Abstract
It remains unknown whether the administration of tyrosine kinase inhibitors (TKIs) targeting BCR-ABL1 after allogeneic hematopoietic cell transplantation (HCT) is associated with improved outcomes for patients with chronic myelogenous leukemia (CML). In this registry study, we analyzed clinical outcomes of 390 adult patients with CML who underwent transplantation between 2007 and 2014 and received maintenance TKI following HCT (n = 89) compared with no TKI maintenance (n = 301), as reported to the Center for International Blood and Marrow Transplant Research. All patients received TKI therapy before HCT. The majority of patients had a disease status of first chronic phase at HCT (n = 240; 62%). The study was conducted as a landmark analysis, excluding patients who died, relapsed, had chronic graft-versus-host disease, or were censored before day +100 following HCT. Of the 89 patients who received TKI maintenance, 77 (87%) received a single TKI and the other 12 (13%) received multiple sequential TKIs. The most common TKIs used for maintenance were dasatinib (n = 50), imatinib (n = 27), and nilotinib (n = 27). As measured from day +100, the adjusted estimates for 5-year relapse (maintenance, 35% versus no maintenance, 26%; P = .11), leukemia-free survival (maintenance, 42% versus no maintenance, 44%; P = .65), or overall survival (maintenance, 61% versus no maintenance, 57%; P = .61) did not differ significantly between patients receiving TKI maintenance or no maintenance. These results remained unchanged in multivariate analysis and were not modified by disease status before transplantation. In conclusion, our data from this day +100 landmark analysis do not demonstrate a significant impact of maintenance TKI therapy on clinical outcomes. The optimal approach to TKI administration in the post-transplantation setting in patients with CML remains undetermined.
Keywords: Allogeneic hematopoietic cell transplantation, Chronic myelogenous leukemia, Maintenance, Tyrosine kinase inhibitor
INTRODUCTION
The introduction of tyrosine kinase inhibitors (TKIs) that target BCR-ABL1 changed the therapeutic landscape for chronic myelogenous leukemia (CML) and significantly improved clinical outcomes, with long-term overall survival (OS) increasing from <20% to 80% to 90% [1,2]. Allogeneic hematopoietic cell transplantation (allo-HCT) remains the sole known curative treatment for CML; however, given the success of TKI therapy and the risks associated with transplantation, allo-HCT is currently reserved for patients with accelerated phase (AP) or blast phase (BP) CML and those with TKI failure or intolerance in chronic phase (CP) disease [3]. Despite the addition of TKIs before allo-HCT, post-transplantation patient outcomes have not changed significantly, with disease relapse the leading cause of transplant failure [4,5].
Maintenance therapy, defined as therapy initiated while the patient remains in complete remission, is a promising approach to reducing the incidence of relapse after HCT [6]. The administration of TKIs as maintenance after allo-HCT for patients with high-risk Philadelphia chromosome-positive (Ph+) leukemia has been investigated in several studies [7–10], and this approach is already being adopted into clinical practice [11]. Although the use of maintenance TKI after HCT was associated with improved leukemia-free survival (LFS) and OS for patients with Ph+ acute lymphoblastic leukemia in an analysis from the European Group for Blood and Marrow Transplantation (EBMT) [12], larger studies investigating maintenance approaches in CML are lacking. Given the changing role of HCT in the management of CML and the increasing use of TKIs in the post-transplantation period, we sought to determine whether maintenance therapy with TKIs following allo-HCT is associated with improved disease control and survival in patients with CML through an analysis of the Center for International Blood and Marrow Research (CIBMTR) registry. We also sought to characterize the use and outcomes of allo-HCT in the management of CML in a modern era of multiple available TKIs.
METHODS
Data Sources
The CIBMTR is a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program comprising a voluntary network of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous transplantations to a centralized statistical center. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information issued in the performance of such research is collected and maintained in the CIBMTR’s capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule.
The CIBMTR collects data that include the following: age, sex, disease type, pretransplantation disease stage, date of diagnosis, graft type, conditioning regimen, post-transplantation disease progression and survival, development of a new malignancy, and cause of death. Data are collected before transplantation, 100 days and 6 months after transplantation, and annually thereafter or until death. The protocol of the present study received a priori approval by the appropriate Institutional Review Committee.
Study Population
The study population identified with the CIBMTR database included patients age ≥18 years who underwent a first allo-HCT for CML between 2007 and 2014. Donors were HLA-identical sibling donors, unrelated donors (URDs), or umbilical cord blood. Patients who had not received TKI therapy before allo-HCT and patients receiving therapies other than TKIs as post-transplantation maintenance were excluded. Also excluded were patients who received ex vivo T cell depletion, CD34 cell selection, or post-transplantation cyclophosphamide as graft-versus-host disease (GVHD) prophylaxis. Published experiences with maintenance TKI therapy have reported a median initiation date between day +30 and day +100 after allo-HCT [7–11].Thus, we chose to conduct this study as a landmark analysis that excluded patients who died, relapsed, had chronic GVHD (cGVHD), or were lost to follow-up before day +100 after allo-HCT.
Variables Included in the Analysis
Our data analysis included patient-related variables were the age at HCT, sex, and Karnofsky Performance Status (KPS). Disease-related variables included disease status at transplantation (CP1 versus CP2+ versus AP versus BP). Transplantation-related variables included conditioning regimen intensity (as defined by the CIBMTR) [13], donor-recipient HLA match (ie, HLA-identical sibling, well-matched URD, partially matched URD, or mismatched URD) [14], GVHD prophylaxis, and the time period in which the transplantation was performed (ie, 2007–2008, 2009–2010, 2011–2012, or 2013–2014). Maintenance therapy was determined based on data recorded from the day +100 post-allo-HCT disease-specific form, which included the choice of TKI but not the date of initiation, dose, or duration of maintenance therapy. Relapse was defined by a report of molecular, cytogenetic, and/or hematologic disease post-HCT.
Study Endpoints and Statistical Analysis
This was a retrospective cohort landmark study from 100 days after HCT to compare outcomes between patients who underwent allo-HCT for CML and received maintenance TKI therapy and controls who received no maintenance therapy.The primary aim was to compare LFS, and the secondary aim was to compare rates of OS, cGVHD, treatment-related mortality (TRM), and relapse in these 2 groups. All the outcomes are reported as time to events with the starting time at 100 days after transplantation.
Descriptive statistics were calculated for all variables. A univariate analysis was performed with the Kaplan-Meier estimates to compute OS and LFS rates. Log-rank tests were used to measure the differences in OS and LFS between the treatment groups. Chronic GVHD, TRM, and relapse rates were estimated using the cumulative incidence functions with consideration of competing risks. Gray’s test was performed to compare the differences in cumulative incidence functions between the treatment groups.
Multivariate Cox proportional hazards regression models for all the endpoints (LFS, OS, relapse TRM, and cGVHD) were used to compare the treatment groups. The assumption of proportional hazards for each factor in the Cox model was tested using time-dependent covariates. There is no variable violating the proportional hazard assumption in this study. Stepwise selection was used to identify significant covariates that influenced outcomes to be included in the final model to get the adjusted treatment effects. The set of adjusting variables for each outcome was decided separately by stepwise selection with inclusion criteria at .05. Statistical significance of the main effects was tested with level .01, accounting for multiple comparisons across the endpoints. Potential interactions between the main effect and significant adjusting covariates and between main effect and donor type were tested, showing no significant interactions at level of .01.
Adjusted survival curves and cumulative incidence curves were generated stratified on the treatment groups and weighted averages of covariate values using the pooled sample proportion as the weight function. These adjusted curves represent the likelihood of outcomes in populations with similar prognostic factors.
RESULTS
Patient and Transplantation Characteristics
A total of 572 patients with CML were initially identified according the defined study population (Supplementary Table S1). Of these patients, 182 were excluded as part of the day +100 landmark criteria (Figure 1; Supplementary Table S2), leaving 390 eligible patients for our analysis. Two groups were identified: those receiving post-allo-HCT maintenance TKI therapy (n = 89) and those not receiving post-allo-HCT maintenance TKI therapy (n = 301). Patient characteristics are summarized inX Table 1. The median duration of follow-up for survivors after day +100 post-allo-HCT was 61 months (range, 7–97 months) in the maintenance TKI group and 68 months (range, 2–98 months) in the no maintenance group. There were no between-group differences in median age, sex, or KPS score at allo-HCT between the 2 groups. Overall, the majority of patients had disease beyond CP1 at HCT (n = 240; 62%). Disease status before allo-HCT differed between the 2 groups, with a higher percentage of patients with CP2+ in the maintenance group and a higher percentage of patients with CP1 in the no maintenance group (P < .001). Transplantation characteristics were similar in the 2 groups in terms of the most common donor types (HLA-matched siblings and unrelated donors), graft source (peripheral blood stem cells), conditioning regimen intensity (myeloablative), and GVHD prophylaxis (calcineurin inhibitor plus methotrexate). The administration of donor lymphocyte infusion Xas prophylaxis or for mixed chime-rism was rare (maintenance group, n = 0; no maintenance group, n = 4).
Figure 1.
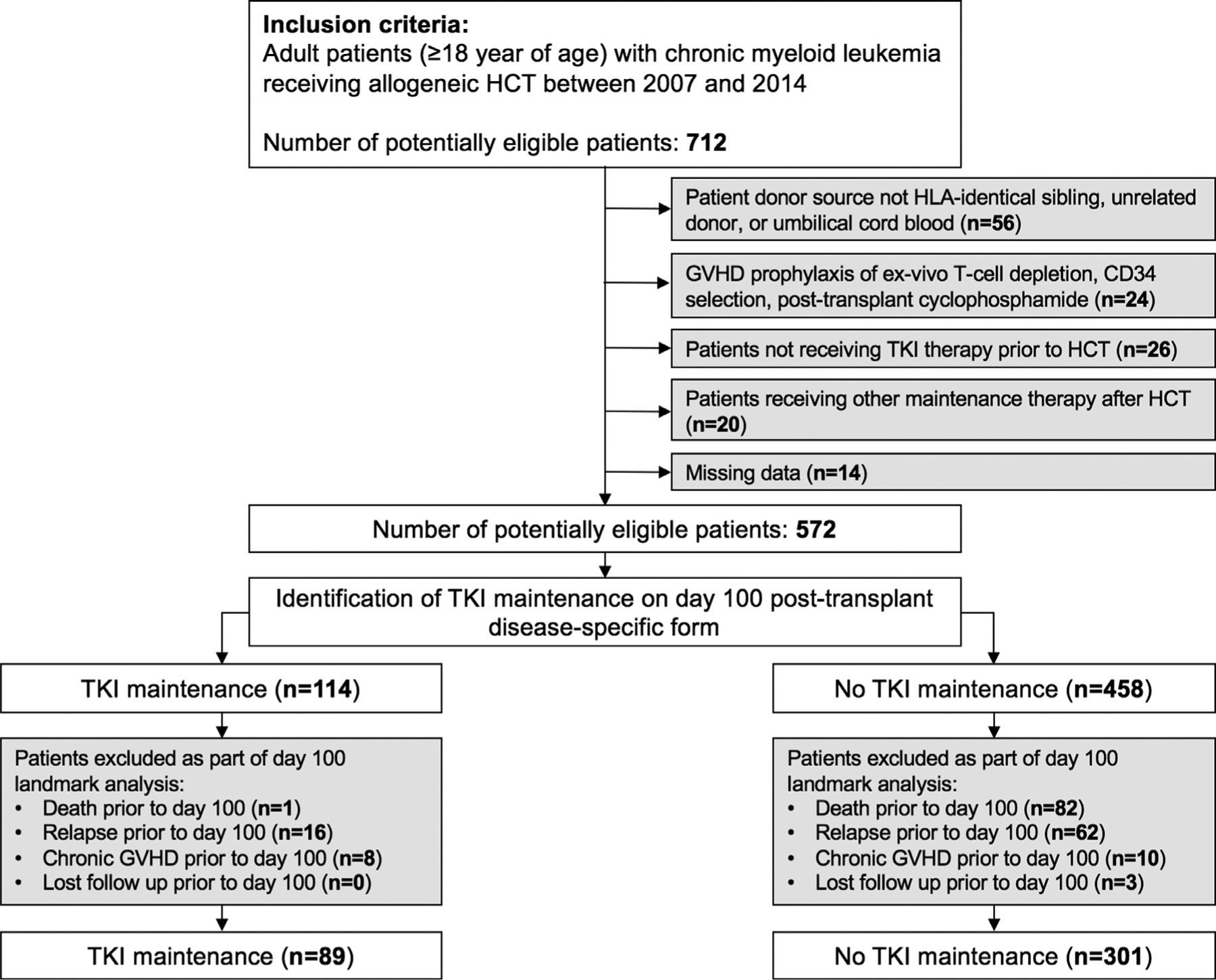
Study flow diagram.
Table 1.
Patient, Disease, and Transplantation Characteristics
| Variable | Post-Allo-HCT Maintenance TKI | No Post-Allo-HCT Maintenance TKI | P Value |
|---|---|---|---|
| Number of patients | 89 | 301 | |
| Number of centers | 45 | 101 | |
| Age, yr, median (range) | 46 (19–64) | 44 (18–76) | .59 |
| Sex, n (%) | .23 | ||
| Male | 56 (63) | 168 (56) | |
| Female | 33 (37) | 133 (44) | |
| KPS score at allo-HCT, n (%) | .91 | ||
| ≥90 | 64 (72) | 218 (72) | |
| <90 | 23 (26) | 74 (25) | |
| Missing | 2 (2) | 9 (3) | |
| Number of TKI therapies before allo-HCT, n (%) | |||
| 1 | 29 (33) | 76 (25) | |
| >1 | 60 (67) | 225 (75) | |
| TKIs used before allo-HCT, n (%) | |||
| Imatinib | 77 (87) | 285 (94) | |
| Dasatinib | 63 (71) | 213 (71) | |
| Nilotinib | 25 (28) | 100 (33) | |
| Ponatinib | 0 | 1 (<1) | |
| Disease status before allo-HCT, n (%) | <.001 | ||
| CP1 | 13 (15) | 137 (46) | |
| CP2+ | 53 (60) | 95 (32) | |
| AP | 12 (13) | 48 (16) | |
| BP | 9 (10) | 14 (5) | |
| Missing | 2 (2) | 7 (2) | |
| Graft source, n (%) | .69 | ||
| Bone marrow | 16 (18) | 66 (22) | |
| Peripheral blood | 62 (70) | 196 (65) | |
| Cord blood | 11 (12) | 39 (13) | |
| Donor type, n(%) | .42 | ||
| HLA-identical sibling | 31 (35) | 96 (32) | |
| Well-matched URD | 32 (36) | 130 (43) | |
| Partially matched URD | 15 (17) | 33 (11) | |
| Mismatched URD | 0 | 3 (<1) | |
| Cord blood | 11 (12) | 39 (13) | |
| Conditioning regimen intensity, n (%) | .51 | ||
| MAC | 76 (85) | 250 (83) | |
| RIC | 8 (9) | 40 (13) | |
| NMA | 5 (6) | 10 (3) | |
| Missing | 0 | 1 (<1) | |
| GVHD prophylaxis, n (%) | .65 | ||
| CNI + MTX | 61 (69) | 182 (60) | |
| CNI + MMF | 23 (26) | 80 (26) | |
| Other | 5 (6) | 39 (13) | |
| Year of HCT, n(%) | .01 | ||
| 2007–2008 | 19 (21) | 120 (40) | |
| 2009–2010 | 45 (51) | 112 (37) | |
| 2011–2012 | 9 (10) | 22 (7) | |
| 2013–2014 | 16 (18) | 47 (16) | |
| Follow-up of survivors after day +100 post-allo-HCT, mo, median (range) | 61 (7–97) | 68 (2–98) | |
CNI indicates calcineurin inhibitor; MAC, myeloablative conditioning; MMF, mycophenolate mofetil; MTX, methotrexate; NMA, nonmyeloablative conditioning; RIC, reduced-intensity conditioning;
TKI Therapy before Allo-HCT
All patients in this group received TKI therapy before allo-HCT, with the majority receiving multiple TKIs in both the maintenance group (67%) and the no maintenance group (75%). The most commonly used TKIs before allo-HCT were imatinib (n = 362), dasatinib (n = 276), and then nilotinib (n = 125). Data on the duration of TKI therapy before allo-HCT, as well as the reason for the use of multiple TKIs (ie, resistance, intolerance, or other), were unavailable.
Maintenance TKI Therapy after HCT
Eighty-nine patients (23%) received TKI maintenance therapy after allo-HCT (Table 2). Of these, 77 (87%) received a single TKI and 12 (13%) received multiple TKIs. In the majority of cases (n = 72; 81%), a different TKI was administered post-allo-HCT than pre-allo-HCT. The most common TKIs used for maintenance were dasatinib (n = 50), imatinib (n = 27), and nilotinib (n = 27). Data on the starting date and duration of maintenance TKI therapy were unavailable.
Table 2.
Characteristics of Post-Transplantation TKI Maintenance
| Variable | Value |
|---|---|
| Number of patients receiving maintenance TKI therapy | 89 |
| Number of TKI maintenance therapies, n (%) | |
| 1 | 77 (87) |
| >1 | 12 (13) |
| Same TKI given pre-HCT and post-HCT, n (%) | |
| No | 72 (81) |
| Yes | 17 (19) |
| TKIs used as maintenance therapy, n | |
| Dasatinib | 50 |
| Imatinib | 27 |
| Nilotinib | 27 |
OS
We observed no significant difference between the 2 groups in the adjusted 2-year and 5-year OS from day +100 (2-year: 76% [95% confidence interval (CI) , 68%−85%) for the maintenance group, 69% [95% CI, 64%−82%] for the no maintenance group [P = .15]); 5-year: 61% [95% CI, 50%−72%] for the maintenance group, 57% [95% CI, 52%−67%] for the no maintenance group [P = .61]) (Figure 2). The leading cause of death was disease relapse in the maintenance group and GVHD in the no mainte nance group was GVHD (Supplementary Table S3). In the multivariate analysis (Table 3), BP disease status before allo-HCT, CP2+ disease status before allo-HCT, cord blood graft source, and peripheral blood graft source were independent adverse risk factors for OS (hazard ratio [HR] for BP, 2.4 [reference, CP1] [95% CI,1.3–4.3; P = .005]; for CP2+, 1.6 [reference, CP1] [95% CI, 1.1–2.4;P = .013]; HR for cord blood, 2.6 [reference, bone marrow] [95% CI, 1.4–4.7; P = .002]; HR for peripheral blood, 2.1 [reference, bone marrow] [95% CI, 1.3–3.4; P = .004]). Maintenance TKI therapy was not a risk factor for OS (HR, .7 [reference, no mainte nance]; 95% CI, .5–1.1; P = .078).
Figure 2.
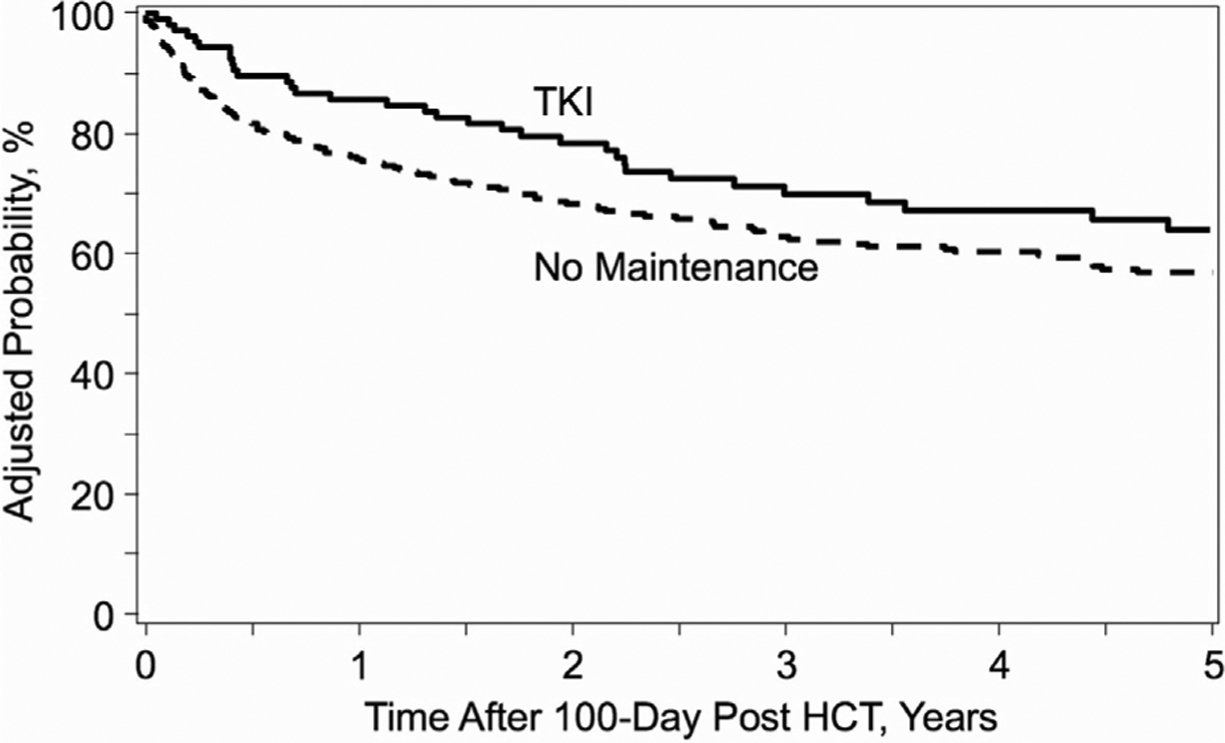
Kaplan-Meier curve of OS according to post-allo-HCT maintenance therapy with TKIs.
Table 3.
Day +100 Landmark Multivariate Analyses of Clinical Outcomes
| Parameter | Outcome, HR, (95% CI) | ||||
|---|---|---|---|---|---|
| cGVHD | TRM | Relapse | LFS | OS | |
| Disease status | |||||
| CP1 (reference) | – | – | – | – | – |
| AP | – | – | – | 1.3 (.8–2.0), P = .284 | 1.0 (.6–1.7), P = .901 |
| BP | – | – | – | 1.8 (1.0–3.1), P = .039 | 2.4 (13–4.3), P = .005 |
| CP2+ | – | – | – | 1.7 (1.2–2.4), P = .003 | 1.6 (1.1–2.4), P = .013 |
| Graft source | |||||
| Bone marrow (reference) | – | – | – | – | – |
| Cord blood | 1.0 (.6–1.6), P = .848 | 2.7 (1.2–5.8), P = .012 | – | – | 2.6 (1.4–4.7), P = .002 |
| Peripheral blood | 2.0 (1.4–2.8), P < .001 | 2.4 (1.3–4.4), P = .006 | – | – | 2.1 (13–3.4), P = .004 |
| GVHD prophylaxis | |||||
| Tacrolimus ± others (reference) | – | – | – | – | – |
| Cyclosporine ± others | .6 (.4–.8), P = .003 | – | – | – | – |
| Others | .5 (.1–2.0), P = .336 | – | – | – | – |
| Age group | |||||
| 18–29 (reference) | – | – | – | – | – |
| 30–39 | – | – | – | 1.0 (.6–1.5), P = .849 | – |
| 40–49 | – | – | – | 1.2 (.8–1.9), P = .353 | – |
| 50–59 | – | – | – | 1.0 (.6–1.5), P = .944 | – |
| ≥60 | – | – | – | 2.3 (1.3–3.9), P = .004 | – |
| KPS score | |||||
| 90–100 (reference) | – | – | – | – | – |
| <90 | .6 (.4–.8), P < .001 | – | 2.0 (1.3–3.0), P = .001 | 1.4 (1.0–1.9), P = .058 | – |
| Maintenance therapy | |||||
| No (reference) | – | – | – | – | – |
| Yes | .8 (.6–1.1), P = .124 | .7 (.4–1.1), P = .130 | 1.4 (.9–2.1), P =.170 | .9 (.6–1.2), P = .356 | .7 (.5–1.1), P = .078 |
Statistically significant values are in bold type.
LFS
We observed no significant between-group difference in the adjusted 2-year and 5-year LFS from day +100 (2-year: 56%[95% CI, 45%−66%] for the maintenance group versus 56% [95% CI, 50%−61%] for the no maintenance group [P = .95]; 5-year: 42% [95% CI, 31%−53%] for the maintenance group versus 44% [95% CI, 39%−47%] for the no maintenance group [P = .65]) (Figure 3). In the multivariate analysis (Table 3), BP disease status before allo-HCT, CP2+ disease status before allo-HCT, and age ≥60 years were independent adverse risk factors for LFS (HR for BP, 1.8 [reference, CP1] [95% CI, 1.0–3.1; P = .039]; HR for CP2+, 1.7 [reference, CP1] [95% CI, 1.2–2.4; P = .003]; HR for age ≥60, 2.3 [reference, age 18–29] [95% CI, 1.3–3.9; P = .004]). Maintenance TKI therapy was not a risk factor for LFS (HR, .9 [reference, no maintenance]; 95% CI, .6–1.2; P = .356).
Figure 3.
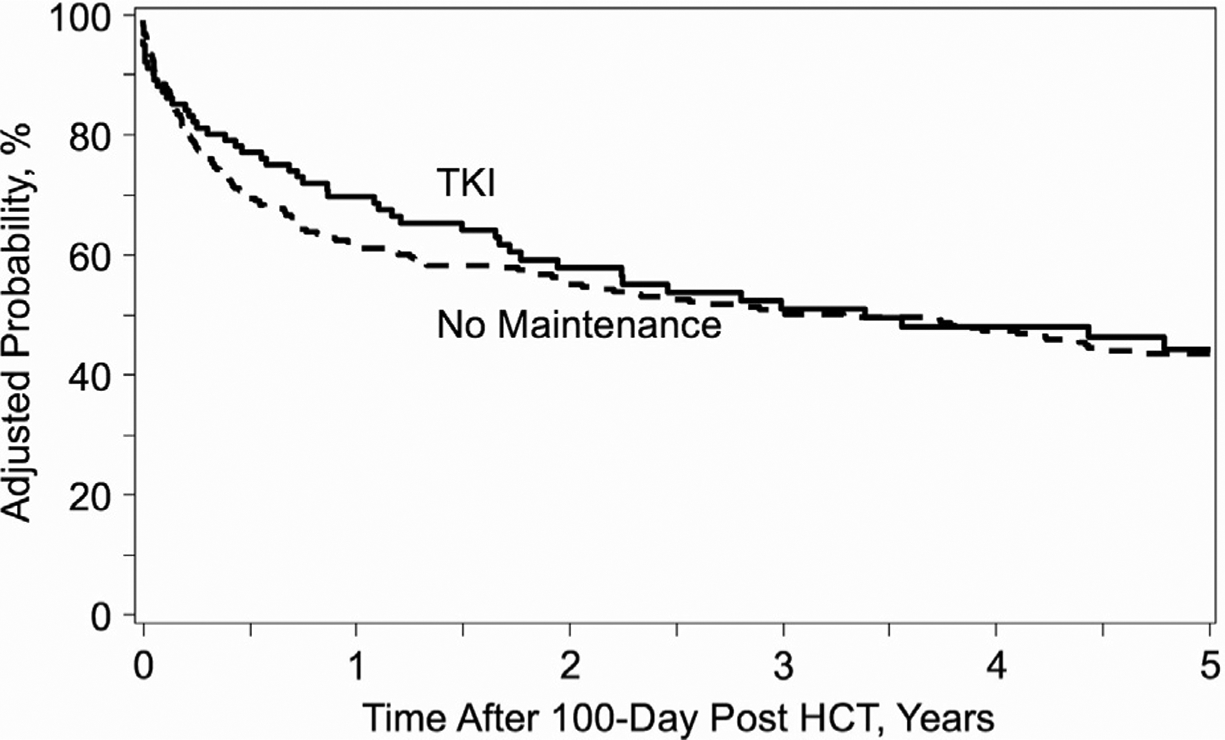
Kaplan-Meier curve of LFS according to post-allo-HCT maintenance therapy with TKIs.
Relapse
The most common cause of relapsed disease in both groups was hematologic relapse (TKI maintenance group, n = 22 [69%]; no maintenance group, n = 50 [68%]). We observed an increased adjusted incidence of relapse at 2 years from day +100 in the TKI maintenance group compared with the no maintenance group (33% [95% CI, 23%−43%] versus 22% [95% CI, 17%−38%]; P = .04). However, no significant difference was observed in 5-year outcome (35% [95% CI, 25%−45%) inX the maintenance group versus 26% [95% CI, 21%−40%] inX the no maintenance group; P = .11) (Figure 4). In the multivariate analysis (Table 3), KPS score <90 was an independent adverse risk factor for relapse (HR for KPS <90, 2.0 [reference, KPS 90–100]; 95% CI, 1.3–3.0; P = .001). Maintenance TKI therapy was not a risk factor for relapse (HR, 1.4 [reference, no main tenance]; 95% CI, .9–2.1; P = .170).
Figure 4.
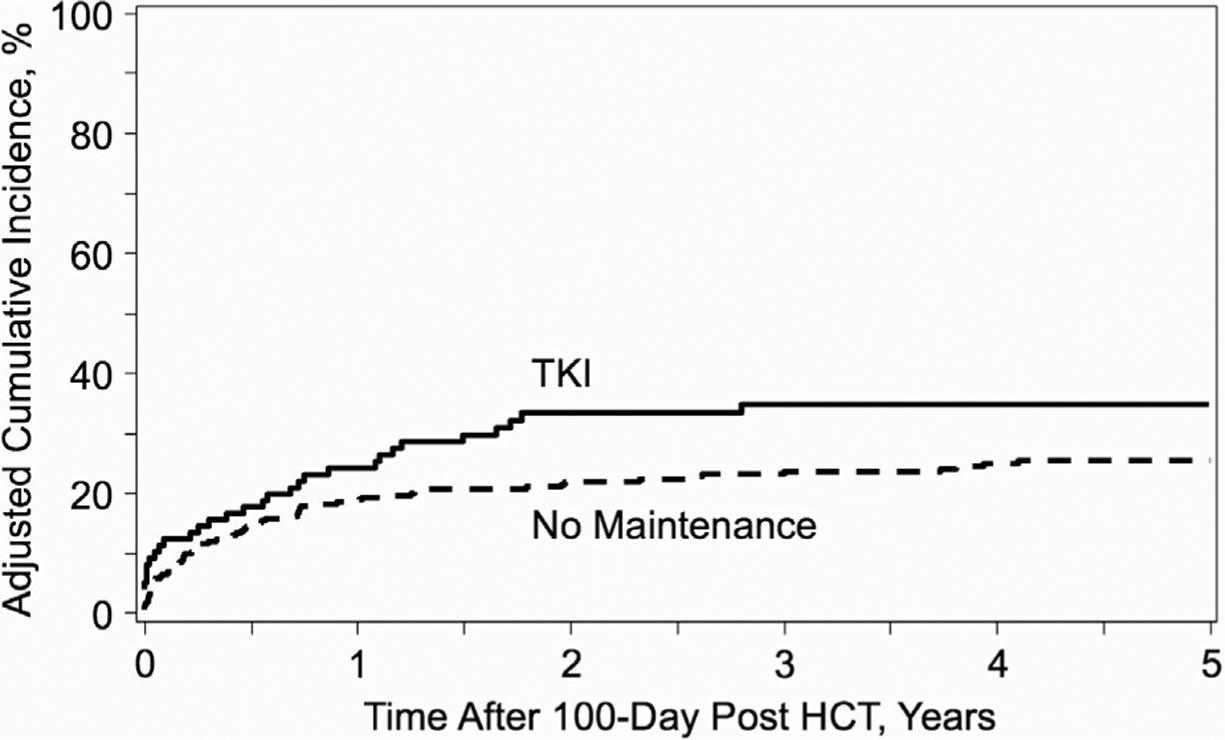
Cumulative incidence curve of relapse according to post-allo-HCT maintenance therapy with TKIs.
TRM
We observed a lower adjusted incidence of TRM at 1-year from day +100 in the TKI maintenance group compared with the no maintenance group (8% [95% CI, 2%−13%] versus 19% [95% CI, 14%−12%]; P = .004). However, there was no significant difference in 5-year outcome (23% [95% CI, 13%−32%] for the maintenance group versus 30% [95% CI, 25%−28%] for the no maintenance group; P = .19 ). In the multivariate analysis (Table 3), cord blood graft source and peripheral blood graft source were independent adverse risk factors for TRM (HR, for cord blood, 2.7 [reference, bone marrow] [95% CI, 1.2–5.8; P = .012]; HR for peripheral blood, 2.4 [reference, bone mar row] [95% CI, 1.3–4.4; P = .006]). Maintenance TKI therapy was not a risk factor for TRM (HR, .7 [reference, no maintenance]; 95% CI, .4–1.1; P = .130).
cGVHD
We observed no significant between-group difference in the adjusted 2-year rate of cGVHD from day +100 (60% [95% CI, 50%−69%] for the maintenance group versus 62% [95% CI, 57%−65%] for the no maintenance group; P = .67). In the multivariate analysis (Table 3), peripheral blood graft source was identified as an adverse risk factor for cGVHD (HR for peripheral blood, 2.0 [reference, bone marrow]; 95% CI, 1.4–2.8; P < .001) and cyclosporine-based GVHD prophylaxis and KPS <90 were independent protective factors against cGVHD (HR for cyclosporine based GVHD prophylaxis, .6 [reference, tacrolimus-based GVHD prophylaxis] [95% CI, .4–.8; P = .003]; HR for KPS <90, .6X [reference, KPS 90–100] [95% CI, .4–.8; P < .001]). Maintenance TKI therapy was not a risk factor for cGVHD (HR, .8 [reference, no maintenance]; 95% CI, .6–1.1; P = .124).
Subgroup Analysis Investigating Disease Status before Allo-HCT
We specifically investigated the interaction of the main effect (TKI maintenance or no maintenance) on the primary outcomes of the study (LFS), according to disease status before allo-HCT. We observed no differential impact of maintenance TKI on LFS (as measured from day +100 post-HCT) based on disease status before allo-HCT (Figure 5).
Figure 5.
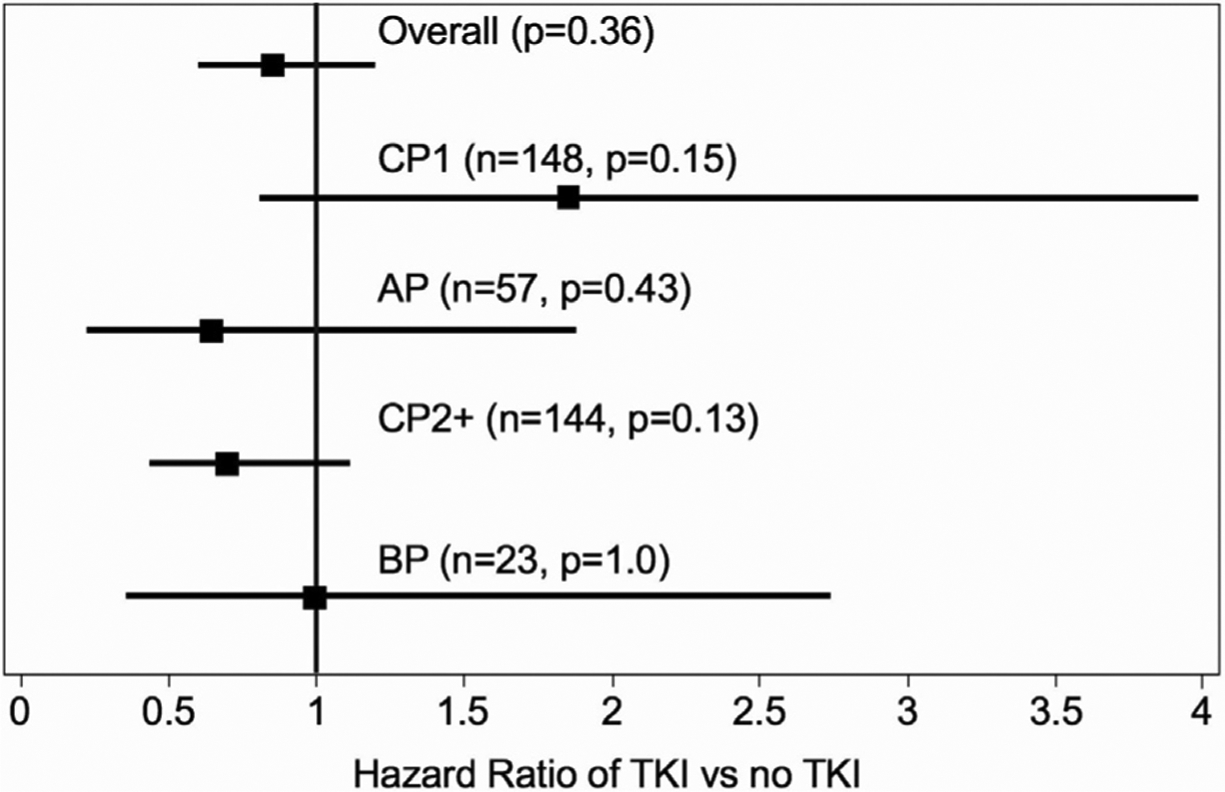
Forest plot of LFS according to disease status before allo-HCT.
We also performed a sensitivity analysis, repeating the aforementioned multivariate analysis after removing patients with CP1 disease. We observed no differential impact of maintenance TKI on major clinical outcomes (OS, LFS, relapse, TRM, and cGVHD) when analyzing patients with disease beyond CP1 at allo-HCT (Supplementary Table S4).
DISCUSSION
In this retrospective registry analysis, we sought to investigate the practice of TKI maintenance therapy following HCT in patients with CML. Our results confirm that in the modern era of TKI therapy, HCT remains a curative option for patients with CML, with encouraging survival. However, the maintenance approach has not been universally adopted, with only 23% of the patients in our cohort receiving TKI maintenance. In a landmark analysis from day +100, we did not demonstrate a benefit in 5-year clinical outcomes (LFS, OS, relapse, TRM, or cGVHD) in the TKI maintenance group compared with the no maintenance group. Although TKI maintenance was associated with a higher incidence of relapse and lower incidence of TRM at earlier time points compared with the no maintenance group, these findings did not maintain significance when analyzing 5-year outcomes or when evaluating the impact of maintenance therapy in the multivariate Cox proportional hazards regression model. The results of this study characterize the clinical outcomes of patients receiving TKI maintenance and call into question the broad application of this approach to all patients with CML. The impact of maintenance TKI did not differ based on disease status before HCT, although we acknowledge that additional differences in disease risk, as well as the physicians’ intent to initiate maintenance therapy, could not be accounted for with this registry analysis. Nevertheless, we believe the results of this study to be important to clinical practice, given the potential toxicities and costs associated with maintenance therapy.
We believe that a number of important factors influenced the outcomes of this study. First, we used a landmark analysis from day +100 following HCT to calculate all follow-up findings and outcomes. Evaluation of maintenance therapies is often associated with inherent selection bias, as patients must be alive and usually without early major complications of allo-HCT (eg, relapse, GVHD, infection, organ toxicity), which can confound clinical perception of the clinical impact of maintenance approaches. By conducting a landmark analysis, we excluded patients with early death, relapse, and cGVHD, and here we attempted to correct for this bias. However, we postulate that this study design also may have in fluenced the findings at earlier time points at which relapse was higher and TRM was lower with TKI maintenance, because an increased number of relapses and deaths before day +100 were excluded from the no maintenance group. Another major factor to consider is the heterogeneity in disease risk and its influence on clinicians’ decision to initiate TKI maintenance. Although we observed differences in disease status before transplantation, we lack additional data on disease risk and TKI sensitivity (including ABL1 domain mutations). We also do not know the reason for the initiation of TKI maintenance, or whether patients in the no maintenance group were originally intended to receive maintenance. Although we observed no significant impact of TKI maintenance on clinical outcomes in this study, we acknowledge that unmeasured cofounders exist that could potentially influence our outcomes. Finally, the majority of patients in this study received myeloablative conditioning, which has been associated with lower rates of relapse early after allo-HCT compared with reduced-intensity conditioning [15]. This conditioning intensity may have influenced clinician decision to initiate post-HCT maintenance therapy. Given the elevated risk for relapse early after allo-HCT with a reduced-intensity conditioning regimen, this may be a population in which TKI maintenance may be of more importance until a maximum GVL effect is seen.
A small number of studies have investigated the use of prophylactic post-transplantation maintenance TKI therapy in patients with Ph+ leukemia. Two early prospective trial of imatinib maintenance in the first year following allogeneic HCT found this approach to be feasible and associated with low rates of relapse [7,8]. As many patients undergoing allogeneic HCT have failed first-line TKIs, there is great interest in the post-HCT use of later generation TKIs. However, the later generation TKIs in particular have been associated with increased toxicities. A phase I/II study investigating nilotinib after allogeneic HCT in 16 patients with high-risk Ph+ leukemia reported 2-year OS and progression-free survival of 69% and 56%, respectively [9]. In this study, 38% of patients had to discontinue therapy because of toxicities, which were predominantly gastrointestinal or hepatic. In a separate phase I/II study, only 32.5% of patients eligible for nilotinib maintenance at engraftment were able to complete the intended 1 year of therapy because of early relapse, toxicities or confounding post-HCT complications [10]. Toxicities can limit duration of TKI maintenance, which is thought to impact the effectiveness of this approach although no studies to date have addressed a minimum or optimal duration of maintenance therapy. No previous registry studies have investigated maintenance TKI use in CML, but in an EBMT analysis of Ph+ acute lymphoblastic leukemia, the use of maintenance TKI post-allo-HCT was identified as a significant factor for improved LFS (HR, .44; P = .002) and OS (HR, .42; P = .004) and lower relapse incidence (HR, .40; P = .01) [12]. Patients with CML undergoing allo-HCT may have increased heterogeneity in disease risk and TKI sensitivity (possibly driven by additional mutations besides BCR-ABL1), which may have limited the extent to which registry analyses are able to detect an impact of TKI maintenance in CML compared with Ph+acute lymphoblastic leukemia.
This study has some additional limitations. A significant limitation is the relatively small size of the TKI maintenance group, although given the current indications for allo-HCT in CML, our cohort represents a comprehensive study group. Given the limits of the data collected in terms of TKI maintenance, we lack information on the starting date, dose, and duration of therapy, as well as the reason why TKI maintenance was stopped. We acknowledge these limitations restrict our ability to assess the impact of this approach, because TKI maintenance therapy may be discontinued after short periods owing to TKI intolerance or other transplantation-related complications [11]. As a result, we analyzed clinical outcomes according to the initiation of maintenance TKI therapy, as reported to the CIBMTR registry, and acknowledge that additional data on maintenance therapy could provide insight into its clinical impact. We also lack data regarding financial burden of maintenance therapy and as well as late effects, 2 important factors that can impact patients’ quality of life. Finally, this study captures transplantations performed during an era of expanding TKI agents. Thus, patients in the earlier years of the study with resistance or intolerance to imatinib or dasatinib before undergoing allo-HCT might not have received the appropriate TKIs for continuing maintenance. Currently, with 5 available TKIs targeting BCR-ABL1, there is no clear optimal choice of TKI following HCT.
In conclusion, the broad application of maintenance TKI following allo-HCT does not seem to be of benefit for patients with CML. Whether there are subpopulations of patients with CML who may benefit from TKI maintenance remains unclear. We believe that the choice to initiate TKIs after allogeneic HCT should be an individualized decision, based on patient-, disease-, and transplantation-related factors.
Supplementary Material
ACKNOWLEDGMENTS
This study is dedicated to the memory of Dr Hanna Jean Khoury, who provided mentorship during the development of the study concept and the early phases of the study design.
Footnotes
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.bbmt.2019.10.017.
REFERENCES
- 1.Björkholm M, Ohm L, Eloranta S, et al. Success story of targeted therapy in chronic myeloid leukemia: a population-based study of patients diagnosed in Sweden from 1973 to 2008. J Clin Oncol. 2011;29 2514–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantarjian H, O’Brien S, Jabbour E, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119:1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gratwohl A, Brand R, Apperley J, et al. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2006;91:513–521. [PubMed] [Google Scholar]
- 5.Khoury HJ, Kukreja M, Goldman JM, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012;47:810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFilipp Z, Chen YB. Strategies and challenges for pharmacological maintenance therapies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:2134–2140. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter PA, Snyder DS, Flowers ME, et al. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007;109:2791–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olavarria E, Siddique S, Griffiths MJ, et al. Posttransplantation imatinib as a strategy to postpone the requirement for immunotherapy in patients under going reduced-intensity allografts for chronic myeloid leukemia. Blood. 2007;110:4614–4617. [DOI] [PubMed] [Google Scholar]
- 9.Shimoni A, Volchek Y, Koren-Michowitz M, et al. Phase 1/2 study of nilotinib prophylaxis after allogeneic stem cell transplantation in patients with advanced chronic myeloid leukemia or Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015;121:863–871. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter PA, Johnston L, Fernandez HF, et al. Posttransplant feasibility study of nilotinib prophylaxis for high-risk Philadelphia chromosome positive leukemia. Blood. 2017;130:1170–1172. [DOI] [PubMed] [Google Scholar]
- 11.DeFilipp Z, Langston AA, Chen Z, et al. Does post-transplant maintenance therapy with tyrosine kinase inhibitors improve outcomes of patients with high-risk Philadelphia chromosome-positive leukemia? Clin Lymphoma Myeloma Leuk. 2016;16 466–471.e1. [DOI] [PubMed] [Google Scholar]
- 12.Brissot E, Labopin M, Beckers MM, et al. Tyrosine kinase inhibitors improve long-term outcome of allogeneic hematopoietic stem cell trans plantation for adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia. Haematologica. 2015;100:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14: 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhabra S, Ahn KW, Hu ZH, et al. Myeloablative vs reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chronic myeloid leukemia. Blood Adv. 2018;2:2922–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


