Summary
Skeletal muscle has the remarkable ability to modulate its mass in response to changes in nutritional input, functional utilization, systemic disease, and age. This is achieved by the coordination of transcriptional and post-transcriptional networks and the signaling cascades balancing anabolic and catabolic processes with energy and nutrient availability. The extent to which alternative splicing regulates these signaling networks is uncertain. Here we investigate the role of the RNA-binding protein hnRNP-U on the expression and splicing of genes and the signaling processes regulating skeletal muscle hypertrophic growth. Muscle-specific Hnrnpu knockout (mKO) mice develop an adult-onset myopathy characterized by the selective atrophy of glycolytic muscle, the constitutive activation of Akt, increases in cellular and metabolic stress gene expression, and changes in the expression and splicing of metabolic and signal transduction genes. These findings link Hnrnpu with the balance between anabolic signaling, cellular and metabolic stress, and physiological growth.
Subject Areas: Cellular Physiology, Molecular Genetics, Molecular Physiology
Graphical Abstract

Highlights
-
•
Hnrnpu mKO mice develop adult-onset myopathy with selective glycolytic muscle atrophy
-
•
Akt is constitutively active in the atrophied muscles of Hnrnpu mKO mice
-
•
Hnrnpu mutants show altered gene expression and alternative splicing patterns
-
•
Induction of genes associated with cellular and metabolic stress
Cellular Physiology; Molecular Genetics; Molecular Physiology
Introduction
Skeletal muscle has the remarkable ability to modulate its mass in response to the physiological changes associated with functional use, nutrient availability, systemic disease, and age. Under normal physiological conditions, skeletal muscle maintains its mass through a complex balance of anabolic and catabolic signaling (Glass, 2005). However, cachexia, metabolic dysfunction, muscular dystrophy, and advanced age lead to a disequilibrium between anabolic and catabolic signaling resulting in muscle dysfunction and atrophy (Cohen et al., 2015; Emery, 2002; Ernste and Reed, 2013; Marcell, 2003; Petruzzelli and Wagner, 2016). IGF-1/PI3K/Akt signaling has emerged as a key regulator of skeletal muscle hypertrophic growth and metabolism through its activation of mTOR-dependent anabolic processes while simultaneously inhibiting Foxo1/3-dependent catabolic processes (Manning and Toker, 2017). Transgenic overexpression of Akt1 in skeletal muscle promotes muscle hypertrophy (Lai et al., 2004), and its selective expression in glycolytic muscles promotes insulin sensitivity in aged mice that display impaired Akt activation (Akasaki et al., 2014). mTOR signals through two complexes, the mTOR containing complex 1 (mTORC1), which both activates anabolic processes while inhibiting autophagy, and the mTOR containing complex 2 (mTORC2), which is the primary Akt1 Ser473 kinase (Manning and Toker, 2017). Although the molecular regulation is complex, changes in Akt and mTOR signaling contribute to metabolic derangements and muscle wasting (Castets et al., 2013; Risson et al., 2009; Saxton and Sabatini, 2017).
Alternative splicing is a key gene regulatory mechanism whereby the differential inclusion of exons results in multiple mRNA isoforms from a single genomic locus. This process plays a crucial role in the post-transcriptional regulation of gene and protein isoform expression (Lee and Rio, 2015) and is differentially regulated in response to cellular stress (Biamonti and Caceres, 2009; Dutertre et al., 2011). Current analyses indicate that >95% of human multi-exon genes are subject to alternative splicing (Pan et al., 2008). Brain, heart, and skeletal muscle have the highest levels of evolutionary conserved alternatively spliced transcripts (Merkin et al., 2012) and are subject to multiple disorders due to mis-splicing events (Faustino and Cooper, 2003; Scotti and Swanson, 2016). Alternative splicing in skeletal muscle plays a crucial role in muscle development, and genetic mutations in both splice sites and the RNA binding proteins that modulate splicing play causal roles in the pathogenesis of neuromuscular disorders (Ho et al., 2005; Lin et al., 2006; Philips et al., 1998; Runfola et al., 2015). In addition, dysregulated splicing is increasingly recognized as a causal factor promoting cellular dysfunction through changes in the binding properties, enzymatic activities, and intracellular localization of a large percentage of the proteome (Tress et al., 2007). However, our understanding of how alternative splicing modulates the activity of IGF-1/PI3K/Akt signaling in skeletal muscle physiology is limited.
The functionally diverse heteronuclear ribonucleoprotein (hnRNP) family of RNA-binding proteins play key roles in RNA metabolism including mRNA stabilization and the regulation of alternative splicing (Geuens et al., 2016; Martinez-Contreras et al., 2007). Genetic mutations and dysregulated expression of hnRNPs A1, E1/2 (PCBP1/2), I (PTBP1), P2 (FUS), and Q (SYNCRIP) are implicated in the molecular pathogenesis of neuromuscular disorders including myotonic and limb girdle muscular dystrophy, amyotrophic lateral sclerosis, and spinal muscle atrophy (Geuens et al., 2016; Li et al., 2020; Vance et al., 2009). HnRNP family member U (hnRNP-U) is the largest member of the hnRNP family, ubiquitously expressed, and known to modulate both mRNA stability and alternative splicing (Huelga et al., 2012; Xiao et al., 2012; Yugami et al., 2007). Clinically, heterozygous loss-of-function mutations in the Hnrnpu gene are associated with craniofacial anomalies, developmental delays, and seizures (Yates et al., 2017). In mice, genetic deletion of Hnrnpu specifically within cardiomyocytes leads to a dilated cardiomyopathy phenotype around postnatal day 14 (Ye et al., 2015). Here, we identify hnRNP-U as being essential for skeletal muscle hypertrophic growth, in part, through modulating the balance between Akt1-dependent anabolic signaling and the energy and nutrient availability to support hypertrophic growth. hnRNP-U expression is repressed with age and necessary for the proper expression and splicing of genes critical for metabolic and growth processes.
Results
The Development and Early Postnatal Growth of Skeletal Muscle Is Phenotypically Normal in the Absence of Hnrnpu
The hnRNP-U protein is expressed in nearly all tissues examined in the adult mouse (Figure 1A). However, the expression of hnRNP-U in skeletal muscle decreases abruptly in aged mice (Figure 1B). To study the role of hnRNP-U in skeletal muscle growth and function, we crossed Hnrnpufl/fl mice (Ye et al., 2015) with a mouse line expressing the Cre recombinase under the control of the human skeletal actin promoter (HSA-Cre). HSA-Cre expression starts at E8.5 and continues into adulthood (Miniou et al., 1999). Consistent with a prior report in which Hnrnpu was deleted in both cardiac and skeletal muscle during development (Ye et al., 2015), Hnrnpufl/fl;HSA-Cre (mKO) mice are born in normal Mendelian ratios with no observable abnormalities (Figure S1). However, in contrast with this prior report, Hnrnpu mKO mice continue to develop normally past postnatal day 14 (P14). Western blot analysis indicates ~50% reduction in hnRNP-U expression in Hnrnpu mutants at P21 (Figure 1C). Laminin stained sections of P21 Hnrnpu mutant gastrocnemius (GSN, Figure 1D) and soleus (Figure 1G) are morphologically indistinguishable from littermate controls. Although subtle differences are observed in the myofiber size distribution curves of the gastrocnemius (Figure 1E) and soleus (Figure 1H), there are no statistically significant differences in the average myofiber cross-sectional areas (Figures 1F and 1I). Furthermore, we observe no differences in the morphology, myofiber size distribution, or the average myofiber cross-sectional area of the tibialis anterior (TA) of Hnrnpu mKO mice at P21 (Figure S2). Collectively, these data indicate embryonic deletion of Hnrnpu does not measurably impact the development or post-natal growth of skeletal muscle up to ~P21.
Figure 1.
Hnrnpu mKO Mice Are Born Phenotypically Normal
(A) Representative western blots of relative hnRNP-U expression in adult mouse tissues. Data are from n = 2 biological replicates.
(B) Representative western blot and quantification of hnRNP-U expression in the gastrocnemius with advancing age. Data are from n = 3 WT male mice at 3 and 12 months of age, and n = 2 WT male mice at 24 months of age.
(C) Western blot of hnRNP-U expression in gastrocnemius muscle at P21.
(D–I) Data are from n = 3 control and n = 3 mKO male mice at P21. (D–F) Immunohistological analyses of gastrocnemius muscle. (G–I) Immunohistological analyses of soleus muscle. (D and G) Laminin staining of muscle transverse sections. The scale bar is 50 μm. (E and H) Myofiber size distribution curves. (F and I) Average myofiber cross-sectional areas. Data are mean ± standard deviation. Significance (∗) was set at p < 0.05. p Values were calculated using the Student's two-tailed t test assuming unequal variances.
Hnrnpu mKO Mice Develop a Severe Adult-Onset Myopathy
To explore the role of Hnrnpu on skeletal muscle and whole-body growth, we performed longitudinal body weight analyses starting at P21. Consistent with our analyses of mice at P21 (Figure 1), male and female mKO mice are indistinguishable by weight from littermate controls up to approximately 4 weeks of age (Figure 2A). Growth curve analyses indicate that Hnrnpu mKO mice reach a body weight plateau at approximately 11–13 weeks of age, with statistically significant reductions in the body weights of males and females by 6- and 12-weeks of age, respectively. Male and female Hnrnpu mutants become visually distinguishable from control littermates by 10–12 weeks and 16–18 weeks, respectively. Functional assessments at 3 months of age indicate that Hnrnpu mutants have significant reductions in grip strength (Figure 2B) and maximal running speed (Figure 2C). Consistent with these analyses, the normalized muscle mass of Hnrnpu mutants is observed to be significantly reduced with respect to littermate controls (Figure 2D).
Figure 2.
Hnrnpu mKO Mice Develop Adult-Onset Myopathy
(A) Growth curves of male and female mice. Data are mean ± SEM from 25–40 mice per genotype, depending on age and gender.
(B) Grip strength.
(C) Maximum running speed.
(D) Normalized muscle wet weight.
(B–D) Data are mean ± standard deviation from n = 8 control and n = 8 mKO male mice. Significance (∗) was set a p < 0.05.
(E–I) Histological analyses of Hnrnpu mutant and control littermates at 3 months of age.
(E) H&E staining of Hnrnpu mutant gastrocnemius muscle. The scale bar is 50 μm.
(F) H&E staining of tibialis anterior. The scale bar is 50 μm.
(G) Quantification of central nuclei in tibialis anterior. Data are from n = 9,785 control and n = 9,921 mutant myofibers from n = 7 male mice per group. Data are mean ± standard deviation. Significance (∗) was set at p < 0.05.
(H) Masson's Trichrome staining of tibialis anterior and gastrocnemius. The scale bar is 50 μm.
(I) Quantification of percent fibrotic area. Data are mean ± standard deviation from n = 3 control and n = 3 male mKO mice. Significance (∗) was set at p < 0.05. p Values were calculated using the Student's two-tailed t test assuming unequal variances.
Histological examination of muscles from Hnrnpu mutants at 3 months of age reveals central nuclei, myofiber degeneration and necrosis, intracellular inclusions, and extensive fibrosis (Figures 2E–2I). H&E staining of Hnrnpu mutant gastrocnemius muscles reveals the accumulation of mononuclear cells and intracellular inclusions (Figure 2E). In the TA we observe myofiber degeneration, intracellular inclusions, and abundant central nuclei (Figure 2F). Quantification of H&E images indicate that 6.45 ± 1.88% of TA myofibers in Hunrnpu mutants contain central nuclei as compared with 0.19 ± 0.24% of control myofibers (Figure 2G). Masson's Trichrome staining of both the TA and gastrocnemius muscles indicate that Hnrnpu mutants have extensive fibrosis (Figure 2H). Quantification of Masson's staining indicate that the fibrotic area of TA and gastrocnemius muscles increase from 4.0 ± 0.67% and 3.4 ± 0.89% in controls to 11.8 ± 2.13% and 20.3 ± 4.78% in Hnrnpu mutants (Figure 2I), respectively. As Hnrnpu mutants age, female mice present with pronounced kyphosis by 5 months, whereas males develop a less severe form by 7 months (Figure S3).
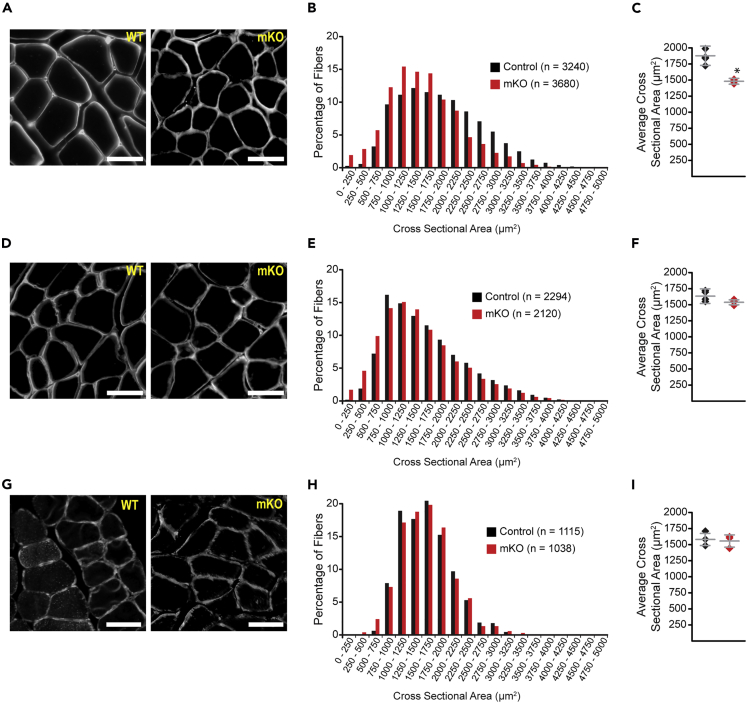
To further characterize the muscle architecture of Hnrnpu mutants, we next performed quantitative analyses of myofiber cross-sectional areas. Immunofluorescence labeling of laminin and quantification of gastrocnemius myofiber cross-sectional areas indicate myofiber atrophy in mutant mice, as indicated by an elevated leftward shift of the myofiber size distribution curve (Figures 3A and 3B). Quantitatively, data indicate a significant 21.2% decrease (1479 ± 29 versus 1878 ± 150 μm2, p = 0.011, mKO versus control) in the average gastrocnemius myofiber cross-sectional area of individual mice (Figure 3C). In contrast, the tibialis anterior has only a subtle leftward shift of the myofiber size distribution curve (Figures 3D and 3E), whereas the soleus is virtually indistinguishable (Figures 3G and 3H) between mKO and littermate controls. Although we observe decreases of 7.9% (1,541 ± 76 versus 1,674 ± 114 μm2, p = 0.058, mKO versus control) and 3.3% (725 ± 94 versus 701 ± 98 μm2, p = 0.537, mKO versus control) in the tibialis anterior (Figure 3F) and soleus (Figure 3I), respectively, these differences fail to reach statistical significance. Consistent with these observations, NMR body composition analyses indicate a significant decrease in the lean muscle mass of Hnrnpu mutants as compared with littermate controls (Table 1). Collectively, these observations indicate that the loss of Hnrnpu results in a multifaceted myopathy characterized by histological features of myofiber necrosis and inclusions, central nuclei, extensive fibrosis, and muscle wasting.
Figure 3.
Selective Muscle Wasting in Hnrnpu Mutants
(A–C) Immunohistological analyses of gastrocnemius muscle.
(D–F) Immunohistological analyses of tibialis anterior muscle.
(F–I) Immunohistological analyses of soleus muscle.
(A, D, and G) Laminin staining of muscle transverse sections. The scale bar is 50 μm.
(B, E, and H) Myofiber size distribution curves.
(C, F, and I) Average myofiber cross-sectional areas.
(A–I) Data are from n = 4 control and n = 4 mKO male mice at 3 months of age. Data are mean ± standard deviation. Significance (∗) was set at p < 0.05. p Values were calculated using the Student's two-tailed t test assuming unequal variances.
Table 1.
NMR Body Composition Parameters of Hnrnpu mKO Mice at 3 Months of Age
| WT (n = 7) | mKO (n = 7) | p Value | |
|---|---|---|---|
| Body weight (g) | 28.61 ± 1.46 | 25.70 ± 1.39 | 0.014 |
| Fat (g) | 3.45 ± 0.52 | 3.44 ± 0.57 | 0.995 |
| Lean (g) | 24.26 ± 1.50 | 21.33 ± 1.51 | 0.019 |
| Free water (g) | 0.17 ± 0.10 | 0.14 ± 0.08 | 0.604 |
| Total water (g) | 18.31 ± 1.03 | 14.94 ± 1.14 | 0.003 |
Related to Figure 2. Data are mean ± standard deviation from n = 7 mice per group. p Values were calculated using the Student's two-tailed t test assuming unequal variances.
Hnrnpu Mutant Mice Have an Impaired Regenerative Response
The decreased lean muscle mass and leftward shift of myofiber size distribution, in conjunction with an increase in myofibers with centrally located nuclei, prompted us to evaluate the capacity for myofiber regeneration following injury in Hnrnpu mutant mice. Morphometric analysis of wild-type (WT) control mice 10 days post injury (DPI) depicts robust regeneration characterized by large well-organized myofibers. In contrast, mKO mice exhibit an impaired regenerative response as indicated by numerous small poorly organized myofibers (Figure 4A). Quantitative analyses of histological sections indicate a leftward shift in the myofiber size distribution curve of newly regenerated myofibers in mKO mice relative to controls (Figure 4B). To explore whether the age-associated loss of hnRNP-U (Figure 1B) contributes to the regenerative deficits of aged muscles (Demontis et al., 2013), we performed the cryoinjury model on young (3 months) and old (24–26 months) C57BL/6 WT mice. Morphometric analyses of H&E-stained sections obtained from young mice exhibit a robust regenerative response at 10 DPI as evidenced by large well-organized regenerated myofibers, whereas old mice exhibit a weak regenerative response characterized by small, poorly organized myofibers (Figure 4C). Quantitative analyses of histological sections indicate a leftward shift in the myofiber size distribution curve of newly regenerated myofibers in aged mice relative to young controls (Figure 4D). Overall, the newly regenerated myofibers of young mKO mice are 32% smaller (903 ± 261 versus 1,339 ± 174, mKO versus control) than littermate controls, whereas the newly regenerated myofibers of old C57BL/6 WT mice are 35% smaller (833 ± 44 versus 1,298 ± 168 μm2, old versus young) than young C57BL/6 controls (Figure 4E). Collectively, these observations indicate that Hnrnpu mutants have a regenerative response with many similarities to that of aged mice, suggesting that the loss of hnRNP-U in advanced age may contribute to regenerative impairments.
Figure 4.
Young Hnrnpu mKO Mice Phenocopy the Regenerative Response of Old Mice
(A) H&E-stained transverse sections of young (3 months) control and mKO tibialis anterior muscle 10 days post injury. The scale bar is 50 μm.
(C) H&E-stained transverse sections of young (3 months) and old (26 months) tibialis anterior muscles 10 days post injury. The scale bar is 50 μm.
(B) Myofiber size distribution curve of regenerating myofibers from (A).
(D) Myofiber size distribution curve of regenerating myofibers from (C).
(E) Dot plot of average regenerated myofiber cross-sectional area from n = 7 young Hnrnpu control (black diamond), n = 7 young male Hnrnpu mKO (red diamond), n = 6 young C57BL/6 (blue diamond), and n = 6 old male C57BL/6 mice (green diamond). Bars are mean ± standard deviation. Significance (∗) was set at p < 0.05. p Values were calculated using the Student's two-tailed t test assuming unequal variances.
Loss of Hnrnpu Leads to Dysregulated Expression and Splicing of Metabolic Genes
To better understand the molecular processes underlying our observations, we performed whole transcriptome gene and splice variant expression analyses on the gastrocnemius muscles of mice at 3 months of age. We obtained >60 million strand-specific 150-bp paired-end reads per sample with at least 91% of reads mapping to the mouse genome. Differential gene expression (log2FoldChange ±1.0, padj <0.01) analysis indicate 1,171 and 786 genes are increased and decreased in expression (mKO versus control), respectively (Figure 5A). Quantitative PCR validation of RNA sequencing (RNA-seq) analyses (data not shown) indicate a marked reduction in the expression of Myostatin, as well as a general repression of the E3 ubiquitin ligases and accessory proteins known to promote muscle atrophy (Bodine et al., 2001; Nowak et al., 2019; Sartori et al., 2013). In addition, genes associated with increased energy expenditures and metabolic stress, including Sln, Asns, Fgf21, Gdf15, Psat1, and Mthfd2 (Balasubramanian et al., 2013; Chung et al., 2017; Fisher and Maratos-Flier, 2016; Maurya et al., 2018; Nilsson et al., 2014), are robustly induced in the muscles of Hnrnpu mutants (Figure 5B). Interestingly, the induction of long non-coding RNAs (lncRNAs) appears to portend muscle atrophy and/or metabolic dysfunction, as we observe >3-fold more lncRNAs to be induced (Log2FoldChange >2, padj <0.01), rather than repressed. To better understand the cellular and molecular processes impacted by the changes in gene expression we performed Gene Ontology enrichment analysis. Our analysis indicates that up-regulated genes are highly associated with inflammatory signaling (Figure S4A), whereas downregulated genes are associated with energy production and metabolic processes (Figure S4B). Specifically, we note the marked repression of Mss51 (Zmynd17), which acts as an inhibitor of oxidative metabolism (Rovira Gonzalez et al., 2019), multiple solute transporters with roles in amino acid and anion/cation (Slc15a5, Slc30a2, Slc40a1, Slc4a10) homeostasis (Lin et al., 2015), as well as multiple enzymes involved in the biogenesis of triacylglycerol (Dgat2l6) (Yen et al., 2008), amino acids (Gadl1, Padi2, Amd1/2), and glycogen (Gys2) (Figure 5C). The top induced and repressed genes in the gastrocnemius of mKO mice are listed in Table S1.
Figure 5.
Gene Expression and Alternative Splicing Are Dysregulated in Hnrnpu mKO Mice
(A) Whole-genome expression profile of male Hnrnpu mutant gastrocnemius. Red and blue dots depict increased and decreased gene expression (mKO versus control, padj <0.01), respectively. Decreased (blue) and increased (red) expression of genes in Hnrnpu mutant gastrocnemius associated with (B) muscle atrophy and metabolic stress, and (C) energy production and nutrient transport.
(B and C) Data are Log2(FoldChange) ± standard error. Significance (∗) was set at p < 0.0001. p Values were calculated using the Wald test corrected for multiple testing using the Benjamini-Hochberg method.
(D) Summary of significant differential splicing events between genotypes. |ΔPSI| > 0.1, FDR < 0.01.
(E) Whole-genome expression profiling of mis-spliced differentially expressed genes in (A). Dots represent individual splicing events occurring on differentially expressed genes. Statistically significant splicing events (FDR < 0.01) occurring on statistically significant differentially expressed genes (padj <0.01) are highlighted in red. p Values were calculated using a likelihood-ratio test.
(F–J) Examples of differential splicing regulated by hnRNP-U: Senp1 (F), Setd3 (G), Mff (H), Sorbs1 (I), and Inpp4a (J). SE, Skipped Exon; RI, Retained Intron; MXE, Mutually Exclusive Exon; A3SS, Alternative 3′ Splice Site; A5SS, Alternative 5′ Splice Site.
We next examined the impact of Hnrnpu deletion on alternative splicing from the strand-specific paired-end RNA-seq data. Using the rMATS program for splicing analysis (Shen et al., 2014), we identified 2,909 significant alternative splicing events (|ΔPSI| > 0.1, false discovery rate [FDR] < 0.01; Table S2). The distribution of these events, which include skipped exons (SEs), retained introns (RIs), mutually exclusive exons (MXEs), and the use of alternative 5’ (A5SS) and 3’ (A3SS) splice sites, is shown in Figure 5D. These data show that loss of hnRNP-U leads to extensive dysregulation of exon utilization in which SE (74.2%) and MXE (11.7%) events far exceed the utilization of alternative exons (A5SS and A3SS), suggesting that hnRNP-U may be involved in defining intron/exon boundaries. This is in contrast with a prior study in the developing heart, in which RI events were the largest share of alternative splicing events following the loss of hnRNP-U (Ye et al., 2015), thus suggesting that the role of hnRNP-U in pre-mRNA splicing modulation is differentially regulated in a cell type- and context-dependent manner. Coupled with the differential gene expression analysis, we observe 1,296 mis-splicing events (FDR < 0.01) across 710 genes whose expression is significantly altered (padj <0.01) in the absence of Hnrnpu (Figure 5E). The majority of these events occur on genes whose expression is decreased (985 events across 529 genes) as compared with those that are increased (311 events across 181 genes). Splicing events in mKO mice are biased toward exclusion (ΔPSI, quadrant I: 228 events across 150 genes, quadrant IV: 653 events across 388 genes) rather than inclusion (-ΔPSI, quadrant II: 83 events across 56 genes, quadrant III: 332 events across 218 genes). To better understand the cellular and molecular processes impacted by the changes in alternative splicing, we again performed Gene Ontology enrichment analysis. Our analysis indicates that genes with the differential inclusion/exclusion of exons (|ΔPSI| > 0.1, FDR < 0.01) are highly associated with metabolic and signal transduction processes (Figure S4C). In addition, Gene Ontology enrichment analysis of genes that are both downregulated and mis-spliced (Figure 5E) similarly indicate that this subset of genes is highly associated with metabolic and signal transduction processes (Figure S4D).
A more detailed examination of altered splicing events revealed that hnRNP-U is necessary for normal exon utilization in multiple genes, including Senp1 (Figure 5F), Setd3 (Figure 5G), Mff (Figure 5H), Sorbs1 (Figure 5I), and Inpp4a (Figure 5J). The Sentrin/SUMO-specific protease 1 (Senp1) has been previously shown to be involved in regulating mitochondrial biogenesis through enhancing PGC-1α activity (Cai et al., 2012), whereas mitochondrial fission factor (Mff) is a critical regulator of mitochondrial dynamics (Otera et al., 2010). Expression of SET domain containing methyltransferase 3 (Setd3) is positively correlated with skeletal muscle hypertrophy (Seaborne et al., 2018) and involved in muscle differentiation via promoting the expression of the myogenic regulatory factors MyoG and Myf6 (Eom et al., 2011). Sorbin and SH3 domain containing protein 1 (Sorbs1) is required for PI3K-independent, insulin-stimulated glucose uptake (Baumann et al., 2000), whereas the inositol polyphosphate-4-phosphatase 1A (Inpp4a) is a PIP2 phosphatase impairing Akt activation (Ivetac et al., 2009). Collectively, these data indicate that Hnrnpu is required for the expression and splicing of genes involved in the signal transduction and metabolic processes crucial for normal skeletal muscle growth.
Akt Signaling Is Constitutively Active in Hnrnpu Mutants
To further explore the impact of Hnrnpu deletion on the signal transduction and metabolic processes underlying the muscle wasting in Hnrnpu mutants, we examined IGF-1/PI3K/Akt signaling. To begin, we injected either saline or recombinant IGF-1 into the gastrocnemius muscles of control and mutant mice at ~4 months of age. Quantitative western blot analyses indicate that IGF-1 receptor (IGF-1R) autophosphorylation in response to IGF-1 administration is similar between Hnrnpu mutant and control mice (Figures 6A and 6B), suggesting that initiation of the IGF-1/PI3K/Akt1 signaling cascade is intact in the absence of Hnrnpu. Upon examination of Akt1 phospho-activation, we observe that IGF-1 stimulation induces a robust induction in Akt1 phosphorylation at Ser473 in control mice (Figures 6A and 6C), indicating that IGF-1 stimulation results in the phospho-activation of Akt1. In contrast, we observe that the level of Akt1 Ser473 phosphorylation in vehicle-treated Hnrnpu mutant mice is comparable with that of IGF-1-stimulated controls (Figure 6A). In addition, IGF-1 stimulation is unable to induce further Akt1 Ser473 phosphorylation (Figure 6A) suggesting that Akt1 is maximumly activated under normal physiological conditions at this time point. Quantification of the phospho-Ser473 to total Akt1 ratio confirms this observation (Figure 6C), although the decreased magnitude of the phospho:total Akt1 ratio relative to IGF-1 stimulated controls is due to the increased expression of Akt1 in Hnrnpu mutant mice (Figure 6D). Collectively, these observations indicate that, in the absence of Hnrnpu, Akt1 becomes constitutively activated and thus desensitized to IGF-1/PI3Ksignaling.
Figure 6.
Constitutive Activation of Akt1 and Impaired Autophagic Flux in Hnrnpu mKO Mice
(A) Representative western blots of gastrocnemius muscle lysates 1 h following injection of IGF-1.
(B–D) Quantification of (A). Data are mean ± standard deviation from n = 3 independent experiments and are normalized to WT vehicle controls. Significance (∗) was set at p < 0.05. p Values were calculated using the Student's two-tailed t test assuming unequal variances.
(E and G) Representative western blots of unstimulated gastrocnemius muscles at 3 months of age.
(F and H) Quantification of (E) and (G), respectively. Data are mean ± standard deviation from n = 3 biological samples per genotype and are normalized to WT controls. Significance (∗) was set at p < 0.05. p Values were calculated using the Student's two-tailed t test assuming unequal variances.
(I) Representative western blots of unstimulated gastrocnemius muscles at P21. Male mice were used for all analyses.
Following up on these observations, we asked whether the signaling downstream of IGF-1R was biased toward Akt1 activation. First, we examined the expression of the PI3K p110 catalytic subunit. Quantitative western blot analyses indicate that Hnrnpu mutant mice express ~70% more p110 than WT controls (Figures 6E and 6F). We next examined PTEN, as it is well established to oppose the Akt1 activating actions of PI3K (Laplante and Sabatini, 2012; Saxton and Sabatini, 2017). Counterintuitively, we observe the expression of PTEN to be increased >4-fold in the gastrocnemius of Hnrnpu mutant mice as compared with controls (Figures 6E and 6F). These observations and the accompanying reductions in body weight (Figure 2C) are consistent with a prior report indicating that increases in PTEN protein expression induce postnatal growth impairments through increases in energy expenditure (Garcia-Cao et al., 2012). Interestingly, the increases in both IGF-1R and Akt1 protein expression are positively correlated with increases in Igf1r (Log2FC = 0.70 ± 0.14, padj = 9.93 × 10−6, mKO versus WT) and Akt1 (Log2FC 0.40 ± 0.16, padj = 0.03, mKO versus WT), whereas the increase in PTEN protein is negatively correlated with Pten (Log2FC −0.65 ± 0.13, p = 9.67 × 10−9, mKO versus WT) expression. Together, these observations suggest that the increase in IGF-1R and Akt1 protein expression contribute to the observed dysregulation of Akt1 activation and that post-transcriptional gene regulation of mature mRNA may also be altered in the absence of Hnrnpu.
Hnrnpu mKO mice share many similarities with the phenotypes of mice in which Tsc1 (Castets et al., 2013) and Atg7 (Masiero et al., 2009) were selectively deleted in skeletal muscle, prompting us to evaluate the downstream effects of Akt signaling on autophagy. Initiated by the essential positive regulator ULK1, and negatively regulated by Akt/mTORC1 signaling, autophagy plays crucial roles in both the maintenance of cellular metabolic homeostasis and the cellular response to stress (Rabinowitz and White, 2010). We observe a nearly 4-fold induction of ULK1 phosphorylation at Ser757 (Figures 6G and 6H) indicating an inhibition of autophagy initiation through mTORC1-dependent mechanism (Kim et al., 2011; Manning and Toker, 2017). To further corroborate this observation, we assayed for the autophagy-specific substrate and cargo receptor SQSTM1/p62, the accumulation of which is used as an indicator of autophagy impairment (Klionsky et al., 2012). Consistent with this, we observe a marked increase in the expression of SQSTM1/p62 (Figure 6G) in Hnrnpu mutants as compared with controls, further corroborating our observations of autophagy impairment in the absence of Hnrnpu. Together, these data indicate that the constitutive activation of Akt leads to an increase in mTORC1-mediated inhibition of autophagy.
The postnatal growth of skeletal muscle up to ~P21 is primarily due to the satellite cell contributions to myofiber volume and myonuclei content, after which myonuclear and satellite cell content stabilizes and hypertrophic signaling begins to dominate (White et al., 2010). As such, we sought to determine whether the satellite cell contributions to muscle growth at this time point masked PI3K/Akt signaling derangements. Quantitative western blot analyses of gastrocnemius muscles of control and mKO mice at P21 indicate no significant differences in the expression or activation of IGF-1R, PI3K p110, Akt1, and PTEN (Figure 6I), indicating the IGF-1/PI3K/Akt signaling is unaltered in Hnrnpu mutants at this time point. In addition, the SQSTM1/p62 protein expression in WT and Hnrnpu mutants at P21 (Figure 6I) is comparable with that of Hnrnpu mutants at 3 months of age (Figures 6G and S5). Collectively, these data indicate that Hnrnpu is essential for the normal physiological activation of Akt signaling and its responsiveness to IGF-1 stimulation.
Discussion
The dysregulation of IGF-1/PI3K/Akt/mTOR signaling is associated with a diverse array of pathological conditions including cancer, cardiovascular disease, inflammatory and autoimmune disorders, and insulin resistance and type 2 diabetes (Manning and Toker, 2017; Saxton and Sabatini, 2017). In skeletal muscle, Akt signaling is a key regulator of glycolytic muscle homeostasis, hypertrophic growth, and metabolism through its activation of mTOR-dependent anabolic processes and simultaneous inhibition of catabolic processes. Alternative splicing plays a crucial role in muscle development, as genetic mutations in both splice sites and RNA-binding proteins involved in splicing regulation playing causal roles in the pathogenesis of neuromuscular disorders (Ho et al., 2005; Lin et al., 2006; Philips et al., 1998; Runfola et al., 2015). We demonstrate that skeletal muscle-specific deletion of Hnrnpu leads to an adult-onset myopathy with selective muscle wasting, fibrosis, and broad changes in both the expression and splicing of genes. In addition, dysregulated splicing is increasingly recognized as a causal factor promoting cellular dysfunction through changes in the binding properties, enzymatic activities, and intracellular localization of a large percentage of the proteome (Tress et al., 2007). Accordingly, our data in the muscles of Hnrnpu mutants demonstrate the constitutive activation of Akt signaling and its desensitization to IGF-1 stimulation. Of the individual muscles analyzed, the soleus was the only muscle without changes in myofiber cross-sectional area. This is in contrast with the non-significant trend and the significantly marked reduction in myofiber cross-sectional areas observed in the tibialis anterior and gastrocnemius, respectively. Although the cellular adaptations underlying the distinct growth responses to Hnrnpu deletion in each of these muscles are unclear, differences in functional utilization (e.g., ankle extensor versus ankle flexor) as well as metabolic adaptations specific to each individual muscle likely contribute.
In mice, both the autophosphorylation of IGF-1R and the sensitivity of Akt1 to IGF-1 are decreased with age (Akasaki et al., 2014). Our results in young Hnrnpu mutants indicate that the autophosphorylation of IGF-1R in response to IGF-1 is unaltered, whereas the phospho-activation of Akt1 is desensitized to IGF-1. These observations indicate that hnRNP-U is essential for the normal signaling activities downstream of IGF-1R and suggest an hnRNP-U-independent mechanism modulates the age-associated changes in IGF-1R activation. In contrast with 1-year-old non-sarcopenic WT mice (Akasaki et al., 2014), we observe Akt1 to be constitutively active under normal physiological conditions in the atrophied muscles of Hnrnpu mutants, suggesting that other processes, in addition to Akt activation, are required for the hypertrophic growth of muscle. Short-term activation of Akt1 signaling induces changes in the expression of genes critical for the activity of pathways involved in the production and storage of energy and nutrients (Wu et al., 2017) in parallel with hypertrophic growth (Izumiya et al., 2008b; Wu et al., 2017). Autophagy is an essential process for the maintenance of cellular homeostasis that may be further induced in response to cellular stress to reduce organelles and macromolecules into amino acids, nucleosides, fatty acids, and sugars in support of cellular growth and survival (Rabinowitz and White, 2010). Although it is impaired by Akt/mTOR hypertrophic signaling (Manning and Toker, 2017), insufficient and excessive autophagy negatively impacts muscle (Mammucari et al., 2007; Masiero et al., 2009), suggesting an inflection point between an autophagic flux that supports muscle hypertrophy and that which promotes muscle wasting. The constitutive activation of Akt in Hnrnpu mutants coincides with both impaired autophagy and an increase in ULK1 inhibitory phosphorylation at S757 and is consistent with prior reports indicating that increased mTORC1 activity is responsible for the observed impairments in autophagy (Castets et al., 2013; Kim et al., 2011).
Muscle energy production may be divided into anaerobic metabolism, which provides energy from glycolysis for rapid movements, and mitochondrial regulated aerobic metabolism for sustained exercise (Zierath and Hawley, 2004). The sensing of nutrient availability and the transport of extracellular nutrients into the cell are tightly regulated by a meshwork of interconnected signaling pathways that include Akt and mTORC1 (Broer and Broer, 2017; Efeyan et al., 2015). Dysregulation of energy metabolism and nutrient insufficiencies are associated with the muscle atrophy and dysfunction known to occur with advancing age, cachexia, and multiple myopathies (Cohen et al., 2015; Koopman et al., 2014; Marcell, 2003; Petruzzelli and Wagner, 2016). The transcriptional profile of Hnrnpu mutants indicates that genes involved in metabolic processes, including energy production, nutrient sensing and transport, and signal transduction are both repressed and mis-spliced, suggesting that their biochemical activities and/or intracellular localization is altered. The observations of constitutively active Akt, and a marked increase in PTEN expression, would suggest an increase in the demand for amino acids necessary to support hypertrophic growth (Wu et al., 2017), as well as an increase in energy expenditures (Garcia-Cao et al., 2012). The significant increases in Asns, Fgf21, and Gdf15 expression are consistent with a state of nutritional and metabolic stress (Broer and Broer, 2017; Fisher and Maratos-Flier, 2016; Patel et al., 2019) and are in agreement with prior analyses of gastrocnemius muscle expressing a constitutively active form of Akt (Wu et al., 2017). In addition, the marked increase in Sln would suggest an increase in mitochondrial biogenesis and oxidative metabolism (Maurya et al., 2018), whereas the increases in Mthfd2 and Psat1 are consistent with compensatory mechanisms to mitigate mitochondrial and oxidative stress (Nilsson et al., 2014; Tyynismaa et al., 2010; Yang and Vousden, 2016).
Tissue-specific and ubiquitously expressed RNA-binding proteins cooperatively interact with the regulatory elements both within a nascent transcript and of the spliceosome for context-dependent tissue-specific mRNA splicing (Fu and Ares, 2014; Wang et al., 2008). Prior reports have demonstrated the importance of skeletal muscle enriched splicing factors Rbfox1/2 (Singh et al., 2018), RBM20 (Guo et al., 2012), and RBM24 (Yang et al., 2014) in the development, functionality, and growth of muscle. Skeletal muscle accounts for approximately 50% of the nearly 50-fold increase in body weight during murine post-natal development (Allen et al., 1979). During this time, a large percentage of the transcriptome undergoes extensive temporal-dependent changes in alternative splicing that occur largely independent of changes in gene expression (Brinegar et al., 2017), suggesting that the temporal changes in protein isoform expression are critical for the post-natal physiological growth of skeletal muscle. In addition, the increasing number of whole transcriptome analyses of physiologically normal, endurance trained, hypertrophic, and atrophying skeletal muscle (Ehmsen et al., 2019; Lindholm et al., 2016; Llano-Diez et al., 2019; Shavlakadze et al., 2019; Wu et al., 2017) provides unique molecular insights into its growth and adaptive responses. Our whole transcriptome analyses describe the changes in gene expression and splicing associated with the myopathy phenotype of Hnrnpu mutants, identifying considerable enrichment for the mis-expression/splicing of genes associated with signal transduction, metabolic processes, and inflammation. Although it may be tempting to draw conclusions based upon these observations given the seemingly ubiquitous enrichment for metabolic and inflammatory genes among these datasets, care must be taken with such interpretations given the differences in experimental models and the uncertainty of whether such transcriptome changes are the primary effect, or a compensatory effect due to changes in muscle physiology. In addition, hnRNP-U is known to enhance the transcript stability of a few select genes (Yugami et al., 2007), which may suggest that gene expression, as influenced by mRNA stability, may be similarly influenced by exon inclusion/exclusion. Although the relationship between mRNA stability and splicing is not well understood and likely regulated on a gene- and intron/exon-specific basis (Clement et al., 1999; Nott et al., 2003), the possibility that this may contribute to our observations is intriguing.
Collectively, our studies link Hnrnpu to the proper expression and splicing of genes underlying the physiological growth of skeletal muscle. Our data would suggest that the muscles of Hnrnpu mutants are under cellular and metabolic stress due to dysregulation of the cellular feedback mechanisms limiting Akt/mTOR signaling in response to energy and/or nutrient insufficiency. Future detailed analyses dissecting the impact of alternative splicing dysregulation on the feedback mechanism limiting Akt/mTOR may provide novel insights to the pathogenesis of muscle wasting disorders.
Limitations of the Study
We demonstrate that loss of Hnrnpu in skeletal muscle causes impaired post-natal hypertrophic growth and changes in the expression and splicing of numerous genes involved in signal transduction and metabolic processes. Our data strongly suggest that impaired muscle growth due to the loss of hnRNP-U is biased toward muscles with a greater content of fast glycolytic, rather than slow oxidative, myofibers but cannot definitively confirm this observation at the level of the individual myofiber subtype. Given the temporal lag between in vivo Cre activation and the time point at which whole transcriptome analyses were performed, we are unable to discern between the primary and secondary effects on the transcriptome, and its impact on muscle physiology, owing to the loss of hnRNP-U. RNA-binding proteins play crucial roles in modulating the stability, structure, and splicing of transcripts (Hentze et al., 2018), making it difficult to discern between these functions as these processes are interrelated. In cell culture model systems, hnRNPU is known to bind a purine-rich consensus sequence (KGRGKRV) to modulate both the splicing of nascent transcripts (Huelga et al., 2012; Xiao et al., 2012) and the stability of mRNA (Yugami et al., 2007). Whether skeletal muscle hnRNP-U binds a similar set of nascent transcripts and enhances mRNA stability is unknown and warrants future investigations. Although our transcriptional data and signaling analyses are consistent with prior reports of cellular and metabolic stress in muscle (Izumiya et al., 2008a; Khan et al., 2017; Suomalainen et al., 2011; Tyynismaa et al., 2010), metabolic profiling and detailed molecular analyses of splice variant functional analyses will be needed to better understand the mechanistic linkages between splice variant expression and the regulation of Akt/mTOR signaling in muscle physiology.
Resource Availability
Lead Contact
Ronald L Neppl (rneppl@bwh.harvard.edu).
Materials Availability
Further information and requests for ressources and reagents should be directed to and will be fullfilled by the Lead Contact.
Data and Code Availability
The accession number for the RNA sequencing data reported in this paper is NCBI Gene Expression Omnibus: GSE145712. No new computational tools were developed in this project. The code for usage of existing tools as described is available upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors would like to thank Dr. Tom Maniatis for the Hnrnpu mouse line and Teri Bowman for her technical assistance with histology processing. This work was supported by the National Institutes of Health K76AG059996 to I.S., a Research Education Component Grant from the National Institutes of Health National Institute on Aging (P30AG031679) funded Boston Claude D. Pepper Older Americans Independence Center to R.L.N., and funds from the Brigham and Women’s Hospital Department of Orthopaedic Surgery to R.L.N.
Author Contributions
D.B., B.D.M., K.B., B.L., E.-J.L., and Y.Z. conducted experiments. D.B., B.D.M., K.B., B.L., E.-J.L., Y.Z., L.K.C., and S.e.R. analyzed data. D.B., B.D.M., K.B., and Y.Z. contributed to writing of the original draft. R.L.N. conceptualized and designed the study. R.L.N. and I.S. performed project administration, supervision, funding acquisition, and review & editing of the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101319.
Supplemental Information
Data are the output from DESeq2. Column headers are: Gene (Ensembl Gene ID), basemean, GeneName (NCBI, Official Symbol), Log2FoldChange, lfcSE (Log2FoldChange Standard Error), p-value, padj, Wald p-value. Data are separated into two tabs for genes increased and decreased in expression.
Data are the output from rMATS. Column headers are: ID (event number), GeneID (Ensembl), gene symbol (NCBI, Official Symbol), chr (chromosome), strand (+or - strand), p-value, FDR, IncLevelDifference (ΔPSI). Data are separated into five tabs, one each for skipped exon (SE), retained intron (RI), mutually exclusive exon (MXE), alternative 5′ splice site (A5SS), and alternative 3′ splice site (A3SS). Additional column headings specific for each type of splicing event are included in their respective tab.
References
- Akasaki Y., Ouchi N., Izumiya Y., Bernardo B.L., Lebrasseur N.K., Walsh K. Glycolytic fast-twitch muscle fiber restoration counters adverse age-related changes in body composition and metabolism. Aging Cell. 2014;13:80–91. doi: 10.1111/acel.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R.E., Merkel R.A., Young R.B. Cellular aspects of muscle growth: myogenic cell proliferation. J. Anim. Sci. 1979;49:115–127. doi: 10.2527/jas1979.491115x. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M.N., Butterworth E.A., Kilberg M.S. Asparagine synthetase: regulation by cell stress and involvement in tumor biology. Am. J. Physiol. Endocrinol. Metab. 2013;304:E789–E799. doi: 10.1152/ajpendo.00015.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C.A., Ribon V., Kanzaki M., Thurmond D.C., Mora S., Shigematsu S., Bickel P.E., Pessin J.E., Saltiel A.R. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. doi: 10.1038/35025089. [DOI] [PubMed] [Google Scholar]
- Biamonti G., Caceres J.F. Cellular stress and RNA splicing. Trends Biochem. Sci. 2009;34:146–153. doi: 10.1016/j.tibs.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Bodine S.C., Latres E., Baumhueter S., Lai V.K., Nunez L., Clarke B.A., Poueymirou W.T., Panaro F.J., Na E., Dharmarajan K. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Brinegar A.E., Xia Z., Loehr J.A., Li W., Rodney G.G., Cooper T.A. Extensive alternative splicing transitions during postnatal skeletal muscle development are required for calcium handling functions. Elife. 2017;6:e27192. doi: 10.7554/eLife.27192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer S., Broer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem. J. 2017;474:1935–1963. doi: 10.1042/BCJ20160822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R., Yu T., Huang C., Xia X., Liu X., Gu J., Xue S., Yeh E.T., Cheng J. SUMO-specific protease 1 regulates mitochondrial biogenesis through PGC-1alpha. J. Biol. Chem. 2012;287:44464–44470. doi: 10.1074/jbc.M112.422626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castets P., Lin S., Rion N., Di Fulvio S., Romanino K., Guridi M., Frank S., Tintignac L.A., Sinnreich M., Ruegg M.A. Sustained activation of mTORC1 in skeletal muscle inhibits constitutive and starvation-induced autophagy and causes a severe, late-onset myopathy. Cell Metab. 2013;17:731–744. doi: 10.1016/j.cmet.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Chung H.K., Ryu D., Kim K.S., Chang J.Y., Kim Y.K., Yi H.S., Kang S.G., Choi M.J., Lee S.E., Jung S.B. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J. Cell Biol. 2017;216:149–165. doi: 10.1083/jcb.201607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement J.Q., Qian L., Kaplinsky N., Wilkinson M.F. The stability and fate of a spliced intron from vertebrate cells. RNA. 1999;5:206–220. doi: 10.1017/s1355838299981190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Nathan J.A., Goldberg A.L. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- Demontis F., Piccirillo R., Goldberg A.L., Perrimon N. Mechanisms of skeletal muscle aging: insights from Drosophila and mammalian models. Dis. Model. Mech. 2013;6:1339–1352. doi: 10.1242/dmm.012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre M., Sanchez G., Barbier J., Corcos L., Auboeuf D. The emerging role of pre-messenger RNA splicing in stress responses: sending alternative messages and silent messengers. RNA Biol. 2011;8:740–747. doi: 10.4161/rna.8.5.16016. [DOI] [PubMed] [Google Scholar]
- Efeyan A., Comb W.C., Sabatini D.M. Nutrient-sensing mechanisms and pathways. Nature. 2015;517:302–310. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmsen J.T., Kawaguchi R., Mi R., Coppola G., Hoke A. Longitudinal RNA-Seq analysis of acute and chronic neurogenic skeletal muscle atrophy. Sci. Data. 2019;6:179. doi: 10.1038/s41597-019-0185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery A.E. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- Eom G.H., Kim K.B., Kim J.H., Kim J.Y., Kim J.R., Kee H.J., Kim D.W., Choe N., Park H.J., Son H.J. Histone methyltransferase SETD3 regulates muscle differentiation. J. Biol. Chem. 2011;286:34733–34742. doi: 10.1074/jbc.M110.203307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernste F.C., Reed A.M. Idiopathic inflammatory myopathies: current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin. Proc. 2013;88:83–105. doi: 10.1016/j.mayocp.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Faustino N.A., Cooper T.A. Pre-mRNA splicing and human disease. Genes Dev. 2003;17:419–437. doi: 10.1101/gad.1048803. [DOI] [PubMed] [Google Scholar]
- Fisher F.M., Maratos-Flier E. Understanding the physiology of FGF21. Annu. Rev. Physiol. 2016;78:223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- Fu X.D., Ares M., Jr. Context-dependent control of alternative splicing by RNA-binding proteins. Nat. Rev. Genet. 2014;15:689–701. doi: 10.1038/nrg3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cao I., Song M.S., Hobbs R.M., Laurent G., Giorgi C., de Boer V.C., Anastasiou D., Ito K., Sasaki A.T., Rameh L. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell. 2012;149:49–62. doi: 10.1016/j.cell.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuens T., Bouhy D., Timmerman V. The hnRNP family: insights into their role in health and disease. Hum. Genet. 2016;135:851–867. doi: 10.1007/s00439-016-1683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass D.J. Skeletal muscle hypertrophy and atrophy signaling pathways. Int. J. Biochem. Cell Biol. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Guo W., Schafer S., Greaser M.L., Radke M.H., Liss M., Govindarajan T., Maatz H., Schulz H., Li S., Parrish A.M. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat. Med. 2012;18:766–773. doi: 10.1038/nm.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M.W., Castello A., Schwarzl T., Preiss T. A brave new world of RNA-binding proteins. Nat. Rev. Mol. Cell Biol. 2018;19:327–341. doi: 10.1038/nrm.2017.130. [DOI] [PubMed] [Google Scholar]
- Ho T.H., Bundman D., Armstrong D.L., Cooper T.A. Transgenic mice expressing CUG-BP1 reproduce splicing mis-regulation observed in myotonic dystrophy. Hum. Mol. Genet. 2005;14:1539–1547. doi: 10.1093/hmg/ddi162. [DOI] [PubMed] [Google Scholar]
- Huelga S.C., Vu A.Q., Arnold J.D., Liang T.Y., Liu P.P., Yan B.Y., Donohue J.P., Shiue L., Hoon S., Brenner S. Integrative genome-wide analysis reveals cooperative regulation of alternative splicing by hnRNP proteins. Cell Rep. 2012;1:167–178. doi: 10.1016/j.celrep.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivetac I., Gurung R., Hakim S., Horan K.A., Sheffield D.A., Binge L.C., Majerus P.W., Tiganis T., Mitchell C.A. Regulation of PI(3)K/Akt signalling and cellular transformation by inositol polyphosphate 4-phosphatase-1. EMBO Rep. 2009;10:487–493. doi: 10.1038/embor.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y., Bina H.A., Ouchi N., Akasaki Y., Kharitonenkov A., Walsh K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008;582:3805–3810. doi: 10.1016/j.febslet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y., Hopkins T., Morris C., Sato K., Zeng L., Viereck J., Hamilton J.A., Ouchi N., LeBrasseur N.K., Walsh K. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7:159–172. doi: 10.1016/j.cmet.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N.A., Nikkanen J., Yatsuga S., Jackson C., Wang L., Pradhan S., Kivela R., Pessia A., Velagapudi V., Suomalainen A. mTORC1 regulates mitochondrial integrated stress response and mitochondrial myopathy progression. Cell Metab. 2017;26:419–428 e415. doi: 10.1016/j.cmet.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Kim J., Kundu M., Viollet B., Guan K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D.J., Abdalla F.C., Abeliovich H., Abraham R.T., Acevedo-Arozena A., Adeli K., Agholme L., Agnello M., Agostinis P., Aguirre-Ghiso J.A. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman R., Ly C.H., Ryall J.G. A metabolic link to skeletal muscle wasting and regeneration. Front. Physiol. 2014;5:32. doi: 10.3389/fphys.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K.M., Gonzalez M., Poueymirou W.T., Kline W.O., Na E., Zlotchenko E., Stitt T.N., Economides A.N., Yancopoulos G.D., Glass D.J. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol. Cell. Biol. 2004;24:9295–9304. doi: 10.1128/MCB.24.21.9295-9304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Rio D.C. Mechanisms and regulation of alternative pre-mRNA splicing. Annu. Rev. Biochem. 2015;84:291–323. doi: 10.1146/annurev-biochem-060614-034316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zhuang Y., Batra R., Thomas J.D., Li M., Nutter C.A., Scotti M.M., Carter H.A., Wang Z.J., Huang X.S. HNRNPA1-induced spliceopathy in a transgenic mouse model of myotonic dystrophy. Proc. Natl. Acad. Sci. U S A. 2020;117:5472–5477. doi: 10.1073/pnas.1907297117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Yee S.W., Kim R.B., Giacomini K.M. SLC transporters as therapeutic targets: emerging opportunities. Nat. Rev. Drug Discov. 2015;14:543–560. doi: 10.1038/nrd4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Miller J.W., Mankodi A., Kanadia R.N., Yuan Y., Moxley R.T., Swanson M.S., Thornton C.A. Failure of MBNL1-dependent post-natal splicing transitions in myotonic dystrophy. Hum. Mol. Genet. 2006;15:2087–2097. doi: 10.1093/hmg/ddl132. [DOI] [PubMed] [Google Scholar]
- Lindholm M.E., Giacomello S., Werne Solnestam B., Fischer H., Huss M., Kjellqvist S., Sundberg C.J. The impact of endurance training on human skeletal muscle memory, global isoform expression and novel transcripts. PLoS Genet. 2016;12:e1006294. doi: 10.1371/journal.pgen.1006294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano-Diez M., Fury W., Okamoto H., Bai Y., Gromada J., Larsson L. RNA-sequencing reveals altered skeletal muscle contraction, E3 ligases, autophagy, apoptosis, and chaperone expression in patients with critical illness myopathy. Skelet. Muscle. 2019;9:9. doi: 10.1186/s13395-019-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C., Milan G., Romanello V., Masiero E., Rudolf R., Del Piccolo P., Burden S.J., Di Lisi R., Sandri C., Zhao J. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Manning B.D., Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcell T.J. Sarcopenia: causes, consequences, and preventions. J. Gerontol. A. Biol. Sci. Med. Sci. 2003;58:M911–M916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- Martinez-Contreras R., Cloutier P., Shkreta L., Fisette J.F., Revil T., Chabot B. hnRNP proteins and splicing control. Adv. Exp. Med. Biol. 2007;623:123–147. doi: 10.1007/978-0-387-77374-2_8. [DOI] [PubMed] [Google Scholar]
- Masiero E., Agatea L., Mammucari C., Blaauw B., Loro E., Komatsu M., Metzger D., Reggiani C., Schiaffino S., Sandri M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Maurya S.K., Herrera J.L., Sahoo S.K., Reis F.C.G., Vega R.B., Kelly D.P., Periasamy M. Sarcolipin signaling promotes mitochondrial biogenesis and oxidative metabolism in skeletal muscle. Cell Rep. 2018;24:2919–2931. doi: 10.1016/j.celrep.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkin J., Russell C., Chen P., Burge C.B. Evolutionary dynamics of gene and isoform regulation in Mammalian tissues. Science. 2012;338:1593–1599. doi: 10.1126/science.1228186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniou P., Tiziano D., Frugier T., Roblot N., Le Meur M., Melki J. Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Res. 1999;27:e27. doi: 10.1093/nar/27.19.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson R., Jain M., Madhusudhan N., Sheppard N.G., Strittmatter L., Kampf C., Huang J., Asplund A., Mootha V.K. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat. Commun. 2014;5:3128. doi: 10.1038/ncomms4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott A., Meislin S.H., Moore M.J. A quantitative analysis of intron effects on mammalian gene expression. RNA. 2003;9:607–617. doi: 10.1261/rna.5250403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M., Suenkel B., Porras P., Migotti R., Schmidt F., Kny M., Zhu X., Wanker E.E., Dittmar G., Fielitz J. DCAF8, a novel MuRF1 interaction partner, promotes muscle atrophy. J. Cell Sci. 2019;132:jcs233395. doi: 10.1242/jcs.233395. [DOI] [PubMed] [Google Scholar]
- Otera H., Wang C., Cleland M.M., Setoguchi K., Yokota S., Youle R.J., Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q., Shai O., Lee L.J., Frey B.J., Blencowe B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- Patel S., Alvarez-Guaita A., Melvin A., Rimmington D., Dattilo A., Miedzybrodzka E.L., Cimino I., Maurin A.C., Roberts G.P., Meek C.L. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 2019;29:707–718.e8. doi: 10.1016/j.cmet.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli M., Wagner E.F. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016;30:489–501. doi: 10.1101/gad.276733.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips A.V., Timchenko L.T., Cooper T.A. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science. 1998;280:737–741. doi: 10.1126/science.280.5364.737. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J.D., White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risson V., Mazelin L., Roceri M., Sanchez H., Moncollin V., Corneloup C., Richard-Bulteau H., Vignaud A., Baas D., Defour A. Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J. Cell Biol. 2009;187:859–874. doi: 10.1083/jcb.200903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira Gonzalez Y.I., Moyer A.L., LeTexier N.J., Bratti A.D., Feng S., Sun C., Liu T., Mula J., Jha P., Iyer S.R. Mss51 deletion enhances muscle metabolism and glucose homeostasis in mice. JCI Insight. 2019;4:e122247. doi: 10.1172/jci.insight.122247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runfola V., Sebastian S., Dilworth F.J., Gabellini D. Rbfox proteins regulate tissue-specific alternative splicing of Mef2D required for muscle differentiation. J. Cell Sci. 2015;128:631–637. doi: 10.1242/jcs.161059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori R., Schirwis E., Blaauw B., Bortolanza S., Zhao J., Enzo E., Stantzou A., Mouisel E., Toniolo L., Ferry A. BMP signaling controls muscle mass. Nat. Genet. 2013;45:1309–1318. doi: 10.1038/ng.2772. [DOI] [PubMed] [Google Scholar]
- Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti M.M., Swanson M.S. RNA mis-splicing in disease. Nat. Rev. Genet. 2016;17:19–32. doi: 10.1038/nrg.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaborne R.A., Strauss J., Cocks M., Shepherd S., O'Brien T.D., van Someren K.A., Bell P.G., Murgatroyd C., Morton J.P., Stewart C.E. Human skeletal muscle possesses an epigenetic memory of hypertrophy. Sci. Rep. 2018;8:1898. doi: 10.1038/s41598-018-20287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavlakadze T., Morris M., Fang J., Wang S.X., Zhu J., Zhou W., Tse H.W., Mondragon-Gonzalez R., Roma G., Glass D.J. Age-related gene expression signature in rats demonstrate early, late, and linear transcriptional changes from multiple tissues. Cell Rep. 2019;28:3263–3273.e3. doi: 10.1016/j.celrep.2019.08.043. [DOI] [PubMed] [Google Scholar]
- Shen S., Park J.W., Lu Z.X., Lin L., Henry M.D., Wu Y.N., Zhou Q., Xing Y. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. U S A. 2014;111:E5593–E5601. doi: 10.1073/pnas.1419161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.K., Kolonin A.M., Fiorotto M.L., Cooper T.A. Rbfox-splicing factors maintain skeletal muscle mass by regulating Calpain3 and proteostasis. Cell Rep. 2018;24:197–208. doi: 10.1016/j.celrep.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen A., Elo J.M., Pietilainen K.H., Hakonen A.H., Sevastianova K., Korpela M., Isohanni P., Marjavaara S.K., Tyni T., Kiuru-Enari S. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 2011;10:806–818. doi: 10.1016/S1474-4422(11)70155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tress M.L., Martelli P.L., Frankish A., Reeves G.A., Wesselink J.J., Yeats C., Olason P.I., Albrecht M., Hegyi H., Giorgetti A. The implications of alternative splicing in the ENCODE protein complement. Proc. Natl. Acad. Sci. U S A. 2007;104:5495–5500. doi: 10.1073/pnas.0700800104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyynismaa H., Carroll C.J., Raimundo N., Ahola-Erkkila S., Wenz T., Ruhanen H., Guse K., Hemminki A., Peltola-Mjosund K.E., Tulkki V. Mitochondrial myopathy induces a starvation-like response. Hum. Mol. Genet. 2010;19:3948–3958. doi: 10.1093/hmg/ddq310. [DOI] [PubMed] [Google Scholar]
- Vance C., Rogelj B., Hortobagyi T., De Vos K.J., Nishimura A.L., Sreedharan J., Hu X., Smith B., Ruddy D., Wright P. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E.T., Sandberg R., Luo S., Khrebtukova I., Zhang L., Mayr C., Kingsmore S.F., Schroth G.P., Burge C.B. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.B., Bierinx A.S., Gnocchi V.F., Zammit P.S. Dynamics of muscle fibre growth during postnatal mouse development. BMC Dev. Biol. 2010;10:21. doi: 10.1186/1471-213X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.L., Satomi Y., Walsh K. RNA-seq and metabolomic analyses of Akt1-mediated muscle growth reveals regulation of regenerative pathways and changes in the muscle secretome. BMC Genomics. 2017;18:181. doi: 10.1186/s12864-017-3548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R., Tang P., Yang B., Huang J., Zhou Y., Shao C., Li H., Sun H., Zhang Y., Fu X.D. Nuclear matrix factor hnRNP U/SAF-A exerts a global control of alternative splicing by regulating U2 snRNP maturation. Mol. Cell. 2012;45:656–668. doi: 10.1016/j.molcel.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Hung L.H., Licht T., Kostin S., Looso M., Khrameeva E., Bindereif A., Schneider A., Braun T. RBM24 is a major regulator of muscle-specific alternative splicing. Dev. Cell. 2014;31:87–99. doi: 10.1016/j.devcel.2014.08.025. [DOI] [PubMed] [Google Scholar]
- Yang M., Vousden K.H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer. 2016;16:650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- Yates T.M., Vasudevan P.C., Chandler K.E., Donnelly D.E., Stark Z., Sadedin S., Willoughby J., Broad Center for Mendelian G., study D.D.D., Balasubramanian M. De novo mutations in HNRNPU result in a neurodevelopmental syndrome. Am. J. Med. Genet. A. 2017;173:3003–3012. doi: 10.1002/ajmg.a.38492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Beetz N., O'Keeffe S., Tapia J.C., Macpherson L., Chen W.V., Bassel-Duby R., Olson E.N., Maniatis T. hnRNP U protein is required for normal pre-mRNA splicing and postnatal heart development and function. Proc. Natl. Acad. Sci. U S A. 2015;112:E3020–E3029. doi: 10.1073/pnas.1508461112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C.L., Stone S.J., Koliwad S., Harris C., Farese R.V., Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008;49:2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugami M., Kabe Y., Yamaguchi Y., Wada T., Handa H. hnRNP-U enhances the expression of specific genes by stabilizing mRNA. FEBS Lett. 2007;581:1–7. doi: 10.1016/S0014-5793(07)01283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath J.R., Hawley J.A. Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS Biol. 2004;2:e348. doi: 10.1371/journal.pbio.0020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data are the output from DESeq2. Column headers are: Gene (Ensembl Gene ID), basemean, GeneName (NCBI, Official Symbol), Log2FoldChange, lfcSE (Log2FoldChange Standard Error), p-value, padj, Wald p-value. Data are separated into two tabs for genes increased and decreased in expression.
Data are the output from rMATS. Column headers are: ID (event number), GeneID (Ensembl), gene symbol (NCBI, Official Symbol), chr (chromosome), strand (+or - strand), p-value, FDR, IncLevelDifference (ΔPSI). Data are separated into five tabs, one each for skipped exon (SE), retained intron (RI), mutually exclusive exon (MXE), alternative 5′ splice site (A5SS), and alternative 3′ splice site (A3SS). Additional column headings specific for each type of splicing event are included in their respective tab.
Data Availability Statement
The accession number for the RNA sequencing data reported in this paper is NCBI Gene Expression Omnibus: GSE145712. No new computational tools were developed in this project. The code for usage of existing tools as described is available upon request.