Abstract
Since the outbreak of the novel coronavirus disease COVID-19, caused by the SARS-CoV-2 virus, it has spread rapidly worldwide and poses a great threat to public health. This is the third serious coronavirus outbreak in <20 years, following SARS in 2002–2003 and MERS in 2012. So far, there are almost no specific clinically effective drugs and vaccines available for COVID-19. Polysaccharides with good safety, immune regulation and antiviral activity have broad application prospects in anti-virus, especially in anti-coronavirus applications. Here, we reviewed the antiviral mechanisms of some polysaccharides, such as glycosaminoglycans, marine polysaccharides, traditional Chinese medicine polysaccharides, and their application progress in anti-coronavirus. In particular, the application prospects of polysaccharide-based vaccine adjuvants, nanomaterials and drug delivery systems in the fight against novel coronavirus were also analyzed and summarized. Additionally, we speculate the possible mechanisms of polysaccharides anti-SARS-CoV-2, and propose the strategy of loading S or N protein from coronavirus onto polysaccharide capped gold nanoparticles vaccine for COVID-19 treatment. This review may provide a new approach for the development of COVID-19 therapeutic agents and vaccines.
Keywords: Polysaccharides, Coronavirus, COVID-19, SARS-CoV-2, Antiviral activity, Mechanism
1. Introduction
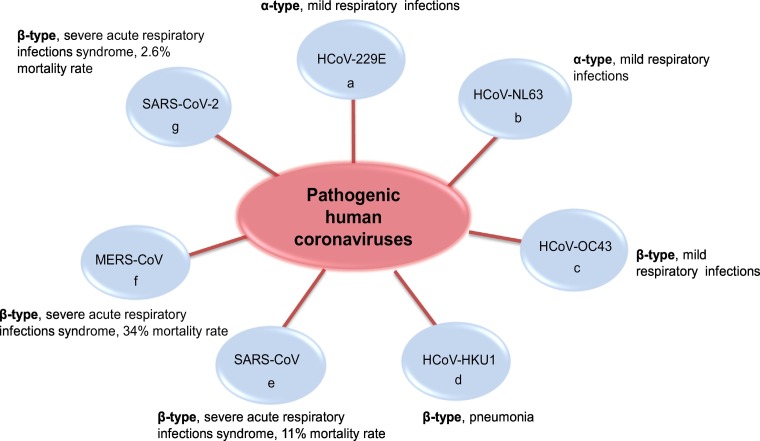
The present outbreak of a coronavirus-associated acute respiratory disease called coronavirus disease 19 (COVID-19) has spread to >210 countries, which is rare among acute infectious diseases in recent years, and caused a great threat to global public health [1]. This disease was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and it is the third documented spillover of an animal coronavirus to humans in only two decades [2,3]. Since the first human coronavirus detected in the 1960s, SARS-CoV-2 is the seventh coronavirus that is known to infect humans (Fig. 1 ) [4]. There are four types of coronaviruses named as α, β, γ and δ, which only α- and β-type contain human pathogenic strains [1,4]. SARS-CoV-2, including severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), are all β-coronal viruses [3].
Fig. 1.
The virus types and symptoms of 7 important pathogenic human coronaviruses.
Coronaviruses (CoVs) are enveloped single-stranded positive-sense RNA viruses (Fig. 2A), which widely infected vertebrates including humans and animals, to cause respiratory and enteric diseases [[5], [6], [7]]. Spike glycoprotein (S protein) plays a major role in the pathogenesis of coronavirus, inducing host immune responses, and is considered a primary target for vaccine preparation [1,8]. Current information indicates that SARS-CoV-2 is more contagious than SARS-CoV including persontoperson spread, which poses a serious threat to human health [3,9].
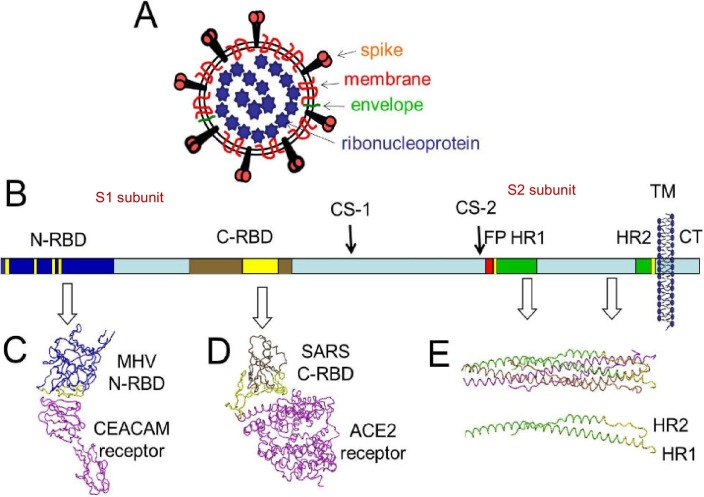
Fig. 2.
The structure of CoV virion and S protein, (A) Depiction of the CoV virion; (B) Depiction of S protein. A single S protein is depicted as a rectangle, and relevant structural features are highlighted as follows: N-terminal receptor binding domain (N-RBD) in dark blue; C-RBD in brown; cleavage sites (CS) 1 and 2, fusion peptide (FP) in red, heptad repeat (HR) regions 1 and 2 in green; transmembrane span (TM) depicted as membrane bilayer; cytoplasmic tail (CT) in light blue.; (C) Structure of the MHV N-RBD in complex with its CEACAM receptor.; (D) Structure of the SARS C-RBD in complex with its ACE2 receptor.; (E) Structure of the post-fusion HR1-HR2 bundle [7].
At present, there are almost no specific drugs for coronavirus therapy. Researchers have been working to inhibit growth of the virus, but the virus may mutate and develop resistance to these therapies [1,10]. It is urgent for the international medical community to develop targeted, high-efficiency and low-toxic drugs to treat the coronavirus based on the structure and property of the coronavirus. Polysaccharides have been used in traditional Chinese herbal medicine for at least 500 years [11], and have advantages of wide sources, low toxicity, good biocompatibility, and immune regulation [12,13]. Some polysaccharides, such as carrageenan, chitosan, fucoidan, and astragalus polysaccharide (APS), have been reported to show strong antiviral activity [[14], [15], [16], [17]]. In particular, sulfated polysaccharides can interfere with the entry process of virus by blocking the positive charge of the pathogen surface receptors, to prevent them from binding to heparan sulfate proteoglycan (HSPGs) on the surface of host cell [18]. Thus, polysaccharides are attractive candidates for developing potential antiviral agents. Here, we summarized and analyzed the antiviral properties, mechanisms, and applications of some polysaccharides and their derivatives in the anti-virus field, aiming to provide a new approach to the development of drugs and vaccines for the treatment of coronavirus, especially for COVID-19.
2. Application prospects of polysaccharides against coronavirus
Polysaccharides are macromolecular compounds obtained mainly from plants, algae, and even animals [15]. The antiviral properties of polysaccharides are not only a simple function of their charge density and chain length, but also their detailed structural characteristics [19]. Coronaviruses, such as SARS-CoV, MERS-CoV, and novel SARS-CoV-2, cause high mortality and pose a severe threat to humans and animals health, creating a need for effective inhibitors [20]. Polysaccharides, which are commonly used active ingredients in traditional Chinese medicine, have a great application prospect in the prevention and treatment of coronavirus based on their broad-spectrum antiviral activities and unique antiviral mechanisms. The presence of carbohydrate-binding agents can strongly inhibit coronaviruses, including transmissible gastroenteritis virus, infectious bronchitis virus (IBV), feline coronaviruses serotypes I and II, mouse hepatitis virus (MHV), and PRRSV [21].
2.1. Source and structure of polysaccharides
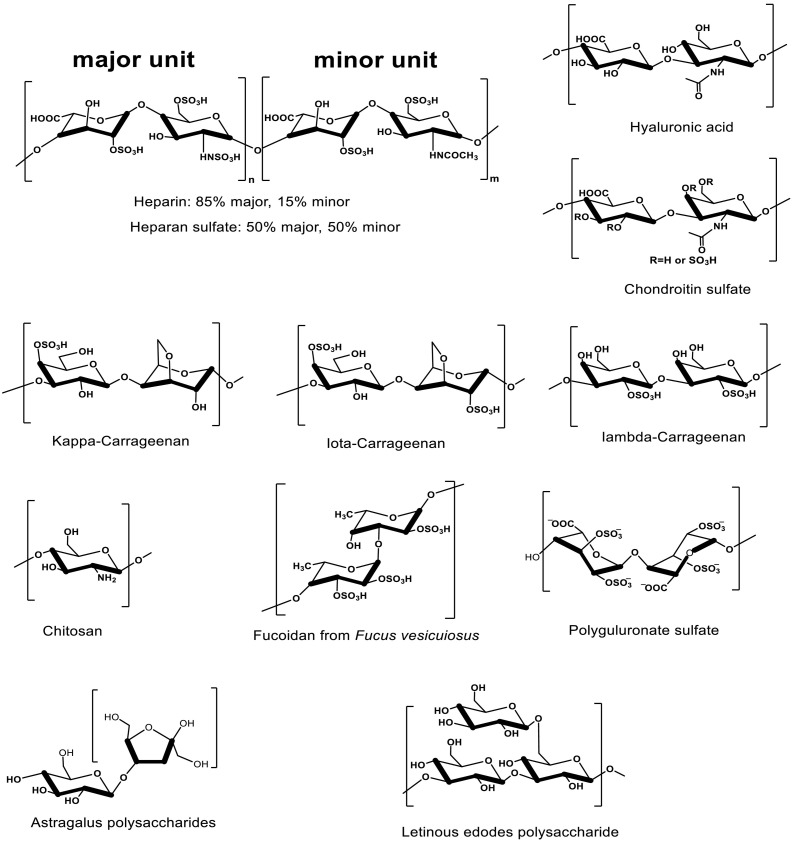
The main sources of polysaccharides are endogenous glycosaminoglycans (GAGs), Marine polysaccharides and terrestrial plant polysaccharides, especially polysaccharides from Chinese herbal medicines. GAGs are naturally-derived linear polysaccharides that are expressed in the intracellular compartments, cell surface, and extracellular environments, and they interact with various molecules to regulate many cellular processes associated with health and disease [22]. GAGs are comprised distinct O-linked disaccharide units, which are typically composed of a combination of iduronic acid, glucuronic acid, glucosamine, galactose or galactosamine monosaccharides [23,24]. The widely studied GAGs mainly include chondroitin sulfate (CS), heparan sulfate (HS) and heparin (HP) in animal tissues (Fig. 3 ) [22,25]. GAG chains are in most cases sulfated, except hyaluronan (HA) (Fig. 3), which are biodegradable and non-immunogenic in the body [26,27]. The chemical structures of typical GAGs are shown in Fig. 3.
Fig. 3.
The structures of several polysaccharides (GAGs, marine polysaccharides, and terrestrial plant polysaccharides).
Marine organisms are rich sources of polysaccharides. Chitosan is a linear, positive-charged, alkaline polysaccharide repeating by glucosamine and N-acetylglucosamine (Fig. 3) [28,29], derived from the shells of shrimps and crustacean or the cell walls of fungi [30,31]. Marine algae products have been applied in traditional Chinese herbal medicine for a long time [11], and contain a variety of polysaccharides, including carrageenan, fucoidan, and alginate. Carrageenans are sulphated linear polysaccharides composed of repeating disaccharide units with alternating 3-linked β-d-galactopyranose (G-units) and 4-linked α-galactopyranose (D-units) or 3,6-anhydro-α- galactopyranose (AnGal-units) [[32], [33], [34]], which are extracted from certain red algae containing 15–40% ester sulfate with an average molecular weight above 100 kDa [35,36]. The three commercial most important and widely distributed carrageenans are iota (ι-, G4S-DA2S), kappa (κ-, G4S-DA) and lambda (λ-, G2S-D2S, 6S)-carrageenan (Fig. 3) [37]. Fucoidan is a fucose-enriched and sulfated polysaccharide extracted from brown algae [11,38], which is composed of L-fucose, sulfate groups and small proportions of D-xylose, D-mannose, D-galactose, and D-glucuronic acid in different sources of brown algae (Fig. 3) [[38], [39], [40]]. Alginate, an acidic and linear polysaccharide extracted from brown algae, is consisted of alternating β-D-mannuronic acid (M) and α- L-guluronic acid (G) residues [41]. Polyguluronate sulfate (PGS) (Fig. 3) is a low molecular weight sulfated brown algae polysaccharide obtained by chemical sulfation of polyguluronate (PG) with about 1.5 sulfate per sugar residue [42,43].
Astragalus polysaccharide (APS) is the most important bioactive component isolated from a Chinese traditional herbal medicine of Astragalus membranaceus, which is composed of glucose, mannose, d-glucose, and D-galactose (Fig. 3) [[44], [45], [46]]. Radix Isatidis (RI) is also a kind of traditional Chinese herbal medicine with significant antiviral effect, and polysaccharide is its main active component [47,48]. The polysaccharide from RI is mainly composed of mannose, glucose, galactose and arabinose [49]. Mushrooms are used as food for long time in China, and also are drugs in the Orient centuries [50]. Lentinus edodes is one of the most widely edible mushrooms, and is popularly consumed as health foods in Asian countries [50,51]. Among the bioactive components of mushrooms, the Lentinus edodes polysaccharide (lentinan, LNT) is the most extensively investigated with many immune processes, which is generally described as biological response modifiers [52,53]. It consists of a β-(1 → 3)-glucan backbone with β-(1 → 6)-glucosyl side-branching units terminated by mannosyl or galactosyl residues (Fig. 3) [50,51]. Recently, LNT has been widely used as an alternative medicine and dietary supplement in the world [50].
2.2. Anti-coronavirus activity of GAGs
Cell surface GAGs serve as co-receptors by increasing the local concentration of pathogens, so that they can more efficiently interact with their entry receptors. Most coronavirus receptors of carbohydrate are mainly negatively charged, such as sulfated GAGs or glycans containing sialic acid [54,55]. S protein concentrated outside the virus contains the receptor binding domains (RBDs) at the N-terminal, such as MHV-CoV N-RBD and SARS C-RBD with their receptor (Fig. 2B–D) [7,56,57]. The coronavirus NL63 (CoV-NL63), and SARS-CoV use angiotensin-converting enzyme 2 (ACE2) as a primary receptor for infection of target cells (Fig. 2) [56,58,59]. Phylogenetically, SARS-CoV-2 is almost identical to SARS-CoV, sharing 79.6% genomic sequence identity [60], and use the same cell entry receptor, ACE2, as SARS-CoV [8,60]. During infection, CoV first binds host cell via interaction between its S1-RBD and the cell membrane receptor, triggering conformational changes in the S2 subunit that result in virus fusion and entry into the target cell. Viral RNA gradually forms mature virions through replication, transcription, and synthesis, and then is released from host cell (Fig. 4 ) [8,[60], [61], [62]]. However, the expression of ACE2 is not sufficient for infection, and HSPGs play important roles in the entry of some pathogens such as SARS-CoV [[63], [64], [65]]. A soluble HS was used to assess whether the attachment of HCoV-NL63 was mediated by HS proteoglycans. Flow cytometric analysis showed that the adhesion of virus to LLC-Mk2 cells was completely inhibited in the presence of soluble HS, indicating the role of this molecule in adhesion to susceptible cells and possible also in cell entry [54]. Both CoV-NL63 and SARS-CoV initially bind to the HS on the cell surface, and virus entry depends on the HS interaction, indicating that HS can inhibit virus attachment and entry [22,54].
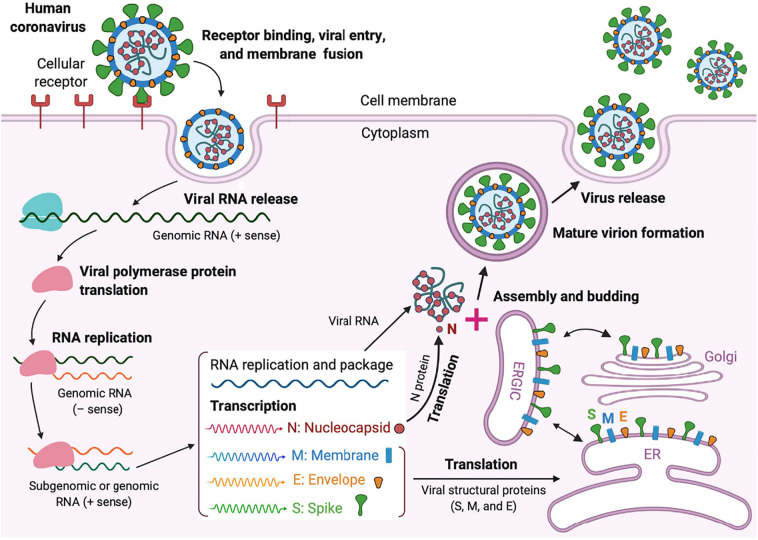
Fig. 4.
Life cycle of highly pathogenic human CoVs. These CoVs enter host cells by first binding to their respective cellular receptors via the surface S protein. Viral genomic RNA is released and translated into viral polymerase proteins. Viral RNA and nucleocapsid (N) structural protein are replicated, transcribed, or synthesized in the cytoplasm, whereas other viral structural proteins, including S, membrane (M), and envelope (E), are transcribed then translated in the endoplasmic reticulum (ER) and transported to the Golgi. The viral RNA–N complex and S, M, and E proteins are further assembled in the ER–Golgi intermediate compartment (ERGIC) to form a mature virion, then released from host cells [62].
Natural products of HS and the allied polysaccharide, heparin, are involved and prevent infection by a range of viruses including S-associated coronavirus strain HSR1 [66]. HS is known to bind CoV surface proteins and to be used by coronavirus for its attachment to target cells [54]. Currently, there are no commercially available medicinal products designed to treat and/or prevent infections associated with the novel SARS-CoV-2 coronavirus outbreak. The surface plasmon resonance and circular dichroism were used to measure the interaction between the SARS-CoV-2 Spike S1 protein RBD (SARS-CoV-2 S1 RBD) and heparin. Additionally, basic amino acids are known to dictate the binding between proteins and heparin. Primary sequence analysis of the expressed protein domain and analysis of the modeled SARS-CoV-2 S1 RBD structure show that there are several potential heparin binding sites, and more importantly, theses patches of basic amino acids are exposed on the protein surface. This study has implications for the rapid development of a first-line therapeutic by repurposing heparin and for next-generation, tailor-made, GAG-based antivirals [66].
2.3. Anti-coronavirus activity of marine polysaccharides
Marine polysaccharides, such as carrageenan, PGS, chitosan, and their derivatives, show good inhibitory activity against various viruses, which provides a reference for their research on coronavirus. Iota-carrageenan containing lozenges show highly active against human rhinovirus (HRV), influenza virus A H1N1, and HCoV OC43 throughout the entire dissolution process, and are a promising therapy against viral infections of throat [67]. The cationically modified chitosan, N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride (HTCC), shows significant inhibition against the human coronavirus HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1, and its hydrophobically modified derivative (HM-HTCC) is a potent inhibitor of the coronavirus HCoV-NL63, indicating that HTCC polymers based on chitosan are effective inhibitors of all low-pathogenic human coronaviruses [68]. Acute viral upper respiratory tract infection, also known as common cold, is mainly caused by respiratory viruses such as rhinovirus, coronavirus, influenza virus [[69], [70], [71]]. Clinical trials applying iota-carrageenan nasal spray have shown to reduce the duration of a virus-confirmed common cold. Carrageenan nasal spray shows significant antiviral efficacy in three virus subgroups, HRV, human coronavirus, and influenza A virus (IAV), and the highest effectiveness was observed in human corona virus-infected patients. The reduced duration of disease was 3 days (p < 0.01), and the number of relapses was three times less (p < 0.01) in carrageenan treated corona-virus -infected patients compared to control patients [70].
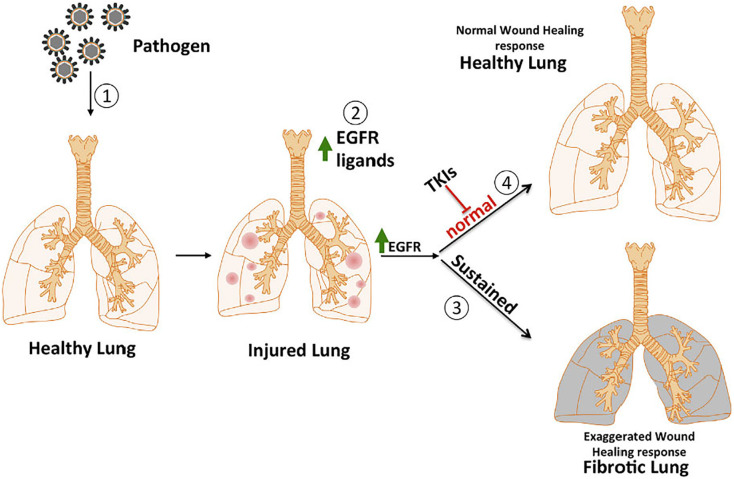
After the outbreak of SARS in 2003, many survivors developed residual pulmonary fibrosis with increased severity in older patients. Pulmonary fibrosis is caused by a hyperactive host response to lung injury mediated by epidermal growth factor receptor (EGFR) signaling in animal models (Fig. 5 ). Inhibition of EGFR signaling can prevent an excessive fibrotic response to SARS-CoV and other respiratory viral infections [72]. Moreover, sulfated polysaccharides such as fucoidan and sulfated rhamnan, can interfere or inhibit the expression and activation of EGFR pathway, which may help to suppress coronavirus [73,74]. The understanding of how polysaccharides play a role in EGFR and other pro-fibrotic pathways after viral infection will provide new ideas for COVID-19 treatment.
Fig. 5.
The illustration about potential role of EGFR in lung fibrosis. Physical injury or a pathogen ① initiates the wound healing response by damaging healthy tissue, releasing EGFR ligands ② and activating the EGFR pathway. This results in an exaggerated wound healing response leading to a fibrotic lung ③. The early use of inhibitors like tyrosine kinase ④ could prevent the normal progress of wound healing and fibrosis [72].
2.4. Anti-coronavirus activity of traditional Chinese medicine polysaccharides
Traditional Chinese herbal medicine is widely used in the prevention and treatment of viral infectious diseases in China [75]. Some Chinese herbs contain potential anti-SARS-CoV-2 active compounds, especially Hedysarum multijugum maxim, coptidis rhizoma, and forsythiae fructus, which have been catalogued for treating viral respiratory infections [76]. This provides a basis for the application of traditional Chinese medicine polysaccharides in coronavirus. The avian coronavirus causes infectious bronchitis (IB), which is one of the most serious diseases affecting the avian industry worldwide. APS can significantly reduce the replication of IBV in infected chicken embryo kidney (CEK) cells in a dose-dependent manner. The titer of IBV-specific antibodies, lymphocyte proliferation, and the expression levels of interleukin (IL)-1β, IL-2, IL-8, and TNF-a in APS treatment groups were higher than those in the control group. These data suggest that APS enhances the immune response to IBV vaccination in chickens, and is a potential therapeutic agent for inhibiting IBV [77,78]. During the outbreak of SARS coronaviruses in China, RI, as a Chinese medicinal herb, was prepared as an antiviral drug [79]. Polysaccharides isolated from RI have been shown to stimulate the expression of cytokines, such as IL-2 and interferon (INF)-γ, thereby regulating and enhancing non-specific immunological function, humoral immunity and cellular immunity in mice to play antiviral effects [48]. Active compounds derived from cultured Lentinula edodes mycelia (AHCC) is an α-glucan-based standardized mushroom extract that has been extensively investigated as an immunostimulant both in animals and in humans affected by influenza virus, herpes virus, avian influenza virus (AIV), human papillomavirus (HPV), hepatitis B virus (HBV), and human immunodeficiency virus (HIV) by promoting a regulated and protective immune response [80]. Due to its action in promoting a protective response to a wide range of viral infections, which can support its use in the prevention of diseases provoked by a human pathogenic coronavirus, including COVID-19 [80].
3. Polysaccharides have unique antiviral mechanisms
The antiviral mechanism of polysaccharides is usually related to its specific structure and virus type. Coronaviruses are enveloped positive-stranded RNA viruses that replicate in the cytoplasm [81]. To deliver the nucleocapsid into host cell, the coronavirus-cell entry procedure involves the fusion of the envelope with the host cell membrane mediated by viral S proteins (Fig. 2) [7,81], which is the main determinant of virus entry.
3.1. Directly interacting with virus
Polysaccharides, especially sulfated polysaccharides, can interact with the surface of virus by negative charge, thereby inhibiting the infectious ability of the virus, or killing the virus directly. Pathogens use GAGs at almost every major entry portal to promote their attachment and invasion of host cells, to move from one cell to another, and to protect themselves from immune attack [22]. For example, fucosylated chondroitin sulfate was effective in blocking laboratory strain HIV-1IIIB entry and replication (4.26 μg/mL and 0.73 μg/mL, respectively), and inhibiting infection by clinic isolate HIV-1KM018 and HIV-1TC-2 (23.75 μg/mL and 31.86 μg/mL, respectively) as well as suppressing HIV-1 drug-resistant virus. Further studies indicated that fucosylated chondroitin sulfate can potently bind the recombinant HIV-1 gp120 protein to inhibit several strains of HIV-1 [82]. A cationically modified chitosan derivative, HTCC, has been shown to be an effective inhibitor of HCoV-NL63 replication. The analysis of the interaction between HTCC polymer and the recombinant ectodomain of the S protein from CoV showed binding, resulting in the formation of protein-polymer complexes. One may assume that such binding will result in the efficient inactivation of the virus [83]. Carrageenan acts primarily by preventing the binding or the entry of virions into cells [84,85]. Iota-carrageenan, a high molecular weight sulfated polysaccharide, is an approved antiviral drug that interacts with the viral surface [67]. The binding and inactivation of virus particles by iota-carrageenan are fast and highly effective. During the residence time of the iota-carrageenan containing lozenge in the mouth, the viral titer is reduced by 85% and 91% for IAV and HCoV-OC43, respectively [67]. Furthermore, animal experiments have shown that iota-carrageenan can reduce the spreading of influenza virus in surface epithelia of infected animals, and thereby provided sufficient benefits for animals to promote survival [86].
3.2. Inhibiting virus adsorption and invasion
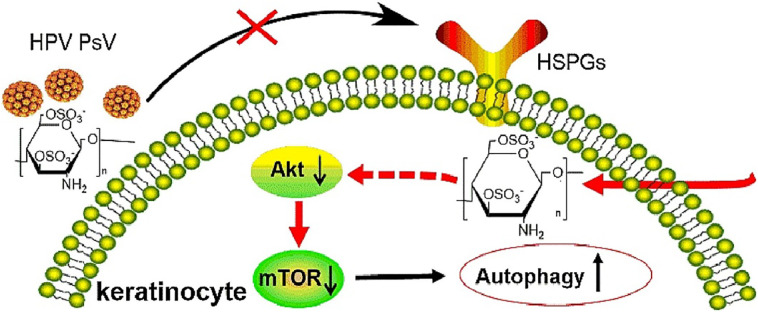
The first step for virus to invade a cell is to bind to the cell surface by electrostatic interaction or a receptor, such as heparan sulfated proteoglycan on the cell surface. Polysaccharides, especially sulfated polysaccharides, have strong polyanionic properties, and can block the positive charge on the cell surface to prevent virus adsorption or invasion [12]. The invasion process of virus is often associated with the endocytosis of virus, the fusion of virus with cell membrane, and the translocation of virus [12]. Heparin or heparin-like materials with broad-spectrum antiviral properties [[87], [88], [89]] have been developed to mimic the cell surface carbohydrates responsible for initial viral attachment, such as HS and carrageenan [34,90]. The sulfated polysaccharide derived from marine microalga showed strong inhibition against IAV infection via the viral adsorption and internalization steps [91]. The antiviral effect of sulfated polysaccharides from seaweeds was mainly exerted during dengue virus (DENV)-2 adsorption and internalization [92]. Iota-carrageenan and its N-sulfonated derivatives of poly (allylamine) hydrochloride showed strong antiviral activities against human metapneumovirus (hMPV), a kind of respiratory infections RNA virus, by blocking virus release from the cellular membrane and inhibiting virus adsorption [93]. Iota-carrageenan also effectively prevents the replication of HRV in primary human nasal epithelial cells in culture. The data suggest that iota-carrageenan acts primarily by preventing the binding or the entry of virions into the cells [85]. Fucoidan can bind to the neuraminidase (NA) of IAV, and inhibit the activity of NA to block the release of IAV. Additionally, fucoidan can also interfere with the activation of EGFR, PKCα, NF-κB, and Akt, and inhibit both IAV endocytosis and EGFR internalization in IAV-infected cells [73]. The antiviral mechanism of the fucoidans may be through blocking herpes simplex virus (HSV)-2 virion adsorption to host cells [94]. Our team found that 3,6-O-sulfated chitosan (36S) possessed broad anti-HPV activities by directly targeting viral capsid protein and host PI3K/Akt/mTOR pathway to inhibit cell autophagy (Fig. 6 ) [95]. Interestingly, using HCoV-NL63 as a model system, it can be determined that HTCC polymer blocks the interaction between S protein and cell receptor, consequently blocking its entry into cells and preventing virus infection [68,96]. The nano/microspheres of N-(2-hydroxypropyl) -3-trimethyl chitosan (HTCC-NS/MS) were used for adsorption of the coronavirus HCoV-NL63 from aqueous virus suspensions. This nano/microspheres can be applied for the removal of coronaviruses and purification of water from pathogenic coronaviruses [97].
Fig. 6.
The mechanism of 3,6-O-sulfated chitosan inhibiting HBV [95].
3.3. Inhibiting viral transcription and replication
Polysaccharides, especially sulfated polysaccharides, can directly interfere with viral replication related enzymes and relevant targets in host cells. Iota-carrageenan can effectively inhibit porcine reproductive and respiratory syndrome virus (PRRSV) replication at mRNA and protein levels in both Marc-145 cells and porcine alveolar macrophages [98]. Carrageenan oligosaccharide and its sulphated derivative have good inhibitory effects on IAV replication both in vitro and in vivo, while not seem to be dependent on the interferon system [99]. Sulfated polysaccharide from Gracilaria lemaneiformis shows anti-influenza virus activities in vitro by inhibiting viral adsorption and replication on host cells [100]. Polysaccharides isolated from Grifola frondosa showed resistance against enterovirus 71, a positive-stranded RNA virus, by blocking viral replication and inhibiting viral VP1 protein expression and genomic RNA synthesis [101]. The virus replication was inhibited by a sulfated polysaccharide from Angelica sinensis, which is a commonly used traditional Chinese herbal medicine, at the dose of 10 and 30 mg/kg (26% and 30% inhibition respectively) [102]. APS has a long-lasting inhibitory effect on HBV replication in vivo, which can be used as a supplementary modality to treat hepatitis B infection [103]. Furthermore, APS can inhibit the replication of avian IBV in vitro in a dose-dependent manner [78].
3.4. Activating host antiviral immunomodulatory system
After the virus invades the host, it will trigger the host's immune response, such as regulating the host NK and macrophages cells, inducing the production of immune cytokines, and indirectly exert antiviral effects by activating innate immunity. Chitosan can enhance antigen-specific immune responses by increasing the induction of regulatory T cells, lung resident T cells, and neutralizing antibodies while reversing Th2-skewed immune responses induced by inactivated respiratory syncytial virus (RSV) vaccine without affecting lung histopathology in mice [13]. The sulphated-carrageenan from red alga showed a strong effect on tobacco mosaic virus (TMV) infection by affecting virus accumulation/infectivity and enhancing locally plant immunity [104]. APS can significantly enhance the immunological function of chicken erythrocytes after infected with infectious bursa disease virus (IBDV) [105]. Additionally, APS can reduce the replication of H9N2 AIV and promote early humoral immune responses in young chickens [17]. LNT can significantly down-regulate the expression level of TNF-α, IL-2 and IL-11, and up-modulate the expression levels of IFN-1 and IFN-γ after challenging with infectious hematopoietic necrosis virus (IHNV), which is an RNA virus. The results indicate that the inhibitory effects of LNT on IHNV infection are possibly attributed to its regulation of the innate immune responses and specific immunity [51].
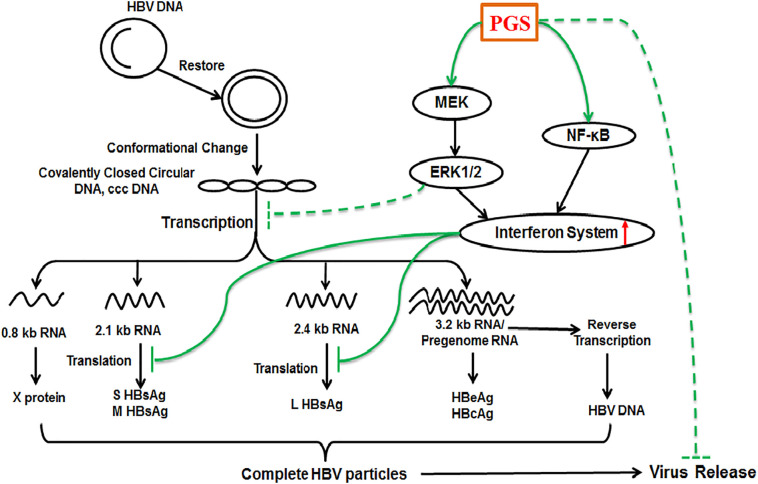
In addition, our team found that PGS, a sulfated derivative of alginate, can effectively inhibit the expression and secretion of HBsAg and HBeAg in HepG2.2.15cells. The anti-HBV mechanism of PGS may be associated with appropriate activation of NF-κB and Raf/MEK/ERK signaling pathways to enhance the interferon system, and interfere with HBV transcription (Fig. 7 ). This study suggested that PGS merits further investigation as a novel anti-HBV agent aimed at modulating the host innate immune system in the future [42]. These studies bring new ideas to the development of current anti-novel coronavirus drugs.
Fig. 7.
The molecular mechanisms of PGS inhibits HBV replication. Cellular NF-κB and Raf/MEK/ERK signaling pathways are associated with the activation of innate immune system such as interferon system. PGS can bind and enter into HepG2.2.15 cells to activate the NF-κB and Raf/MEK/ERK pathways to enhance the interferon system, and indirectly suppress HBV transcription [42].
4. Polysaccharide adjuvant for antiviral vaccine
Vaccination is the most successful and effective medical intervention to prevent infectious diseases, which can reduce mortality, prolong life expectancy and improve quality of life [106]. The development of a vaccine for coronavirus is a critical step in prevention, but it may not be effective for future strains, and we must be ready for the next epidemic [3]. Polysaccharide adjuvant can enhance the immune effect of a vaccine, thus promoting body-specific immunity and non-specific immunity, cellular immunity, humoral immunity and mucosal immunity [107]. Chitosan is effective in stimulating humoral and cell-mediated immune responses with a proven safety record in animals and humans, which has been used as adjuvant for improving vaccine efficacy, especially in RNA virus vaccines [13,108,109]. Chitosan can only modestly protect animals against RSV infection when given post-infection, while it can significantly reduce RSV infection in mice when combined with inactivated RSV vaccine before infection. This study suggested that chitosan can be applied as a potential treatment/adjuvant for RSV infection [13]. Chitosan-adjuvanted vaccines can enhance antibody titers against A- and B-type human influenza viruses 4 to 6 times compared with the vaccines without chitosan. Inactivated AIV A/H5N2 admixed with chitosan, when administered to mice challenged afterwards with the same virus, showed higher immunogenicity and protective efficacy compared with the antigen without chitosan [110]. Chitosan adjuvanted vaccine stored at 4 °C can preserve its adjuvant properties for at least 8 months. Chitosan can stimulate proliferative and cytotoxic activity of splenic mononuclear leukocytes in mice [111]. Thus, chitosan is a promising adjuvant candidate for inactivated influenza vaccines, which provides a reference for the development of anti-novel coronavirus vaccines.
Although SARS-CoV vaccines can protect against lethal infection, the addition of delta inulin-based polysaccharide adjuvant on day 3 post-challenge can significantly increase serum neutralizing-antibody titers and reduce lung virus titers. It also shows that immunity achieved with delta inulin adjuvants is long-lived, thereby overcoming the natural tendency for rapidly waning coronavirus immunity. This suggests that delta inulin polysaccharide adjuvants have the potential to develop more effective coronavirus vaccines [20]. In addition, some Chinese herbal medicinal polysaccharides have been used as safe and effective adjuvants [77,107,112,113]. APS as an adjuvant combined with influenza vaccine can improve the immune response and systemic humoral response to H5N1 virus infection [46,114]. APS can potentially be used as an immunomodulator for a foot-and-mouth disease virus (FMDV), which is an RNA virus vaccine, and provide better protection against FMDV [115]. APS is also a potent adjuvant for hepatitis B DNA vaccine, and can enhance the immune responses of HBV DNA vaccine via promoting dendritic cells maturation and inhibit the regulatory T cells frequency [116]. Polysaccharide extract from RI exerts potent anti-IAV activity against human seasonal influenza viruses (H1N1 and H3N2) and AIV (H6N2 and H9N2) in vitro [49]. The polysaccharides also significantly reduced the expression of pro-inflammatory cytokines (IL-6) and strongly inhibited the protein expression of TLR-3 induced by PR8. The polysaccharide extract from RI, therefore, has the potential to be used as an adjunct to antiviral therapy for the treatment of IAV infection [49].
Additionally, peptide-based vaccines have become as a potentially important strategy for the development of therapeutic vaccination [117,118]. They do not require in vitro culture, making them biologically safe, and their selectivity can accurately activate the immune responses [119,120]. For example, a hydrocarbon-stapled short α-helical peptide can effectively inhibit MERS-CoV infection and its S protein-mediated cell-cell fusion [121]. The epitopes selected from the S glycoprotein of SARS-COV-2 can be used to design and prepare immunogenic multi-epitopic peptide vaccine against novel coronavirus disease caused by SARS-CoV-2 [122,123]. The T cell multi epitopes-based peptide vaccine was designed for COVID-19 using the envelope protein of SARS-CoV-2 as an immunogenic target [124]. Carrageenan and its structurally related compounds may serve as innovative adjuvants for enhancing peptide-based vaccine potency through immune enhancement [118]. The glycopeptides prepared by the combination of selected polysaccharides with peptides, and the peptide vaccines with polysaccharide adjuvants, will have important application prospects for inhibiting coronaviruses.
5. Antiviral nanomaterials and delivery systems of polysaccharides
Nanobiotechnology provides a variety of solutions for the prevention, diagnosis, and treatment of infectious diseases such as viruses. Nanoparticles can be designed as an efficient delivery system to increase the activity of low toxicity vaccines against the host [125]. The application of nanoparticles in vaccine formulations can not only improve the immunogenicity and stability of antigen, but also achieve targeted delivery and sustained release [126]. Nanomaterials may have intrinsic immunomodulatory functions, acting as adjuvants or immune potentiators [127]. The chitosan/alginate nanoparticle encapsulated bee venom (BV) with slow-releasing properties and mucosal adhesiveness has been developed, which can effectively induce non-specific immune stimulation, particularly those related to Th1 responses and viral clearance activities against PRRSV infection [128]. The effectiveness of a novel nanoparticle vaccine is supported by stimulating effective neutralizing antibody and antigen-specific T cell responses in mice immunized with a MERS-CoV nanoparticle vaccine candidate. Using a MERS-CoV-permissive transgenic mouse model, it is shown that the mice immunized with this nanoparticle-based MERS-CoV vaccine can protect against a lethal challenge of MERS-CoV without triggering undesirable eosinophilic immunopathology. The biocompatible hollow nanoparticle may accelerate the development of effective and safe vaccines against emerging coronavirus pathogens [129]. Bovine coronavirus (BCV) N protein-loaded chitosan nanoparticles (CNP) can significantly increase both IgA and IgG levels after the second immunization comparable to the control group. The IgM content in serum increased after the second immunization in the BCV N protein-loaded CNP group. These findings indicate that CNP can be used as a novel adjuvant in veterinary vaccine field [130].
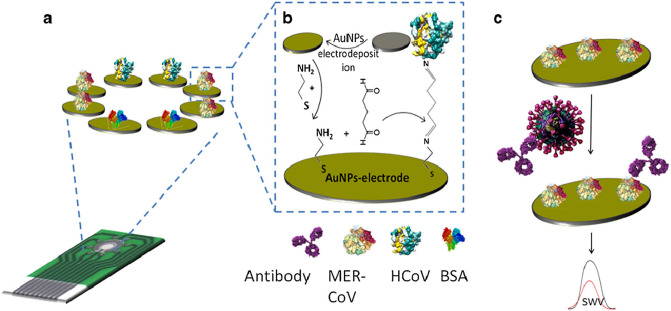
In addition, the gold nanoparticles (AuNPs)-based nanomaterials also have good application prospects in anti-novel coronavirus. An electrochemical immunosensor based on an array of AuNPs-modified carbon electrodes was used for the determination of MERS-CoV (Fig. 8 ) [131]. Highly sulfonated gold nanoparticles and heparin coated AuNPs with no cytotoxicity displayed broad-spectrum virucidal properties against HSV, HPV, RSV, dengue and lentivirus in vitro [132]. S protein plays a key role in the pathogenesis of coronavirus, and it is considered a primary target for vaccine preparation [8,133]. Virus like particles (sVLPs) were prepared by protein corona formation with IBV S protein as a model antigen and incubated with 100 nm AuNPs in a solution containing an optimized concentration of viral proteins [133]. As compared to inoculation with free proteins, vaccination with the sVLPs showed enhanced lymphatic antigen delivery, stronger antibody titers, increased splenic T-cell response, and reduced infection-associated symptoms in an avian model of coronavirus infection [133]. The study demonstrates a simple and reliable method in bridging viral antigens with synthetic nanoparticles for improved vaccine application [133].
Fig. 8.
COV immunosensor array chip (a). The immunosensor fabrication steps (b), the detection process of the competitive immunosensor for the virus (c) [131].
Additionally, polysaccharides or their derivatives, such as carrageenan oligosaccharide and fucoidan derived from marine algae with biodegradability, abundance, and non-toxicity, can be used as biocompatible reductants for green synthesis AuNPs [134,135]. Based on our previous works, we hypothesize that the S or N protein from coronavirus can be designed to be loaded onto polysaccharides capped AuNPs by protein corona formation for coronavirus nanoparticles vaccine application (Fig. 9 ).
Fig. 9.
The proposed schematics illustrating the S or N protein from coronavirus loaded onto polysaccharides capped AuNPs.
6. Conclusions & future perspectives
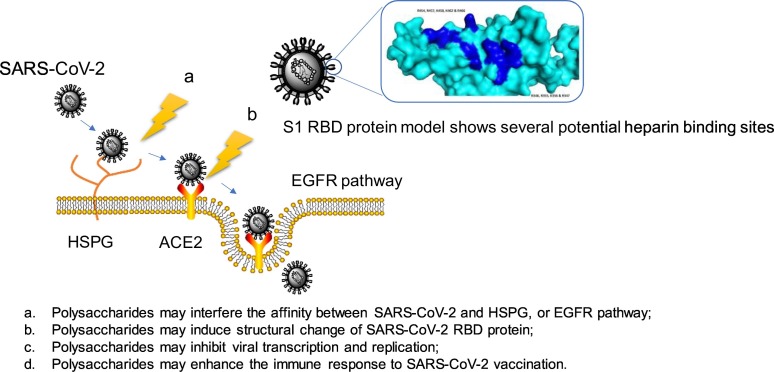
Polysaccharides have a long history to be used as important active ingredients in traditional Chinese medicine due to their extensive activity and reliable safety. Polysaccharides have broad spectrum of antiviral activities and unique antiviral mechanisms. They can exert antiviral effects by interfering with the life cycle of virus, or can indirectly exert antiviral activities by enhancing the body's immunity, which makes them have a great application prospect in anti-coronavirus. GAGs, marine polysaccharides such as carrageenan and chitosan, traditional Chinese medicine polysaccharides such as APS and LNT, and their derivatives, have shown potent anti-coronavirus activity and multiple anti-coronavirus mechanisms. We speculate that polysaccharides will exert anti-SARS-CoV-2 effects by the possible mechanisms as shown in Fig. 10 . More importantly, polysaccharide-based vaccine adjuvants, nanomaterials, and drug delivery systems will play important roles in anti-coronavirus field. Based on the immunomodulation and antiviral activity of polysaccharides, combined with nanotechnology, we put forward the strategy of loading S or N protein of coronavirus onto polysaccharide capped AuNPs vaccine for COVID-19 treatment (Fig. 9). This review may provide a novel idea for the development of anti-COVID-19 drugs and vaccines.
Fig. 10.
The proposed mechanisms of polysaccharides anti-SARS-CoV-2.
Acknowledgments
Acknowledgments
This work was supported by NSFC-Shandong Joint Fund (U1606403), and Innovation Project of Qingdao National Laboratory for Marine Science and Technology (No.2015ASKJ02).
Declaration of competing interest
The authors have declared no conflict of interest.
References
- 1.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S., Albaiu D. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6(3):315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.V. Coronaviridae Study Group of the International Committee on Taxonomy of The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y., Peng F., Wang R., Guan K., Jiang T., Xu G., Sun J., Chang C. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthony S.J., Johnson C.K., Greig D.J., Kramer S., Che X., Wells H., Hicks A.L., Joly D.O., Wolfe N.D., Daszak P., Karesh W., Lipkin W.I., Morse S.S., P. Consortium, Mazet J.A.K., Goldstein T. Global patterns in coronavirus diversity. Virus Evol. 2017;3(1) doi: 10.1093/ve/vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv. Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heald-Sargent T., Gallagher T. Ready, set, fuse! The coronavirus spike protein and acquisition of fusion competence. Viruses. 2012;4(4):557–580. doi: 10.3390/v4040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang B., Bragazzi N.L., Li Q., Tang S., Xiao Y., Wu J. An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov) Infect Dis Model. 2020;5:248–255. doi: 10.1016/j.idm.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chase G., Wunderlich K., Reuther P., Schwemmle M. Identification of influenza virus inhibitors which disrupt of viral polymerase protein-protein interactions. Methods. 2011;55(2):188–191. doi: 10.1016/j.ymeth.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Dutot M., Grassin-Delyle S., Salvator H., Brollo M., Rat P., Fagon R., Naline E., Devillier P. A marine-sourced fucoidan solution inhibits toll-like-receptor-3-induced cytokine release by human bronchial epithelial cells. Int. J. Biol. Macromol. 2019;130:429–436. doi: 10.1016/j.ijbiomac.2019.02.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L., Huang G. The antiviral activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018;115:77–82. doi: 10.1016/j.ijbiomac.2018.04.056. [DOI] [PubMed] [Google Scholar]
- 13.Muralidharan A., Russell M.S., Larocque L., Gravel C., Sauve S., Chen Z., Li C., Chen W., Cyr T., Rosu-Myles M., Wang L., Li X. Chitosan alters inactivated respiratory syncytial virus vaccine elicited immune responses without affecting lung histopathology in mice. Vaccine. 2019;37(30):4031–4039. doi: 10.1016/j.vaccine.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Hao C., Yu G., He Y., Xu C., Zhang L., Wang W. Marine glycan-based antiviral agents in clinical or preclinical trials. Rev. Med. Virol. 2019;29(3) doi: 10.1002/rmv.2043. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y.E., Kim H., Seo C., Park T., Lee K.B., Yoo S.Y., Hong S.C., Kim J.T., Lee J. Marine polysaccharides: therapeutic efficacy and biomedical applications. Arch. Pharm. Res. 2017;40(9):1006–1020. doi: 10.1007/s12272-017-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H., Huang G., Chen X. Chemical modifications and biological activities of polysaccharides. Curr. Drug Targets. 2016;17:1799–1803. doi: 10.2174/1389450117666160502151004. [DOI] [PubMed] [Google Scholar]
- 17.Kallon S., Li X., Ji J., Chen C., Xi Q., Chang S., Xue C., Ma J., Xie Q., Zhang Y. Astragalus polysaccharide enhances immunity and inhibits H9N2 avian influenza virus in vitro and in vivo. Journal of Animal Science and Biotechnology. 2013;4 doi: 10.1186/2049-1891-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurienzo P. Marine polysaccharides in pharmaceutical applications: an overview. Mar Drugs. 2010;8(9):2435–2465. doi: 10.3390/md8092435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bimalendu R. Focus on antivirally active sulfated polysaccharides: from structure–activity analysis to clinical evaluation. Glycobiology. 2008;1:1–15. doi: 10.1093/glycob/cwn092. [DOI] [PubMed] [Google Scholar]
- 20.Honda-Okubo Y., Barnard D., Ong C.H., Peng B.H., Tseng C.T., Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J. Virol. 2015;89(6):2995–3007. doi: 10.1128/JVI.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Meer F.J., de Haan C.A., Schuurman N.M., Haijema B.J., Peumans W.J., Van Damme E.J., Delputte P.L., Balzarini J., Egberink H.F. Antiviral activity of carbohydrate-binding agents against Nidovirales in cell culture. Antivir. Res. 2007;76(1):21–29. doi: 10.1016/j.antiviral.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.P.W. Park, Glycosaminoglycans and infection, Front. Biosci. 21(6) 1260–1277. [DOI] [PMC free article] [PubMed]
- 23.Gandhi N.S., Mancera R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008;72(6):455–482. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 24.Miller T., Goude M.C., McDevitt T.C., Temenoff J.S. Molecular engineering of glycosaminoglycan chemistry for biomolecule delivery. Acta Biomater. 2014;10(4):1705–1719. doi: 10.1016/j.actbio.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hachim D., Whittaker T.E., Kim H., Stevens M.M. Glycosaminoglycan-based biomaterials for growth factor and cytokine delivery: making the right choices. J. Control. Release. 2019;313:131–147. doi: 10.1016/j.jconrel.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh E.J., Park K., Kim K.S., Kim J., Yang J.A., Kong J.H., Lee M.Y., Hoffman A.S., Hahn S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release. 2010;141(1):2–12. doi: 10.1016/j.jconrel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Valcarcel J., Novoa-Carballal R., Perez-Martin R.I., Reis R.L., Vazquez J.A. Glycosaminoglycans from marine sources as therapeutic agents. Biotechnol. Adv. 2017;35(6):711–725. doi: 10.1016/j.biotechadv.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Wang H., Qian J., Ding F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018;66(2):395–413. doi: 10.1021/acs.jafc.7b04528. [DOI] [PubMed] [Google Scholar]
- 29.Yen M.-T., Yang J.-H., Mau J.-L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009;75(1):15–21. [Google Scholar]
- 30.K. Kurita, Chitin and chitosan: functional biopolymers from marine crustaceans, Mar. Biotechnol. 8(3) 203–226. [DOI] [PubMed]
- 31.Asier S., Rene H.D., María A., Susana F., Jalel L. The antifungal activity of functionalized chitin nanocrystals in poly (lactid acid) films. Materials. 2017;10(5):546–561. doi: 10.3390/ma10050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coviello T., Matricardi P., Marianecci C., Alhaique F. Polysaccharide hydrogels for modified release formulations. J. Control. Release. 2007;119(1):5–24. doi: 10.1016/j.jconrel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Jiao G., Yu G., Zhang J., Ewart H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. 2011;9(2):196–223. doi: 10.3390/md9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Necas J., Bartosikova L. Carrageenan: a review. Veterinãrnã Medicãna. 2013;58(4):187–205. [Google Scholar]
- 35.Robal M., Brenner T., Matsukawa S., Ogawa H., Truus K., Rudolph B., Tuvikene R. Monocationic salts of carrageenans: preparation and physico-chemical properties. Food Hydrocoll. 2017;63:656–667. [Google Scholar]
- 36.Sedayu B.B., Cran M.J., Bigger S.W. A review of property enhancement techniques for carrageenan-based films and coatings. Carbohydr. Polym. 2019;216:287–302. doi: 10.1016/j.carbpol.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 37.Campo V.L., Kawano D.F., Silva D.B.d., Carvalho I. Carrageenans: biological properties, chemical modifications and structural analysis–a review. Carbohydr. Polym. 2009;77(2):167–180. [Google Scholar]
- 38.Wu L., Sun J., Su X., Yu Q., Yu Q., Zhang P. A review about the development of fucoidan in antitumor activity: progress and challenges. Carbohydr. Polym. 2016;154:96–111. doi: 10.1016/j.carbpol.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Ale M.T., Maruyama H., Tamauchi H., Mikkelsen J.D., Meyer A.S. Fucose-containing sulfated polysaccharides from brown seaweeds inhibit proliferation of melanoma cells and induce apoptosis by activation of caspase-3 in vitro. Mar Drugs. 2011;9(12):2605–2621. doi: 10.3390/md9122605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zvyagintseva T.N. Structural characteristics and biological activity of fucoidans from the brown algae Alaria sp. and Saccharina japonica of different reproductive status. Chem. Biodivers. 2012;9(4):817–828. doi: 10.1002/cbdv.201100266. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda A., Takemura A., Ono H. Preparation of low-molecular weight alginic acid by acid hydrolysis. Carbohydr. Polym. 2000;42(4):421–425. [Google Scholar]
- 42.Wu L., Wang W., Zhang X., Zhao X., Yu G. Anti-HBV activity and mechanism of marine-derived polyguluronate sulfate (PGS) in vitro. Carbohydr. Polym. 2016;143:139–148. doi: 10.1016/j.carbpol.2016.01.065. [DOI] [PubMed] [Google Scholar]
- 43.Zhao X., Yu G., Yue N., Guan H. Effects of low-molecular-weight polyguluronate sulfate on experimental urolithiasis in rats. Urol. Res. 2007;35(6):301–306. doi: 10.1007/s00240-007-0113-5. [DOI] [PubMed] [Google Scholar]
- 44.Li R., Chen W.C., Wang W.P., Tian W.Y., Zhang X.G. Antioxidant activity of Astragalus polysaccharides and antitumour activity of the polysaccharides and siRNA. Carbohydr. Polym. 2010;82(2):240–244. [Google Scholar]
- 45.Wang Y., Chen Y., Du H., Yang J., Ming K., Song M., Liu J. Comparison of the anti-duck hepatitis A virus activities of phosphorylated and sulfated Astragalus polysaccharides. Experimental Biology & Medicine. 2016;344 doi: 10.1177/1535370216672750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdullahi A.Y., Kallon S., Yu X., Zhang Y., Li G. Vaccination with astragalus and ginseng polysaccharides improves immune response of chickens against H5N1 avian influenza virus. Biomed. Res. Int. 2016;2016 doi: 10.1155/2016/1510264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z., Wang Y., Zheng Z., Zhao S., Zhao J., Lin Q., Li C., Zhu Q., Zhong N. Antiviral activity of Isatis indigotica root-derived clemastanin B against human and avian influenza A and B viruses in vitro. Int. J. Mol. Med. 2013;31(4):867–873. doi: 10.3892/ijmm.2013.1274. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Y.L., Wang J.B., Shan L.M., Jin C., Ma L., Xiao X.H. Effect of Radix isatidis polysaccharides on immunological function and expression of immune related cytokines in mice. Chin J Integr Med. 2008;14(3):207–211. doi: 10.1007/s11655-008-0207-2. [DOI] [PubMed] [Google Scholar]
- 49.Li Z., Li L., Zhou H., Zeng L., Chen T., Chen Q., Zhou B., Wang Y., Chen Q., Hu P., Yang Z. Radix isatidis polysaccharides inhibit influenza a virus and influenza a virus-induced inflammation via suppression of host TLR3 signaling in vitro. Molecules. 2017;22(1) doi: 10.3390/molecules22010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Li S., Wang X., Zhang L., Cheung P.C.K. Advances in lentinan: isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011;25(2):196–206. [Google Scholar]
- 51.Ren G., Xu L., Lu T., Yin J. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int. J. Biol. Macromol. 2018;115:1202–1210. doi: 10.1016/j.ijbiomac.2018.04.132. [DOI] [PubMed] [Google Scholar]
- 52.Ren G., Li K., Hu Y., Yu M., Qu J., Xu X. Optimization of selenizing conditions for Seleno-Lentinan and its characteristics. Int. J. Biol. Macromol. 2015;81:249–258. doi: 10.1016/j.ijbiomac.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 53.Volman J.J., Ramakers J.D., Plat J. Dietary modulation of immune function by beta-glucans. Physiol. Behav. 2008;94(2):276–284. doi: 10.1016/j.physbeh.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 54.Milewska A., Zarebski M., Nowak P., Stozek K., Potempa J., Pyrc K. Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J. Virol. 2014;88(22):13221–13230. doi: 10.1128/JVI.02078-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olofsson S., Bergstrom T. Glycoconjugate glycans as viral receptors. Ann. Med. 2005;37(3):154–172. doi: 10.1080/07853890510007340. [DOI] [PubMed] [Google Scholar]
- 56.Peng G., Sun D., Rajashankar K.R., Qian Z., Holmes K.V., Li F. Crystal structure of mouse coronavirus receptor-binding domain complexed with its murine receptor. Proc. Natl. Acad. Sci. U. S. A. 2011;108(26):10696–10701. doi: 10.1073/pnas.1104306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu K., Peng G., Wilken M., Geraghty R.J., Li F. Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. J. Biol. Chem. 2012;287(12):8904–8911. doi: 10.1074/jbc.M111.325803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hofmann H., Pyrc K., van der Hoek L., Geier M., Berkhout B., Pohlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. U. S. A. 2005;102(22):7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dimitrov D.S. The secret life of ACE2 as a receptor for the SARS virus. Cell. 2003;115(6):652–653. doi: 10.1016/S0092-8674(03)00976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du L., Yang Y., Zhou Y., Lu L., Li F., Jiang S. MERS-CoV spike protein: a key target for antivirals. Expert Opin. Ther. Targets. 2016;21(2):131–143. doi: 10.1080/14728222.2017.1271415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lang J., Yang N., Deng J., Liu K., Yang P., Zhang G., Jiang C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sureau C., Salisse J. A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus a-determinant. Hepatology. 2013;57(3):985–994. doi: 10.1002/hep.26125. [DOI] [PubMed] [Google Scholar]
- 65.Longarela O.L., Schmidt T.T., Schoeneweis K., Romeo R., Wedemeyer H., Urban S., Schulze A. Proteoglycans act as cellular hepatitis delta virus attachment receptors. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mycroft-West C., Su D., Elli S., Guimond S., Miller G., Turnbull J., Yates E., Guerrini M., Fernig D., Lima M., Skidmore M. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding. bioRxiv. 2020 doi: 10.1101/2020.02.29.971093. [DOI] [Google Scholar]
- 67.Morokutti-Kurz M., Graf C., Prieschl-Grassauer E. Amylmetacresol/2,4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat. Int J Gen Med. 2017;10:53–60. doi: 10.2147/IJGM.S120665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milewska A., Kaminski K., Ciejka J., Kosowicz K., Zeglen S., Wojarski J., Nowakowska M., Szczubialka K., Pyrc K. HTCC: broad range inhibitor of coronavirus entry. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0156552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ludwig M., Enzenhofer E., Schneider S., Rauch M., Mueller C.A. Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial. Respir. Res. 2013;14(1):124. doi: 10.1186/1465-9921-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koenighofer M., Lion T., Bodenteich A., Prieschl-Grassauer E., Grassauer A., Unger H., Mueller C.A., Fazekas T. Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials. Multidisciplinary Respiratory Medicine. 2014;9(1) doi: 10.1186/2049-6958-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monto A.S., Fendrick A.M., Sarnes M.W. Respiratory illness caused by picornavirus infection: a review of clinical outcomes. Clin. Ther. 2001;23(10):1615–1627. doi: 10.1016/S0149-2918(01)80133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Venkataraman T., Frieman M.B. The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis. Antivir. Res. 2017;143:142–150. doi: 10.1016/j.antiviral.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang W., Wu J., Zhang X., Hao C., Zhao X., Jiao G., Shan X., Tai W., Yu G. Inhibition of influenza a virus infection by fucoidan targeting viral neuraminidase and cellular EGFR pathway. Sci. Rep. 2017;7 doi: 10.1038/srep40760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang S., Wang W., Hao C., Yu Y., Qin L., He M., Mao W. Antiviral activity against enterovirus 71 of sulfated rhamnan isolated from the green alga Monostroma latissimum. Carbohydr. Polym. 2018;200:43–53. doi: 10.1016/j.carbpol.2018.07.067. [DOI] [PubMed] [Google Scholar]
- 75.Li T., Peng T. Traditional Chinese herbal medicine as a source of molecules with antiviral activity. Antivir. Res. 2013;97(1):1–9. doi: 10.1016/j.antiviral.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang D.H., Wu K.L., Zhang X., Deng S.Q., Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J Integr Med. 2020;18(2):152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang P., Wang J., Wang W., Liu X., Liu H., Li X., Wu X. Astragalus polysaccharides enhance the immune response to avian infectious bronchitis virus vaccination in chickens. Microb. Pathog. 2017;111:81–85. doi: 10.1016/j.micpath.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 78.Zhang P., Liu X., Liu H., Wang W., Liu X., Li X., Wu X. Astragalus polysaccharides inhibit avian infectious bronchitis virus infection by regulating viral replication. Microb. Pathog. 2018;114:124–128. doi: 10.1016/j.micpath.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liao H.-F., Lu M.-C., Chang H.-C., Wei C.-C., Kao C.-H., Chen Z.-H., Huang C.-C., Li C. Effects of herbal medicinal formulas on suppressing viral replication and modulating immune responses. Am. J. Chin. Med. 2010;38(1):173–190. doi: 10.1142/S0192415X10007749. [DOI] [PubMed] [Google Scholar]
- 80.Di Pierro F., Bertuccioli A., Cavecchia I. Possible therapeutic role of a highly standardized mixture of active compounds derived from cultured Lentinula edodes mycelia (AHCC) in patients infected with 2019 novel coronavirus. Minerva Gastroenterol. Dietol. 2020;66(2) doi: 10.23736/S1121-421X.20.02697-5. [DOI] [PubMed] [Google Scholar]
- 81.Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang N., Wu M.Y., Zheng C.B., Zhu L., Zhao J.H., Zheng Y.T. The depolymerized fucosylated chondroitin sulfate from sea cucumber potently inhibits HIV replication via interfering with virus entry. Carbohydr. Res. 2013;380:64–69. doi: 10.1016/j.carres.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 83.Milewska A., Ciejka J., Kaminski K., Karewicz A., Bielska D., Zeglen S., Karolak W., Nowakowska M., Potempa J., Bosch B.J., Pyrc K., Szczubialka K. Novel polymeric inhibitors of HCoV-NL63. Antivir. Res. 2013;97(2):112–121. doi: 10.1016/j.antiviral.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buck C.B., Thompson C.D., Roberts J.N., Muller M., Lowy D.R., Schiller J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;2(7):e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grassauer A., Weinmuellner R., Meier C., Pretsch A., Prieschl-Grassauer E., Unger H. Iota-carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008;5:107. doi: 10.1186/1743-422X-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leibbrandt A., Meier C., Konig-Schuster M., Weinmullner R., Kalthoff D., Pflugfelder B., Graf P., Frank-Gehrke B., Beer M., Fazekas T., Unger H., Prieschl-Grassauer E., Grassauer A. Iota-carrageenan is a potent inhibitor of influenza a virus infection. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0014320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baram-Pinto D., Shukla S., Gedanken A., Sarid R. Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles. Small. 2010;6(9):1044–1050. doi: 10.1002/smll.200902384. [DOI] [PubMed] [Google Scholar]
- 88.Rusnati M., Vicenzi E., Donalisio M., Oreste P., Landolfo S., Lembo D. Sulfated K5 Escherichia coli polysaccharide derivatives: a novel class of candidate antiviral microbicides. Pharmacol. Ther. 2009;123(3):310–322. doi: 10.1016/j.pharmthera.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 89.Scordi-Bello I.A., Mosoian A., He C., Chen Y., Cheng Y., Jarvis G.A., Keller M.J., Hogarty K., Waller D.P., Profy A.T., Herold B.C., Klotman M.E. Candidate sulfonated and sulfated topical microbicides: comparison of anti-human immunodeficiency virus activities and mechanisms of action. Antimicrob. Agents Chemother. 2005;49(9):3607–3615. doi: 10.1128/AAC.49.9.3607-3615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jones S.T., Cagno V., Janecek M., Ortiz D., Gasilova N., Piret J., Gasbarri M., Constant D.A., Han Y., Vukovi L., Kral P., Kaiser L., Huang S., Constant S., Kirkegaard K., Boivin G., Stellacci F., Tapparel C. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 2020;6(5) doi: 10.1126/sciadv.aax9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim M., Yim J.H., Kim S.Y., Kim H.S., Lee W.G., Kim S.J., Kang P.S., Lee C.K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012;93(2):253–259. doi: 10.1016/j.antiviral.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 92.Pujol C.A., Ray S., Ray B., Damonte E.B. Antiviral activity against dengue virus of diverse classes of algal sulfated polysaccharides. Int. J. Biol. Macromol. 2012;51(4):412–416. doi: 10.1016/j.ijbiomac.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 93.Ciejka J., Botwina P., Nowakowska M., Szczubialka K., Pyrc K. Synthetic sulfonated derivatives of poly(allylamine hydrochloride) as inhibitors of human metapneumovirus. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0214646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun Q.L., Li Y., Ni L.Q., Li Y.X., Cui Y.S., Jiang S.L., Xie E.Y., Du J., Deng F., Dong C.X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2020;229 doi: 10.1016/j.carbpol.2019.115487. [DOI] [PubMed] [Google Scholar]
- 95.Gao Y., Liu W., Wang W., Zhang X., Zhao X. The inhibitory effects and mechanisms of 3,6-O-sulfated chitosan against human papillomavirus infection. Carbohydr. Polym. 2018;198:329–338. doi: 10.1016/j.carbpol.2018.06.096. [DOI] [PubMed] [Google Scholar]
- 96.Szczubialka K., Ciejka J., Milewska A., Golda A., Kaminski K., Wytrwal M., Pyrc K., Nowakowaka M. Antiviral polymers. Engineering of Biomaterials. 2016;138 [Google Scholar]
- 97.Ciejka J., Wolski K., Nowakowska M., Pyrc K., Szczubialka K. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;76:735–742. doi: 10.1016/j.msec.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo C., Zhu Z., Yu P., Zhang X., Dong W., Wang X., Chen Y., Liu X. Inhibitory effect of iota-carrageenan on porcine reproductive and respiratory syndrome virus in vitro. Antivir. Ther. 2019;24(4):261–270. doi: 10.3851/IMP3295. [DOI] [PubMed] [Google Scholar]
- 99.Wang W., Zhang P., Yu G.-L., Li C.-X., Hao C., Qi X., Zhang L.-J., Guan H.-S. Preparation and anti-influenza A virus activity of κ-carrageenan oligosaccharide and its sulphated derivatives. Food Chem. 2012;133(3):880–888. [Google Scholar]
- 100.Chen M.Z., Xie H.G., Yang L.W., Liao Z.H., Yu J. In vitro anti-influenza virus activities of sulfated polysaccharide fractions from Gracilaria lemaneiformis. Virol. Sin. 2010;25(5):341–351. doi: 10.1007/s12250-010-3137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao C., Gao L., Wang C., Liu B., Jin Y., Xing Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr. Polym. 2016;144:382–389. doi: 10.1016/j.carbpol.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 102.Yang T., Jia M., Zhou S., Pan F., Mei Q. Antivirus and immune enhancement activities of sulfated polysaccharide from Angelica sinensis. Int. J. Biol. Macromol. 2012;50(3):768–772. doi: 10.1016/j.ijbiomac.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 103.Dang S.S., Jia X.L., Song P., Cheng Y.A., Zhang X., Sun M.Z., Liu E.Q. Inhibitory effect of emodin and Astragalus polysaccharide on the replication of HBV. World J. Gastroenterol. 2009;15(45):5669–5673. doi: 10.3748/wjg.15.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghannam A., Abbas A., Alek H., Al-Waari Z., Al-Ktaifani M. Enhancement of local plant immunity against tobacco mosaic virus infection after treatment with sulphated-carrageenan from red alga (Hypnea musciformis) Physiol. Mol. Plant Pathol. 2013;84:19–27. [Google Scholar]
- 105.Jiang J., Wu C., Gao H., Song J., Li H. Effects of astragalus polysaccharides on immunologic function of erythrocyte in chickens infected with infectious bursa disease virus. Vaccine. 2010;28(34):5614–5616. doi: 10.1016/j.vaccine.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 106.Xia Y., Fan Q., Hao D., Wu J., Ma G., Su Z. Chitosan-based mucosal adjuvants: sunrise on the ocean. Vaccine. 2015;33(44):5997–6010. doi: 10.1016/j.vaccine.2015.07.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun B., Yu S., Zhao D., Guo S., Wang X., Zhao K. Polysaccharides as vaccine adjuvants. Vaccine. 2018;36(35):5226–5234. doi: 10.1016/j.vaccine.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 108.Zaharoff D.A., Rogers C.J., Hance K.W., Schlom J., Greiner J.W. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25(11):2085–2094. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Illum L., Jabbal-Gill I., Hinchcliffe M., Fisher A.N., Davis S.S. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Del Rev. 2001;51(1–3):81–96. doi: 10.1016/s0169-409x(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 110.Ghendon Y., Markushin S., Krivtsov G., Akopova I. Chitosan as an adjuvant for parenterally administered inactivated influenza vaccines. Arch. Virol. 2008;153(5):831–837. doi: 10.1007/s00705-008-0047-4. [DOI] [PubMed] [Google Scholar]
- 111.Ghendon Y., Markushin S., Vasiliev Y., Akopova I., Koptiaeva I., Krivtsov G., Borisova O., Ahmatova N., Kurbatova E., Mazurina S., Gervazieva V. Evaluation of properties of chitosan as an adjuvant for inactivated influenza vaccines administered parenterally. J. Med. Virol. 2009;81(3):494–506. doi: 10.1002/jmv.21415. [DOI] [PubMed] [Google Scholar]
- 112.Sun J.L., Hu Y.L., Wang D.Y., Zhang B.K., Liu J.G. Immunologic enhancement of compound Chinese herbal medicinal ingredients and their efficacy comparison with compound Chinese herbal medicines. Vaccine. 2006;24(13):2343–2848. doi: 10.1016/j.vaccine.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 113.Yang L., Hu Y., Xue J., Wang F., Wang D., Kong X., Li P., Xu W. Compound Chinese herbal medicinal ingredients can enhance immune response and efficacy of RHD vaccine in rabbit. Vaccine. 2008;26(35):4451–4455. doi: 10.1016/j.vaccine.2008.06.075. [DOI] [PubMed] [Google Scholar]
- 114.Bolhassani A., Talebi S., Anvar A. Endogenous and exogenous natural adjuvants for vaccine development. Mini Reviews in Medicinal Chemistry. 2017;17(15):1442–1456. doi: 10.2174/1389557517666170228115801. [DOI] [PubMed] [Google Scholar]
- 115.Li J., Zhong Y., Li H., Zhang N., Ma W., Cheng G., Liu F., Liu F., Xu J. Enhancement of Astragalus polysaccharide on the immune responses in pigs inoculated with foot-and-mouth disease virus vaccine. Int. J. Biol. Macromol. 2011;49(3):362–368. doi: 10.1016/j.ijbiomac.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 116.Du X., Zhao B., Li J., Cao X., Diao M., Feng H., Chen X., Chen Z., Zeng X. Astragalus polysaccharides enhance immune responses of HBV DNA vaccination via promoting the dendritic cell maturation and suppressing Treg frequency in mice. Int. Immunopharmacol. 2012;14(4):463–470. doi: 10.1016/j.intimp.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 117.van der Burg S.H., Ressing M.E., Kwappenberg K.M.C., de Jong A., Straathof K., de Jong J., Geluk A., van Meugaarden K.E., Franken K., Ottenhoff T.H.M., Fleuren G.J., Kenter G., Melief C.J.M., Offringa R. Natural T-HELPER immunity against human papillomavirus type 16 (HPV16) E7-derived peptide epitopes in patients with HPV16-positive cervical lesions: identification of 3 human leukocyte antigen class II-restricted epitopes. Int. J. Cancer. 2001;91(5):612–618. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1119>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Y.-Q., Tsai Y.-C., Monie A., Hung C.-F., Wu T.C. Carrageenan as an adjuvant to enhance peptide-based vaccine potency. Vaccine. 2010;28(32):5212–5219. doi: 10.1016/j.vaccine.2010.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nadine P.P., Dudek L., Aguilar Marie-Isabel, Croft Nathan P., Purcell Anthony W. Epitope discovery and their use in peptide based vaccines. Curr. Pharm. Des. 2010;16(28):3149–3157. doi: 10.2174/138161210793292447. [DOI] [PubMed] [Google Scholar]
- 120.Purcell A.W., McCluskey J., Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat. Rev. Drug Discov. 2007;6(5):404–414. doi: 10.1038/nrd2224. [DOI] [PubMed] [Google Scholar]
- 121.Wang C., Xia S., Zhang P., Zhang T., Wang W., Tian Y., Meng G., Jiang S., Liu K. Discovery of hydrocarbon-stapled short alpha-helical peptides as promising middle east respiratory syndrome coronavirus (MERS-CoV) fusion inhibitors. J. Med. Chem. 2018;61(5):2018–2026. doi: 10.1021/acs.jmedchem.7b01732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bhattacharya M., Sharma A.R., Patra P., Ghosh P., Sharma G., Patra B.C., Lee S.S., Chakraborty C. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): immunoinformatics approach. J. Med. Virol. 2020;92(6):618–631. doi: 10.1002/jmv.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li L., Sun T., He Y., Li W., Fan Y., Zhang J. Epitope-based peptide vaccine design and target site characterization against novel coronavirus disease caused by SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.02.25.965434. [DOI] [Google Scholar]
- 124.Abdelmageed M.I., Abdelmoneim A.H., Mustafa M.I., Elfadol N.M., Murshed N.S., Shantier S.W., Makhawi A.M. Design of multi epitope-based peptide vaccine against E protein of human COVID-19: an immunoinformatics approach. bioRxiv. 2020 doi: 10.1101/2020.02.04.934232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Torres-Sangiao E., Holban A.M., Gestal M.C. Advanced nanobiomaterials: vaccines, diagnosis and treatment of infectious diseases. Molecules. 2016;21(7) doi: 10.3390/molecules21070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kheirollahpour M., Mehrabi M., Dounighi N.M., Mohammadi M., Masoudi A. Nanoparticles and vaccine development. Pharm Nanotechnol. 2020;8(1):6–21. doi: 10.2174/2211738507666191024162042. [DOI] [PubMed] [Google Scholar]
- 127.Irvine D.J., Hanson M.C., Rakhra K., Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 2015;115(19):11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee J., Kim Y.M., Kim J.H., Cho C.W., Jeon J.W., Park J.K., Lee S.H., Jung B.G., Lee B.J. Nasal delivery of chitosan/alginate nanoparticle encapsulated bee (Apis mellifera) venom promotes antibody production and viral clearance during porcine reproductive and respiratory syndrome virus infection by modulating T cell related responses. Vet. Immunol. Immunopathol. 2018;200:40–51. doi: 10.1016/j.vetimm.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 129.Lin L.C.W., Huang C.Y., Yao B.Y., Lin J.C., Agrawal A., Algaissi A., Peng B.H., Liu Y.H., Huang P.H., Juang R.H., Chang Y.C., Tseng C.T., Chen H.W., Hu C.M.J. Viromimetic STING agonist-loaded hollow polymeric nanoparticles for safe and effective vaccination against middle east respiratory syndrome coronavirus. Adv. Funct. Mater. 2019;29(28) doi: 10.1002/adfm.201807616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun Q.S., Zhang J.L., Han D.Q., Yang Y.B., Zhu L., Yu L. Characterization and immunological evaluation of chitosan nanoparticles as adjuvants for bovine coronavirus N protein. Appl. Mech. Mater. 2012;161:113–120. [Google Scholar]
- 131.Layqah L.A., Eissa S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Mikrochim Acta. 2019;186(4):224. doi: 10.1007/s00604-019-3345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cagno V., Andreozzi P., D’Alicarnasso M., Jacob Silva P., Mueller M., Galloux M., Le Goffic R., Jones S.T., Vallino M., Hodek J., Weber J., Sen S., Janeček E.-R., Bekdemir A., Sanavio B., Martinelli C., Donalisio M., Rameix Welti M.-A., Eleouet J.-F., Han Y., Kaiser L., Vukovic L., Tapparel C., Král P., Krol S., Lembo D., Stellacci F. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2017;17(2):195–203. doi: 10.1038/nmat5053. [DOI] [PubMed] [Google Scholar]
- 133.Chen H.W., Huang C.Y., Lin S.Y., Fang Z.S., Hsu C.H., Lin J.C., Chen Y.I., Yao B.Y., Hu C.M. Synthetic virus-like particles prepared via protein corona formation enable effective vaccination in an avian model of coronavirus infection. Biomaterials. 2016;106:111–118. doi: 10.1016/j.biomaterials.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen X., Han W., Zhao X., Tang W., Wang F. Epirubicin-loaded marine carrageenan oligosaccharide capped gold nanoparticle system for pH-triggered anticancer drug release. Sci. Rep. 2019;9(1):6754. doi: 10.1038/s41598-019-43106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Manivasagan P., Bharathiraja S., Bui N.Q., Jang B., Oh Y.O., Lim I.G., Oh J. Doxorubicin-loaded fucoidan capped gold nanoparticles for drug delivery and photoacoustic imaging. Int. J. Biol. Macromol. 2016;91:578–588. doi: 10.1016/j.ijbiomac.2016.06.007. [DOI] [PubMed] [Google Scholar]