Abstract
Background
Stent thrombosis (ST) is a dreaded complication after stent implantation and is associated with a mortality between 5% and 45%. The mechanisms by which ST arises are complex. Because of the seriousness of this situation, all physicians should have at least basic knowledge of it. In this article, we present the risk factors for ST and discuss some innovative approaches to its treatment.
Methods
This review is based on pertinent articles retrieved by a selective search in PubMed, and on current international guidelines and expert recommendations.
Results
The frequency of ST has been markedly lowered by technical advances in coronary stenting and by the implementation of modern implantation techniques, including the introduction of coverage with dual antiplatelet therapy (DAPT). Both patient-related risk factors and procedural aspects can elevate the risk of ST. The independent risk factors for ST include premature termination of DAPT (hazard ratio [HR] 26.8; 95% confidence interval [8.4; 85.4]; p <0.0001), malignant disease (odds ratio [OR]: 17.45; [4.67; 65.26]; p <0.0001), and diabetes mellitus (OR: 3.14; [1.33; 7.45]; p = 0.0093). In comparison to angiographically guided procedures, the use of intracoronary imaging techniques in patients with acute coronary syndrome lowers the frequency of ST (0.6% versus 1.2%; p = 0.005). These techniques enable the detection of many findings in the coronary arteries that are associated with the development of ST. In such cases, countermeasures such as secondary stent dilatation or prolongation of DAPT can help prevent ST.
Conclusion
As the pathophysiology of ST is multifactorial, research in this area presents a special challenge. Prospective clinical trials will be needed to determine whether the systematic use of imaging techniques can lower the frequency of ST.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. The questions on this article can be found at http://daebl.de/RY95. The deadline for submissions is 30 April, 2021.
Participation is possible at cme.aerztebatt.de
More than 300 000 percutaneous coronary interventions (PCIs) are carried out annually in Germany, with the worldwide figure reaching over five million (e1). As such, PCI represents one of the most frequently performed interventions in modern medicine. Although complications are rare, there are some that can have a significant impact on the patient‘s treatment outcome. Stent thrombosis (ST) represents a potentially life-threatening and fatal outcome following PCI (e2) which is associated with a mortality rate of between 5 and 45%, as well as a recurrence rate of 15–20% at 5 years (1– 3). Given the high number of PCIs performed each year, although rare, ST is therefore an important complication and topic of research. The classification of ST is made on the basis of the Academic Research Consortium (ARC) definition and takes into consideration not only the time elapsed since stent implantation, but also the likelihood of ST recurrence (4) (table 1). A meta-analysis (5) showed rates of definite, probable, or possible ST of 2.4% (95% confidence interval [CI]: 2.0%; 2.9%) with follow-up over a median period of 22 months. Lemesle et al. (3) reported that very late (more than 12 months following stent implantation; Table 1) ST was responsible for 20% of myocardial infarctions (MI) in 2816 patients with previous stent implantation. Of the patients with MI due to very late ST, 59% presented with the clinical picture of ST-segment elevation myocardial infarction (STEMI).
Table 1. Clinical classification of stent thrombosis (ST) based on the criteria of the Academic Research Consortium (ARC) and according to (4, 5).
| Time after stent implantation | Early ST | Late ST | Very late ST |
| – Acute ST: 0–24 h – Subacute ST: Between 24 h and 30 days |
More than 30 days up to 1 year | More than 1 year | |
| Incidence | – Acute ST: 0.4% (0.2%; 0.6%) (5) – Subacute ST: 1.1% (1.0%; 1.3%) (5) |
0.5% (0.4%; 0.6%) (5) | 0.6% (0.4%; 0.8%) (5) |
| Probability | Definite ST | Probable ST | Possible ST |
| ● Angiographic confirmation of ST:
The presence of a thrombus in the region of the stent or 5 mm proximal or distal to the stent, as well as the occurrence of at least one of the following criteria within 48 h – Acute symptoms of ischemia at rest – New ECG changes typical of ischemia – Typical rise and fall in cardiac biomarkers – Non-occlusive thrombus – Occlusive thrombus with TIMI 0 or TIMI 1 flow in the ‧region of the stent or proximal to a stent up to the ‧adjacent side or main branch ● Documentation of ST based on pathological confirmation: Thrombus detection within the stent following autopsy or tissue analysis following thrombectomy |
– Any unexplained death within 30 days of stent implantation – Any myocardial infarction with acute ischemia in the region of the implanted stent without angiographic confirmation of ST in the absence of another identifiable cause |
– Any unexplained death ‧occurring later than 30 days after stent implantation |
TIMI 0 flow, no coronary blood flow; TIMI 1 flow, sharply slowed coronary blood flow; the categories range from TIMI 0 to TIMI 3
The mechanisms underlying the development of ST are multifactorial and risk stratification is complex at the individual patient level (e3). Patient-related characteristics as well as features of the lesion to be treated, including procedural aspects, affect the occurrence of ST, as do mechanical effects and premature discontinuation of antithrombotic therapy (6). The type of stent implanted also plays an important role in terms of the risk of ST. This article describes the history of coronary stents in relation to the development of ST.
Methods
A selective literature search was conducted in PubMed and took into consideration current international guidelines and specialist recommendations. The search criteria and search terms used are shown in the Box.
BOX. Literature search.
The literature search for this study was carried out using the PubMed database. The search criteria mentioned below were used for the basic search; the search was extended according to the specific subtopics (“IVUS”/“OCT”/“angiography”) at the relevant point.
Date: 01/01/1989–29/02/2020
Language: English
Terms: (thromb*[tiab]) AND coronar*[tiab]) AND stent[tiab]) OR (“coronary artery disease” [tiab] OR CAD[tiab] OR “coronary heart disease” [tiab] OR CHD[tiab] OR “acute myocardial infarction”[tiab] OR AMI[tiab] OR “acute coronary syndrome”[tiab] OR ACS[tiab] OR NSTEMI[tiab] OR STEMI[tiab] OR “unstable angina”[tiab]) AND (“coronary intervention”[tiab] OR PCI[tiab] OR “coronary stenting”[tiab] OR “coronary artery stent”[tiab] OR “drug-eluting stent”[tiab] OR DES[tiab] OR “drug eluting stent” [tiab] OR bare-metal stent[tiab] OR BMS[tiab] OR “bare metal stent” [tiab] OR scaffold[tiab]) AND (thromb*[tiab]).
Stent technologies and risks of stent thrombosis
In 1987, Sigwart and Puel described for the first time the use of a self-expanding bare metal stent (BMS) in the setting of acute vessel occlusion during balloon angioplasty (e4). Due to better angiographic and clinical outcomes compared to balloon angioplasty alone, the use of BMS was long considered the preferred treatment method; however, it resulted in an up to 30% increased rate of in-stent thrombosis (7). Further advances in implantation techniques and the introduction of dual antiplatelet therapy (DAPT) reduced the risk of thrombosis. Both Schömig et al. and Leon et al. showed a significant reduction in ST when using DAPT following BMS implantation (p = 0.005) (8, 9). Stent thrombosis occurred in 16 patients (2.9%) following the administration of aspirin and in 15 patients (2.7%) following the administration of aspirin and warfarin. After receiving DAPT consisting of aspirin and ticlopidine, three patients (0.5%) exhibited ST.
The first drug-eluting stent (DES) was implanted by J. Eduardo Sousa in 1999 (e5). Although the then novel development was supposed to reduce the occurrence of restenosis (7) and other complications following the implantation of BMS, it was initially associated with a renewed rise in ST (e6). The controlled release of antiproliferative agents resulted in a marked reduction in in-stent restenosis (e7). However, the desired effect led to delayed integration of the stent in the vessel wall and an increased risk for the development of late ST (e7). Second-generation DES were subsequently coated with antiproliferative drugs that were less toxic, polymer coatings that were more biocompatible, and thinner stent struts made of modern alloys. Compared to older DES, these improvements resulted in a reduced risk for the occurrence of late and very late ST. The COMPARE study (10) revealed a significant reduction in definite and probable ST 12 months after implantation from 3% to 0.7% when a switch was made from paclitaxel-eluting to everolimus-eluting stents (p = 0.002). The development of biodegradable polymer coatings that remained temporarily on the stent surface was also hailed as a promising strategy (e8). However, there is a lack of data collected over a longer period of time on the implantation of new DES with thin stent struts and biodegradable polymers. In 2012, fully resorbable vascular scaffolds (bioresorbable scaffolds, BRS), which were designed to reduce the long-term effects of the metal implant, were introduced. Following the use of the ABSORB scaffold, increased rates of scaffold thrombosis were seen compared to everolimus-eluting stents (11). Meta-analyses supported these results, showing a 2-year incidence of thrombosis of 2.3% compared to 0.7% ST following implantation of everolimus-eluting DES (12). In the light of this negative result, the scaffold was no longer used in routine practice from that time onwards and was withdrawn from the market in 2017. Recent studies on the use of new-generation BRS found them to have an improved safety profile over an observation period of 12 months, as well as stable angiographic parameters at 6–12 months following implantation (e9). However, due to the lower number of patients included and the lack of longer-term data, the validity of these findings is limited (e9).
Drug therapy to reduce stent thrombosis
Dual antiplatelet therapy
The key element in the prevention of stent thrombosis lies in the prescription of dual antiplatelet therapy (DAPT) following PCI (7). Unplanned discontinuation of therapy is a leading risk factor for the development of ST (13). For patients with stable angina, the guidelines on chronic coronary heart disease (14) recommend DAPT for a 6-month period following PCI. If patients are at high risk for life-threatening bleeding, DAPT of shorter duration can be considered in view of the low risk of ST after 1–3 months. In patients with acute coronary syndrome (ACS), a 12-month treatment duration is advised; in the case of a high risk of bleeding, a shorter duration of 6 months can be prescribed (15). If DAPT needs to be prematurely discontinued in patients at increased risk of bleeding in order to reduce hemorrhagic complications, discontinuation in this context appears to be safer following implantation of new-generation DES compared to BMS (16).
On the other hand, patients implanted with first-generation DES can benefit from prolonged treatment duration, as can patients with complex coronary lesions who have well tolerated a first period of DAPT therapy. Here, one can consider a treatment duration of more than 12 months (17, 18). Particularly in patients at increased risk for bleeding and low risk for ischemia, a shorter treatment duration can be contemplated. A meta-analysis that included patients with ACS showed that shorter treatment duration compared to 12-month DAPT was associated with a comparable number of ischemic events and a reduced number of bleeding events (19). However, patients with multivessel coronary disease following ACS are at increased risk for ischemia and could benefit from prolonged DAPT (e10). In order to evaluate the optimal treatment, the guidelines on dual antiplatelet therapy (15, 20) recommend an assessment of the individual ischemia and bleeding risk (e11). A number of risk assessment systems, such as the PRECISE-DAPT score, are available to this end (21).
Predictors of stent thrombosis
Numerous risk factors are associated with the occurrence of ST. Table 2 summarizes these factors for the occurrence of early and late ST in a patient-related manner. The strongest predictor for the development of early stent thrombosis is premature discontinuation of DAPT in the first 30 days following stent implantation (7, 22). A prospective study (2) that looked at 2229 patients following placement of a DES showed increased rates of subacute (hazard ratio [HR]: 161.17; [26.03; 997.94], p <0.001) and late ST (HR: 57.13; [14.84; 219.96], p <0.001) after premature discontinuation of DAPT. Van Werkum et al. (23) demonstrated that procedural aspects also need to be considered as risk factors for ST. Late ST correlates highly with the presence of malignant disease (odds ratio [OR]: 17.45; [4.67; 65.26]; p <0.0001), as well as diabetes mellitus (OR: 3.14; [1.33; 7.45]; p = 0.0093). A left ventricular ejection fraction of less than 30% is a powerful risk factor for early ST (OR: 2.71; [1.61; 4.57]; p = 0.0002) (7, 23). In addition, a multitude of findings associated with an increased risk for ST can be determined with the help of intracoronary imaging techniques (Figure 1): malapposition of the stent struts as well as discontinuity and fractures in the implanted stents modify the flow characteristics and affect the local level of blood viscosity (e12).
Table 2. Risk factors for the development of stent thrombosis (ST).
| Early ST | Late ST |
| – Premature discontinuation of DAPT (7) – Genetic polymorphisms (7) |
– Malignant disease (23) |
| – Reduced left ventricular function (7, 23) – Malignant disease (23) |
– Peripheral arterial occlusive disease (23) |
| – Thrombocytosis (39) – Diabetes mellitus (39) |
– Diabetes mellitus (7, 23) – Reduced left ventricular function (7, 23) – Younger age (23) – Smoking (39) |
DAPT, dual antiplatelet therapy
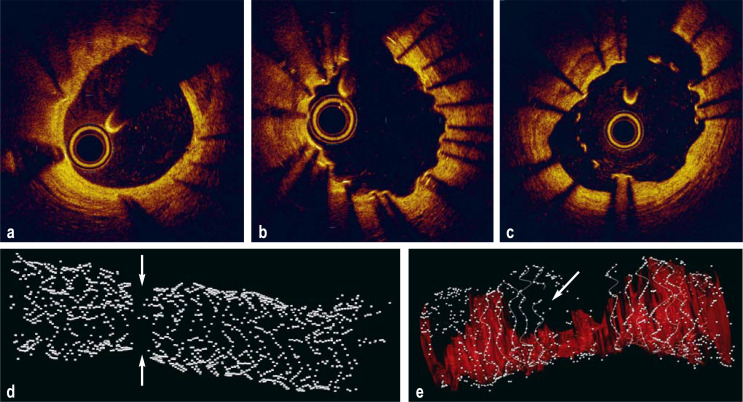
Figure 1:
Predictors of stent thrombosis: selected findings in optical coherence tomography
a) Normal finding: good result following implantation of a drug-eluting stent (DES), neointimal coverage of stent struts
b) Evaginations: outward bulging of the luminal vessel contour between the struts of a DES
c) Malapposition: lack of contact between the abluminal stent surface and the vessel wall, including incomplete neointimal coverage of the stent struts
d) 3D reconstruction of a stent fracture: the arrows point to the broken, dislocated struts of a DES
e) 3D reconstruction of a stent fracture: the arrow points to stenosis within the fractured stent, resulting in reduced blood flow (red)
An increased incidence of very late ST (24, 25) following implantation of first-generation DES led to a modification of the alloy, geometry, drugs eluted, and polymer coating. In this regard, a meta-analysis found a significant reduction in ST following implantation of modern DES compared to BMS and first-generation DES (26). The thickness of the stent struts also has an impact on the risk for the development of ST. The SORT OUT VII study (27) compared a sirolimus-eluting stent (60-µm stent strut thickness) with a biolimus-eluting stent (120-µm stent strut thickness) and demonstrated lower rates of definite ST when the thinner struts were used (0.4 % versus 1.2%; p = 0.034). Therefore, the current guidelines on myocardial revascularization (28) recommend the exclusive use of newer-generation DES.
Prevention by using modern intracoronary imaging techniques
Coronary artery angiography is considered the standard imaging method in the diagnosis and treatment of coronary pathologies. Furthermore, the use of modern intravascular imaging techniques enables an individual treatment approach and has a long-term impact on the incidence of cardiovascular events, including ST. A meta-analysis (29) with 17 882 patients compared imaging-guided with purely angiography-guided PCI: the former resulted in a significant reduction in myocardial infarctions (OR: 0.72; [0.52; 0.93] and repeat target vessel revascularization (OR: 0.74 [0.58; 0.90]), and ST (OR: 0.42 [0.20; 0.72]). The use of intracoronary imaging in patients with ACS reduced the incidence of ST (0.6% versus 1.2%; p = 0.005) (e13).
Intravascular ultrasound (IVUS) was long considered the cornerstone of diagnosis of stent thrombosis. The introduction of optical coherence tomography (OCT), which uses infrared light and has an approximately ten-fold higher axial resolution, significantly increases discriminatory power in the diagnosis of ST (30). The prospective PESTO registry (31) investigated patients with ST using OCT and identified morphological abnormalities, such as malapposition of stent struts or stent underexpansion, in 97% of cases. Whilst malapposition was strongly associated with the development of both early (48%) and late (31%) ST, stent underexpansion correlated with the occurrence of early ST in 26% of cases. These findings highlight the importance of the optimal implantation technique. A meta-analysis (32) covering 4946 patients reported a prevalence of 16% (95% CI: [12%; 20%]) for incomplete stent apposition; the incidence of late or very late ST (incidence rate ratio [IRR]: 4.81 [2.68; 8.62] and MI (IRR: 3.09; [1.72; 5.55]) was significantly increased. Modern imaging techniques make it possible for the pathomechanisms underlying the development of ST to be detected early on and for the relevant countermeasures to be taken (figure 2). Jang et al. (33) showed that IVUS-guided implantation of DES is associated with a significant reduction in overall mortality (OR: 0.64; [0.51; 0.81]; p <0.001), as well as significantly lower rates of MI (OR: 0.57; [0.42; 0.78], p <0.001) and ST (OR: 0.59; [0.42; 0.82]; p = 0.002). In contrast, data for the comparison of angiographic and OCT-guided interventions are lacking. The results of the CLI-OPCI study suggest that the use of OCT in the setting of PCI improve the clinical treatment outcome of patients. Using OCT, it was possible to detect suboptimal stent implantation in over 30% of lesions (34). The randomized studies OPINION (35) and ILUMIEN III (36) compared the two imaging methods and came to the conclusion that the use of OCT is not inferior to IVUS in terms of treatment outcome. Both the use of IVUS and the use of OCT can improve the outcome of coronary interventions. These imaging methods enable adequate stent sizing, including selection of the optimal stent length. Acute complications such as stent strut malapposition or dissection can be identified with high sensitivity and specificity. (30).
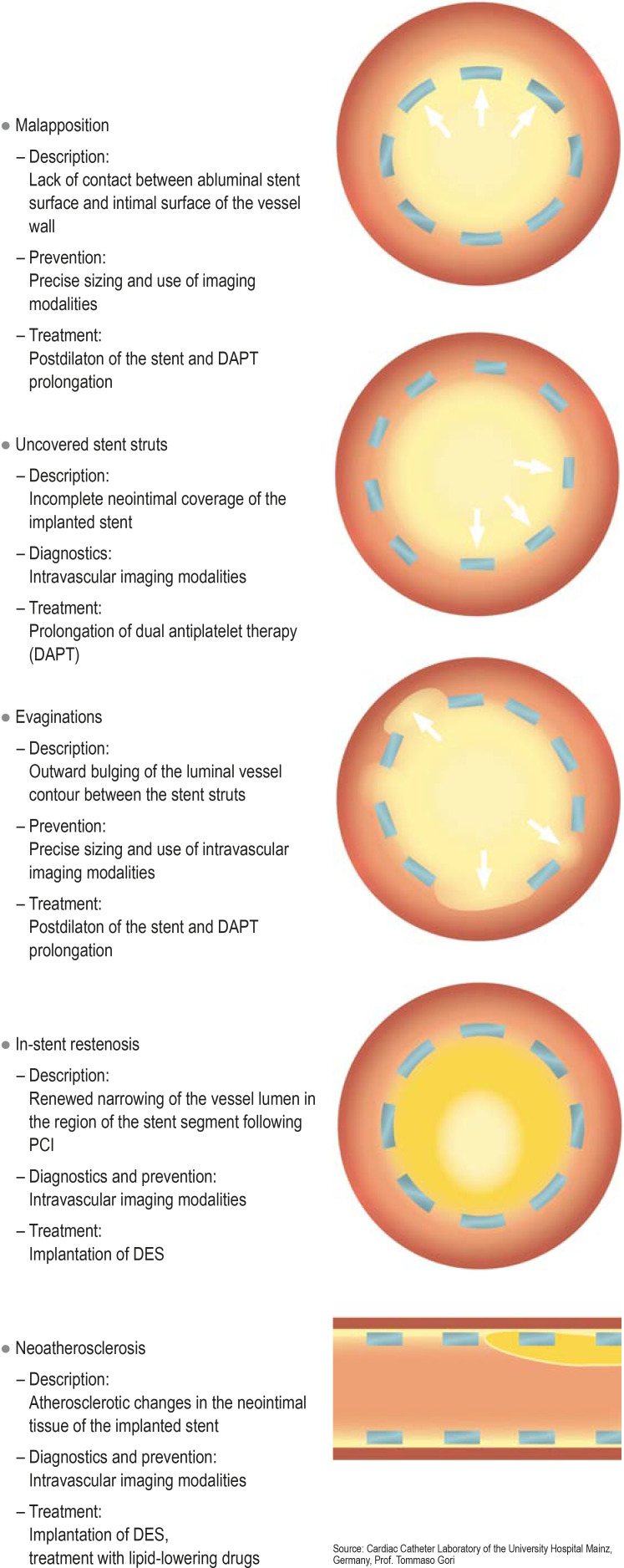
Figure 2.
Mechanism of development as well as targeted preventive and treatment measures for stent thrombosis (ST)
The benefit of intravascular imaging is particularly evident in high-risk patients and patients with complex coronary lesions (30). However, the data come from retrospective analyses, and prospective data are lacking. The results of the ILUMIEN IV study, which is investigating the benefit of OCT in high-risk patients with complex coronary heart disease, should close this gap. Table 3 compares the advantages and disadvantages of the two imaging modalities.
Table 3. Advantages and disadvantages of the imaging modalities intravascular ultrasound (IVUS) and optical coherence tomography (OCT).
| IVUS | Advantages | Disadvantages |
| ● Decades of clinical experience ● High tissue penetration as well as visualization of the external elastic membrane enables optimization of stent size ● Extensive studies on the effect of IVUS-guided intervention on the clinical treatment outcome, including data from meta-analyses ● Existence of established predictors for the development of restenosis and availability of clear guidelines and threshold values |
● Acquired images are more challenging to interpret, poorer resolution for structures adjacent to the lumen ● Limited tissue characterization (better with virtual histology) ● Detection of thrombus material is challenging, dissections are difficult to measure ● Limited assessment of stent strut malapposition ● Limited penetration and measurement of calcified lesions |
|
| OCT | Advantages | Disadvantages |
| ● 10-Fold higher resolution facilitates detection of fine details for structures adjacent to the lumen (dissections, malapposition) ● Better tissue characterization with greater precision compared to histology ● Clear visualization and classification (white versus red) of thrombus material ● Existence of established predictors for the development of restenosis and stent thrombosis |
● Contrast medium required ● Limited tissue penetration ● No randomized data (a new clinical trial is currently in the process of recruiting participants) |
Table modified from Räber et al. (40)
Practical implications for clinical routine
Due to their greater safety and efficacy, new-generation DES should be preferred over older DES (28). The current guidelines recommend the preferential implantation of DES irrespective of clinical presentation, the lesion to be treated, and the expected duration of DAPT (28). Precise matching of the stent to the target vessel, including optimization of the implantation technique while correcting morphological abnormalities at the time of placement, can reduce the risk for the development of ST (37). Data from the Syntax II study (38) support the use of a state-of-the-art treatment strategy. The clinical treatment outcome of patients with three vessel coronary artery disease was improved by using innovative measurement methods to visualize coronary physiology, the implantation of modern DES with thin stent struts, and intracoronary imaging techniques. Compared to conventional coronary interventions, a significant reduction in severe cardiovascular and cerebrovascular events (13.2% versus 21.9%; p = 0.001), as well as a significant reduction in definite ST (0.9% versus 2.9%; p = 0.048) was seen at 2 years. Intracoronary imaging techniques yield precise information on the mechanistic aspects underlying the development of ST (figure 2).
Conclusion
The causes for the development of stent thrombosis are multifactorial. Risk factors at the level of the patient, the lesion to be treated, and the interventional approach need to be promptly identified and appropriate countermeasures initiated. The current guidelines on myocardial revascularization recommend a differentiated treatment decision that takes into consideration all influencing factors, as well as the use of modern DES and individual implantation techniques. Rigid treatment regimes are increasingly being relegated to the background. In terms of selecting the optimal antiplatelet therapy, the guidelines on dual platelet aggregation inhibition speak for a modern treatment concept that is based on the individual ischemia and bleeding risk of the patient. The guidelines recommend the use of intravascular imaging in order to optimize stent implantation on the one hand and to identify mechanistic features that could underlie the development of ST on the other. These imaging methods are becoming increasingly important and are opening up new perspectives to optimize treatment outcome. The combination of our growing understanding of the etiology of ST, the development of new stents, and the possibilities offered by modern imaging techniques enables individual treatment approaches that have long-term effects on the incidence of stent thrombosis.
Key Messages.
The mechanisms and risk factors for the development of stent thrombosis are multifactorial and require a differentiated risk stratification.
Antiplatelet therapy concepts should be guided by the individual ischemia and bleeding risk of the patient.
The use of modern drug-eluting stents and the optimization of the implantation technique reduce the risk for the development of ST.
Modern imaging modalities yield information on coronary findings associated with the development of ST and improve the outcome of PCI.
Innovative treatment concepts enable a multimodal treatment approach with long-term effects on the incidence of ST.
Coronary Stent Thrombosis—Predictors and Prevention. The submission deadline is 30 April 2021. Only one answer per question is possible.
Please select the most applicable answer.
Question 1
Approximately how many percutaneous coronary interventions are carried out annually in Germany?
100 000
300 000
750 000
1 million
2 million
Question 2
Which criterion does the Academic Research Consortium (ARC) definition take into account for the classification of stent thrombosis?
Time elapsed since stent implantation
Patient age
Comorbidities
Patient sex
Vessel wall thickness
Question 3
After what period of time following stent implantation is the Academic Research Consortium (ARC) definition met for very late stent thrombosis?
After 4 months
After 6 months
After 8 months
After 10 months
After more than 1 year
Question 4
Which finding, in addition to the presence of a thrombus in the region of the stent or 5 mm proximal or distal to the stent, needs to be present within 48 h in order for the criterion of definite stent thrombosis to be met?
Tachycardia
Bradycardia
Atrial fibrillation
Acute symptoms of ischemia at rest
Reduction in ejection fraction by at least 50%
Question 5
What is the strongest predictor for the development of early stent thrombosis?
Malignant disease
Premature discontinuation of dual antiplatelet therapy in the first 30 days following stent implantation
Reduced left ventricular function
Peripheral arterial occlusive disease
Advanced age
Question 6
Which imaging modality has the best axial resolution for the diagnosis of stent thrombosis?
Optical coherence tomography
Positron emission tomography
Computed tomography
Angiography
Doppler ultrasound
Question 7
What is meant by evagination in stent thrombosis?
Lack of contact between abluminal stent surface and intimal surface of the vessel wall
Incomplete neointimal coverage of the implanted stent
Outward bulging of the luminal vessel contour between the stent struts
Atherosclerotic change in the neointimal tissue of the implanted stent
Narrowing of the vessel lumen in the region of the stent segment following percutaneous coronary intervention
Question 8
What is the advantage of intravascular ultrasound in the diagnosis of stent thrombosis?
High tissue penetration and visualization of the external elastic membrane enable stent size optimization.
Clear visualization and classification of thrombus material
Better tissue characterization with greater accuracy compared to histology
The existence of established predictors for the prevention of stent thrombosis
Simple interpretation of images and excellent resolution of structures adjacent to the lumen
Question 9
Which type of stent does the current ESC/EACTS guideline recommend for implantation?
The bare metal stent with balloon angioplasty
The new-generation drug-eluting stent
The bioresorbable scaffold stent
The everolimus-eluting stent
The covered vascular stent
Question 10
Which patients may benefit from prolonged dual antiplatelet therapy?
Patients with left ventricular hypertrophy
Patients with left atrial dilation
Patients with a normal ejection fraction
Patients with right heart failure
Patients with multivessel disease following acute coronary syndrome
Acknowledgments
Translated from the original German by Christine Rye.
Footnotes
Conflict of interests
Prof. Gori received honoraria for consultancy activities (Advisory Board) from Abbott Vascular and Daiichi Sankyo. He received lecture honoraria from Abbott Vascular, Boston Sci, SMT, and Bayer.
The remaining authors declare that no conflicts of interest exist.
References
- 1.Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv. 2014;7:1081–1092. doi: 10.1016/j.jcin.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 3.Lemesle G, Tricot O, Meurice T, et al. Incident myocardial infarction and very late stent thrombosis in outpatients with stable coronary artery disease. J Am Coll Cardiol. 2017;69:2149–2156. doi: 10.1016/j.jacc.2017.02.050. [DOI] [PubMed] [Google Scholar]
- 4.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 5.D‘Ascenzo F, Bollati M, Clementi F, et al. Incidence and predictors of coronary stent thrombosis: evidence from an international collaborative meta-analysis including 30 studies, 221,066 patients, and 4276 thromboses. Int J Cardiol. 2013;167:575–584. doi: 10.1016/j.ijcard.2012.01.080. [DOI] [PubMed] [Google Scholar]
- 6.Cuculi F, Puricel S, Jamshidi P, et al. Optical coherence tomography findings in bioresorbable vascular scaffolds thrombosis. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.114.002518. e002518. [DOI] [PubMed] [Google Scholar]
- 7.Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur Heart J. 2015;36:3320–3331. doi: 10.1093/eurheartj/ehv511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084–1089. doi: 10.1056/NEJM199604253341702. [DOI] [PubMed] [Google Scholar]
- 9.Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting Stent anticoagulation restenosis study investigators. N Engl J Med. 1998;339:1665–1671. doi: 10.1056/NEJM199812033392303. [DOI] [PubMed] [Google Scholar]
- 10.Kedhi E, Joesoef KS, McFadden al., et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375:201–209. doi: 10.1016/S0140-6736(09)62127-9. [DOI] [PubMed] [Google Scholar]
- 11.Ali ZA, Gao R, Kimura T, et al. Three-year outcomes with the absorb bioresorbable scaffold: individual-patient-data meta-analysis from the ABSORB randomized trials. Circulation. 2018;137:464–479. doi: 10.1161/CIRCULATIONAHA.117.031843. [DOI] [PubMed] [Google Scholar]
- 12.Polimeni A, Anadol R, Münzel T, Indolfi C, De Rosa S, Gori T. Long-term outcome of bioresorbable vascular scaffolds for the treatment of coronary artery disease: a meta-analysis of RCTs. BMC Cardiovasc Disord. 2017;17:147. doi: 10.1186/s12872-017-0586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz S, Schuster T, Mehilli J, et al. Stent thrombosis after drug-eluting stent implantation: incidence, timing, and relation to discontinuation of clopidogrel therapy over a 4-year period. Eur Heart J. 2009;30:2714–2721. doi: 10.1093/eurheartj/ehp275. [DOI] [PubMed] [Google Scholar]
- 14.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2019;00:1–71. doi: 10.1093/eurheartj/ehz425. doi:10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 15.Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 16.Varenne O, Cook S, Sideris G, et al. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet. 2018;391:41–50. doi: 10.1016/S0140-6736(17)32713-7. [DOI] [PubMed] [Google Scholar]
- 17.Valgimigli M, Borghesi M, Tebaldi M, et al. Should duration of dual antiplatelet therapy depend on the type and/or potency of implanted stent? A pre-specified analysis from the PROlonging Dual antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY (PRODIGY) Eur Heart J. 2013;34:909–919. doi: 10.1093/eurheartj/ehs460. [DOI] [PubMed] [Google Scholar]
- 18.Yeh RW, Kereiakes DJ, Steg PG, et al. Lesion complexity and outcomes of extended dual antiplatelet therapy after percutaneous coronary intervention. J Am Coll Cardiol. 2017;70:2213–2223. doi: 10.1016/j.jacc.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verdoia M, Kedhi E, Ceccon C, Suryapranata H, De Luca G. Duration of dual antiplatelet therapy and outcome in patients with acute coronary syndrome undergoing percutaneous revascularization: a meta-analysis of 11 randomized trials. Int J Cardiol. 2018;264:30–38. doi: 10.1016/j.ijcard.2018.02.095. [DOI] [PubMed] [Google Scholar]
- 20.Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS - Web Addenda. https://academic.oup.com/DocumentLibrary/EHJ/SupplementaryData/ehx419web.pdf (last accessed on 2 April 2020) [Google Scholar]
- 21.Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025–1034. doi: 10.1016/S0140-6736(17)30397-5. [DOI] [PubMed] [Google Scholar]
- 22.Généreux P, Rutledge DR, Palmerini T, et al. Stent thrombosis and dual antiplatelet therapy interruption with everolimus-eluting stents: insights from the Xience V Coronary Stent System trials. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.114.001362. pii: e001362. [DOI] [PubMed] [Google Scholar]
- 23.van Werkum JW, Heestermans AA, Zomer AC, et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol. 2009;53:1399–1409. doi: 10.1016/j.jacc.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 24.Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115:1440–1455. doi: 10.1161/CIRCULATIONAHA.106.666800. [DOI] [PubMed] [Google Scholar]
- 25.McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- 26.Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–1402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 27.Jensen LO, Thayssen P, Maeng M, et al. Randomized comparison of a biodegradable polymer ultrathin strut sirolimus-eluting stent with a biodegradable polymer biolimus-eluting stent in patients treated with percutaneous coronary intervention: The SORT OUT VII Trial. Circ Cardiovasc Interv. 2016;9 doi: 10.1161/CIRCINTERVENTIONS.115.003610. pii: e003610. [DOI] [PubMed] [Google Scholar]
- 28.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 29.Buccheri S, Franchina G, Romano S, et al. Clinical outcomes following intravascular imaging-guided versus coronary angiography-guided percutaneous coronary intervention with stent implantation: a systematic review and bayesian network meta-analysis of 31 studies and 17,882 patients. JACC Cardiovasc Interv. 2017;10:2488–2498. doi: 10.1016/j.jcin.2017.08.051. [DOI] [PubMed] [Google Scholar]
- 30.Maehara A, Matsumura M, Ali ZA, Mintz GS, Stone GW. IVUS-guided versus OCT-guided coronary stent implantation: a critical appraisal. JACC Cardiovasc Imaging. 2017;10:1487–1503. doi: 10.1016/j.jcmg.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Souteyrand G, Amabile N, Mangin L, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J. 2016;37:1208–1216. doi: 10.1093/eurheartj/ehv711. [DOI] [PubMed] [Google Scholar]
- 32.Sethi A, Singbal Y, Rastogi U, Prasad VS. Late incomplete stent apposition is associated with late/very late stent thrombosis: a meta-analysis. Catheter Cardiovasc Interv. 2018;91:365–375. doi: 10.1002/ccd.27102. [DOI] [PubMed] [Google Scholar]
- 33.Jang JS, Song YJ, Kang W, et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. JACC Cardiovasc Interv. 2014;7:233–243. doi: 10.1016/j.jcin.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Prati F, Romagnoli E, La Manna A, et al. Long-term consequences of optical coherence tomography findings during percutaneous coronary intervention: the Centro Per La Lotta Contro L‘infarto-Optimization Of Percutaneous Coronary Intervention (CLI-OPCI) LATE study. EuroIntervention. 2018;14:e443–e451. doi: 10.4244/EIJ-D-17-01111. [DOI] [PubMed] [Google Scholar]
- 35.Kubo T, Shinke T, Okamura T, et al. Optical frequency domain imaging vs intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J. 2017;38:3139–3147. doi: 10.1093/eurheartj/ehx351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali ZA, Maehara A, Généreux P, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388:2618–2628. doi: 10.1016/S0140-6736(16)31922-5. [DOI] [PubMed] [Google Scholar]
- 37.Pasceri V, Pelliccia F, Pristipino C, et al. Clinical effects of routine postdilatation of drug-eluting stents. Catheter Cardiovasc Interv. 2014;83:898–904. doi: 10.1002/ccd.24999. [DOI] [PubMed] [Google Scholar]
- 38.Serruys PW, Kogame N, Katagiri Y, et al. Clinical outcomes of state-of-the-art percutaneous coronary revascularisation in patients with three-vessel disease: two-year follow-up of the SYNTAX II study. EuroIntervention. 2019;15:e244–e252. doi: 10.4244/EIJ-D-18-00980. [DOI] [PubMed] [Google Scholar]
- 39.Dangas GD, Claessen BE, Mehran R, et al. Development and validation of a stent thrombosis risk score in patients with acute coronary syndromes. JACC Cardiovasc Interv. 2012;5:1097–1105. doi: 10.1016/j.jcin.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Räber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39:3281–3300. doi: 10.1093/eurheartj/ehy285. [DOI] [PubMed] [Google Scholar]
- E1.MedMarket Diligence, LLC. Global dynamics of surgical and interventional cardiovascular procedures, 2015-2022. (Report #C500) MedMarket Diligence. 2016 [Google Scholar]
- E2.Iqbal J, Sumaya W, Tatman V, et al. Incidence and predictors of stent thrombosis: a single-centre study of 5,833 consecutive patients undergoing coronary artery stenting. EuroIntervention. 2013;9:62–69. doi: 10.4244/EIJV9I1A10. [DOI] [PubMed] [Google Scholar]
- E3.Amabile N, Trouillet C, Meneveau N, et al. Mechanical abnormalities associated with first- and second-generation drug-eluting stent thrombosis analyzed by optical coherence tomography in the national PESTO French registry. Int J Cardiol. 2017;227:161–165. doi: 10.1016/j.ijcard.2016.11.084. [DOI] [PubMed] [Google Scholar]
- E4.Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316:701–706. doi: 10.1056/NEJM198703193161201. [DOI] [PubMed] [Google Scholar]
- E5.Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol. 2010;56:S1–S42. doi: 10.1016/j.jacc.2010.06.007. [DOI] [PubMed] [Google Scholar]
- E6.Chen KY, Rha SW, Li YJ, et al. Comparisons of everolimus and paclitaxel-eluting stents in patients with acute myocardial infarction. J Interv Cardiol. 2015;28:147–156. doi: 10.1111/joic.12187. [DOI] [PubMed] [Google Scholar]
- E7.Stefanini GG, Taniwaki M, Windecker S. Coronary stents: novel developments. Heart. 2014;100:1051–1061. doi: 10.1136/heartjnl-2012-303522. [DOI] [PubMed] [Google Scholar]
- E8.Yin Y, Zhang Y, Zhao X. Safety and efficacy of biodegradable drug-eluting vs bare metal stents: a meta-analysis from randomized trials. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099648. e99648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Haude M, Ince H, Abizaid A, et al. Sustained safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de novo coronary lesions: 12-month clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial. Eur Heart J. 2016;37:2701–2709. doi: 10.1093/eurheartj/ehw196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Bansilal S, Bonaca MP, Cornel JH, et al. Ticagrelor for secondary prevention of atherothrombotic events in patients with multivessel coronary disease. J Am Coll Cardiol. 2018;71:489–496. doi: 10.1016/j.jacc.2017.11.050. [DOI] [PubMed] [Google Scholar]
- E11.Deutsche Gesellschaft für Kardiologie - Herz-und Kreislaufforschung e.V. ESC pocket guidelines. Duale antithrombozytäre Therapie (DAPT) bei Koronarer Herzkrankheit. Version 2017. Gründwald: Börm Bruckmeier Verlag GmbH. 2018 [Google Scholar]
- E12.Poon EKW, Thondapu V, Hayat U, et al. Elevated blood viscosity and microrecirculation resulting from coronary stent malapposition. J Biomech Eng. 2018;140 doi: 10.1115/1.4039306. doi: 10.1115/1.4039306. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita T, Sakamoto K, Tabata N, et al. Imaging-guided PCI for event suppression in Japanese acute coronary syndrome patients: community based observational cohort registry. Cardiovasc Interv Ther. 2020 doi: 10.1007/s12928-020-00649-3. doi: 10.1007/s12928-020-00649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]