorescent light. Environ. Sci. Technol. 2003;37:4494–4499. doi: 10.1021/es0209464. [DOI] [PubMed] [Google Scholar]
- Subramani M., Sepperumal U. FTIR analysis of bacterial mediated chemical changes in Polystyrene foam. Ann. Biol. Res. 2016;7:55–61. [Google Scholar]
- Suwanchawalit C., Buddee S., Wongnawa S. Triton X-100 induced cuboid-like BiVO4 microsphere with high photocatalytic performance. J. Environ. Sci. 2017;55:257–265. doi: 10.1016/j.jes.2016.04.030. [DOI] [PubMed] [Google Scholar]
- Szabó-Bárdos E., Somogyi K., Törő N., Kiss G., Horváth A.J.A. Photocatalytic decomposition of l-phenylalanine over TiO2: identification of intermediates and the mechanism of photodegradation. Appl. Catal. B Environ. 2011;101:471–478. [Google Scholar]
- Thomas R.T., Nair V., Sandhyarani N. TiO2 nanoparticle assisted solid phase photocatalytic degradation of polythene film: a mechanistic investigation. Colloids Surf. A. Physicochem. Eng. Asp. 2013;422:1–9. [Google Scholar]
- Thomas R.T., Sandhyarani N. Enhancement in the photocatalytic degradation of low density polyethylene–TiO2 nanocomposite films under solar irradiation. RSC Adv. 2013;3:14080–14087. [Google Scholar]
- Truc N.T.T., Lee C.-H., Lee B.-K., Mallampati S.R. Development of hydrophobicity and selective separation of hazardous chlorinated plastics by mild heat treatment after PAC coating and froth flotation. J. Hazard. Mater. 2017;321:193–202. doi: 10.1016/j.jhazmat.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Vahl A., Veziroglu S., Henkel B., Strunskus T., Polonskyi O., Aktas O.C., Faupel F. Pathways to tailor photocatalytic performance of TiO2 thin films deposited by reactive magnetron sputtering. J. Mater. 2019;12:2840. doi: 10.3390/ma12172840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weert S., Redondo-Hasselerharm P.E., Diepens N.J., Koelmans A.A. Effects of nanoplastics and microplastics on the growth of sediment-rooted macrophytes. Sci. Total Environ. 2019;654:1040–1047. doi: 10.1016/j.scitotenv.2018.11.183. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhang J., Liu L., Yang F., Zhang Y. Evaluation of cooling property of high density polyethylene (HDPE)/titanium dioxide (TiO2) composites after accelerated ultraviolet (UV) irradiation. Sol. Energy Mater. Sol. Cells. 2015;143:120–127. [Google Scholar]
- Wang W.-K., Chen J.-J., Zhang X., Huang Y.-X., Li W.-W., Yu H.-Q. Self-induced synthesis of phase-junction TiO2 with a tailored rutile to anatase ratio below phase transition temperature. Sci. Rep. 2016;6:20491. doi: 10.1038/srep20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A.J., Lewis C., Goodhead R.M., Beckett S.J., Moger J., Tyler C.R., Galloway T.S. Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ. Sci. Technol. 2014;48:8823–8830. doi: 10.1021/es501090e. [DOI] [PubMed] [Google Scholar]
- Wright S.L., Levermore J.M., Kelly F.J. Raman spectral imaging for the detection of inhalable microplastics in ambient particulate matter samples. Environ. Sci. Technol. 2019;53:8947–8956. doi: 10.1021/acs.est.8b06663. [DOI] [PubMed] [Google Scholar]
- Yang Y., Yang J., Wu W.-M., Zhao J., Song Y., Gao L., Yang R., Jiang L. Biodegradation and mineralization of polystyrene by plastic-eating mealworms: part 2. Role of gut microorganisms. Environ. Sci. Technol. 2015;49:12087–12093. doi: 10.1021/acs.est.5b02663. [DOI] [PubMed] [Google Scholar]
- Yuan K., Cao Q., Lu H.-L., Zhong M., Zheng X., Chen H.-Y., Wang T., Delaunay J.-J., Luo W., Zhang L. Oxygen-deficient WO3−x@ TiO2−x core–shell nanosheets for efficient photoelectrochemical oxidation of neutral water solutions. J. Mater. Chem. A. 2017;5:14697–14706. [Google Scholar]
- Zan L., Tian L., Liu Z., Peng Z. A new polystyrene–TiO2 nanocomposite film and its photocatalytic degradation. Appl. Catal. A Gen. 2004;264:237–242. [Google Scholar]
- Zan L., Wang S., Fa W., Hu Y., Tian L., Deng K. Solid-phase photocatalytic degradation of polystyrene with modified nano-TiO2 catalyst. Polymer. 2006;47:8155–8162. [Google Scholar]
- Zhao X., Li Z., Chen Y., Shi L., Zhu Y. Enhancement of photocatalytic degradation of polyethylene plastic with CuPc modified TiO2 photocatalyst under solar light irradiation. Appl. Surf. Sci. 2008;254:1825–1829. [Google Scholar]
- Zhou L., Wang L., Zhang J., Lei J., Liu Y. Well-dispersed Fe2O3 nanoparticles on g-C3N4 for efficient and stable photo-fenton photocatalysis under visible-light irradiation. Eur. J. Inorg. Chem. 2016;34:5387–5392. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate a new code. All relevant data are available from the Lead Contact upon reasonable request.