Abstract
Diet and lifestyle-induced dysregulated lipid metabolism have been implicated in fatty liver disease. Chronic redox modulation and hepatic inflammation are key pathological mediators and hallmarks of fatty liver disease associated liver steatosis and steatohepatitis. In this context, owing to the beneficial phytochemical properties such as optimal omega-6: omega-3 PUFA ratio of hempseed, we aimed to explore its potential anti-inflammatory and antioxidant properties against high-fat diet (HFD)-induced experimental model of fatty liver disease. The hempseed lipid fractions (HEMP) were prepared and their ameliorating effects on HFD induced morphological changes, lipid profiles, liver function markers (LFT), markers of oxidative stress and inflammation were studied. Results indicated that HEMP administration to hypercholesterolemic rats resolved the morphological, histopathological, and biochemical indicators of fatty liver diseases. Further, the mechanistic evidence revealed that these hepatoprotective effects of HEMP are mediated through inhibition of oxidative stress and inflammatory mediators such as Cox-2, hPGDS, mPGES, IL-4, TNF-α and sEH. In conclusion, current study suggests the plausible antioxidant and anti-inflammatory role of HEMP in alleviating pathophysiological conditions including fatty liver disease, where oxidative stress and inflammation are key mediators.
Keywords: Biochemistry, Plant products, Diet, Inflammation, Oxidative stress, Hepatology, Evidence-based medicine, High-fat diet, Fatty liver, Hempseeds
Biochemistry; Plant Products; Diet; Inflammation; Oxidative Stress; Hepatology; Evidence-Based Medicine; High-fat diet; fatty liver; Hemp Seeds
1. Introduction
The liver is the primary site for the regulation of serum cholesterol and triglycerides (TGs) via modulating their biosynthesis and metabolism as well as packaging, reuptake, and export of lipoproteins. Dysregulated lipid metabolism, including hypercholesterolemia (HC), can trigger Non-Alcoholic fatty liver disease (NAFLD) [1]. Multiple phases of NAFLD, such as fatty liver, steatohepatitis, and liver cirrhosis, have been characterized in individuals with no history of substantial alcohol consumption [2, 3]. Additionally, the fatty residues in the liver have also been implicated in fibrosis, hepatocellular carcinoma (HCC), liver failure, and death [4].
Owing to globalization, lifestyle factors, including consumption of processed, refined foods, NAFLD has emerged as the most prevalent hepatic disease in developing countries and continued to increase in other countries as well [5]. Further, metabolic syndrome, diabetes, obesity, and insulin resistance leading to accretion of TGs and free fatty acids in the liver synergistically contributed to making NAFLD a global burgeoning epidemic [6]. Since NAFLD is predominantly associated with lifestyle, changing the dietary habits remain the first line of therapy for restoring NAFLD and NASH. Nevertheless, oxidative stress emerged as one of the most critical pathological events and hallmarks during NAFLD and hepatic steatosis (NASH) [7, 8]. Despite these understandings, not only the redox-sensitive pathogenic mechanism of NAFLD remains elusive, but also there is a lack of adequate treatment for NAFLD.
In this regard, prevention of de novo cholesterol synthesis using statins as well-known HMG CoA reductase inhibitors is well established. Statins are generally the first option medications for patients with HC, especially those with liver and cardiovascular disease. However, chronic use of statins not only induces resistance amongst patients but is also associated with unwanted side effects such as myalgia, myositis, kidney or liver failure, metabolic, and neurological side effects [9, 10]. These outcomes warrant the urgent need to explore safe therapeutic/preventive alternatives.
Multiple attempts have been made in this regard to explore new salubrious agents. For example, both polyunsaturated fatty acids (PUFAs) and phytosterols can prevent NAFLD [11] by dramatically decreasing the total and LDL-cholesterol levels. Hempseed (Cannabis sativa) oil contains 80% PUFAs, high concentrations of phytosterols (e.g., sitosterol and campesterol), essential fatty acids along with α, γ-linoleic acids [12]. Due to these properties, they can be identified as useful phytotherapeutics against a vast range of lipid-associated diseases [12]. Further, lignanamides in the hempseed are potent antioxidant and anti-inflammatory agents [13]. Thus, currently, we examined the ameliorative potential of a hempseed lipid fraction (HEMP) in a high-fat diet (HFD) induced fatty liver.
2. Materials and methods
2.1. Chemicals
All the chemicals used in the study were of analytical grade and were purchased from Sigma (India), HiMedia (India), SRL (India), and MP Biomedical (India). Organic raw hempseeds of premium quality were procured from Petnest, produced, and packed by G K Engineers, Bhavnagar, Gujarat, India.
2.2. Animal procurement
Female Wistar rats were obtained from the Central Animal House, Panjab University, Chandigarh, and housed in the Departmental Animal Room in polypropylene cages at 12/12-hour light/dark cycle. All the experiments were performed following the guidelines of the institutional ethical committee (Approval # PU/45/99/CPCSEA/IAEC/2017/59).
2.3. Diet preparation and experimental design
High-fat diets (HFD) to induce experimental HC and diets containing HEMP were prepared as described earlier [14]. Briefly, animals were divided into five groups viz Control, HFD, HEMP, HFD+HEMP, and HFD/HEMP. Control rats were fed regular pellet diet and water ad libitum for two months, whereas HFD rats were fed diet that contained 2% cholesterol, 1% cholic acid, 10% peanut oil, 40% sucrose, 47% pellet diet for two months. HEMP rats were fed diet containing 10% hempseed lipid fraction and 90% pellet diet for two months. HFD + HEMP group animals were fed HFD supplemented with HEMP (10%) for two months, whereas for HFD/HEMP treatment, rats were fed HFD for one month, followed by HEMP diet for one month.
The changes in body weight were checked weekly. After the completion of diet/treatment schedules, animals were sacrificed under Ketamine anesthesia, followed by cervical dislocation. Blood was obtained by puncturing the retro-orbital plexus. Serum was isolated from blood and was used for lipid profile experiments.
2.4. Defatting of hempseed by soxhlet extraction
The hempseed powder was defatted using soxhlet extraction, and lipid and protein fractions were isolated as described previously [14]. Based on the previous studies suggesting the benefits of crude extracts over purified components and published results from our lab, only lipid fraction of hempseeds i.e. HEMP was used in the current study [14].
2.5. Analysis of serum lipid profile
Serum was separated from blood and level of total cholesterol, HDL/LDL cholesterol, and triglycerides levels were measured using the kits from RECKON (Reckon diagnostics P. LTD. Vadodara INDIA) as per manufacturer's protocol.
2.6. Analysis of liver function test
Serum glutamic pyruvic transaminase (SGPT), serum glutamic-oxaloacetic transaminase (SGOT), and alkaline phosphatase (ALP) were measured using the kits from RECKON as per manufacturer's protocol.
2.7. Histopathological studies
Formalin-fixed and paraffin-embedded 5–7 μm thick liver tissue sections were stained with hematoxylin and eosin for histopathological examination using the standard protocol.
2.8. Estimation of oxidative stress markers
10% of liver homogenates were prepared in RIPA buffer (pH 7.4) and used for evaluations of oxidative stress.
2.8.1. Non-enzymatic markers of oxidative stress
Total ROS levels in the liver homogenates were estimated using the fluorescent probe DCFH-DA [15].
The levels of lipid peroxidation (LPO), total protein carbonyl content and redox ratio were measured in freshly prepared liver homogenate by the respective methods described earlier [16, 17, 18].
2.8.2. Activities of antioxidant enzymes
The specific activities of antioxidant enzymes Catalase, glutathione peroxidase (GPx), Glutathione reductase (GR) and Glutathione-S-Transferase (GST) in the liver were estimated by the methods of [19, 20, 21, 22] respectively after normalization with total protein levels measured by the method of Lowry [23].
2.9. Protein expression studies
The protein expressions of Cox-2, hPGDS, mPGES, IL-4, TNF-α, and sEH were studied by Enzyme-Linked Immunosorbent Assay (ELISA) as described earlier using β-actin protein as an internal control [14].
2.10. Statistical analysis
The data were analyzed using one-way analysis of variance (ANOVA) and multiple post hoc test (Tukey) to compare various treatment groups using GraphPad Prism 5.0 program (GraphPad Software, San Diego, CA). The significance was set at p < 0.05. All data were expressed as mean ± SD of at least n = 4–6 independent observations (for protein expression studies n = 4).
3. Results and discussion
Lifestyle changes have been implicated as the leading cause of dysregulated lipid metabolism, which can culminate in HC and associated pathologies. Also, sociocultural characteristics and endocrine derangements also contribute to the sexual dimorphism responsible for differences in the prevalence, risk factors, and clinical outcomes of NAFLD. Despite the understanding that hormonal status of women affects the risk factors for NAFLD, men are found to be more susceptible and at greater risk. However recent inconclusive and conflicting reports suggest higher prevalence of NAFLD in women than in men. Thus, adequate consideration of sex differences, sex hormones/menopausal status and age are needed to fill current gaps to understand pathogenesis of NAFLD [24, 25].
At the molecular level, HC associated oxidative insults have been implicated in various hepatic pathologies, including NAFLD, steatohepatitis, liver cirrhosis and fibrosis. Thus, reducing free radical burden can be a solution against these adverse changes. The risk factor and side effects of allopathic drugs warrant the consideration of plant-derived pharmaceuticals as effective alternatives. Hempseeds lipid fraction being an excellent source of essential fatty acids and phytosterols [12] and effective antioxidants [14] are evaluated for their hepato-protective effects.
3.1. HEMP ameliorates markers of fatty liver
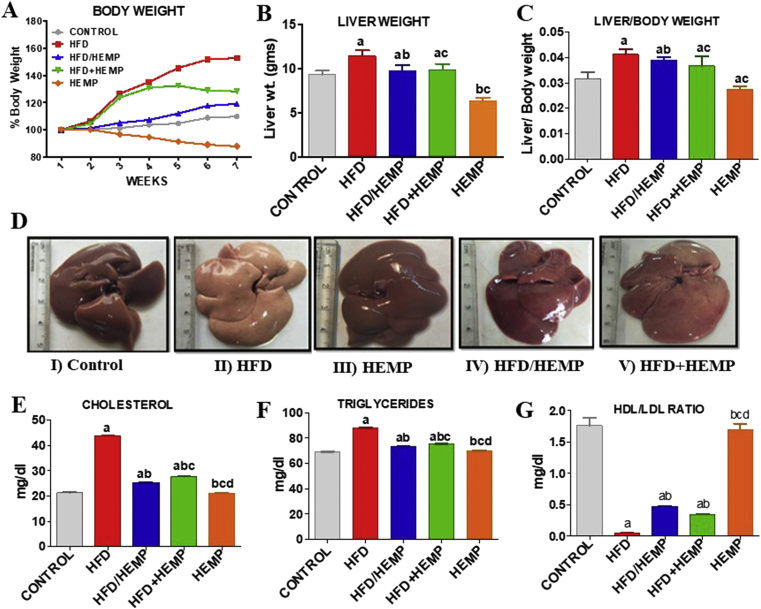
HFD can lead to enhanced fat accumulation in the liver due to HC dependent altered lipid metabolism. In the present study, increased body weights, liver weights, and liver: body weight ratio (Figure 1A-C) of animals fed on the HFD diet were observed compared with other groups. Alternatively, HEMP administration, along with HFD, i.e., in both HFD/HEMP and HFD+HEMP groups, showed slight gain or reduced liver: body weights (Figure 1C).
Figure 1.
Changes in (A) Body weights (B) liver weights (C) liver/body weight ratios (D) Representative photographs illustrating the gross morphological changes in the liver of different groups; I–V, (E–G), Changes in lipid profile, i.e., cholesterol, triglycerides, and LDL/HDL ratios. Data are expressed as mean ± SD of at least 6 independent observations. a:represents p < 0.05 when compared between CONTROL vs. HFD, HFD/HEMP, HFD+HEMP, HEMP; b: represents p < 0.05 when compared between HFD vs. HFD/HEMP, HFD+HEMP, HEMP; c: represents p < 0.05 when compared between HFD/HEMP vs. HFD+HEMP, HEMP; d: represents p < 0.05 when compared between HFD+HEMP vs. HEMP: represents the liver photograph of CONTROL, HFD, HEMP, HFD/HEMP, and HFD+HEMP groups, respectively.
Morphologically also, the livers of rats demonstrated severe changes (Figure 1D I-V). The livers of control, HEMP, and HFD/HEMP showed normal deep red coloration and morphology (Figure 1D I, III, IV). Though the liver from HFD+HEMP fed rats showed changes similar to HFD (Figure 1D V), the livers of HFD fed animals were enlarged and appeared pale, indicating the fatty liver (Figure 1D II). Studies have suggested that HFD is a crucial factor in the pathogenesis of fatty liver or hepatic steatosis associated with obesity depicted via ballooning degeneration [26]. Consistent with this, the histopathological analysis also suggests that elevation of cholesterol may increase the risk of hepatic steatosis. Although these effects can be reversed by lowering the cholesterol and fat deposition in the liver, hempseed administration can significantly reduce the fat accumulation in the liver [27].
In agreement with previous literature, the current study also validates that HFD induced altered HDL-C/LDL-C ratio and hypertriglyceridemia are an indicative risk for NAFLD [28]. Figure 1 (E-G), shows that serum levels of cholesterol, TG, and LDL/HDL ratio significantly increased in rats fed with HFD when compared with other groups. These changes in HFD group animals can be attributed to the increased gene expressions of the apolipoprotein B & E (APO- B, APO-E) (data not shown). These increased expressions of APO-B and APO-E suggested a disrupted secretion of APO-E as well as disequilibrium of fatty acid metabolism, and lipidosis in the liver [29]. Alternatively, HEMP, HFD+HEMP, and HFD/HEMP fed groups demonstrated a considerable decrease in the levels of cholesterol, LDL, and TGs and concordant increase in HDL compared to HFD group (Figure 1E-G). Previous studies [30] also reported the ability of hempseed to counterbalance the deleterious effects of LDL by increasing the functional impact of the HDL. Thus, HEMP induced increase in HDL, in turn, can not only antagonize the cellular and molecular effects of LDL but can also enhance the removal of cholesterol.
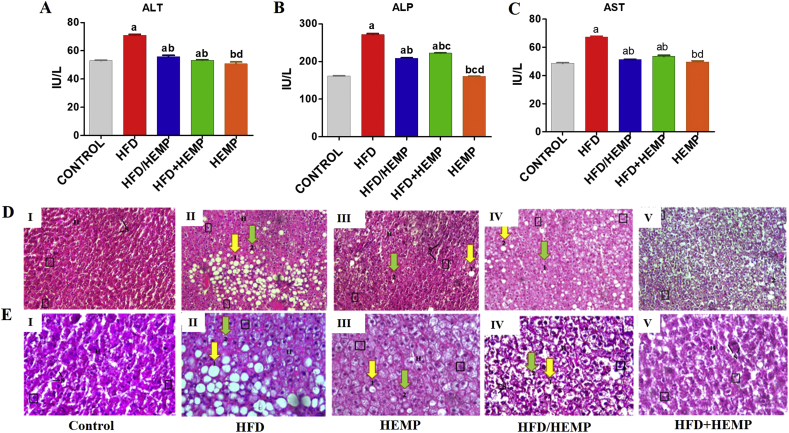
The anti-dyslipidemic hepatic effects of HEMP were also validated in terms of changes in the liver function markers where enzymes SGOT, SGPT, and ALP were significantly increased in the mice fed with HFD diet as compared to other groups (Figure 2A-C). These lipids associated increase in the liver function markers are reflective of HC/HFD associated hepatocellular damage and correlate well with the fatty changes in the liver morphology [31]. Contrary to this, decreased levels of liver function markers were observed in rats fed with HFD/HEMP and HFD+HEMP (Figure 2A-C) compared to HFD group. A trend of significant normalization of liver function markers towards normal control rats was also observed in HFD/HEMP and HFD+HEMP group rats (Figure 2A-C). These findings suggest the anti-hypercholesterolemic effects of hempseed [14], which can be owed to the presence of optimal proportions of n3 and n6 PUFAs [32] and the high ALA content that augments omega-3 fatty acids biosynthesis [33].
Figure 2.
Graphs (A–C) illustrating changes in the Liver function markers (D, E) Photomicrographs of liver illustrating the histological changes in the liver of different groups; I–V, at 20x and 40x magnifications, respectively. H- Hepatocyte, S- sinusoidal cells, 1- macrovesicular fat cells, 2-microvesicular fat cells, Square- Binucleate cells. Data are expressed as mean ± SD of at least 4–6 independent observations. a-d represents p < 0.05 when compared between CONTROL vs. HFD, HFD/HEMP, HFD+HEMP, HEMP; b: represents p < 0.05 when compared between HFD vs. HFD/HEMP, HFD+HEMP, HEMP; c: represents p < 0.05 when compared between HFD/HEMP vs. HFD+HEMP, HEMP; d: represents p < 0.05 when compared between HFD+HEMP vs. HEMP: represents the liver photograph of CONTROL, HFD, HEMP, HFD/HEMP, and HFD+HEMP groups, respectively as described in the legend of Figure 1.
Histopathological investigations of H&E stained sections of the liver of the control and HEMP rats (Figure: 2D, E I & V) revealed the normal histological architectures as evidenced by maintained lobular architecture with normal hepatocytes, few binucleate and sinusoidal cells. HFD led to inflammation along with signs of macrovesicular steatosis in perivenular areas (labeled as '1′), and few markers of microvesicular steatosis (labeled as '2′) were also seen (Figure 2D, E II). Further, sinusoidal cells appear irregular and narrow because of fat loaded cells suggesting that elevation of cholesterol may increase the risk of steatosis. Contrary to this, HFD/HEMP and HFD+HEMP (Figure 2D, E III & IV) demonstrated few binucleate cells and a comparatively lesser number of macro and microvesicular steatosis when compared with HFD suggesting HEMP mediated significant reduction in hepatic fat accumulation culminating in amelioration of macrovesicular steatosis [27].
3.2. HEMP resolves fatty liver associated hepatic inflammation through modulation of redox homeostasis
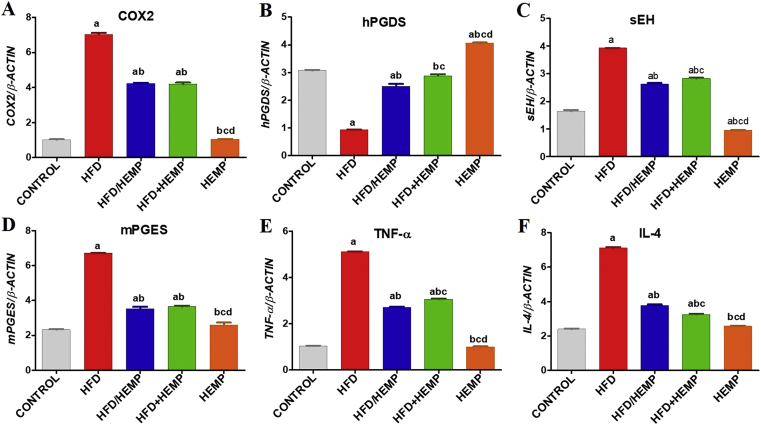
Fatty liver is characterized by a marked activation of inflammatory cells and upregulation of soluble inflammatory mediators, including cytokines and chemokines. These mediators play an active role in NAFLD and are considered as potential therapeutic targets [34]. Among these factors, currently, the protein levels of inflammation inducible COX-2, sEH, IL-4, and TNF-α increased significantly in HFD fed rats compared to control rats suggest activation of pathways contributing to the prostaglandin (PG)-E2 development. Increased mPGES and a concomitant downregulation of anti-inflammatory hPGDS in HFD rats corroborate these findings (Figure 3A-F). Consistent with these observations, studies [35] also reported exaggerated and unresolved inflammation mediated through hPGDS deficiency, as observed in HFD groups in the current study. In contrast, HEMP administration in rats fed with HFD/HEMP and HFD+HEMP reversed the HFD induced changes in the expressions of COX-2, sEH, IL-4, and TNF-α and a remarkable improvement in hPGDS expression, which was comparable to control (Figure 3A-F) indicating the pro-resolution effects of HEMP.
Figure 3.
Graphs (A–F) illustrating changes in the protein expressions of various inflammatory factors. Data are expressed as mean ± SD of at least 4 independent observations. a:represents p < 0.05 when compared between CONTROL vs. HFD, HFD/HEMP, HFD+HEMP, HEMP; b: represents p < 0.05 when compared between HFD vs. HFD/HEMP, HFD+HEMP, HEMP; c: represents p < 0.05 when compared between HFD/HEMP vs. HFD+HEMP, HEMP; d: represents p < 0.05 when compared between HFD+HEMP vs. HEMP: represents the liver photograph of CONTROL, HFD, HEMP, HFD/HEMP, and HFD+HEMP groups, respectively.
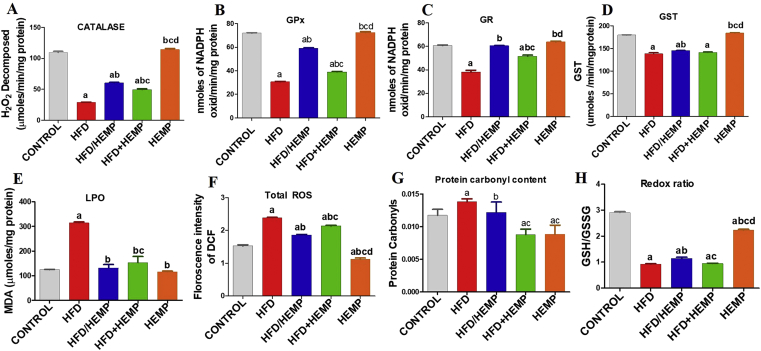
On a mechanistic level, a direct association of increased hepatic free cholesterol pool to the mitochondrial dysfunction and consequent sensitization to TNF-α induced hepatotoxicity has been established [36]. Thus, considering the central role of the redox component and its involvement in the regulation of inflammatory pathways prompted us to investigate this axis during fatty liver disease. Both enzymatic and non-enzymatic markers of oxidative stress indicated that hepato-protective anti-inflammatory effects of HEMP might be mediated through its redox modulatory activities. A statistically significant decreased activity of enzymatic antioxidants such as catalase, GPx, GR, and GST was observed in the HFD fed rats when compared to other groups (Figure 4A-D). These results indicated HC induced accumulation of ROS and the inadequacy of these enzymes to counteract such oxidative insults [37]. Alternatively, an increase in LPO, levels of ROS, and protein carbonyls in HFD group animals along with decreased redox ratio (GSH: GSSG) corroborate these findings (Figure 4E-H). This suggested oxidative stress-mediated NAFLD complications [38] in the HFD group rats when compared to the rest of the groups. On the other hand, the rats fed with HFD/HEMP and HFD+HEMP showed significantly reduced activities of these enzymes, which were significantly higher as compared to the HFD fed rats and comparable to HEMP fed rats and control animals (Figure 4A-D). Corroborating with the activities of antioxidant enzymes, the HFD/HEMP and HFD+HEMP groups showed a statistically significant decrease in the levels of free radicals suggesting the beneficial redox modulatory activities of Hemp (Figure 4E-H) due to its unique nutritional and phytochemical composition [14]. These free radical scavenging activities of hempseeds have been previously reported [14, 32, 33].
Figure 4.
Graphs (A–H) illustrating changes in the enzymatic and non-enzymatic markers of oxidative stress. Data are expressed as mean ± SD of at least 4 independent observations. a:represents p < 0.05 when compared between CONTROL vs. HFD, HFD/HEMP, HFD+HEMP, HEMP; b: represents p < 0.05 when compared between HFD vs. HFD/HEMP, HFD+HEMP, HEMP; c: represents p < 0.05 when compared between HFD/HEMP vs. HFD+HEMP, HEMP; d: represents p < 0.05 when compared between HFD+HEMP vs. HEMP: represents the liver photograph of CONTROL, HFD, HEMP, HFD/HEMP, and HFD+HEMP groups, respectively.
In conclusion, lipid fraction of hempseeds (HEMP) suppresses high-fat diet-associated fatty liver through positive redox modulatory effects on lipid metabolism and hepatic inflammation.
Declarations
Author contribution statement
N. Kaushal: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
E. Kulshreshtha: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
M. Gupta: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Department of Science and Technology, Ministry of Science and Technology (DST-FIST (SR/FST/LS1-645)) and University Grants Commission (UGC-SAP (F.4-1/2015/DSA-1 (Sap-II)).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors thank Dr Pulkit Rastogi, Department of Histopathology, PGIMER, Chandigarh for histopathological analyses.
References
- 1.Shashikiran U., Sudha V., Jayaprakash B. What is obesity. Med. J. Malaysia. 2004;59(1):131. [PubMed] [Google Scholar]
- 2.Ratziu V., Bellentani S., Cortez-Pinto H., Day C., Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2010;53(2):372–384. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Le Michael H. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PloS One. 2017;12(3) doi: 10.1371/journal.pone.0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luedde Tom, Schwabe Robert F. NF-κB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011;8(2):108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed Mohamed H., Byrne Christopher D. Modulation of sterol regulatory element binding proteins (SREBPs) as potential treatments for non-alcoholic fatty liver disease (NAFLD) Drug Discov. Today. 2007;12(17-18):740–747. doi: 10.1016/j.drudis.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Benedict Mark., Zhang Xuchen. Non-alcoholic fatty liver disease: an expanded review. World J. Hepatol. 2017;9(16):715–732. doi: 10.4254/wjh.v9.i16.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spahis Schohraya. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxidants Redox Signal. 2017;26(10):519–541. doi: 10.1089/ars.2016.6776. [DOI] [PubMed] [Google Scholar]
- 8.Polimeni Licia. Oxidative stress: new insights on the association of non-alcoholic fatty liver disease and atherosclerosis. World J. Hepatol. 2015;7(10):1325. doi: 10.4254/wjh.v7.i10.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson Paul D. Statin-associated side effects. J. Am. Coll. Cardiol. 2016;67(20):2395–2410. doi: 10.1016/j.jacc.2016.02.071. [DOI] [PubMed] [Google Scholar]
- 10.Ramkumar Satish, Raghunath Ajay, Raghunath Sudhakshini. Statin therapy: review of safety and potential side effects. Acta Cardiol. Sin. 2016;32(6):631. doi: 10.6515/ACS20160611A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Ziwei. Effect of omega-3 polyunsaturated fatty acids to reverse biopsy-proven parenteral nutrition-associated liver disease in adults. Clin. Nutr. 2012;31(2):217–223. doi: 10.1016/j.clnu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Callaway J.C. Hempseed as a nutritional resource: an overview. Euphytica. 2004;140(1-2):65–72. [Google Scholar]
- 13.Luo Qian. Anti-neuroinflammatory effects of grossamide from hemp seed via suppression of TLR-4-mediated NF-κB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. Mol. Cell. Biochem. 2017;428(1-2):129–137. doi: 10.1007/s11010-016-2923-7. [DOI] [PubMed] [Google Scholar]
- 14.Kaushal Naveen, Dhadwal Shallu, Kaur Parminder. Ameliorative effects of hempseed (Cannabis sativa) against hypercholesterolemia associated cardiovascular changes. Nutr. Metabol. Cardiovasc. Dis. 2020;30(2):330–338. doi: 10.1016/j.numecd.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Driver Amy S., Kodavanti Prasada Rao S., Mundy William R. Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol. Teratol. 2000;22(2):175–181. doi: 10.1016/s0892-0362(99)00069-0. [DOI] [PubMed] [Google Scholar]
- 16.Wills EoD. Mechanisms of lipid peroxide formation in animal tissues. Biochem. J. 1966;99(3):667. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine Rodney L. [37] Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. Academic Press. [DOI] [PubMed] [Google Scholar]
- 18.Hilf Russell, Hissin P.J. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976;74(1):214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 19.Luck H. Academic Press; New York, London: 1963. "Methods of enzymatic analysis." Enzyme assays in vivo. [Google Scholar]
- 20.Barrera Lawrence N. Epigenetic and antioxidant effects of dietary isothiocyanates and selenium: potential implications for cancer chemoprevention. Proc. Nutr. Soc. 2012;71(2):237–245. doi: 10.1017/S002966511200016X. [DOI] [PubMed] [Google Scholar]
- 21.Massey Vincent, Williams Charles H. On the reaction mechanism of yeast glutathione reductase. J. Biol. Chem. 1965;240(11):4470–4480. [PubMed] [Google Scholar]
- 22.Habig William H., Pabst Michael J., Jakoby William B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 23.Lowry O.H. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Ballestri Stefano. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv. Ther. 2017;34:1291–1326. doi: 10.1007/s12325-017-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonardo Amedeo. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70(4):20192. doi: 10.1002/hep.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorrentino Paolo. Silent non-alcoholic fatty liver disease—a clinical–histological study. J. Hepatol. 2004;41(5):751–757. doi: 10.1016/j.jhep.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Gårdebjer Emelie M. Effects of periconceptional maternal alcohol intake and a postnatal high-fat diet on obesity and liver disease in male and female rat offspring. Am. J. Physiol. Endocrinol. Metabol. 2018;315(4):E694–E704. doi: 10.1152/ajpendo.00251.2017. [DOI] [PubMed] [Google Scholar]
- 28.Pirola Carlos J., Silvia Sookoian. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: a meta-analysis. Hepatology. 2015;62(6):1742–1756. doi: 10.1002/hep.28142. [DOI] [PubMed] [Google Scholar]
- 29.Chen Xueying. Plasma PLTP activity is inversely associated with HDL-C levels. Nutr. Metabol. 2009;6(1):49. doi: 10.1186/1743-7075-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann Alexander F., Hagey L.R. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci. 2008;65(16):2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy Janardan K., Sambasiva Rao M. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290(5):G852–G858. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- 32.Gavel N. The effect of dietary hempseed on atherogenesis and contractile function in aortae from hypercholesterolemic rabbits. Acta Physiol. Hung. 2011;98(3):273–283. doi: 10.1556/APhysiol.98.2011.3.4. [DOI] [PubMed] [Google Scholar]
- 33.Prociuk M.A. Cholesterol-induced stimulation of platelet aggregation is prevented by a hempseed-enriched diet. Can. J. Physiol. Pharmacol. 2008;86(4):153–159. doi: 10.1139/Y08-011. [DOI] [PubMed] [Google Scholar]
- 34.Braunersreuther Vincent. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J. Gastroenterol.: WJG. 2012;18(8):727. doi: 10.3748/wjg.v18.i8.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajakariar Ravindra. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyΔ12–14 PGJ2. Proc. Natl. Acad. Sci. Unit. States Am. 2007;104(52):20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manco Melania. Correlation of serum TNF-α levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am. J. Clin. Pathol. 2007;127(6):954–960. doi: 10.1309/6VJ4DWGYDU0XYJ8Q. [DOI] [PubMed] [Google Scholar]
- 37.Hassanzadeh Taghi. Taq1B polymorphism of cholesteryl ester transfer protein (CETP) gene in primary combined hyperlipidaemia. Indian J. Med. Res. 2009;129(3):293–298. [PubMed] [Google Scholar]
- 38.Ohara Yuichi, Peterson Timothy E., Harrison David G. Hypercholesterolemia increases endothelial superoxide anion production. J. Clin. Invest. 1993;91(6):2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]