Abstract
The present hypothesis suggests an innovative therapeutic strategy to cease Covid 19 infection. It is based on the inhibition of Spike glycoprotein and ACE-2 receptor interaction that provides the entry of virus in human host cells, by targeting the S protein with a recombinant molecule made of the ACE-2 receptor ectodomain and an opsonin, the formed complex would enhance its phagocytosis.
Keywords: Recombinant, ACE-2 ectodomain, Spike, SARS-CoV-2, Opsonin, Phagocytosis
Introduction
A novel SARS-like coronavirus (SARS-CoV-2) is currently emerging and rapidly spreading in humans; to cease this pandemic is to understand how the virus could infect host human cells. Coronavirus spike (S) glycoproteins promote entry into cells via interaction with angiotensin-converting enzyme 2 (ACE-2) [1]. The ACE-2 receptor is expressed unevenly in many human tissues [2]. The crystal structure of the SARS-CoV-2 receptor binding domain in complex with Human ACE-2 was determined [3]. The present article suggests a therapeutic approach to inhibit this interaction and refrain this infection.
The hypothesis
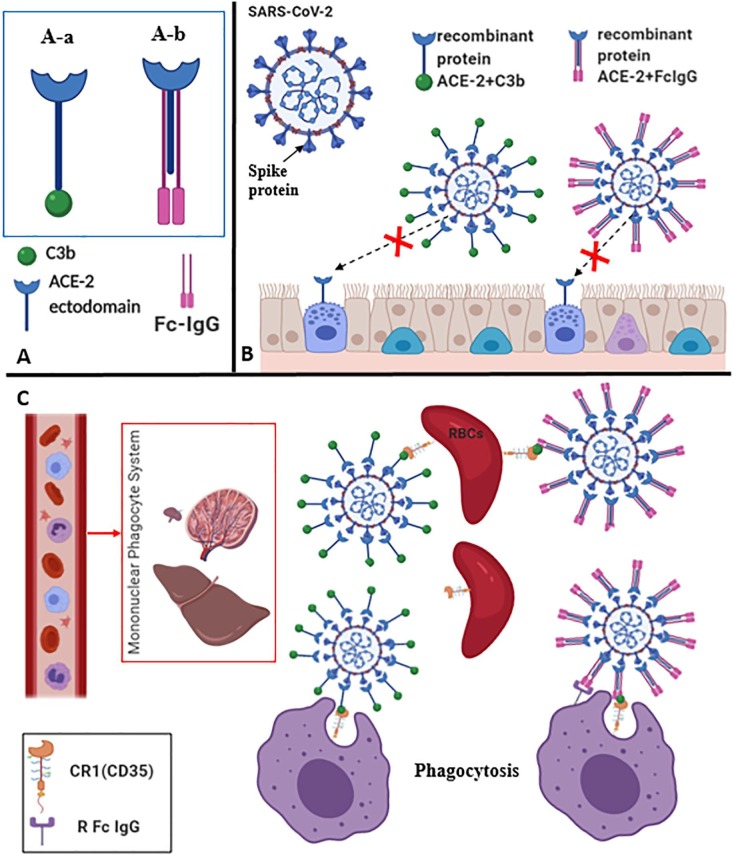
We should design a molecule that has the same affinity of ACE-2 to S protein; in a manner that the formed complex of its combination to S protein will be eliminated from the body. To that end, the author thought to produce a recombinant human protein constituted of two main parts with two possible designs (Fig. 1 -A).
Fig. 1.
Recombinant protein proposed designs and their pathways to induce SARS-CoV-2 phagocytosis The recombinant human protein is constituted of two main parts; the first represent the extracellular domain (ectodomain) of human ACE2 which is in a soluble form, the second is an opsonin molecule which is either the human C3b protein fragment (A-a), or the Fc fragment/CH1 domain of human IgG (A-b). The conjugated molecule targets S protein and inhibits its attachment to ACE-2 receptor on epithelial cells of respiratory or gastrointestinal tract (B). The immune complex generated by the recombinant protein two forms: ACE-2 + C3b and ACE-2 + FcIgG (and transported by RBCs) that enhance its phagocytosis with two distinct pathways: direct and indirect respectively.
Evaluation and discussion of the idea impact
The designed structures can not only target the virus by covering its surface and preventing its attachment to ACE-2 receptor (Fig. 1-B) but also opsonizing and enhancing phagocytosis with two distinct pathways, directly and indirectly (Fig. 1-C). The direct pathway is mediated by the C3b fragment (the larger of two elements formed by the cleavage of complement component 3) that binds to its receptor CR1 (CD35) expressed on red blood cells (RBCs) surface, RBCs transport the complex to the reticuloendothelial system (RES)/mononuclear phagocyte system (MPS) (liver, spleen, kidney, peyer's patches, bone morrow…etc.) to be phagocytized. The indirect pathway is activated by the Fc/CH1 domain of human IgG that binds to C3b, this later combines to its receptor expressed on RBCs surface, RBCs transport the complex to the RPS/ MPS to be phagocytized and eliminated. The receptor of Fc-IgG (RFc-IgG) and CR1 located on phagocyte surface help them to receive the complex. Within clinical administration of these molecules, they will imminently enter in competition with ACE-2 receptor for binding to S viral protein, the adaptation of an optimal dose (of these recombinant proteins) have to be taking into account to avoid this competition and rapidly overcoming the virus. In order to promote the indirect pathway, the blood C3b amount should be sufficient to convey the immune complex towards lymphoid tissues via RBCs, therefore we could simultaneously inject human C3b to ensure the activation of this pathways. In the other hand, this may prevent the deposit of the immune complex on some preferred tissues according to their high affinity or high blood pressure. This approach has many advantages that are briefly summarized (Table 1 ).
Table 1.
Advantages of the suggested recombinant protein.
| Advantage | Explanation |
|---|---|
| Gain of specificity at 100% | The use of ACE-2 ectodomain guaranties the same binding affinity |
| It avoids side effects | No chemical compound unlike drugs |
| It is based on innate immune system reactions | Opsonization and phagocytosis |
| It doesn’t create a cross reactivity of the immune system against the recombinant protein | Both of proteins are human; part of the body |
| It doesn’t require many technologies neither a lot of stages | Monoclonal antibodies production holds many steps and take more time |
| It could be applied for eventual new receptors engaged as an entry way of SARS-CoV-2 | The ectodomain will be changed according to the new receptor |
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110108.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Walls A.C. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li M.-Y., Li L., Zhang Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 94; 2020. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.