Abstract
In recent years, the problem of microplastic pollution has begun to receive more attention. Currently, it is known that these particles, less than 5 mm in diameter, can lead to problems for both ecosystems and human health due to the toxicity of their components. In spite of this, research on this topic has focused mainly on the oceans, leaving aside rivers, which are the main source of these pollutants to oceans. Additionally, information is limited to certain rivers in countries of the northern hemisphere where wastewater treatment plants can retain up to 80% of microplastics. In South America, microplastic pollution is practically unknown, and wastewater treatment in several areas is still limited. This study focused on quantifying the microplastics present in the upper basin of the Guayllabamba River, in the Tropical Andes, a biodiversity hotspot. This basin is where the capital city of Ecuador, Quito, is located. Less than 10% of the wastewater in Quito is treated and the rest is dumped to rivers without treatment. We performed a physical analysis of microplastics, by weight and by category of microplastic, in various sampling points before and after urban areas. We found microplastic pollution beginning in the headwaters of the basin, with significant increases in urban areas of the Metropolitan District of Quito. Values of suspended microplastics in rivers after urban areas were higher than those recorded in the literature. Plastic levels in sediment were also higher after urban areas. Microplastics were highly correlated with other water pollutants, showing the prevailing necessity of wastewater treatment plants, because all of this pollution is dumped into rivers that flow from 2800 m a.s.l. to highly diverse freshwater ecosystems and human populations located downstream that depend on these aquatic sources, and finally to the Pacific Ocean.
Keywords: Pollution, Rivers, Plastics, Quito, Freshwater, Tropical Andes, Environmental analysis, Environmental assessment, Environmental health, Environmental impact assessment, Environmental pollution, Environmental science
Pollution; rivers; Plastics; Quito; Freshwater; Tropical Andes; Environmental Analysis; Environmental Assessment; Environmental Health; Environmental Impact Assessment; Environmental Pollution; Environmental Science
1. Introduction
Plastics have become a ubiquitous pollutant, present in all ecosystems, with aquatic ecosystems being one of the most affected (Khan et al., 2018). Microplastic particles, defined as plastic particles of less than 5 mm in diameter, have shown to be an important issue for human and ecosystem health (Wagner et al., 2014; Wright and Kelly, 2017). In 2004, the first reports of microplastics accumulating in marine ecosystems showed that these particles could easily be incorporated in marine food-webs (Thompson et al., 2004). They easily enter food chains by ingestion at the first trophic levels (Talvitie et al., 2017; Scherer et al., 2017). Despite the fact that about 80% of microplastic particles found in oceans come from fluvial sources, most of the research done in microplastics is mainly focused on the dynamics of microplastics in the oceans with limited information for rivers (Mani et al., 2015).
Due to urban expansion, microplastics presence has become more noticeable in urban rivers (Khan et al., 2018). This is an ever-increasing problem, given that rivers are the main source of microplastics that reach the ocean (Eerkes-Medrano & Thompson 2018). Many studies have revealed that the problem of microplastics does not end there, but that even freshwater organisms are being affected by these particles (Scherer et al., 2017). Measures such as wastewater treatment offer partial solutions to this problem. In fact, water treatment plants can retain about 80% of the microplastic particles of an effluent (Hoellein et al., 2017). Despite this, the percentage of retained microplastic particles will depend on the efficiency of the treatment plant, which is why these plants can function as sources of microplastics for the rivers, depending on how the retained material is disposed of (Talvitie et al., 2017).
Microplastics have a high toxicity, because when they enter living organisms they can cause stress due to ingestion, leakage of additives, and exposure to associated pollutants (Anderson et al., 2016 & Kramm and Völker, 2018), which can lead to a bioaccumulation or biomagnification of compounds such as polycyclic aromatic hydrocarbons that are carcinogens or endocrinogens (Chae et al., 2015). Pollution by these particles has even reached ecosystems where salt is extracted for human consumption and therefore have been found in table salt (Yang et al., 2015).
In addition, microplastics are easily fragmented, and thus their particles can be found not only in almost all aquatic ecosystems, but also in drinking water. Around the world, drinking water contains between 0-57 particles per liter, giving on average 4 particles with a size between 0.1 and 5 mm, which is a very significant value considering that a person consumes an average of 2 L of water per day (Browne et al., 2011). A study performed in 14 countries, including Ecuador, showed that microplastic particles were found in 75% of tap water collected from different cities, including Quito (Browne et al., 2011). In cities such as Quito, the growth of the urban area has generated problems in aquatic ecosystems, including the direct disposal of wastewater and the urbanization of riparian habitats (da Cruz e Souza & Ríos-Touma, 2018). Moreover, the increase in population and industry, with the waste produced by both, has over time generated sanitary problems which have endangered the use of water reserves (Guerrero-Latorre et al., 2018). In Quito, lack of wastewater treatment could be one of the main contributors of plastic reaching the Pacific Ocean, since less than 10% of the city's wastewater is treated (EPMAPS, 2016). Besides the problem of the lack of wastewater treatment, the mismanagement of garbage should be added, because in the city, many streams are treated as dump sites and effluents of wastewater (Egas and Ordóñez, 2015). When runoff water reaches rivers and streams, it contains a large load of pollutants, thus also contributing to the microplastics content of rivers (Mani et al., 2015). Many important Andean cities are located at the headwaters of basins that drain to the Pacific or the Amazon-Atlantic basin and therefore they contribute with pollution loads from the upper parts of the basins. The Upper Guayllabamba River Basin, where Quito is located, is part of the Esmeraldas Basin that drains to the Pacific Ocean. Pollution in this basin generates several problems downstream in terms of water quality, because its waters have multiple uses in different populations across the basin, including the provision of water to the city of Esmeraldas, located in the lower Esmeraldas Basin. Understanding the sources, dynamics, types and amounts of microplastics in high altitude Andean streams is of the utmost importance in order to generate adequate plans to avoid the arrival of these particles to rivers, trophic chains, humans, and the sea (Célleri & Freyen 2009). Our aim with this study is to quantify microplastics in the rivers of the Metropolitan District of Quito. Specifically, we sought to: Determine differences in the amount and type of microplastics found in rivers before and after urban areas, and compare the values found with other rivers worldwide to understand the magnitude of the microplastic problem in an Andean city without wastewater treatment.
2. Material and methods
2.1. Study area
The Upper Guayllabamba River Basin is located in the inter-Andean valley of northern Ecuador. It has four main rivers: the Machángara, with a length of 22.5 km, the Monjas with 24.3 km, the San Pedro with 53.9 km and Guayllabamba with 175.3 km. The Metropolitan District of Quito is located along the basin, which has an area of 4230 km2 and a population of more than 2.5 million inhabitants (Landázuri et al., 2014). The Upper Guayllabamba River Basin is located between the provinces of Pichincha, Napo and Cotopaxi and its rivers supply water to the Metropolitan District of Quito. In addition, its waters play an important role for agriculture and livestock in the basin (Tucci, 2009). However, the basin also suffers from water extraction and pollution due to agriculture, livestock and urban wastewater discharge directly into the rivers (EPMAPS, 2011). Both wastewater and municipal solid waste are very high, with the upper Guayllabamba accounting for around the 14% of the country's municipal solid waste (MSW) (Table 1, values from Castillo, 2012 and Hoornweg, & Bhada-Tata, 2012).
Table 1.
Location, riparian quality index result (QBR), habitat quality index result (IHF) and the generation rate of wastewater and Municipal Solid Waste (MSW) of studied sites in the Upper Guayllabamba river basin.
| River/point code | Elevation | Length | Latitude | Key | QBR1 | IHF1 | MSW2 Generation (ton/day) | Wastewater3 Generation (m3/day) |
|---|---|---|---|---|---|---|---|---|
| San Pedro/ SP7 | 2923 | -78.552438 | -0.533825 | SP7 | 20 | 75 | 21.5 | 6444.6 |
| Pita/ 3.2 PI | 2844 | -78.383733 | -0.400956 | 3.2PI | 95 | 90 | 9.1 | 2725.3 |
| Pita/ SP3 | 2465 | -78.459882 | -0.296142 | SP3 | 20 | 54 | 35.7 | 10202.4 |
| San Pedro/ SP2 | 2386 | -78.452697 | -0.264217 | SP2 | 35 | 58 | 67.5 | 19290.3 |
| Guayllabamba/ M5 | 1945 | -78.373258 | -0.068651 | M5 | 15 | 54 | 1173.5 | 335296.2 |
The values of the QBR and IHF show the quality of the water system of the studied points being rated above a value of 100 points.
Values for MSW generation rate come from Castillo (2012) where they provide a rate of domestic urban waste generation of 0.532 and for rural areas of 0.508 kg ∗ inhab/day.
Values for wastewater generation rate come from EPMAPS (2011) and INEC (2010) where they provide a rate generation of 0.8 and endowment of 190 l ∗ inhab/day.
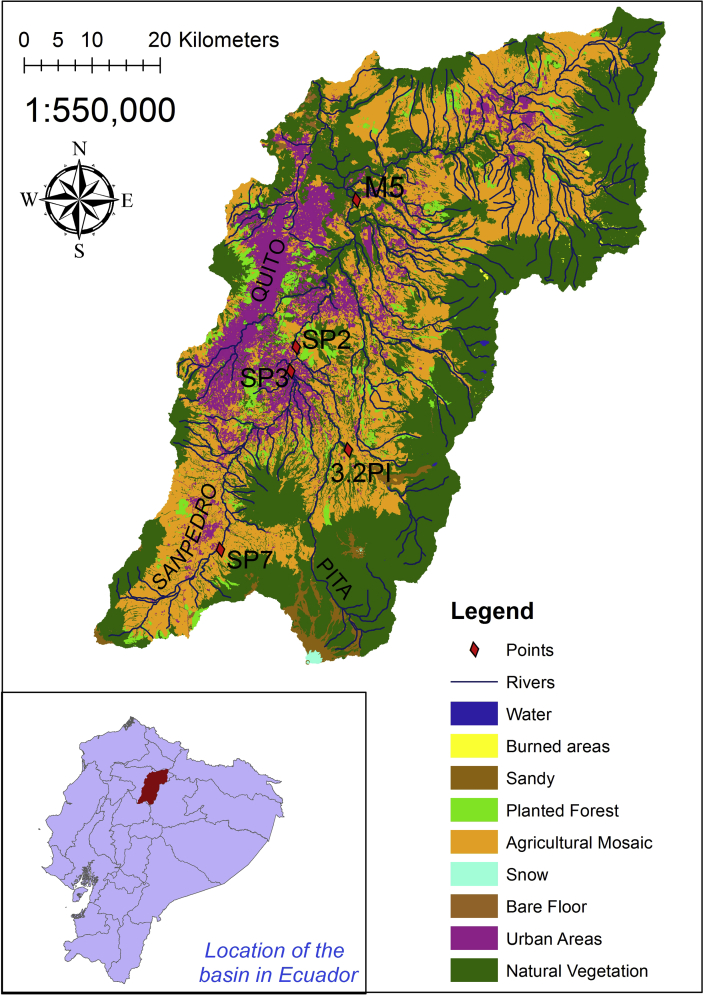
We chose five sampling points in the upper Guayllabamba river basin near the city of Quito, located in the Pita, San Pedro and Guayllabamba river basins (Figure 1). Points include areas before, in, and after urbanized areas. We chose the first 2 points (SP7 and 3.2PI) because they served as reference indicators of the headwaters of the San Pedro river in Machachi and those of the of Pita river near the Pintag area. The third point (SP3) is located at the town of San Rafael, where the Pita river has been heavily affected by human activities and urbanization. The fourth point (SP2) is located further downstream on the San Pedro River, in Guangopolo, near a power station and after several urban areas. The fifth point (M5) is on the Guayllabamba River, where the river receives pollution coming from the entire upper Guayllabamba basin, including most of polluted waters of the city of Quito (Tables 1 and 2). We visited each point four times, from March to June 2018, during different rainfall conditions in order to include this seasonal variation in our samples.
Figure 1.
The Upper Guayllabamba River Basin with the sampled points.
Table 2.
Amount and weight of microplastics by watershed area and number of inhabitants in the sampled points. Each value is the average of four sampling dates. Values in parentheses are from point SP2 and show results without considering the last sampling, in an unexpected discharge event occurred. Mp = microplastic.
| Point | #Mp/m3 | g. Mp/m3 | Drainage area (Km2) | Habitants1 | Rel ((#Mp/m3)/Km2) | Rel ((#Mp/m3)/hab) | #Mp/Kg |
|---|---|---|---|---|---|---|---|
| SP7 | 1.42 | 0.00012 | 209 | 42399 | 0.0068 | 0.00003 | 32.6 |
| 3.2PI | 0.72 | 0.00005 | 58.5 | 17930 | 0.0122 | 0.00004 | 14.3 |
| SP3 | 26.96 | 0.00477 | 1338.2 | 67121 | 0.0202 | 0.00040 | 112.5 |
| SP2 | 3704.64 (168.12) | 0.53387 (0.01071) | 1405.5 | 126910 | 2.6358 (0.11) | 0.02919 (0.0013) | 186.5 (102.3) |
| M5 | 1186.34 | 0.15395 | 2578.2 | 2205901 | 0.4601 | 0.00054 | 126.5 |
The values in parentheses represent the results without the last study date, that is, the values without counting the unexpected event.
The relationship between the amount of microplastics and the area show the number of particles (#Mp/m3) per km2, where the most polluted points have the highest values, once again the last date of sampling in SP2 causes this point to have the highest values in the study. We can also see the relationship between the amount of microplastics (#Mp/m3) and the number of inhabitants where we can see that while the population is larger, more amount of microplastic particles exist.
Information from National Institute of Statistics and Censuses Ecuador, INEC (2010). www.ecuadorencifras.gob.ec
2.2. Environmental variables
We measured in situ: Temperature (°C), Oxygen saturation (%), Dissolved Oxygen (DO, mg/L), conductivity (μS/cm), pH and total dissolved solids (TDS) using the YSI Pro 1030 Model (Conductivity, TDS, pH, temperature) and the YSI Pro ODO Model (Oxygen saturation % and dilution mg/L DO). We collected samples in amber bottles which we stored at 4 °C until their arrival at the lab (less than 4 h), where analysis or appropriate fixation was performed. Parameters measured at lab were: color, nitrites, ammonium, phosphates, Biological Oxygen Demand (BOD5), Chemical Oxygen Demand (COD) and chlorides, using standard analysis methods (Rice et al., 2017).
We measured flow only at points SP7, 3.2PI, SP3 and SP2 (only on the first three dates because on our last sampling date, flow was too strong to measure discharge in a safe way). We applied a transect method, using a GlobalWater Flow meter (GlobalWater, TX, USA). Flow was calculated by measuring the width of the river and the speed of the water at several points of a transect (Turnipseed and Sauer, 2010). We could not measure flow at M5 due to channel incision and high flows at this point. For this point, reference flow information was gathered from publicly available historical records of FONAG (Tucci, 2009).
To evaluate the quality of the riparian vegetation, we followed the Andean adaptation of the QBR quality Index (QBR-And, Acosta et al., 2009). For this, we observed 100 linear meters of the river analyzing 4 aspects: 1) Degree of cover of riparian area, 2) Structure of the cover, 3) Cover quality and 4) Fluvial channel. The index values are distributed in five ranges of quality: >95: natural state, riparian forest without alterations; 90-75: good quality, slightly disturbed forest; 70-55: acceptable quality, start of major alteration; 30–50: bad quality, strong alteration; <25: poor quality (Acosta et al., 2009). We evaluated the physical river habitat quality based on the River Habitat Index (IHF, Acosta et al., 2009). This index measures 7 different aspects of the fluvial channel: 1) Substrate Composition and Size; 2) Speed/depth regimes; 3) inclusion in rapids - sedimentation in ponds; 4) Frequency of rapids; 5) Percentage of shade in the channel; 6) Elements of heterogeneity; 7) Coverage and diversity of aquatic vegetation.
2.3. Microplastics sampling
For the sampling and collection of microplastics in water samples we used a 250 μm mesh drift net composed of 4 sub-nets with dimensions of 35cm in height and 17cm in width each. Sampling was started at the highest elevation points in the basin and continued down along the basin (Table 1). Sampling time was 20 min per point to standardize with previous studies on streams (Hoellein et al., 2017; McCormick et al., 2016). During the sampling we measured the speed of the water that passed through the drift net and the height of water in each sub-net to be able to ascertain the quantity of particles per volume of water (Hoellein et al., 2017; McCormick et al., 2016). After surface-water sampling, we collected sediment with a ponar grab sampler, approximately 1 L per point. All samples were transported to the laboratory at 4 °C, where they were stored at 4 °C until processing for microplastic counts (Hidalgo-Ruz et al., 2012 & Leslie et al., 2017).
2.4. Processing of microplastics
We followed NOAA methods for microplastic processing (Masura et al., 2015), which were previously used in rivers (Hoellein et al., 2017; McCormick et al., 2016). We started by passing surface water through stacked sieves of 5 mm, 1.1 mm and 0.3 mm. All samples were passed through 0.3 mm, and only the highly polluted samples (SP3, SP2 and M5) were passed through 5 and 1.1 mm before. We placed the retained material from the 0.3 mm sieve into previously weighed and dried precipitation beakers, and left them in the stove for 24 h at 90 °C. We then weighed them to obtain the dry net material per sample (Masura et al., 2015).
For the digestion of organic material, we placed the dry sample in a beaker and added 20 ml of 30% H2O2 and 20 mL of 0.05 M Fe (II). We proceeded to shake on a thermal plate at 75 °C until the organic matter was degraded. Then we added 5 mL of 5M NaCl solution to the beaker, where it was stirred until homogenized (Masura et al., 2015). For the separation of microplastics based on density, we placed the solution in a glass funnel for separation. Each sample was left in the funnels overnight for the separation of the particles. The remaining sediment was discarded after verifying that no microplastic particle had gone to the bottom of the funnel. The suspended material, containing floating microplastics, was collected and passed through Whatman glass fiber filters of 0.7 μm and 47 mm (Masura et al., 2015).
With each filter, we counted five categories of microplastics: fragment, foam, pellet, film and fiber (Hoellein et al., 2017; McCormick et al., 2016) using 4X and 10X amplification with an CX31 Olympus Microscope. At the moment of counting the microplastic particles, we used the keys provided by NOAA (Masura et al., 2015) where an explanation is given of how to distinguish microplastics from other microparticles. To discard these microparticles we dragged the tweezers through the particles, if these were pulverized or unmade, then the pieces were not plastic. Similarly, if the particles retained their shape, then they were correctly identified as plastic. We also became familiar with the plastic reference materials (Masura et al., 2015). Fibers were the most numerous, therefore we counted them using a sub-sampled approach for counting the number of particles, using 3 out of 8 fields of the view of each filter. The films and fragments were counted in all the filters, except in the last date in point SP2, where we counted the films equally by quadrants. The number of particles in each field was averaged and then expanded to the entire filter using the relative areas of the field of view and filter area. For surface water samples, the number of microplastic particles per volume of river water sampled was calculated, and the values were presented as number (#) of microplastics (Mp)/per cubic meter (m3). For large samples (most from downstream sites), we counted 3 of 16 fields due to the large amount of microplastics (Hoellein et al., 2017; McCormick et al., 2016).
2.5. Data analysis
To know the relationship between environmental variables and the quantity, type and weight of microplastics, we used a Spearman rank correlation. Differences in amount of microplastics in water and sediment among points were evaluated using Kruskal Wallis H tests. For all statistical analysis we used STATISTICA 10.0 (Tulsa, USA).
3. Results
3.1. Quantity of microplastics in the rivers of Quito
We found that the amount and weight of particles per m3 increased with drainage size, and therefore with human presence in the basin (Table 2 & Figures 2a, 2b). Regarding the environmental variables and their relation with the quantity and weight of microplastics per m3, Spearman Rank correlations showed that dissolved oxygen and oxygen saturation had a significant inversely proportional correlation with microplastics (Figure 3). For other parameters related to pollution, such as turbidity, nitrites and nitrates, ammonium, phosphates, alkalinity, chlorides, BOD5, COD, and conductivity, among others (Figure 3), there was a positive correlation, meaning that at higher concentrations of pollutants, the amount and weight of microplastics found was greater (Figure 3).
Figure 2.
a. Number of Microplastics (quantity) per cubic meter found in the Upper Guayllabamba River Basin. Y axis values are shown in logarithmic scale to allow to see the significant differences in each point. Note: One of the reasons why we use this scale is that the difference in the number of particles between the first points (SP7 and 3.2PI) and the mid and final points (SP3, SP2 and M5) was very noticeable and in another type of graphic we could not observe the differences. b. Weight of microplastics (g) per cubic meter found in the Upper Guayllabamba River Basin. Note: In the graph we can see that the weight of the microplastic particles is very low (even very close to 0.0) but as it goes down in the basin these values start to vary. We can observe that point SP2 initially had a value close to SP3 but after the last sampling date SP2 it came to surpass M5.
Figure 3.
Heatmap of Spearman Rank correlations values of the amount and weight of microplastics in water in relation to water quality parameters. All correlations in bold are significant p>0,05. Certain pollutants have high correlations with microplastics in water especially Chlorides, Nitrites, and conductivity. Heat colors represent stronger correlations.
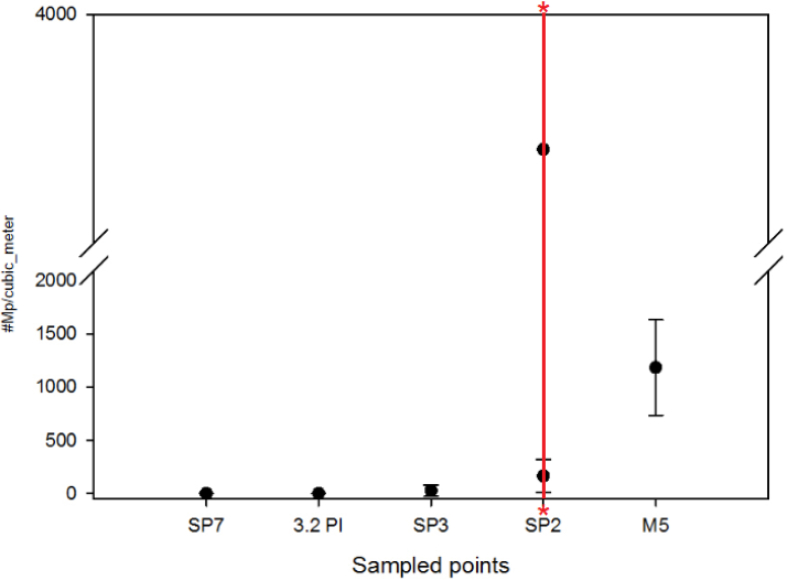
Point M5 (Guayllabamba River, downstream site) accumulates the effect of all the upper Guayllabamba basin, and therefore the whole contribution of microplastics of the upper part of the basin (Figure 4). Despite this, on the last sampling date, point SP2 showed a notable variation in the amount of microplastics (Figure 5c; Figure 4), with higher values of microplastics. Although the source of this discharge is uncertain (but was probably a discharge from a drinking water treatment plant facility) all the pollutants, including microplastics, showed an incredible increase in the SP2 site during this date (see Table 2 for values and Figure 5 for pictures).
Figure 4.
Amount of microplastics per point found in the Upper Guayllabamba River Basin where the 2SE (standard error) is shown. The red line and asterisks show the values of the standard error at point SP2 which are outside the graph (10889 & -3354.22).
Figure 5.
Microplastics in samples from Guayllabamba river basin. a & b from site at San Pedro River (SP2) at Upper Guayllabamba River Basin. c filtered sample from (SP2) at the event of sediment discharge.
The amount of microplastics in sediments (items per Kg of sediment) found in the Upper Guayllabamba River Basin showed that points located in the headwaters of the basin (3.2PI and SP7) had the lowest amounts, while points SP3, M5 and SP2 had the highest values respectively (Figure 6).
Figure 6.
Items of microplastics per Kg in river sediments at studied sites in the Upper Guayllabamba River Basin.
3.2. Effect of urbanization in the amount and type of river microplastics
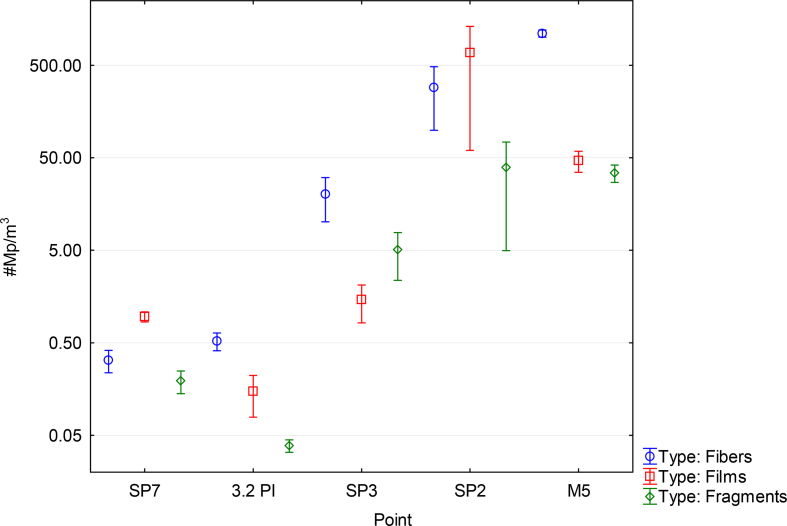
The Pita and San Pedro rivers at their upstream points, 3.2 PI and SP7 respectively, were used as reference sites because these points are far from the urban areas, and are composed mostly of a mosaic of Andean forest, agriculture and low-intensity cattle herding. These 2 points had the smallest amount of microplastic particles per cubic meter. On the other hand, these same basins at their lowest points, SP3 and SP2 respectively, had a considerable increase in microplastics (Figures 4, 6, and 7). Finally, we have the M5 point (Guayllabamba River), which captures the wastewater of more than 2 million people and receives pollution from an area of 2578.2 km2. Throughout the Upper Guayllabamba River Basin, the presence of particles such as fragments, films and fibers were evidenced. The presence of fibers increased tremendously in urban areas (Figures 7 and 8).
Figure 7.
Types of microplastic particles found in water at studied sites of the Upper Guayllabamba River Basin.
Figure 8.
Types of microplastic particles found in sediment at studied sites of the Upper Guayllabamba River Basin. Bars are Standard Errors of the mean.
The amount of microplastics in sediments certainly showed that urban areas had a greater amount of these particles (Figures 6 and 8). The microplastic particles found in sediments at our upstream points (SP7 and 3.2PI) (with 36.6 and 14.3 particles per dry kilogram respectively) presented very low values even when compared with the literature (Table 3). The points SP2 and M5 had the highest values in the study (186.5 and 126.5 particles per kilogram respectively).
Table 3.
Number of microplastics (# Mp/Kg) found in sediments at studied sites compared with other rivers worldwide.
| River/Point | Amount of Mp/Kg (dw)1 | Country1 |
|---|---|---|
| Guayllabamba/M5 | 126 | Ecuador |
| San Pedro/SP2 | 186.5 | Ecuador |
| Pita/SP3 | 112.5 | Ecuador |
| San Pedro/SP7 | 32.6 | Ecuador |
| Pita/3.2PI | 14.3 | Ecuador |
| Meuse, Eijsden/P1.1 | 1760 | The Netherlands |
| Meuse, Eijsden/P1.2 | 1030 | The Netherlands |
| Rhine, Lobith/P2.1 | 5280 | The Netherlands |
| Rhine, Lobith/P2.2 | 4520 | The Netherlands |
| Rhine, Bimmen/P3.1 | 1970 | The Netherlands |
| Rhine, Bimmen/P3.2 | 1420 | The Netherlands |
| Amsterdam Urban Canal A1 | <68 | Germany |
| Amsterdam Urban Canal A2 | 1050 | Germany |
| Amsterdam Urban Canal A3 | 146 | Germany |
| Amsterdam Urban Canal A4 | 10500 | Germany |
| Amsterdam Urban Canal A5 | 527 | Germany |
| Amsterdam Urban Canal A6 | 132 | Germany |
Information from Leslie, Brandsma, Van Velzen & Vethaak, 2017. These authors used different collection methodologies from the ones used in this study.
We found significant differences in the type of microplastic particles per m3 across sampling points. Fibers were the most abundant particles across all points, except for the last sampling date for point SP2, in which films showed the highest values. For fibers (KW-H (4,20) = 15.6143, p = 0.0036) and fragments (KW-H (4,20) = 16.078, p = 0.0029) there were differences across sites, with higher values being recorded as watershed area increased (Figure 7), but not for films (KW-H (4,20) = 3.1214, p = 0.5377).
Microplastics in sediments had a similar pattern to microplastics in water. Fibers were more abundant than other types of particles except for the last sampling date, where films showed the highest values of the study at point SP2. Our results show a significant difference in sediments by type of particle and by point (Figure 8). In the case of fibers (KW-H (4,20) = 14.1676, p = 0.0068) and fragments (KW-H (4,20) = 11.5139, p = 0.0214) there was an increasing abundance of them with increasing watershed area, but not in the case of films (KW-H (4,20) = 3.2285, p = 0.5203).
When relating the amount of microplastics found by drainage area at each point, it can be noticed that the number of particles per square kilometer of watershed at the initial points (SP7 and 3.2 PI) is much lower than at the final point, which is M5 (except for point SP2 during the last sampling date) (Table 2). The drainage areas of points 3.2PI and SP7 are under minimum influence from urban populations (Figure 1, Table 4), however, microplastics were detected at these points. On the other hand, in the following points, SP3, SP2 and M5, the amount of microplastics per square kilometer of drainage area increases as urban areas increase. The amount of microplastics found also increases as the number of inhabitants in the basin increase. Point 3.2PI presents the lowest values of the study because the influence of the urban area is almost zero. On the other hand, points SP2 and M5 present the highest values of the study, and have the largest number of microplastics in relation to the number of inhabitants, with SP2 showing a higher amount of microplastics in relation to existing population than M5 (Figure 2a).
Table 4.
Amount of microplastics (# Mp/m3) found in water at studied sites compared with other rivers worldwide.
| River/Point | # Mp/m3 in water | Drainage Area (Km2) | Habitants | Amount of Mp/m3 in water after presence of WWTP | Country |
|---|---|---|---|---|---|
| Guayllabamba/M5 | 1584.23 | 2578.2 | 2205901 | - | Ecuador |
| San Pedro/SP2 | 168.12 | 1405.5 | 126910 | - | Ecuador |
| Pita/SP3 | 30.18 | 1338.2 | 67121 | - | Ecuador |
| San Pedro/SP7 | 2.23 | 209 | 42399 | - | Ecuador |
| Pita/3.2PI | 0.73 | 58.5 | 17930 | - | Ecuador |
| Higgen's Cr. | 0.57 | 168.863 | 2640003 | 11.22 | USA1 |
| Springbrook Cr. | 1.17 | 173.52 | 63000 | 5.39 | USA |
| L Kickapoo Cr. | 1.24 | NF | NF | 0.80 | USA |
| N. Shore Ch. | 3.36 | 67.97 | 45000 | 6.60 | USA |
| Goose Cr. | 4,37 | 370.36 | 1300000 | 2.53 | USA |
| DuPage R. | 5.92 | NF | NF | 10.28 | USA |
| W Br DuPage R. | 0.93 | NF | 24000 | 2.96 | USA |
| Salt Cr. | 0.48 | NF | NF | 3.73 | USA |
| E Br DuPage R. | 3.14 | NF | NF | 8.86 | USA |
| Rin River (Duisburg) | NF | 232.8 | 499845 | 8.84 | Germany2 |
| Rin River (Rees) | NF | 109,7 | 22267 | 11.05 | Germany |
Note: The values in blank “-” mean that within Ecuador the points studied do not have Wastewater Treatment Plant (WWTP), while the values with "NF" mean that the information were not found.
The values in USA come from McCormick et al. (2016).
The values in Germany come from Mani et al. (2015).
The values for habitants and drainage area in other river worldwide come from each wastewater system official site.
3.3. Microplastics in the rivers of Quito in a global context
Quito has around 2.5 million inhabitants, but about 88% of its inhabitants discharge their wastewater through the sewer system without treatment (EPMAPS, 2011). We compared our results with others worldwide, using studies from: the Rhine River at the Netherlands, Germany and France (Mani et al., 2015) and nine creeks in Illinois, USA (McCormick et al., 2016). We found striking differences with the values previously published for the amount of microplastic particles found per m3 (Figure 9). This is because the studies available for comparison come from places where wastewater discharge goes to treatment plants or recirculation, so the items and amount of microplastics show lower values than those found in the upper Guayllabamba basin. Almost all points studied (with the exception of 3.2PI) have higher values of microplastics per m3 than those previously reported for rivers. Moreover, an abnormal event recorded at point SP2 on the last sampling date produced a value that exceeded the maximum values registered for our last point, M5.
Figure 9.
Number of microplastic items per meter cubic found in the study basin compared to other rivers around the world. A cut is indicated between the black lines in Guayllabamba (M5) due to the amount of microplastics found. Values for other studies come from: 1) McCormick et al., (2016), that studied streams the following in the United States: Higgen's Cr., Springbrook Cr., L Kickapoo Cr., N. Shore Ch., Goose Cr., DuPage R., W Br DuPage R., Salt Cr. & DuPage R; and 2) Mani et al., (2015) the Rhine River in Germany studied in the towns of Duisburg and Rees.
For the values reported in the Rhine River (Table 4), it could be noted that at Rees, the amount of microplastic particles in relation to the area of influence is similar to the values obtained in points 3.2 PI and SP7. On the other hand, for the values reported at Desiburg, it could be noted that the density of microplastics per square kilometer is much lower, with values are similar to those found in the oceans (Mani et al., 2015). Likewise, if the ratio of microplastics per inhabitant is considered, the Deinsburg point has similar values to points 3.2PI and SP7. This does not happen in Rees, since the number of microplastics per inhabitant is similar to point SP3, which shows that there is already pollution due to the influence of urban areas (Table 4). The presence of water treatment plants causes a considerable decrease in the number of microplastics per cubic meter. If we compare the values found in Goose creek (McCormick et al., 2016) with those of SP2, with a similar number of habitants in the drainage area, our point has 40 times more microplastics than Goose creek.
When we compare microplastic amounts in sediments in upper Guayllabamba river basin (values between 14.3- 186.5 Kg-1) with the values obtained in sediments in other rivers (Leslie et al., 2017; values between 1030-5280 kg-1) and urban canals worldwide (<68–10500 Kg-1) we can notice that our values are not as high as those reported in the Netherlands (Table 3). Values found in points SP7 and 3.2PI are low compared to published values (Leslie et al., 2017). Higher values found on point SP2 can be related to its proximity to a potable water treatment plant that could empty sediments in this reach but also, at this point the San Pedro River has a lower slope compared to M5 point that has a pronounced slope.
4. Discussion
The amount of microplastics in both water and in sediments found in the Upper Guayllabamba Basin showed increasing values as the basin area increased. Much of this can be related to the characteristics of the area of the studied points. The amount of microplastics is highly correlated to the concentration of pollutants in water (Figure 3), showing a relationship between rivers’ pollution and microplastics, mainly with increasing urbanization, as has been shown elsewhere (McCormick et al., 2016). Points located at the headwaters of the basin (SP7 and 3.2PI) had a relatively low amount of microplastics, while the most polluted points (M5, SP2 and SP3), had the greatest amounts of microplastics.
Population density was a main factor in affecting microplastic abundance. Points 3.2PI and SP7, which have 0.71 and 1.41 microplastic particles per cubic meter respectively, are located at the headwaters of the basin and showed a smaller amount of microplastic particles in their waters. On the other hand, points SP3 and SP2 have a greater amount of microplastic particles, and both drain several urban areas in the basin. Finally, point M5, located in the Guayllabamba river, which receives water from all the upper basin and around 2.5 million inhabitants, had 1,186,339 microplastic particles per cubic meter. We could not find a similar value in the literature. All our impacted sites had more microplastics per cubic meter (SP3, SP2 and M5), than those reported elsewhere (p.e: Mani et al., 2015 & McCormick et al., 2016).
Differences in the content of microplastics in sediments when compared to other studies may be due to geomorphological characteristics of the study area, which features pronounced slopes. The methodology with which the sediments were collected could be another reason for this difference. We used a ponar grab, while in the Netherlands they used a centrifugation methodology when collecting the sediments (Leslie et al., 2017). However, the distribution of the microplastics in sediments is not yet clear (Martin et al., 2017).
Within the study, the majority of particles reported at all points were fibers (Figures 7 and 8). These fibers are related to synthetic clothing products, and have generated great concern due to their alarming quantity in other parts of the world as well (Anderson et al., 2017; Jönsson et al., 2018). Although in early studies fibers did not seem to be a source of pollution, it is now known that they are the most easily fractionated form of microplastic, and their presence increases significantly in areas where the population is greater (Hartline et al., 2016; Blair et al., 2017). At points SP7 and 3.2PI, with lower inhabitants per square meter, the number of fibers found was similar to other particles such as films or fragments (Costa et al., 2010), at points SP3, SP2 and M5, fibers increased significantly, even surpassing known values for other parts of the world (McCormick et al., 2016; Mani et al., 2015). Point M5 was the most polluted in the study because it received water from all the upper basin, therefore it has the largest number of fibers. Despite this, an unexpected event in the last sampling date at SP2, related to a cleaning procedure of a water tank from a potabilization plant, contributed with a huge amount of microplastics, making this value the highest from the study period. This may also be due to the fact that point SP2 has the largest amount of population in relation to the size of the basin, so it is possible to think that these particles were retained in the WTP upstream (the cause was not clear in base of information provided by authorities but they suggested it might be related to a tank cleaning event) and at the time of maintenance were released, causing the values to exceed any other value in the study or in the bibliography. This means that activities such as sediment cleaning of water treatment plants or similar activities can also produce pulses of microplastics entering rivers (Cole, 2016).
At our upstream sites, points SP7 and 3.2 PI, which have agricultural and livestock uses in the watershed, very low microplastic values were found, both in weight and number. One of the reasons for the presence of plastic particles in these points is runoff and poor management of solid waste, because the areas surrounding these rivers related to these industries (agricultural and livestock) do not have adequate soil or riparian vegetation coverture to prevent pollutants such as plastic containers or pieces from reaching the water (Acosta, 2015 & da Cruz e Souza & Ríos-Touma, 2018). Also, is known that rain can transport and deposit microplastics in remote areas, away from pollution centers (Allen et al., 2019) and this could be a source of microplastics for these sites. Our values are not alarming until reaching the urban areas where the values of microplastics begin to increase significantly and are even higher than those previously reported (Anderson et al., 2016; Blair et al., 2017).
Problems such as the removal of riparian forests, loss of vegetation cover, and changes in land use have occurred along the Upper Basin of the Guayllabamba River for several decades (Table 1 and Figure 3, Acosta et al., 2009), and are also important factors for pollutant runoff, including microplastics. The lack of well-preserved riparian areas and pollution by runoff is well known (Dehghani et al., 2017 & Murphy et al., 2016) however, when compared with existing data on microplastics input (McCormick et al., 2016; Mani et al., 2015), our own data from the Upper Guayllabamba River Basin reveals larger and more alarming patterns. This is related with the direct discharge of wastewaters to the rivers in this basin as we move downstream, with pollutants increasing as industries and urbanization populations increase.
It is difficult to compare the high values we found with the low quantity of microplastics found in other countries. Basically, we are comparing our values with values from countries that have wastewater treatment plants, like Germany, where several policies have been established over the years to avoid pollution problems and recover the ecological health, especially the Rhine that was in a critical condition (Mani et al., 2015 and Vaughan et al., 2017). The situation in Ecuador, where there is a lack of enforcement of wastewater management policies, a deficient management of solid waste, and likewise a poor management of water resources, is widespread across the Andean ranges. No data was found for other Andean countries regarding plastic pollution in rivers, but it is probably similar to values found in the Upper Guayllabamba basin, making a regional effort to improve the ecological quality of rivers urgent, especially in urban streams, to stop contributing with plastic and other pollution to the sea (Graca et al., 2017).
Lack of wastewater treatment has always been a health hazard. This is true also in Quito (Guerrero-Latorre et al., 2018) but now we know that microplastics are also an important factor to consider. Freshwater biodiversity in tropical countries is highly unknown and diverse, so the effects of pollutants in this diversity are hard to measure, but it is probable that we already have lost or endangered several species. Also, we have to consider that in highly biodiverse areas like Ecuador, where the hotspot of the Tropical Andes is an important part of the country, the effects at the ecosystem level can be devastating. Drainages like the Esmeraldas, which receives all the pollution from the Upper Guayllabamba basin, arrive to the Pacific Ocean and are therefore, in direct contact with other endangered ecosystems like mangrove forests and the Galapagos Islands.
It is urgent that high-altitude cities, located on the upper parts of basins, adopt measures to control pollution. Regarding plastic disposal, the city of Quito is currently working on laws to prevent single-use plastics. This is an important step, but solid waste management should be improved, as it is expected that by 2025, MSW in Ecuador will have doubled from the values recorded in 2012 (Hoornweg and Bhada-Tata, 2012). Furthermore, the implementation of wastewater treatment plants is imperative in these cities, not only because of microplastics, but because of all the health hazards for humans and the impacts on the biodiversity of freshwater mountain ecosystems (Guerrero-Latorre et al., 2018; Encalada et al., 2019).
5. Conclusions
The data on the amount of microplastic particles found in the rivers of the Upper Guayllabamba River Basin represent the first data of this type for rivers in Ecuador and Andean countries. The concentrations of these particles in some cases are relatively low, as is the case with headwater streams such as points 3.2PI and SP7, but as the rivers pass through urban areas, these concentrations become very alarming, exceeding by far the data found in the literature.
Wastewater discharge to rivers is highly related to the amount of microplastics found in these rivers. This relationship becomes more noticeable in urban areas due to the population increase, poor management of urban waste, the loss of riparian plant cover, and the lack of wastewater treatment, and thus the amount of microplastics in the last points is alarmingly high. Our points below urban areas show strikingly high values, which represent a threat for both human and ecosystem health. Freshwater biodiversity and the ecosystem services provided by Andean streams make wastewater treatment plants and wise watershed management implementation an urgent and imperative need in Andean cities.
Declarations
Author contribution statement
Blanca Rios-Touma: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mishell Donoso: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Universidad de Léas Américas, Quito- Ecuador (Dirección de Investigación, Project: AMB·BRT.17.01).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank María Genoveva Granda, Indira Black, Gabriela Jijón, Andrés Arguello, Daniel Valencia, Juan Manuel Guerra, Christian Villamarín, Nicole Gutierrez and Xavier Amigo for field and laboratory assistance. We also thank Juan Esteban Suárez for English revisions.
References
- Acosta R., Ríos B., Rieradevall M., Prat N. Propuesta de un protocolo de evaluación de la calidad ecológica de ríos andinos (CERA) y su aplicación a dos cuencas en Ecuador y Perú. Limnética. 2009;28(1):35–64. [Google Scholar]
- Acosta G.F. Caracterización físico-química y microbiológica del agua del río Soacha, Cundinamarca, Colombia/Physico-chemical and microbiological characterization of Soacha river water Cundinamarca, Colombia/Caracterização microbiológica físico-químicas da água de rio Soacha, Cundinamarca, Colombia. Revista De Investigación Agraria y Ambiental. 2015;6(2):119–144. [Google Scholar]
- Allen S., Allen D., Phoenix V.R., Le Roux G., Durántez Jiménez P., Simonneau A., Binet S., Galop D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019;12:339–344. [Google Scholar]
- Anderson J., Park B., Palace B. Microplastics in aquatic environments: implications for Canadian ecosystems. Environ. Pollut. 2016;218:269–280. doi: 10.1016/j.envpol.2016.06.074. [DOI] [PubMed] [Google Scholar]
- Anderson P.J., Warrack S., Langen V., Challis J.K., Hanson M.L., Rennie M.D. Microplastic contamination in lake Winnipeg, Canada. Environ. Pollut. 2017;225:223–231. doi: 10.1016/j.envpol.2017.02.072. [DOI] [PubMed] [Google Scholar]
- Blair R., Waldron S., Phoenix V., Gauchotte L. Micro- and nanoplastic pollution of freshwater and wastewater treatment systems. Springer Sci. Rev. 2017:2213–7793. [Google Scholar]
- Browne M.A., Crump P., Niven S.J., Teuten E., Tonkin A., Galloway T., Thompson R. Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ. Sci. Technol. 2011;45(21):9175. doi: 10.1021/es201811s. [DOI] [PubMed] [Google Scholar]
- Castillo M. Consultoría para la realización de un estudio de caracterización de residuos sólidos urbanos domésticos y asimilables a domésticos para el distrito metropolitano de quito. EMASEO. 2012 [Google Scholar]
- Célleri R., Feyen J. The hydrology of tropical andean ecosystems: importance, knowledge status, and perspectives. Mt. Res. Dev. 2009;29(4):350–355. [Google Scholar]
- Chae D., Kim I., Kim S., Song Y.K., Shim W.J. Abundance and distribution characteristics of microplastics in surface seawaters of the Incheon/Kyeonggi coastal region. Arch. Environ. Contam. Toxicol. 2015;69(3):269–278. doi: 10.1007/s00244-015-0173-4. [DOI] [PubMed] [Google Scholar]
- Cole M. A novel method for preparing microplastic fibers. Sci. Rep. 2016;6:34519. doi: 10.1038/srep34519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M.F., Sul I.D., Silva-cavalcanti J., Araújo M.C., Spengler B., Tourinho P.S. On the importance of size of plastic fragments and pellets on the strandline: a snapshot of a brazilian beach. Environ. Monit. Assess. 2010;168(1-4):299–304. doi: 10.1007/s10661-009-1113-4. [DOI] [PubMed] [Google Scholar]
- da Cruz e Sousa R., Ríos-Touma B. Stream restoration in Andean cities: learning from contrasting restoration approaches. Urban Ecosyst. 2018;21:281–290. [Google Scholar]
- Dehghani S., Moore F., Akhbarizadeh R. Microplastic pollution in deposited urban dust, tehran metropolis. Iran. Environ. Sci. Pollut. Res. Int. 2017;24(25):20360–20371. doi: 10.1007/s11356-017-9674-1. [DOI] [PubMed] [Google Scholar]
- Eerkes-Medrano D., Thompson R. Chapter 4 - Occurrence, Fate, and Effect of Microplastics in Freshwater Systems. In: Zeng Eddy Y., editor. Microplastic Contamination in Aquatic Environments An Emerging Matter of Environmental Urgency. Elsevier; 2018. pp. 95–132. [Google Scholar]
- Egas J., Ordóñez J. NOVUM, Asesora y Consultora Ambiental. 2015. Plan de Intervención Ambiental Integral en las Quebradas de Quito. [Google Scholar]
- Encalada A.C., Flecker A.S., Poff N.L., Suárez E., Herrera-R G.A., Ríos-Touma B., Jumani S., Larson E.I., Anderson E.P. A global perspective on tropical montane rivers. Science (80-. ) 2019;365:1124 LP–1129. doi: 10.1126/science.aax1682. [DOI] [PubMed] [Google Scholar]
- EPMAPS . 2011. Estudios de Actualización del Plan Maestro Integrado de Agua Potable y Alcantarillado Para El Distrito Metropolitano de Quito. [Google Scholar]
- Graca B., Szewc K., Zakrzewska D., Dołęga A., Szczerbowska-boruchowska M. Sources and fate of microplastics in marine and beach sediments of the southern baltic Sea—a preliminary study. Environ. Sci. Pollut. Res. Int. 2017;24(8):7650–7661. doi: 10.1007/s11356-017-8419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Romero B., Bonifaz E., Timoneda N., Rusiñol M., Girones R., Ríos-Touma B. Quito’s virome: Metagenomic analysis of viral diversity in urban streams of Ecuador’s capital city. Sci. Total Environ. 2018 doi: 10.1016/j.scitotenv.2018.07.213. [DOI] [PubMed] [Google Scholar]
- Hartline N.L., Bruce N.J., Karba S.N., Ruff E.O., Sonar S.U., Holden P.A. Microfiber masses recovered from conventional machine washing of new or aged garments. Environ. Sci. Technol. 2016;50(21):11532–11538. doi: 10.1021/acs.est.6b03045. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Ruz V., Gutow L., Thompson R.C., Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ. Sci. Technol. 2012;46(6):3060–3075. doi: 10.1021/es2031505. [DOI] [PubMed] [Google Scholar]
- Hoellein T., McCormick A., Hittie J., London M., Scott J., Kelly J. Longitudinal patterns of microplastic concentration and bacterial assemblages in surface and benthic habitats of an urban river. Freshw. Sci. 2017;36(3) 000–000. [Google Scholar]
- Hoornweg D., Bhada-Tata P. Urban Development Series Knowledge Papers #15. World Bank; 2012. What a waste: a global review of solid waste management. [Google Scholar]
- Jönsson C., Arturin O.L., Hanning A., Landin R., Holmström E., Roos S. Microplastics shedding from Textiles—developing analytical method for measurement of shed material representing release during domestic washing. Sustainability. 2018;10(7):2457. [Google Scholar]
- Khan F.R., Mayoma B.S., Biginagwa F.J., Syberg K. Microplastics in inland African waters: Presence, sources, and fate. In: Wagner M, Lambert S, editors; Barceló D, Kostianoy A, editors. Freshwater Microplastics Emerging Environmental Contaminants? Springer; 2018. pp. 101–124. (The Handbook of Environmental Chemistry 58.). [Google Scholar]
- Kramm J., Völker C. Understanding the risks of microplastics: a social-ecological risk perspective. In: Wagner M., Lambert S., editors. vol. 58. Springer; Cham: 2018. (Freshwater Microplastics. The Handbook of Environmental Chemistry). [Google Scholar]
- Landázuri A., Quevedo J., Torres M., Mayorga A., Gómez L. Programa de Descontaminación de los Ríos de Quito. 2014. Muestreo y caracterización de la Descarga “iñaquito”, representativa de la Cuenca urbana de la Quebrada el batán: Quito-Ecuador. [Google Scholar]
- Leslie HA. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environment International. 2017 doi: 10.1016/j.envint.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Mani T., Hauk A., Walter U., Burkhardt-Holm P. Microplastics profile along the Rhine river. Sci. Rep. 2015;5:17988. doi: 10.1038/srep17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick A., Hoellein T., London M., Hittie J., Scott J., Kelly J. Microplastic in surface waters of urban rivers: concentration, sources, and associated bacterial assemblages. Ecosphere. 2016;7(11):e01556. [Google Scholar]
- Martin J., Lusher A., Thompson R.C., Morley A. The deposition and accumulation of microplastics in marine sediments and bottom water from the Irish continental shelf. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-11079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masura J., Baker J., Foster G., Courtney A. NOAA Technical Memorandum NOS-OR&R-48. 2015. Laboratory methods for the analysis of microplastics in the marine environment: recommendations for quantifying synthetic particles in waters and sediments. [Google Scholar]
- Murphy F., Ewins C., Carbonnier F., Quinn B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016;50(11):5800–5808. doi: 10.1021/acs.est.5b05416. [DOI] [PubMed] [Google Scholar]
- Rice A., Baird E., Eaton R. Standard Methods for Examination of Water and Wastewater. APHA: American Public Health Association, American Water Works Association, and Water Env. Federation; Washington: 2017. [Google Scholar]
- Scherer C., Brennholt N., Reifferscheid G., Wagner M. Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-17191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talvitie J., Mikola A., Koistinen A., Setal O. Solutions to microplastic pollution. removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017;123:401–407. doi: 10.1016/j.watres.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Thompson R.C., Olsen Y., Mitchell R.P., Davis A. Lost at sea: where is all the plastic? Science. 2004;304(5672):838. doi: 10.1126/science.1094559. [DOI] [PubMed] [Google Scholar]
- TUCCI C.E.M. 2009. Plan de Manejo Integrado de los Recursos Hídricos en la Cuenca Alta del Río Guayllabamba. BID Banco Interamericano de Desarrollo Económico y FONAG Fondo para la Protección del Agua. 147p. [Google Scholar]
- Turnipseed D.P., Sauer V.B. 2010. Discharge measurements at gaging stations: U.S. Geological Survey Techniques and Methods book 3. chap. A8, 87 p. [Google Scholar]
- Vaughan R., Turner S.D., Rose N.L. Microplastics in the sediments of a UK urban lake. Environ. Pollut. 2017;229:10–18. doi: 10.1016/j.envpol.2017.05.057. [DOI] [PubMed] [Google Scholar]
- Wagner M., Scherer C., Alvarez-Muñoz D., Brennholt N., Bourrain X., Buchinger S., Fries E., Grosbois C., Klasmeier J., Marti T., Rodriguez-Mozaz S., Urbatzka R., Vethaak A.D., Winther-Nielsen M., Reifferscheid G. Microplastics in freshwater ecosystems: what we know and what we need to know. Environ. Sci. Eur. 2014;2014(26):12. doi: 10.1186/s12302-014-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S.L., Kelly F.J. Plastic and human health: a micro issue? Environ. Sci. Technol. 2017;51(12):6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- Yang D., Shi H., Li L., Li J., Jabeen K., Kolandhasamy P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015;49(22):13622–13627. doi: 10.1021/acs.est.5b03163. [DOI] [PubMed] [Google Scholar]