Abstract
Lumbar disc herniation (LDH) elicits low back pain (LBP) and lower-limb symptoms. Paralumbar spine disease (PLSD), for example, superior cluneal nerve/middle cluneal nerve entrapment (SCN-EN, MCN-EN) and sacroiliac joint pain (SIJ), may be attributable to LDH whose treatment may not ameliorate their symptoms. We treated LDH patients and addressed their coexisting PLSDs. We retrospectively analyzed the effects of targeted block therapy for PLSD in 47 patients with LDH. They were 23 men and 24 women ranging in age from 21 to 79 years. They were seen between August 2014 and October 2018, within 3 weeks of LDH onset. PLSD was diagnosed based on the symptoms of patients whose pain was not controlled by oral medications. The treatment outcome was assessed by comparing the numerical rating scale (NRS) and the Roland-Morris Disability Questionnaire (RDQ) score recorded before and 2 weeks after last block treatment. Of the 47 patients with LDH, 2 suffered no LBP and 30 reported tenderness in the low back. We performed block therapy in 13 patients; 9 (19.1%) had concurrent PLSD and experienced pain relief. Their NRS improved from 8.1 ± 1.8 before- to 1.3 ± 0.9 after treatment; their RDQ score fell from 11.2 ± 6.0 to 0.9 ± 1.2 (both, p < 0.01). In an LDH patient with MCN-EN alone, MCN neurolysis was performed 2 weeks after a single MCN block proved to be only transiently effective. Paralumbar diseases may coexist in patients with LDH; treatment of the former may alleviate their LBP.
Keywords: low back pain, lumbar disc herniation, middle cluneal nerve, superior cluneal nerve, paralumbar spine disease
Introduction
Low back pain (LBP) and lower-limb pain are common symptoms of lumbar disc herniation (LDH). When conservative treatment with oral medications, rehabilitation, and root block are ineffective, surgery may be indicated. Some operated patients experience failed back surgery syndrome (FBSS).
Paralumbar spine disease (PLSD) is defined as a disease that leads to LBP around the lumbar spine.1) PLSD, for example, superior cluneal nerve/middle cluneal nerve entrapment (SCN-EN, MCN-EN) and sacroiliac joint pain (SIJ) elicit LBP and leg symptoms1-6) mimicking the symptoms of LDH.7) In some patients with FBSS, treatment for PLSD improves some symptoms.1,8) We report the effective block treatment of coexisting PLSD in patients with LDH.
Patients and Methods
Patients
This retrospective study was approved by the institutional ethics committee of Kushiro Rosai Hospital; prior consent for inclusion in this study was obtained from all patients. Included were 47 consecutive patients with LDH seen between August 2014 and October 2018 at our institutions; one patient had been operated in 2014 and was part of an earlier investigation.1) They were 23 men and 24 women (mean age 55.4 years, range 21–79 years). LDH was diagnosed based on subjective symptoms and magnetic resonance imaging (MRI) and neurological findings; pain and/or numbness in the affected dermatome coincided with MRI findings. We focused on the acute phase of LDH in this study. All patients experienced lower-limb pain and/or LBP, defined in this study as low back- and buttock pain, within 3 weeks of LDH onset. The LDH level was L2/L3 (n=5), L3/L4 (n=4), L4/L5 (n=20), and L5/S1 (n=18). No patients reported chronic LBP; all suffered acute symptoms.
Treatment strategy
Our first treatment choice was oral medications (acetaminophen, non-steroidal anti-inflammatory drugs, tramadol, pregabalin). Patients with lower-limb paresis or bladder-rectal dysfunction were candidates for early surgical treatment. When intractable LBP persisted, we considered the coexistence of PLSD, for example, SCN-EN, MCN-EN, and SIJ pain, and performed block therapy. Surgery for LDH was performed when medications and block treatments were ineffective. To evaluate the effect of each block accurately, only one block was performed per day, and repeat blocks were basically performed every other day as needed.
Diagnostic criteria
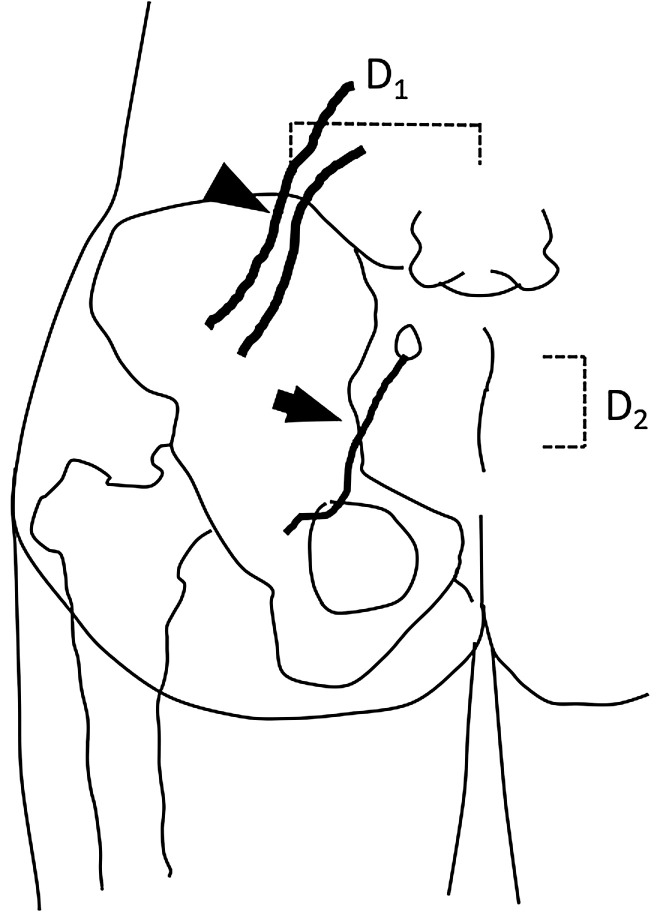
Figure 1 is a schema of the left lumbar area and hip. SCN-EN was diagnosed as reported by others.9-11) LBP involves the iliac crest and buttocks. The trigger point is at a site in the upper iliac crest 70 mm from the midline at a depth near the iliac bone (SCN entrapment point). Blocks at the site of the trigger point using 2 mL of 1% lidocaine alleviate LBP.
Fig. 1.
Schematic of the left lumbar area and hip. The trigger point (arrowhead) at the SCN-EN site is located 70 mm (D1, dashed line) lateral to the midline on the iliac crest. The trigger point (arrow) at the MCN-EN site is located 35 mm (D2, dashed line) caudal to the PSIS and at a slightly lateral point at the edge of the iliac crest. MCN-EN: middle cluneal nerve entrapment; PSIS: posterior superior iliac spine; SCN-EN: superior cluneal nerve entrapment.
The diagnosis of MCN-EN was also based on earlier reports.11-14) LBP involves the buttocks. The trigger point is 35 mm behind the posterior superior iliac spine (PSIS) and slightly beyond the termination of the iliac crest at a depth near the iliac bone (MCN entrapment point). Blocks at the site of the trigger point using 2 mL of 1% lidocaine improve LBP. We took care to prevent the block from reaching the SIJ.
We used the SIJ score to diagnose SIJ pain.15-17) When at least four sites with SIJ dysfunction were suspected and when pain disappeared after SIJ block (1% lidocaine), SIJ pain was diagnosed.
Radiological findings
Before block treatment for PLSD, three lateral lumbar spine radiographs (flexed, neutral, and extended) were obtained. Neutral-position films were acquired at the patients’ natural posture without any instructions. The lumbar lordosis angle was measured from the superior endplate between L-1 and S-1. The criteria for instability were slippage by more than 4 mm and/or an angle change of more than 10° on flexion and extension.18)
Evaluation of treatment outcomes
Treatment outcomes were assessed based on the numerical rating scale (NRS) and the Roland-Morris Disability Questionnaire (RDQ) scores recorded before and 2 weeks after last block treatment. The NRS 2 weeks post-treatment and at the last follow-up visit after discharge were also assessed. When the NRS score fell by 3 or more points, block therapy was considered effective in LDH patients with coexisting PLSD.19)
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan),20) a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria, version 3.5.1). EZR is a modified version of R commander (version 2.5-1) designed to add statistical functions frequently used in biostatistics. Differences of p <0.05 were considered statistically significant.
Results
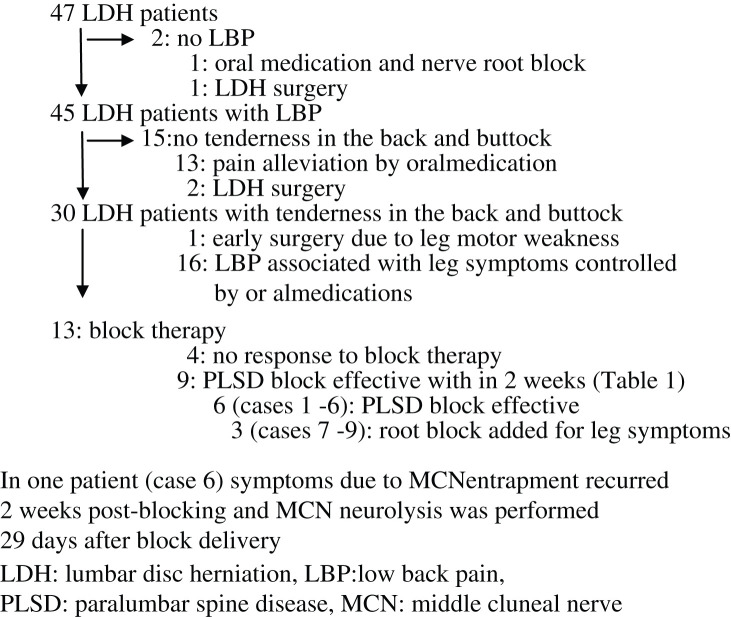
Of the 47 patients, 2 reported no LBP; their leg symptoms were controlled by oral medication and nerve root block or by LDH surgery (Fig. 2). Among 15 patients who did not suffer tenderness in the back and buttock, 13 experienced pain alleviation by oral medication and 2 underwent LDH surgery. Of the remaining 30 patients, all suffered tenderness in the low back or buttock and we suspected the coexistence of PLSD. One of these patients underwent early surgery due to leg motor weakness in the right S1 area and in 16, oral medications controlled LBP associated with leg symptom due to LDH.
Fig. 2.
Workflow diagram of patient recruitment and treatment.
Consequently, 13 of the 47 patients (27.7%) underwent block therapy. Of these 13 patients, 4 did not respond to block therapy; their NRS before and 2 weeks after treatment was 7.0 ± 1.4 and 5.8 ± 1.7, respectively. In the other 9, the NRS fell from 8.1 ± 1.8 before- to 1.3 ± 0.9 after block treatment and their RDQ score fell from 11.2 ± 6.0 to 0.9 ± 1.2 (both, p < 0.01, paired t-test). Table 1 lists the 9 LDH patients with concurrent PLSD whose LBP was found to have responded to block therapy at 2-week post-last blocking assessment. Their average age was 60.5 years, and not significantly different from the other patients (54.1 years). Their mean lumbar sagittal alignment (L1-S1) before treatment was 32.8° (16.1–43.8°) with no instability. There were two patients with instability (cases 5 and 8) manifested slippage by more than 4 mm and no angle change of more than 10° on flexion and extension. No patients presented with chronic vertebral fracture or Parkinson disease that may play a role in cluneal nerve entrapment.
Table 1.
Patients with LDH and coexisting PLSD who showed a response to block therapy at 2-week post-blocking assessment
| Case | Age | Sex | LDH | SCN-EN | MCN-EN | SIJ pain | Root block |
|---|---|---|---|---|---|---|---|
| level | number of blocks | number of blocks | number of blocks | number of blocks | |||
| 1 | 64 | F | L4/5 | 0 | 1 | 0 | 0 |
| 2 | 43 | M | L5/S | 0 | 1 | 0 | 0 |
| 3 | 65 | F | L5/S | 0 | 3 | 0 | 0 |
| 4 | 61 | F | L4/5 | 3 | 0 | 0 | 0 |
| 5 | 64 | F | L5/S | 0 | 3 | 1 | 0 |
| 6 | 52 | F | L5/S | 0 | 1* | 0 | 0 |
| 7 | 44 | F | L5/S | 3 | 0 | 0 | 1 |
| 8 | 73 | F | L4/5 | 1 | 3 | 1 | 1 |
| 9 | 79 | F | L3/4 | 0 | 0 | 1 | 1 |
In case six symptoms due to MCN entrapment recurred 2-week post-blocking and MCN neurolysis was performed 29 days after block delivery. LDH: lumbar disc herniation, MCN-EN: middle cluneal nerve entrapment, PLSD: paralumbar spine disease, SCN-EN: superior cluneal nerve entrapment, SIJ: sacroiliac joint.
Of the nine LDH patients with concurrent PLSD, one suffered pain from concomitant SCN-EN only, four from MCN-EN only, and one reported SIJ pain only. Three of the other patients presented with multiple concurrent PLSD. None of our patients manifested piriformis muscle tenderness. In six of the nine patients (cases 1–6), blocks plus oral medications controlled LBP and their leg symptoms were alleviated 2 weeks after therapy. In the other cases (cases 7–9), block treatment improved their LBP but their leg symptoms failed to respond sufficiently and they required nerve root blocks within 2 weeks of the first blocking. Above block therapies during this periods required an average 7.2 days (1–14 days).
The mean follow-up term was 5.8 months (range 2–8 months) in the nine LDH patients whose concomitant PLSD responded to block therapy. In one patient (case 6), symptoms are due to MCN entrapment recurred 2 weeks post-blocking, and MCN neurolysis was performed 29 days after the single first block delivery. Medication, taken on an as-needed basis delivered adequate pain control to the patients (mean NRS 1.0, range 0–3). The last follow-up visit was a mean of 25.3 months post-treatment (range 8–52 months). During that period, two patients with concurrent MCN-EN required additional blocks; they were delivered 11 (case 5) and 38 months (case 4) after the first treatment. Their remaining pain was also controlled using medication as needed and their mean NRS was 1.0 (range 0–3).
Discussion
The SCN is a sensory nerve; the dorsal branches at Th11 to L5 pass through the lumbar region, penetrate the thoracolumbar fascia near the iliac crest, and reach the buttocks. The MCN is also a sensory nerve; its dorsal branches at S1 to S3 pass below the PSIS and reach the buttocks. The SIJ is innervated by its dorsal branches at L4 to S3. When these nerves are compressed due to LDH, only the nerves at the LDH level tend to be damaged. However, as we found that nerves other than those directly compressed by LDH were affected, we suspected that unknown factors were involved in LBP elicitation.
According to Morimoto et al.,2) an increase in the paravertebral muscle tonus and tightness throughout the sinuvertebral nerves that supply the vertebral structure (ligaments, facets, intervertebral discs) may elicit SCN-EN, as may SCN stretching with posture and motion. The etiology of MCN-EN may be related to an increase in the gluteus maximus muscle tonus and MCN stretching due to posture and movement.6,14) In addition, repeat loading of the SIJ may affect structures around this joint and its dysfunction may result in SIJ pain.15) When the MCN is entrapped around the SIJ, slight but repetitive SIJ loading and minor subluxation may elicit MCN-EN.6,14) Under such conditions, SCN-EN, MCN-EN, and SIJ pain can coexist in patients with LDH.5,7,14) Consequently, various changes due to LDH may lead to coexisting PLSDs.
Patients with LDH report LBP, leg pain, motor weakness, and bladder- and rectal disorders21,22) that can significantly affect their activities of daily living.23) Conservative treatment with oral medications, epidural block, nerve root block, and physical therapy24) aims at pain reduction. When it fails, surgery may be necessary. LDH patients with cauda equina syndrome and severe or progressive neurological symptoms are also surgical candidates.8)
We considered SCN-EN, MCN-EN, and SIJ pain to be PLSDs. Their symptoms are similar to those of undiagnosed lumbar spine diseases.5,7) For the diagnosis and treatment of PLSD, we first identify the trigger point of each disease5,15) and then deliver a block at the trigger point using a local anesthetic for further symptom assessments. We suggest that in patients with LBP associated with LDH, careful symptom assessment must be performed to determine whether local blocks or surgical treatments are appropriate.
Among our 47 LDH patients, 45 reported LBP; 13 also suffered tenderness in the low back and buttock; they underwent block treatment. While it was ineffective in four patients, in nine with coexisting PLSD it contributed to LBP control.
Elsewhere,8) we reported that LDH patients with FBSS benefited from treatment of their peripheral nerve disease; their persistent or recurrent LBP and leg pain improved. These findings suggest that block treatment for coexisting PLSD may be effective in patients with LDH.
Limitations
This study has some limitations. Our study population was small and the outcomes were first evaluated 2 weeks after last block. Because our findings were made in patients whose LBP occurred within 3 weeks of LDH onset, they cannot be extrapolated to all patients with LDH. In addition, although normal saline nerve blockage may help to identify the placebo effect of block therapy, this approach raises ethical issues. In the current series, we did not include facet pain and para-vertebral muscle pain. Although various changes due to LDH and unknown factors may lead to PLSD, we were unable to pinpoint the eliciting changes and factors.
In this study, we focused on the cases with the acute phase within 3 weeks of the onset, and we evaluated the short-term outcome at 2 weeks after last block. In some cases, outcome was assessed more than 3 weeks after onset, and it cannot be denied the possibility of the effect of natural improvement.
Footnotes
Conflicts of Interest Disclosure
The authors declare no conflict of interest.
References
- 1).Matsumoto J, Isu T, Kim K, et al. : Impact of additional treatment of paralumbar spine and peripheral nerve diseases after lumbar spine surgery. World Neurosurg 112: e778–e782, 2018 [DOI] [PubMed] [Google Scholar]
- 2).Morimoto D, Isu T, Kim K, et al. : Long-term outcome of surgical treatment for superior cluneal nerve entrapment neuropathy. Spine (Phila Pa 1976) 42: 783–788, 2017 [DOI] [PubMed] [Google Scholar]
- 3).Iwamoto N, Isu T, Kim K, et al. : Treatment of low back pain elicited by superior cluneal nerve entrapment neuropathy after lumbar fusion surgery. Spine Surg Relat Res 1: 152–157, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Morimoto D, Isu T, Shimoda Y, et al. : Assessing the treatment for sacroiliac joint dysfunction, piriformis syndrome and tarsal tunnel syndrome associated with lumbar degenerative disease. No Shinkei Geka 37: 873–879, 2009. (Japanese) [PubMed] [Google Scholar]
- 5).Isu T, Kim K, Morimoto D, Iwamoto N: Superior and middle cluneal nerve entrapment as a cause of low back pain. Neurospine 15: 25–32, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Kim K, Isu T, Matsumoto J, Yamazaki K, Isobe M: Low back pain due to middle cluneal nerve entrapment neuropathy. Eur Spine J 27: 309–313, 2018 [DOI] [PubMed] [Google Scholar]
- 7).Kim K, Isu T, Morimoto D, et al. : Common diseases mimicking lumbar disc herniation and their treatment. Mini-inv Surg 1: 43–51, 2017 [Google Scholar]
- 8).Yamauchi T, Kim K, Isu T, et al. : Undiagnosed peripheral nerve disease in patients with failed lumbar disc surgery. Asian Spine J 12: 720–725, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Lu J, Ebraheim NA, Huntoon M, Yeasting RA: Anatomic considerations of superior cluneal nerve at posterior iliac crest region. Clin Orthop Relat Res 347: 224–228, 1998 [PubMed] [Google Scholar]
- 10).Maigne JY, Doursounian L: Entrapment neuropathy of the medial superior cluneal nerve. Nineteen cases surgically treated, with a minimum of 2 years’ follow-up. Spine (Phila Pa 1976) 22: 1156–1159, 1997 [DOI] [PubMed] [Google Scholar]
- 11).Tubbs RS, Levin MR, Loukas M, Potts EA, Cohen- Gadol AA: Anatomy and landmarks for the superior and middle cluneal nerves: application to posterior iliac crest harvest and entrapment syndromes. J Neurosurg Spine 13: 356–359, 2010 [DOI] [PubMed] [Google Scholar]
- 12).Aota Y: Entrapment of middle cluneal nerves as an unknown cause of low back pain. World J Orthop 7: 167–170, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Morimoto D, Isu T, Kim K, et al. : Surgical treatment of superior cluneal nerve entrapment neuropathy. J Neurosurg Spine 19: 71–75, 2013 [DOI] [PubMed] [Google Scholar]
- 14).Matsumoto J, Isu T, Kim K, Iwamoto N, Morimoto D, Isobe M: Surgical treatment of middle cluneal nerve entrapment neuropathy: technical note. J Neurosurg Spine 29: 208–213, 2018 [DOI] [PubMed] [Google Scholar]
- 15).Murakami E, Aizawa T, Noguchi K, Kanno H, Okuno H, Uozumi H: Diagram specific to sacroiliac joint pain site indicated by one-finger test. J Orthop Sci 13: 492–497, 2008 [DOI] [PubMed] [Google Scholar]
- 16).Kurosawa D, Murakami E, Aizawa T: Referred pain location depends on the affected section of the sacroiliac joint. Eur Spine J 24: 521–527, 2015 [DOI] [PubMed] [Google Scholar]
- 17).Kurosawa D, Murakami E, Ozawa H, et al. : : A diagnostic scoring system for sacroiliac joint pain originating from the posterior ligament. Pain Med 18: 228–238, 2017 [DOI] [PubMed] [Google Scholar]
- 18).Iwamoto N, Isu T, Kim K, et al. : Treatment of low back pain elicited by superior cluneal nerve entrapment neuropathy after lumbar fusion surgery. Spine Surg Relat Res 1: 152–157, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Lee CH, Chung CK, Kim CH: The efficacy of conventional radiofrequency denervation in patients with chronic low back pain originating from the facet joints: a meta-analysis of randomized controlled trials. Spine J 17: 1770–1780, 2017 [DOI] [PubMed] [Google Scholar]
- 20).Kanda Y: Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48: 452–458, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Taher F, Essig D, Lebl DR, et al. : Lumbar degenerative disc disease: current and future concepts of diagnosis and management. Adv Orthop 2012: 970752, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Ahn UM, Ah NU, Buchowski JM, Garrett ES, Sieber AN, Kostuik JP: Cauda equina syndrome secondary to lumbar disc herniation: a meta-analysis of surgical outcomes. Spine (Phila Pa 1976) 25: 1515–1522, 2000 [DOI] [PubMed] [Google Scholar]
- 23).Yiengprugsawan V, Hoy D, Buchbinder R, et al. : Low back pain and limitations of daily living in Asia: Longitudinal findings in the Thai cohort study. BMC Musculoskelet Disord 18: 19, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Gregory DS, Seto CK, Wortley GC, Shugart CM: Acute lumbar disk pain: navigating evaluation and treatment choices. Am Fam Physician 78: 835–842, 2008 [PubMed] [Google Scholar]