Summary
Methylation is envisioned as a promising way to rationally improve key pharmacokinetic characteristics of lead compounds. Although diverse tailoring enzymes are found to be clustered with cyclodipeptide synthases (CDPSs) to perform further modification reactions on the diketopiperazine (DKP) rings generating complex DKP-containing compounds, so far, a limited number of methyltransferases (MTs) co-occurring with CDPS have been experimentally characterized. Herein, we deciphered the methylation steps during drimentines (DMTs) biosynthesis with identification and characterization of DmtMT2-1 (from Streptomyces sp. NRRL F-5123) and DmtMT1 (from Streptomyces youssoufiensis OUC6819). DmtMT2-1 catalyzes N4-methylation of both pre-DMTs and DMTs; conversely, DmtMT1 recognizes the DKP rings, functioning before the assembly of the terpene moiety. Notably, both MTs display broad substrate promiscuity. Their combinatorial expression with the dmt1 genes in different Streptomyces strains successfully generated eight unnatural DMT analogs. Our results enriched the MT tool-box, setting the stage for exploring the structural diversity of DKP derivatives for drug development.
Subject Areas: Kinetic Chemistry, Enzyme Engineering, Structural Biology
Graphical Abstract
Highlights
-
•
The methylation steps during drimentines biosynthesis were unraveled
-
•
Two N-MTs with different regioselectivities were identified
-
•
The substrate promiscuities of DmtMT1 and DmtMT2-1 were probed
-
•
Combinatorial biosynthesis expanded the chemical space of drimentines
Kinetic Chemistry; Enzyme Engineering; Structural Biology
Introduction
Natural products with 2,5-diketopiperazine (DKP) scaffolds are a large class of specialized metabolites with structural diversity and notable bioactivities (Borthwick, 2012). The DKP rings confer structural stability and rigidity against proteolysis, making them attractive in pharmaceutical development (Borthwick, 2012). From the biosynthetic point of view, the DKP ring is assembled via a traditional non-ribosomal peptide synthetase (NRPS) or a recently characterized cyclodipeptide synthase (CDPS) machinery (Belin et al., 2012). Notably, diverse tailoring enzymes are often found to be clustered with CDPSs to perform further modification reactions on the DKP rings such as oxidation (Cryle et al., 2010; Meng et al., 2018; Patteson et al., 2018), methylation (Giessen et al., 2013a, 2013b; Li et al., 2019; Liu et al., 2019; Shi et al., 2019), prenylation (Yao et al., 2018), as well as cyclization (Yao et al., 2018), generating complex DKP-containing compounds. Although relatively few tailoring enzymes co-occurring with CDPS clusters have been experimentally characterized, most of them exhibit broad substrate scopes (Giessen et al., 2013a, 2013b; Li et al., 2019; Liu et al., 2019; Tian et al., 2018; Yao et al., 2018). The small sizes and substrate promiscuities of the CDPSs and their associated tailoring enzymes highlight the CDPS pathway enzymes as potential powerful tools for the generation of structurally unique DKP compounds by combinatorial biosynthetic approaches. The use of CDPSs as “biosynthetic hooks” is thus considered an effective strategy for identifying genes modifying 2,5-DKP rings to expand the chemical space of DKPs (Borgman et al., 2019; Canu et al., 2020).
Methylation of O-, C-, N-, and S-centered nucleophiles are ubiquitous tailoring reactions during the biosynthesis of small molecules, which increases the lipophilicity and membrane permeability of small-molecule scaffolds, enhancing their membrane transport, oral bioavailability, absorption, and excretion (Barreiro et al., 2011; Liscombe et al., 2012). N-methylation is envisioned as a promising way to rationally improve key pharmacokinetic characteristics of cyclic peptides (Chatterjee et al., 2008). Although bioinformatics analyses indicate the presence of a large number of methyltransferases (MTs) genetically associated with CDPSs (Skinnider et al., 2018), most of them remain unexplored. Up to now, only five MTs from CDPS-dependent pathways have been functionally characterized: O-methyltransferase Ndas_1149 from the nocazine pathway methylating phenolic hydroxyl groups of cyclo (L-Phe-L-Tyr) (cFY) (Δ3), cFY(Δ3, Δ6) and cyclo (L-Tyr-L-Tyr) (cYY) (Δ3, Δ6) (Giessen et al., 2013a), N-methyltransferase Amir_4628 catalyzing two successive N-methylations at the cWW ring to generate Me2-cWW (Giessen et al., 2013b), GutE/PcmE from guanitrypmycin pathway transferring a methyl group onto the guaninyl residue (Liu et al., 2019; Shi et al., 2019), and C-methyltransferase StspM1 mediating C3-methylation of indole ring and cyclization between the indole C2 of the Trp residue and the α-nitrogen (Li et al., 2019). These facts drive us to explore novel MTs from CDPS-dependent pathways for the generation of diverse DKP derivatives. We previously identified three homologous CDPS loci dmt1-3 encoding drimentines (DMTs) (Figure 1, Yao et al., 2018), which are a family of terpenylated diketopiperazine alkaloids with antibacterial, antifungal, and anthelmintic activities (Che et al., 2012; Lacey et al., 1998). The CDPS DmtBs synthesize cyclo (L-Trp-L-Xaa) (cWX) (X = Val, Pro, Leu, Ile or Ala), followed by prenylation and cyclization, which are, respectively, accomplished by the phytoene-synthase-like (PSL) prenyltransferase DmtCs and the membrane terpene cyclase DmtAs to afford DMTs (Yao et al., 2018). Noticeably, three putative MT genes are located adjacent to the dmt2 locus (from Streptomyces sp. NRRL F-5123) and the dmt3 locus (from S. aidingensis CGMCC 4.5739), suggesting that these two clusters might encode more complicated methylated DMTs. In contrast, no putative MT gene is found around the dmt1 locus from S. youssoufiensis OUC6819, although this strain is able to produce drimentine F (DMT F), which harbors N15-methyl group (Che et al., 2012). Herein, on one hand, we characterized the function of the dmt2- and dmt3-associated MT genes; on the other hand, we deciphered the N15-methylation step during the biosynthesis of DMT F; thereby, two S-adenosylmethionine (SAM)-dependent N-MTs (DmtMT2-1 and DmtMT1) with different regioselectivities were obtained. Their substrate spectra were probed, revealing that both of them exhibited considerable substrate promiscuities. Mixing and matching dmtMT2-1 and dmtMT1 with other DMT biosynthetic genes afforded unnatural methylated DMTs, providing novel effective CDPS-associated MTs for compound structural diversification.
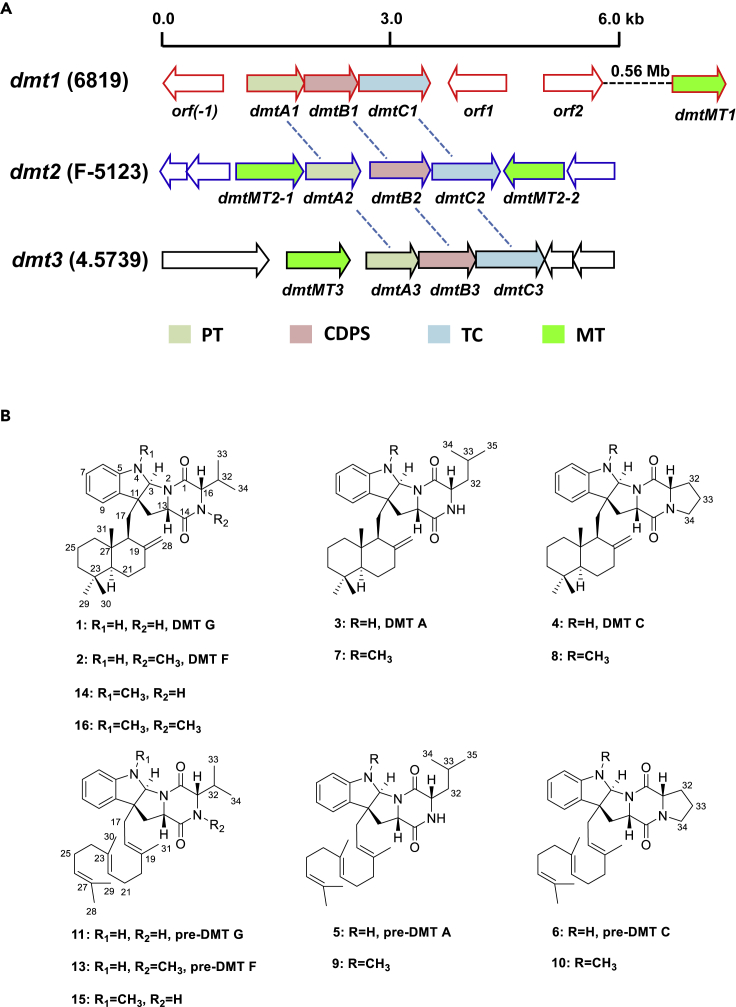
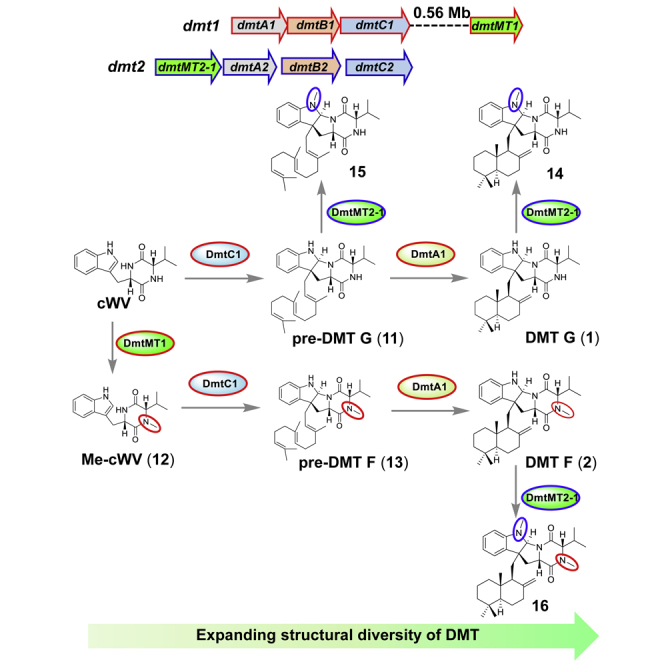
Figure 1.
Genetic Organization of the dmt1-3loci and Structures of Their Encoding Compounds
(A) dmt1-3loci from S. youssoufiensis OUC6819, Streptomyces sp. NRRL F-5123, and S. aidingensis CGMCC 4.5739. PT, prenyltransferase; CDPS, cyclodipeptide synthase; TC, terpene cyclase; MT, methyltransferase.
(B) Chemical structures of drimentines, pre-drimentines, and their methylated derivatives. Compounds 7–10 and 13–16 are generated in this study.
Results
Function of the MT Genes Adjacent to the dmtLoci
As indicated in Figure 1 and Table S3, DmtMT2-1 shows 36.7% identity/49.8% similarity to MitM (AAD28459.1), which functions as an aziridine N-methyltransferase during the biosynthesis of mitomycin (Varoglu et al., 2001); DmtMT2-2 shows 16.4% identity/25.3% similarity to UbiE (YP_026269.1), which catalyzes the carbon methylation reaction in the biosynthesis of ubiquinone and menaquinone (Lee et al., 1997); DmtMT3 shows 24.2% identity/34.6% similarity to PrmC (AY600244.1) of Chlamydia trachomatis involving in methylation of the class 1 peptide chain release factors (Pannekoek et al., 2005).
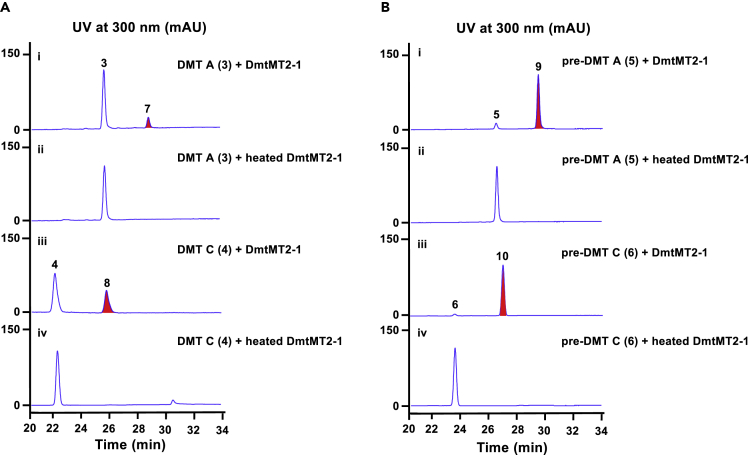
To investigate the function of these MT genes during the biosynthesis of DMTs, we solubly expressed them in E. coli (Figure S1) and tested their enzymatic activities in vitro. Given methylation may occur at different phases during DMTs biosynthesis, different substrates were subjected to assays based on the encoding products of dmt2/3 (Yao et al., 2018). HPLC analysis of the reactions showed: (1) DmtMT2-1 was able to recognize both DMT A (3)/C (4) (Figure 2Ai and iii) and pre-DMT A (5)/C (6) (Figure 2Bi and iii), generating compounds 7–10, but could not recognize cWL or cWP (Figure S2A), which indicated that a pyrroloindoline ring is necessary for the activity of DmtMT2-1; (2) neither DmtMT2-2 (Figures S2B–S2D) nor DmtMT3 (Figures S2E and S2F) recognized the tested substrates.
Figure 2.
In Vitro Methyltransferase Activity of DmtMT2-1
In the presence of SAM, DmtMT2-1 converted DMT A (3)/C (4) (A) and pre-DMT A (5)/C (6) (B) to N4-methyl-DMT A (7)/C (8) and N4-methyl-pre-DMTA (9)/C (10), respectively. The red-tinted peaks represent the methylated products.
Subsequently, large volume (30 mL) of reactions followed by chemical isolation was performed, leading to identification of compounds 7–10. High-resolution electrospray ionization mass spectrometry (HR-ESI-MS) analysis indicated the molecular formula of 7 as C33H47N3O2 (m/z [M + H]+ 518.3778, calcd 518.3747, Data S1), 8 as C32H43N3O2 (m/z [M + H]+ 502.3434, calcd 502.3434, Data S1), 9 as C33H47N3O2 (m/z [M + H]+ 518.3776, calcd 518.3747, Data S1), and 10 as C32H43N3O2 (m/z [M + H]+ 502.3476, calcd 502.3434, Data S1), which are 14 mass units bigger than that of DMT A (3), DMT C (4), pre-DMT A (5), and pre-DMT C (6), respectively (Yao et al., 2018). Comparison of the 1H and 13C NMR data between 7 and 3 (Yao et al., 2018) revealed that they share similar structure except the presence of an additional methyl group (δH 2.87, δC 33.2) in 7 (Data S1). Based on HMBC correlation observed from the methyl proton H-36 (δH 2.87) to C3 (δC 83.6) and C5 (δC 150.6), we determined the methyl group is attached to the nitrogen of the dihydroindole system instead of the DKP ring (Data S1). Thus, compound 7 was determined as N4-methyl-DMT A. Similarly, compounds 8–10 are similar to DMT C (4), pre-DMT A (5), and pre-DMT C (6), respectively, but carry an additional methyl group (δH 2.88, δC 33.5 for 8, δH 2.91, δC 33.2 for 9, and δH 2.92, δC 33.5 for 10) attached to the nitrogen of dihydroindole (Data S1). Thereby, 8–10 were characterized as N4-methyl derivatives of compounds 4–6. The above results supported that DmtMT2-1 is responsible for the N4-methylation during DMTs biosynthesis, which may happen before or after cyclization. It was worth to mention that methylation occurs only at N15 in all the reported methylated DMTs (DMT F, H, and I). Thus, the N4-methyltransferase DmtMT2-1 would expand the diversity of DMTs compounds.
Probing the MT Gene Involved in N15-Methylation of DMT F
Although no MT gene is found adjacent to dmt1, S. youssoufiensis OUC6819 is able to produce DMT F, which harbors a methyl group at N15 position. This fact indicates that the responding MT gene is located in another place of the genome. With comparative liquid chromatography-mass spectrometry (LC-MS) analysis of the fermentation products from the OUC6819 strains, a compound peak with molecular weight of 14 Daltons bigger than that of cWV (m/z [M + H]+ calcd 286.1556; Figure S3B) was observed in ΔdmtC1 (the prenyltransferase gene in charge of the assembly of farnesyl group onto cWV) (Figure S3Aii) and the dmtB1 overexpression strain (Figure S3Aiii) but not in the wild-type strain (Figure S3Ai). This phenotype led us to hypothesize that methylation may happen before the assembly of the terpene moiety. Therefore, Amir_4628, which conducts two successive N-methylations at the DKP ring (Giessen et al., 2013b), was subjected to BLAST against the OUC6819 genome, revealing a homologous gene 6880MT (renamed as dmtMT1; 33.5% identity/45.0% similarity to Amir_4628) at 0.56 Mb upstream of the dmt1 locus (Figure 1, Table S3).
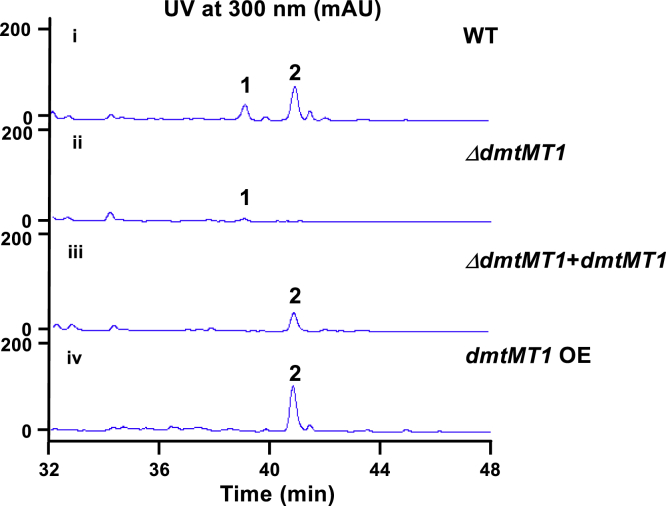
To detect if dmtMT1 is involved in the biosynthesis of DMT F, in-frame deletion was performed, generating ΔdmtMT1 (Figure S4). HPLC analysis revealed that the production of DMT F was completely abolished; conversely, DMT G was still produced in ΔdmtMT1 albeit in small amount (Figure 3ii). Moreover, complementation of ΔdmtMT1 restored the production of DMT F (Figure 3iii); overexpression of dmtMT1 resulted in increased production of DMT F by about 2-fold (Figure 3iv). These results ambiguously demonstrated dmtMT1 to be responsible for the N15-methylation of DMT F.
Figure 3.
HPLC Analysis of the Fermentation Products from OUC6819 Strains
(i) WT, wild-type strain; (ii) ΔdmtMT1; (iii) ΔdmtMT1 + dmtMT1, genetic complementation strain of ΔdmtMT1; (iv) dmtMT1 OE, overexpression strain of dmtMT1.
Timing of the N15-Methylation during DMT F Biosynthesis
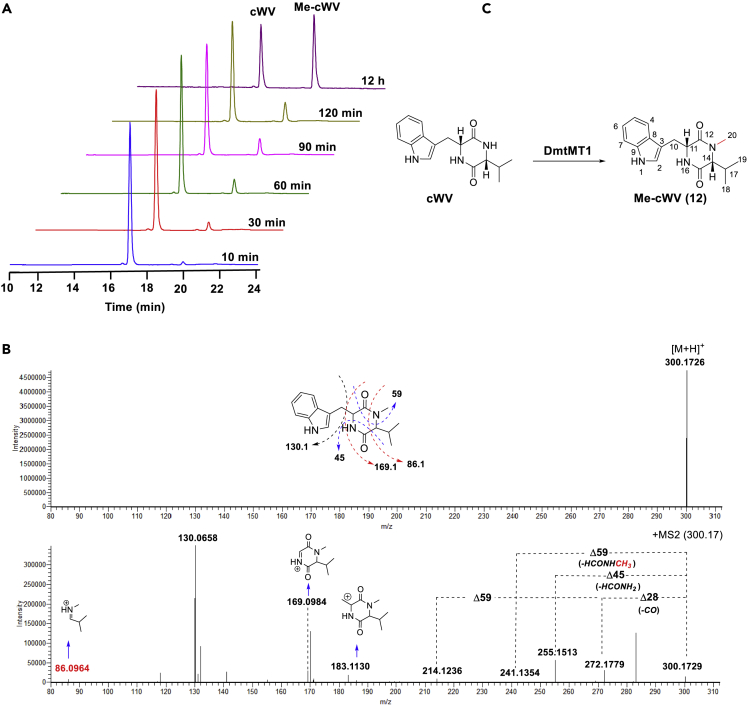
To identify the exact timing of the methylation step, in vitro biochemical reactions were carried out using cWV, pre-DMT G (11), and DMT G (1) as substrates. As indicated in Figure 4, DmtMT1 was capable of recognizing cWV to give a new peak (Figure 4A) but could not accept pre-DMT G or DMT G (Figure S5), supporting the methyl group is assembled right after the formation of cWV. Time course analysis showed that only a single methylation takes place, as indicated by the presence of the sole product with a molecular ion peak [M + H]+ at m/z 300.1726, which was 14 units bigger than that of cWV (m/z [M + H]+ calcd 286.1556) (Figures 4A and 4B).
Figure 4.
In Vitro Methyltransferase Activity of DmtMT1
(A) Time course experiment of the DmtMT1-catalyzed reaction using cWV as substrate. Chromatograms show the absorbance at 280 nm.
(B) HR-ESI-MS2 fragmentation spectrum of Me-cWV (m/z 300.1726).
(C) Schematic representation of DmtMT1-catalyzed reaction illustrated by using cWV as substrate.
See also Figure S5.
To clarify the position of the methylation, HR-ESI-MS2 analysis was conducted. As shown in Figure 4B, in addition to the characteristic fragmentation pattern of 2,5-DKP with neutral losses of 28 Da (-CO) and 45 Da (-HCONH2) (Guo et al., 2009), successive neutral loss of 59 Da corresponding to a HCONHCH3 fragment could also be detected, suggesting that the methylation takes place at the DKP ring; the protonated substituent ion at m/z 130 indicated the presence of tryptophan (Guo et al., 2009); the ions at m/z 169.0984, 183.1130 resulting from elimination of 3-methyl-1-H-indole and indole, respectively, combined with m/z 86.0964 from sequential losses of CO and Trp residue proved that the methyl group is attached to the α-nitrogen of Val in cWV, which was further confirmed by the HMBC correlation of Me-cWV (12) from the methyl proton H-20 (δH 2.82) to C12 (δC 166.0) and C14 (δC 66.6) (Figure 4C and Data S1).
This fact means the PSL-family prenyltransferase DmtC1, which transfers farnesyl group onto cWV to generate pre-DMT G (Yao et al., 2018), should be able to recognize Me-cWV as well. Thus, we incubated DmtC1 with Me-cWV (12) in the presence of farnesyl diphosphate (FPP), and a new compound (13) appeared as expected (Figure S6), with a molecular ion peak [M + H]+ at m/z 504.3579, which was 14 units bigger than that of pre-DMT G (Data S1). The 1H and 13C NMR spectra of 13 provided a dataset similar to that of pre-DMT G, with the chemical shift difference observed for a methyl group (δH 2.81, δC 32.7) (Data S1). The absence of one-proton singlet at δH 7.92 in pre-DMT G indicated that the methyl group was attached to N15 as confirmed by the strong HMBC correlation from the methyl proton H-35 (δH 2.81) to C14 (δC 165.1) and C16 (δC 66.3) (Data S1). Considering the biosynthetic assembly line of DMT G, we proposed that compound 13 is the biosynthetic precursor of DMT F, and thus it was named as pre-DMT F, which would be further cyclized by the terpene cyclase DmtA1 to afford DMT F.
Probing Substrate Promiscuity of DmtMT2-1 and DmtMT1
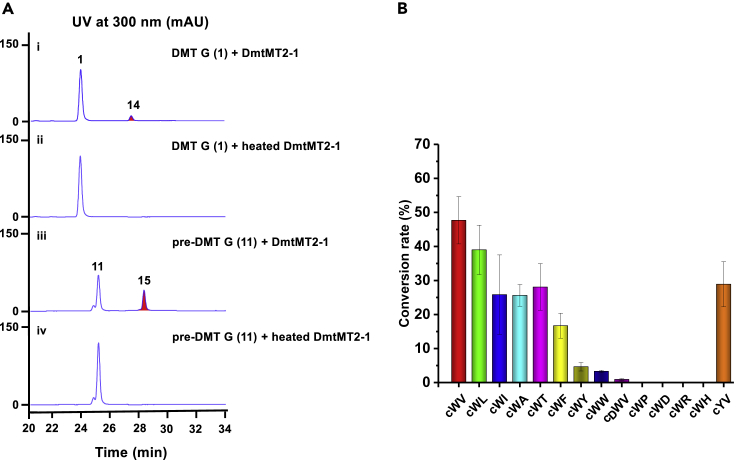
With the two methyltransferases (DmtMT2-1 and DmtMT1) in hand, we evaluated their potentials as tool enzymes to diversify structures of DMT compounds. For DmtMT2-1, another two substrates, DMT G (1) and pre-DMT G (11), were tested. As shown in Figure 5A, both of them were recognized by DmtMT2-1, transforming 1 into 14 and 11 into 15 as expected. All the products were isolated and their structures elucidated by HR-ESI-MS and NMR analyses (Data S1). Finally, 14 (m/z [M + H]+ 504.3630, calcd 504.3590) and 15 (m/z [M + H]+ 504.3647, calcd 504.3590) were determined to be N4-methyl-DMT G and N4-methyl-pre-DMT G as indicated by the HMBC correlations from the methyl proton H-35 (δH 2.86 for 14 and δH 2.91 for 15) to C3 (δC 83.6 for 14 and δC 84.2 for 15) and C5 (δC 150.8 for 14 and δC 150.9 for 15) (Data S1).
Figure 5.
Substrate Promiscuities of DmtMT2-1 and DmtMT1
(A) HPLC traces of DmtMT2-1-catalyzed reactions using DMT G (1) and pre-DMT G (11) as substrates.
(B) Histogram of the conversion rates of DmtMT1 regarding different cyclodipeptides. The relative production amounts are obtained by comparison with the respective substrate calculated using UV trace integrals. Error bars represent ±SD of three independent experiments. HPLC traces and HR-ESI-MS2 spectra of each reaction are shown in Data S2.
To probe the substrate promiscuity of DmtMT1, a series of DKPs were tested. Delightedly, DmtMT1 exhibited broad substrate flexibility and was able to recognize a bunch of DKPs, including cWL, cWI, cWA, cWT, cWF, cWY, cWW, and noticeably cyclo (D-Trp-L-Val) (cDWV) and cYV as well (the nomenclature of a cyclodipeptide is indicated here by the one-letter code for the two L-configured amino acids) (Figure 5B and Data S2). The responding methylated DKPs were identified by HR-ESI-MS2 analysis (Data S2). Their fragmentation patterns were in agreement with that of Me-cWV (Figure 4B), supporting that each N-methylation regio-specifically occurs at the position derived from the second amino acid (from the biosynthetic point of view). Based on the relative catalytic efficiencies, we can clearly see DmtMT1 prefers cWXs with X being aliphatic over aromatic amino acids (Figure 5B). Delightedly, DmtMT1 was capable of transferring the methyl group onto cDWV and cYV, albeit at much lower efficiencies, implying it has certain flexibility toward the first amino acid as well.
The above results indicated that both DmtMT2-1 and DmtMT1 are N-methyltransferases with broad substrate promiscuity and stringent regiospecificity, which would potentially serve as efficient catalysts for structural diversification in drug development.
Generation of Unnatural DMTs by Using the Logic of Synthetic Biology
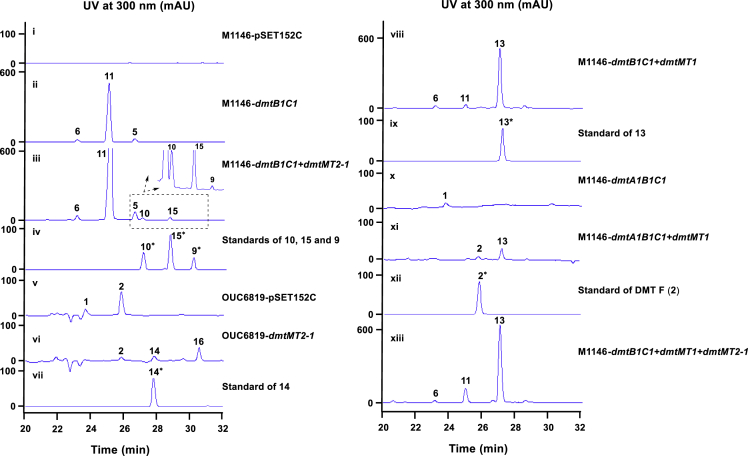
The availability of these two MTs drove us to further generate unnatural DMT analogs by using the logic of synthetic biology. Based on the biosynthetic machinery of DMTs, we introduced dmtMT2-1 or/and dmtMT1 into different cells, including DMTs producer S. youssoufiensis OUC6819, S. coelicolor M1146/dmtB1C1 (accumulating pre-DMTs), and S. coelicolor M1146/dmtA1B1C1 (producing DMTs) (Yao et al., 2018).
As shown in Figure 6, expression of dmtMT2-1 in S. coelicolor M1146/dmtB1C1 led to the appearance of three small peaks, which were identified to be methylated pre-DMT compounds 10, 15, and 9, respectively (Figure 6iii and iv). When dmtMT2-1 was introduced into S. youssoufiensis OUC6819, in addition to 14, another compound peak 16 showed up (Figure 6vi and vii). Considering the regioselectivity of dmtMT2-1, we speculated 16 might be N4-methyl-DMT F, which was confirmed by HR-ESI-MS (Data S1) and NMR data with the presence of the methyl group (δH 2.85, δC 31.4), along with its strong HMBC correlations to C3 (δC 82.8) and C5 (δC 151.5) (Data S1).
Figure 6.
Combinatorial Expression of dmtMT1 and/or dmtMT2-1 with dmt1 Genes
Expression of dmtMT1 in S. coelicolor M1146/dmtB1C1 led to decreased amount of 11 and simultaneous accumulation of pre-DMT F (13) (Figure 6viii and ix). When introducing dmtMT1 into S. coelicolor M1146/dmtA1B1C1, DMT F (2) was accumulated as expected (Figure 6xi and xii). Unfortunately, no new product was observed after further introduction of dmtMT2-1 into S. coelicolor M1146/dmtB1C1 + dmtMT1 (Figure 6xiii).
Discussion
CDPSs are small enzymes that utilize two aminoacyl-tRNAs to catalyze the formation of a 2,5-DKP ring system (Gondry et al., 2009). To date, over 110 CDPSs have been functionally characterized and usually exhibit a certain degree of substrate promiscuity, making CDPSs intriguing members of Nature's biosynthetic repertoire (Canu et al., 2020). The resulting cyclodipeptides are generally further modified by at least one tailoring enzyme adjacent to CDPS, including formation of C-C bond and C-O bond, and regioselective methylation and prenylation (Borgman et al., 2019). In this study, we identified and characterized two promiscuous N-methyltransferases and elucidated the methylation machinery during DMTs biosynthesis through in vivo and in vitro experiments, providing significant insights into generation of DKP derivatives by using synthetic biology approaches.
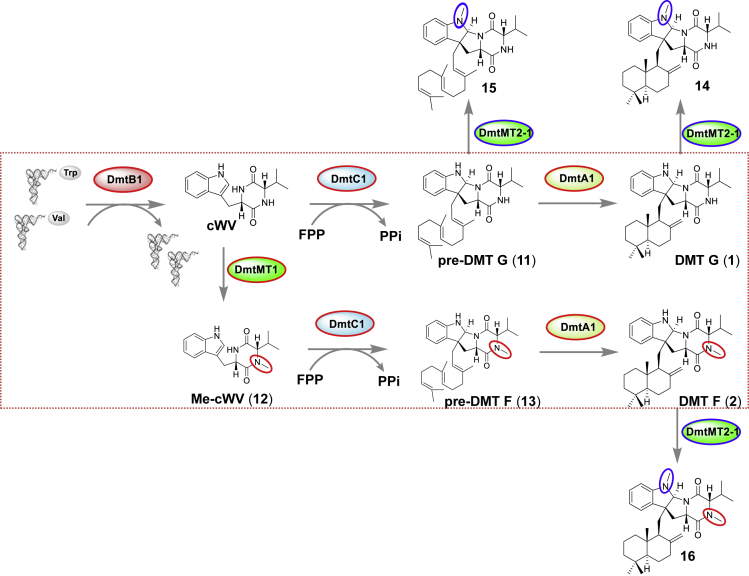
Although both DmtMT2-1 and DmtMT1 are involved in DMT biosynthesis, they act at different timings with different manners of function. As indicated in Figure 7, in OUC6819, the methyl group is assembled right after the formation of cWV instead of happening as the last step, and the following prenylation and cyclization steps occur in parallel, indicating both DmtC1 and DmtA1 display flexible substrate promiscuity as well, which should be exploited further. Conversely, as a novel dihydroindole N-methyltransferase, DmtMT2-1 from Streptomyces sp. NRRL F-5123 functions after indole C3-normal prenylation, recognizing pre-DMTs, DMTs, and N15-methyl-DMTs. Such big structural differences between their substrates are in accordance with the low similarity/identity (29.5%/17.4%) between DmtMT2-1 and DmtMT1. Notably, mixing and matching of dmtMT2-1 and dmtMT1 with other DMT biosynthetic genes leads to generation of “unnatural” natural products N4-methyl-DMT G (14), N4-methyl-pre-DMT G (15), and N4-methyl-DMT F (16).
Figure 7.
Assembly Lines of Drimentines Occurring in S. youssoufiensis OUC6819 (highlighted in red rectangle box) and S. coelicolor M1146/dmtB1C1+dmtMT2-1
In microbes, the genes responsible for production of a specialized metabolite are mostly found in close proximity to another in dedicated biosynthetic gene clusters. However, dmtMT1 is located at 0.56 Mb downstream of the dmt1 locus. In comparison with its homolog Amir_4628, which doubly methylates DKPs constituting two identical aromatic amino acids (Giessen et al., 2013b), DmtMT1 prefers cWX with X being aliphatic amino acids and strictly methylates nitrogen originating from the X residue. Noticeably, DmtMT1 displays remarkable substrate promiscuity. It allows the second amino acid to be Phe and Tyr and even admits the first amino acid to be D-configured Trp and Tyr, making DmtMT1 a very promising tool enzyme for compound diversification.
With the advent of next-generation sequencing, massive microbial genome sequence data have been uploaded to the public domain. Simple BLAST-based searches reveal large numbers of MTs associated with CDPSs in prokaryotic genomes. Hence, we constructed a sequence similarity network from selected MT sequences and assigned them to known MTs. As indicated in Figure S7, three MT homologs (WP_055513754.1, WP_029387245.1, and WP_078513178.1) clustered with DmtMT2-1 are associated with a CDPS and a PSL-prenyltransferase, indicating that they might be involved in the biosynthesis of novel DMT-like compounds; noticeably, another four DmtMT2-1 homologs (StspM2/StflM2/5971M2/StalM2) are proposed to be involved in the N-methylation step during nocardioazine biosynthesis, but no in vivo nor in vitro results are provided (Li et al., 2019). Our results would enlighten deciphering of the methylation steps of nocardioazine-like natural products. Moreover, there are still several MTs that are not clustered with any known MTs, suggesting the untapped potentials of MTs as sources of new catalysts.
Apart from methyltransferases, we note that several other ORFs are in close proximity to CDPSs in cluster dmt2/3, such as the cyclodipeptide oxidases (CDOs), which have been reported to catalyze Cα-Cβ dehydrogenations on diverse cyclodipeptides (Giessen et al., 2013a; Lautru et al., 2002). Thus, further experiments are required to completely characterize the functions of these proteins and gain a broader understanding of DMTs biosynthesis.
Limitations of the Study
The molecular mechanism underlying the promiscuity of DmtMT1 and DmtMT2-1 has not been elucidated in the present study. Future crystallographic studies and systematic structure-guided mutagenesis would shed light on these issues.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Prof. Dr. Wenli Li (email: liwenli@ouc.edu.cn).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact without restriction.
Data and Code Availability
The accession number for the dmtMT1 reported in this paper is GenBank: MK894429 (https://www.ncbi.nlm.nih.gov/nuccore/MK894429.1). All relevant data supporting the findings of this study are available within the paper and its Supplemental Information files. Additional data are provided upon reasonable request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by grants from the National Key R&D Program of China (2019YFC0312501), the National Natural Science Foundation of China (U1706206, 81991525, 31900049, 31570032, 31711530219 & 31171201), and the China Postdoctoral Science Foundation (2019M652483).
Author Contributions
Conceptualization, W.L.; Methodology, J.L. and Z.L.; Investigation, T.Y. and E.J.; Formal Analysis, H.L.; Writing – Original Draft, W.L. and T.Y.; Writing – Review & Editing, W.L. and T.Y.; Funding Acquisition, W.L. and T.Y.; Resources, Q.C., T.Z., and D.L.; Supervision, W.L.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101323.
Supplemental Information
Document S1. Transparent Methods, Figures S1–S7, and Tables S1 –S3
Data S2. HPLC and HR-ESI-MS2 Data of DmtMT1-Cataylzed Reactions, Related to Figure 5
Data S3. Methyltransferases Used for Sequence Similarity Network Construction, Related to Figures 1 and S7.
References
- Barreiro E.J., Kümmerle A.E., Fraga C.A. The methylation effect in medicinal chemistry. Chem. Rev. 2011;111:5215–5246. doi: 10.1021/cr200060g. [DOI] [PubMed] [Google Scholar]
- Belin P., Moutiez M., Lautru S., Seguin J., Pernodet J.L., Gondry M. The nonribosomal synthesis of diketopiperazines in tRNA-dependent cyclodipeptide synthase pathways. Nat. Prod. Rep. 2012;29:961–979. doi: 10.1039/c2np20010d. [DOI] [PubMed] [Google Scholar]
- Borgman P., Lopez R.D., Lane A.L. The expanding spectrum of diketopiperazine natural product biosynthetic pathways containing cyclodipeptide synthases. Org. Biomol. Chem. 2019;17:2305–2314. doi: 10.1039/c8ob03063d. [DOI] [PubMed] [Google Scholar]
- Borthwick A.D. 2,5-Diketopiperazines: synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012;112:3641–3716. doi: 10.1021/cr200398y. [DOI] [PubMed] [Google Scholar]
- Canu N., Moutiez M., Belin P., Gondry M. Cyclodipeptide synthases: a promising biotechnological tool for the synthesis of diverse 2,5-diketopiperazines. Nat. Prod. Rep. 2020;37:312–321. doi: 10.1039/c9np00036d. [DOI] [PubMed] [Google Scholar]
- Chatterjee J., Gilon C., Hoffman A., Kessler H. N-methylation of peptides: a new perspective in medicinal chemistry. Acc. Chem. Res. 2008;41:1331–1342. doi: 10.1021/ar8000603. [DOI] [PubMed] [Google Scholar]
- Che Q., Zhu T., Qi X., Mandi A., Kurtan T., Mo X., Li J., Gu Q., Li D. Hybrid isoprenoids from a reeds rhizosphere soil derived actinomycete Streptomyces sp. CHQ-64. Org. Lett. 2012;14:3438–3441. doi: 10.1021/ol301396h. [DOI] [PubMed] [Google Scholar]
- Cryle M.J., Bell S.G., Schlichting I. Structural and biochemical characterization of the cytochrome P450 CypX (CYP134A1) from Bacillus subtilis: a cyclo-L-leucyl-L-leucyl dipeptide oxidase. Biochemistry. 2010;49:7282–7296. doi: 10.1021/bi100910y. [DOI] [PubMed] [Google Scholar]
- Giessen T.W., von Tesmar A.M., Marahiel M.A. Insights into the generation of structural diversity in a tRNA-dependent pathway for highly modified bioactive cyclic dipeptides. Chem. Biol. 2013;20:828–838. doi: 10.1016/j.chembiol.2013.04.017. [DOI] [PubMed] [Google Scholar]
- Giessen T.W., von Tesmar A.M., Marahiel M.A. A tRNA-dependent two-enzyme pathway for the generation of singly and doubly methylated ditryptophan 2,5-diketopiperazines. Biochemistry. 2013;52:4274–4283. doi: 10.1021/bi4004827. [DOI] [PubMed] [Google Scholar]
- Gondry M., Sauguet L., Belin P., Thai R., Amouroux R., Tellier C., Tuphile K., Jacquet M., Braud S., Courcon M. Cyclodipeptide synthases are a family of tRNA-dependent peptide bond-forming enzymes. Nat. Chem. Biol. 2009;5:414–420. doi: 10.1038/nchembio.175. [DOI] [PubMed] [Google Scholar]
- Guo Y., Cao S., Zong X., Liao X., Zhao Y. ESI-MSn study on the fragmentation of protonated cyclic-dipeptides. Spectroscopy. 2009;23:131–139. [Google Scholar]
- Lacey E., Power M., Wu Z., Rickards R. Patent Int. Appl.; 1998. Terpenylated Diketopiperazines,(drimentines) WO9809968 A1. [Google Scholar]
- Lautru S., Gondry M., Genet R., Pernodet J. The albonoursin gene cluster of S. noursei: biosynthesis of diketopiperazine metabolites independent of nonribosomal peptide synthetases. Chem. Biol. 2002;9:1355–1364. doi: 10.1016/s1074-5521(02)00285-5. [DOI] [PubMed] [Google Scholar]
- Lee P.T., Hsu A.Y., Ha H.T., Clarke C.F. A C-methyltransferase involved in both ubiquinone and menaquinone biosynthesis: isolation and identification of the Escherichia coli ubiE gene. J. Bacteriol. 1997;179:1748–1754. doi: 10.1128/jb.179.5.1748-1754.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Qiu Y., Guo C., Han M., Zhou Y., Feng Y., Luo S., Tong Y., Zheng G., Zhu S. Pyrroloindoline cyclization in tryptophan-containing cyclodipeptides mediated by an unprecedented indole C3 methyltransferase from Streptomyces sp. Hph0547. Chem. Commun. 2019;55:8390–8393. doi: 10.1039/c9cc03745d. [DOI] [PubMed] [Google Scholar]
- Liscombe D.K., Louie G.V., Noel J.P. Architectures, mechanisms and molecular evolution of natural product methyltransferases. Nat. Prod. Rep. 2012;29:1238–1250. doi: 10.1039/c2np20029e. [DOI] [PubMed] [Google Scholar]
- Liu J., Xie x., Li S.-M. Guanitrypmycin biosynthetic pathways imply Cytochrome P450-mediated regio-and stereospecific guaninyl transfer reactions. Angew. Chem. 2019;58:11534–11540. doi: 10.1002/anie.201906891. [DOI] [PubMed] [Google Scholar]
- Meng S., Han W., Zhao J., Jian X., Pan H., Tang G. A six-oxidase cascade for tandem C−H bond activation revealed by reconstitution of bicyclomycin biosynthesis. Angew. Chem. 2018;57:719–723. doi: 10.1002/anie.201710529. [DOI] [PubMed] [Google Scholar]
- Pannekoek Y., Heurgue-Hamard V., Langerak A.A., Speijer D., Buckingham R.H., van der Ende A. The N5-glutamine S-adenosyl-L-methionine-dependent methyltransferase PrmC/HemK in Chlamydia trachomatis methylates class 1 release factors. J. Bacteriol. 2005;187:507–511. doi: 10.1128/JB.187.2.507-511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patteson J.B., Cai W., Johnson R.A., Santa Maria K.C., Li B. Identification of the biosynthetic pathway for the antibiotic bicyclomycin. Biochemistry. 2018;57:61–65. doi: 10.1021/acs.biochem.7b00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Xu X., Zhao E.J., Zhang B., Li W., Zhao Y., Jiao R.H., Tan R.X., Ge H.M. Genome mining and enzymatic total biosynthesis of purincyclamide. Org. Lett. 2019;21:6825–6829. doi: 10.1021/acs.orglett.9b02461. [DOI] [PubMed] [Google Scholar]
- Skinnider M.A., Johnston C.W., Merwin N.J., Dejong C.A., Magarvey N.A. Global analysis of prokaryotic tRNA-derived cyclodipeptide biosynthesis. BMC Genom. 2018;19:45. doi: 10.1186/s12864-018-4435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W., Sun C., Zheng M., Harmer J.R., Yu M., Zhang Y., Peng H., Zhu D., Deng Z., Chen S.-L. Efficient biosynthesis of heterodimeric C3-aryl pyrroloindoline alkaloids. Nat. Commun. 2018;9:1–9. doi: 10.1038/s41467-018-06528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoglu M., Mao Y., Sherman D.H. Mapping the mitomycin biosynthetic pathway by functional analysis of the MitM aziridine N-methyltransferase. J. Am. Chem. Soc. 2001;123:6712–6713. doi: 10.1021/ja015646l. [DOI] [PubMed] [Google Scholar]
- Yao T., Liu J., Liu Z., Li T., Li H., Che Q., Zhu T., Li D., Gu Q., Li W. Genome mining of cyclodipeptide synthases unravels unusual tRNA-dependent diketopiperazine-terpene biosynthetic machinery. Nat. Commun. 2018;9:4091. doi: 10.1038/s41467-018-06411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Document S1. Transparent Methods, Figures S1–S7, and Tables S1 –S3
Data S2. HPLC and HR-ESI-MS2 Data of DmtMT1-Cataylzed Reactions, Related to Figure 5
Data S3. Methyltransferases Used for Sequence Similarity Network Construction, Related to Figures 1 and S7.
Data Availability Statement
The accession number for the dmtMT1 reported in this paper is GenBank: MK894429 (https://www.ncbi.nlm.nih.gov/nuccore/MK894429.1). All relevant data supporting the findings of this study are available within the paper and its Supplemental Information files. Additional data are provided upon reasonable request.