Abstract
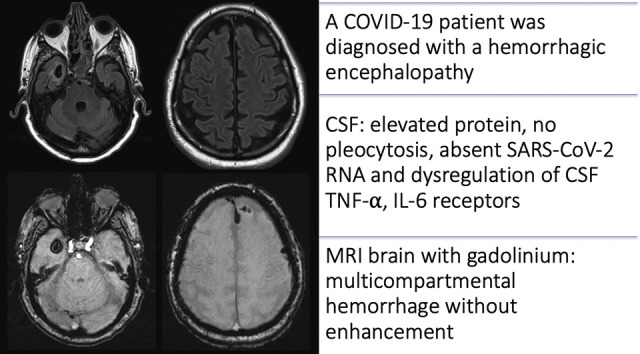
The mechanisms for neurological complications of COVID-19, the disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), are not yet well understood. We present a critically ill man with a COVID-19-associated hemorrhagic encephalopathy. SARS-CoV-2 RNA was not detected in cerebrospinal fluid (CSF) or blood. CSF analyses suggested dysregulation of pro-inflammatory cytokine pathways, particularly tumor necrosis factor-α and interleukin-6, consistent with a cytokine release syndrome. The patient gradually recovered with supportive care and neurological rehabilitation. Awareness of this clinical entity may facilitate the identification of patients with a potentially remediable cause of encephalopathy in COVID-19.
Keywords: COVID-19, Encephalopathy, SARS-CoV-2, Coronavirus, Intracerebral hemorrhage
Graphical abstract
Highlights
-
•
COVID-19 is a rapidly evolving and deadly global pandemic.
-
•
Cytokine storm syndromes have been proposed as a cause for severe disease.
-
•
Neurological manifestations of COVID-19 are an area of active research.
-
•
Pro-inflammatory CSF cytokine dysregulation may mediate hemorrhagic encephalopathy.
-
•
Vigilance for this clinical condition is required as COVID-19 continues to spread.
1. Introduction
COVID-19, the disease caused by infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has become a rapidly evolving and deadly global pandemic. Clinical presentations of COVID-19 range from mild nonspecific symptoms to critical illness from multi-organ failure. Neurological manifestations such as loss of taste or smell, seizure, and stroke have been observed and occur more frequently in more severely affected patients (Mao et al., 2020). Mechanisms for central nervous system (CNS) injury in the context of SARS-CoV-2 infection are an area of active discovery. Here we report the case of a patient with COVID-19 who presented with altered mental status progressing to severe cortical dysfunction. We describe brain magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) findings suggestive of a parainfectious hemorrhagic encephalopathy.
2. Case description
A 69 year-old man with a history of coronary artery disease, hypertension, and type 2 diabetes mellitus was admitted to hospital with acute encephalopathy and respiratory distress. Twelve days before admission he returned to Canada from a populous city in the United States, the last stop on an international journey. Four days before admission, symptoms of cough and fatigue prompted a nasopharyngeal swab for COVID-19, which tested positive for SARS-CoV-2 RNA by reverse transcriptase polymerase chain reaction (RT-PCR). The patient's neighbour called emergency services for increasing confusion over two days prior to admission.
On presentation the patient was hemodynamically stable, tachypneic, and hypoxemic requiring 3 L/min supplemental oxygen. He was alert and disoriented without lateralizing neurological deficits, but required sedation and intubation due to severe agitation. His chest radiograph was consistent with viral pneumonia from COVID-19. Initial evaluation for encephalopathy included an unremarkable computed tomography (CT) scan of the head (Fig. 1 ). CSF protein was elevated without pleocytosis or evidence of a culprit pathogen (Supplementary Table S1). Prerenal acute kidney injury, euglycemic diabetic ketoacidosis, E. coli urinary tract infection, and moderate hypoxemia were managed and improved. The patient required minimal vasopressor support while in intensive care.
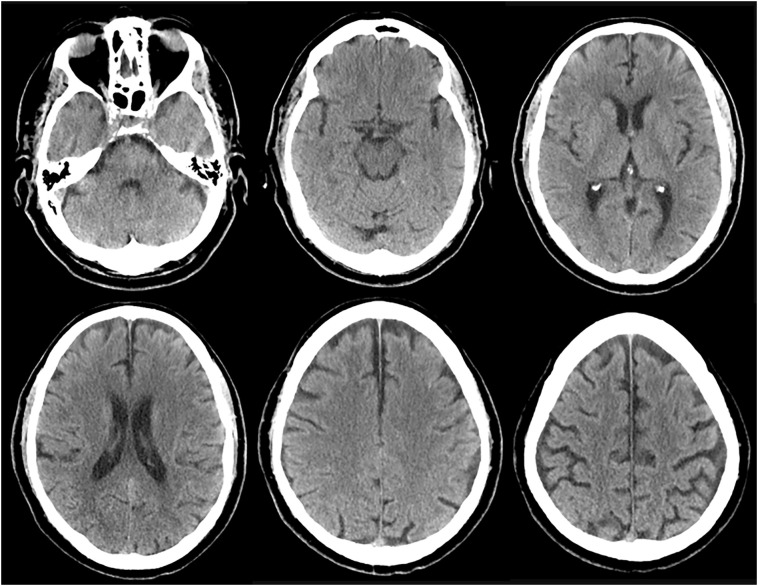
Fig. 1.
Non-contrast CT of the head on admission to hospital (day 0).
Axial images show no acute pathology to explain the clinical presentation. Chronic microangiopathic disease can be appreciated, along with age-appropriate mild generalized brain atrophy.
Thirteen days into the admission, the patient progressed from inconsistently following commands to being unresponsive and diffusely paretic without sedation. Neurological examination showed limited spontaneous respirations requiring controlled ventilation. His eyes were open spontaneously, conjugate and in midline. He did not follow commands to move his eyes or extremities. Pupillary, corneal, oculocephalic, gag, and cough responses were present and normal. He grimaced to central pain and had absent motor responses in the extremities to noxious stimuli. There were spontaneous, brief, flexion and pronation movements involving the arms. His tone was flaccid in the extremities with areflexia apart from a preserved left triceps reflex.
A gadolinium-enhanced cranial MRI (Fig. 2 ) showed multicompartmental hemorrhages with mild surrounding vasogenic edema and no abnormal enhancement. CT/CT angiogram of the head revealed no underlying vasculopathy (Supplementary Fig. S1). MRI of the spine with gadolinium was unremarkable. Laboratory results showed uremia (blood urea nitrogen 38 mmol/L, creatinine 107 μmol/dL), hypernatremia (150 mmol/L), and ongoing hypoxemia (PaO2/FiO2 ratio 165), but did not explain the degree of encephalopathy. Laboratory parameters did not show a bleeding diathesis. The platelet count was normal. International normalized ratio was borderline elevated at 1.1–1.3, with a normal partial thromboplastin time and no biochemical evidence of disseminated intravascular coagulation. Although serologies for lupus anticoagulant and beta-2-glycoprotein-1 were not evaluated, serum studies for anticardiolipin antibodies were negative. Electroencephalography showed generalized slowing with no focal abnormalities or epileptiform discharges. Electromyography/nerve conduction studies were confounded by extremity edema and could not rule in an axonal or demyelinating neuropathy.
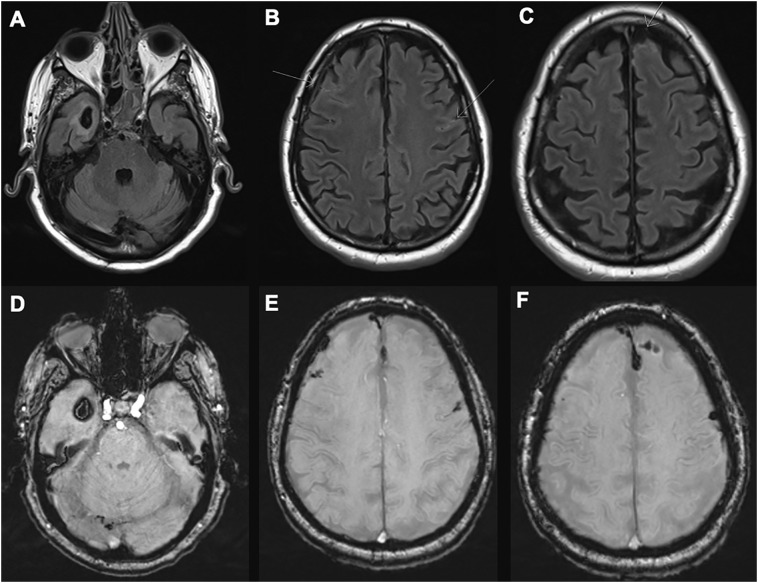
Fig. 2.
MRI of the brain on day 13.
Axial fluid-attenuated inversion recovery (FLAIR) [A-C] and susceptibility-weighted images (SWI) [D-F] demonstrate multifocal, multicompartmental hemorrhages with peri-hemorrhage vasogenic edema. Arrows show small hemorrhages in the subarachnoid space. MRI brain with gadolinium (not shown) demonstrated no abnormal contrast enhancement.
On day 14, CSF and blood tested negative for SARS-CoV-2 RNA by RT-PCR (Alberta Provincial Virology Laboratory), while nasopharyngeal and endotracheal samples remained positive. We examined soluble cytokine receptor levels in CSF from day 1 and 14 with a multiplex assay (Human Soluble Cytokine 14-Plex Clinical RUO Discovery Assay, Eve Technologies, Calgary, Canada). The samples showed high levels of the soluble receptors for interleukin-1 (sIL-1R), interleukin-6 (sIL-6R), soluble glycoprotein-130 (sgp130), and tumor necrosis factor-α (sTNFR) (Table 1 ). Based on the absence of pleocytosis and viral RNA in the CSF, we diagnosed a parainfectious encephalopathy. Off-label treatment with oral hydroxychloroquine was prescribed for a total of seven days based on in vitro data suggesting it can attenuate cytokine production in pre-treated microglia (Koch et al., 2015). A repeat MRI of the brain with gadolinium on day 21 demonstrated expected temporal evolution of the hemorrhagic lesions (Supplementary Fig. S2). Two months following admission, the patient gradually recovered. He was transferred to a neurorehabilitation unit with mild residual physical and cognitive impairments.
Table 1.
Cerebrospinal fluid (CSF) soluble cytokine receptor profile results from day 1 and day 14.
| Value | Units | Day 1 | Day 14 | 95% Range* |
|---|---|---|---|---|
| sCD30 | pg/ml | 3.88 | 0.01 | 0–27.7 |
| sEGFR | pg/ml | 7735.16 | 8048.82 | 1852–7958 |
| sIL-1RI | pg/ml | 6.13 | 3.36 | 0–15.2 |
| sIL-1RII | pg/ml | Not detectable | Not detectable | 0–364 |
| sIL-2Ra | pg/ml | 5.35 | 12.08 | 0–240 |
| sIL-4R | pg/ml | 0.01 | 0.01 | 0–35.2 |
| sIL-6R | pg/ml | 1445.88 | 1627.28 | 396–1553 |
| sgp130 | pg/ml | 47,407.19 | 67,658.05 | 15,223–62,771 |
| sRAGE | pg/ml | 1.36 | 0.27 | 0–4.1 |
| sTNFRI | pg/ml | 1597.91 | 2402.78 | 253–1309 |
| sTNFRII | pg/ml | 2513.66 | 3684.47 | 308–2760 |
| sVEGFR1 | pg/ml | 98.02 | 132.99 | 0–286.7 |
| sVEGFR2 | pg/ml | 2121.42 | 2700.49 | 388–2102 |
| sVEGFR3 | pg/ml | 12.96 | 7.92 | 0–65.1 |
Human Soluble Cytokine Receptor Panel (MILLIPLEX® assay, EMD MilliporeSigma). *Range depicts the 5th and 95th percentile based on the normal distribution provided by Eve Technologies, Calgary, Alberta, Canada. This distribution is derived from 124 patient CSF samples submitted to the laboratory (22 for research use, 102 for experimental clinical diagnostic use). Note that this is not a true ‘reference range’ since none of the patient samples are from healthy controls. The application of this assay is for research purposes only. Inter-assay coefficient of variation (CV) is less than 15% according to EMD MilliporeSigma. sCD30 = soluble CD30; sEGFR = soluble epidermal growth factor receptor; sgp130 = soluble glycoprotein 130; sIL-1R = soluble interleukin-1 receptor; sIL-2Ra = soluble interleukin-2 receptor-a; sIL-4R = soluble interleukin-4 receptor; sIL-6R = soluble interleukin-6 receptor; sRAGE = soluble receptor for advanced glycation endproducts; sTNFR = soluble tumor necrosis factor-α receptor; sVEGFR = soluble vascular endothelial growth factor receptor.
3. Discussion
Early experience with COVID-19 suggests that non-specific neurologic manifestations can occur in upwards of one-third of patients (Mao et al., 2020). Here, we report encephalopathy with multifocal cerebral hemorrhages in a COVID-19 patient with severe, otherwise unexplained cortical dysfunction. The combined absence of pleocytosis and SARS-CoV-2 RNA in CSF suggest against encephalitis, and no competing etiology was identified. A CSF cytokine receptor assay used experimentally showed pro-inflammatory cytokine dysregulation, particularly of the TNF-α and IL-6 systems. These findings led us to suspect parainfectious inflammation as a key disease mechanism. With supportive care and rehabilitation, the patient gradually recovered.
Acute necrotizing encephalopathy (ANE) is a rare parainfectious encephalopathy which presents with hemorrhagic brain lesions and mostly affects children with influenza. ANE is thought to be caused by a cytokine release syndrome, including dysregulation of the TNF-α and IL-6 systems (Aiba et al., 2001). Recently, a case of an adult with COVID-19 who presented with imaging features of ANE was published (Poyiadji et al., 2020). Unfortunately, a traumatic lumbar puncture limited CSF analysis in that case. While our patient did have some imaging features resembling ANE, his MRI did not demonstrate the typical medial thalamic lesions or abnormal contrast enhancement (Wong et al., 2006).
Direct invasion of CNS tissue by SARS-CoV-2 has been confirmed by ultrastructural analysis of a patient autopsy (Bulfamante et al., 2020). As reported by others, patients with neurological manifestations of COVID-19 have yielded mostly negative results with respect to CSF RT-PCR for SARS-CoV-2 RNA (Helms et al., 2020; Li et al., 2020), with positive results in a single case (Moriguchi et al., 2020). Reasons for this may relate to test sensitivity, timing, and whether neurological involvement is due to direct CNS injury or an epiphenomenon of severe systemic disease. A theoretical model suggests that clinical illness from COVID-19 may occur after the peak period of SARS-CoV-2 neuroinvasion (Panciani et al., 2020); where viral RNA titres within CSF fall below assay detection limits despite evolving CNS injury. SARS-CoV-2 RNA was not detected in the CSF of our patient during his established encephalopathy, yet pro-inflammatory cytokine dysregulation was apparent. Therefore, parainfectious inflammation may be a contributor. We cannot exclude direct viral neuroinvasion as a factor which triggers or sustains the illness.
A macrovascular explanation for our patient's intracranial hemorrhages was not found; however microvascular inflammation may play a role. SARS-CoV-2 infiltration and subsequent inflammation of vascular endothelial cells has been observed pathologically in multiple extracerebral tissues (Varga et al., 2020). Furthermore, reports of acute cerebrovascular events in otherwise healthy COVID-19 patients suggest vascular endothelial dysfunction as a potential disease mechanism (Saiegh et al., 2020; Oxley et al., 2020). This raises the possibility that our patient's concomitant CSF cytokine dysregulation and intracranial hemorrhages may relate to unique aspects of SARS-CoV-2 pathophysiology. As for hemorrhage precipitants, we did not find laboratory evidence of a coagulopathy in our patient. Combined pro-thrombotic and pro-hemorrhagic states have been reported in the context of an antiphospholipid antibody syndrome in COVID-19 patients (Zhang et al., 2020). Unlike the cases in this report, anticardiolipin antibodies were negative in our patient. The status of the other antiphospholipid antibodies is unknown.
Severe presentations of COVID-19 are associated with elevated systemic inflammatory markers, mediating a ‘cytokine storm’ that contributes to tissue damage (Mehta et al., 2020). Several soluble cytokine receptors were elevated in our patient's CSF, most notably the TNF-α (sTNFR1 and sTNFR2) and IL-6 (sIL-6R and sgp130) signaling systems (Table 1). Although this finding is intriguing, CSF reference ranges for the cytokine receptor assay have not been validated using data from healthy controls (see Table 1 for details). In addition, no serum samples were evaluated. TNF-α and IL-6 are pro-inflammatory cytokines elevated in the CSF of patients with inflammatory neurologic diseases when compared to controls (Pranzatelli, 2018). Both cytokine systems have pleotropic effects, systemically and within the nervous system (Probert, 2015; Wolf et al., 2014). As putative therapeutic targets in severe COVID-19 (Mehta et al., 2020), the impact of these cytokine systems on CNS injury merits further investigation. The use of hydroxychloroquine in COVID-19 remains controversial, and its application here was strictly off-label. Hydroxychloroquine use was based on data showing that pre-treatment of lipopolysaccharide-exposed microglia can attenuate the production of multiple cytokines in vitro (Koch et al., 2015). These data do not allow for extrapolation regarding hydroxychloroquine's influence on established cytokine release syndromes in severe COVID-19.
4. Conclusion
Cytokine dysregulation requires further investigation as a mechanism for CNS injury associated with COVID-19. The true incidence of parainfectious encephalopathy associated with COVID-19 is unknown. As the pandemic sweeps the globe, vigilance for this clinical entity is needed.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent for publication
Informed consent was obtained from the patient to publish this report.
Author contributorship
JDK, GAEJ, CEL, KF, SA and MWK participated in the clinical care for this patient. JDK and GAEJ wrote the first draft of the manuscript. All authors participated in the writing and interpretation of the clinical and investigation findings, and in the writing and revision of the manuscript.
Declaration of Competing Interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jneuroim.2020.577326.
Appendix A. Supplementary data
References
- Aiba H., Mochizuki M., Kimura M., Hojo H. Predictive value of serum interleukin-6 level in influenza virus-associated encephalopathy. Neurology. 2001;57:295–299. doi: 10.1212/wnl.57.2.295. [DOI] [PubMed] [Google Scholar]
- Bulfamante G., Chiumello D., Canevini M.P., Priori A., Mazzanti M., Centanni S., Felisati G. First ultrastructural autoptic findings of SARS-Cov-2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020 doi: 10.23736/S0375-9393.20.14772-2. [DOI] [PubMed] [Google Scholar]
- Helms, J., Kremer, S., Merdji, H., Clere-Jehl, R., Schenck, M., Kummerlen, C., Collange, O., Boulay, C., Fafi-Kremer, S., Ohana, M., Anheim, M., Meziani, F., 2020. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. NEJMc2008597–2. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed]
- Koch M.W., Zabad R., Giuliani F., Hader W., Jr., Lewkonia R., Metz L., Yong V.W. Hydroxychloroquine reduces microglial activity and attenuates experimental autoimmune encephalomyelitis. J. Neurol. Sci. 2015;358:131–137. doi: 10.1016/j.jns.2015.08.1525. [DOI] [PubMed] [Google Scholar]
- Li H., Xue Q., Xu X. Involvement of the nervous system in SARS-CoV-2 infection. Neurotox. Res. 2020:1–7. doi: 10.1007/s12640-020-00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020:1–8. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, U.K COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020:1–11. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., Skliut M., Weinberger J., Dangayach N.S., Bederson J.B., Tuhrim S., Fifi J.T. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panciani P.P., Saraceno G., Zanin L., Renisi G., Signorini L., Battaglia L., Fontanella M.M. SARS-CoV-2: “three-steps” infection model and CSF diagnostic implication. Brain Behav. Immun. 2020:1–3. doi: 10.1016/j.bbi.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranzatelli M.R. Advances in biomarker-guided therapy for pediatric- and adult-onset neuroinflammatory disorders: targeting chemokines/cytokines. Front. Immunol. 2018;9:229–231. doi: 10.3389/fimmu.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert L. TNF and its receptors in the CNS: the essential, the desirable, and the deleterious effects. Neuroscience. 2015;302:2–22. doi: 10.1016/j.neuroscience.2015.06.038. [DOI] [PubMed] [Google Scholar]
- Saiegh AlF, Ghosh R., Leibold A., Avery M.B., Schmidt R.F., Theofanis T., Mouchtouris N., Philipp L., Peiper S.C., Wang Z.-X., Rincon F., Tjoumakaris S.I., Jabbour P., Rosenwasser R.H., Gooch M.R. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J. Neurol. Neurosurg. Psychiatry. 2020 doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J., Rose-John S., Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Wong A.M., Simon E.M., Zimmerman R.A., Wang H.S., Toh C.H., Ng S.H. Acute necrotizing encephalopathy of childhood: correlation of MR findings and clinical outcome. AJNR Am. J. Neuroradiol. 2006;27:1919–1923. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H., Wang C., Zhao J., Sun X., Tian R., Wu W., Wu D., Ma J., Chen Y., Zhang D., Xie J., Yan X., Zhou X., Liu Z., Wang J., Du B., Qin Y., Gao P., Qin X., Xu Y., Zhang W., Li T., Zhang F., Zhao Y., Li Y., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.