Abstract
Background
Electrocardiogram (ECG) is considered the initial screening method for the detection of left ventricular hypertrophy (LVH) despite its low sensitivity. However, there are no data on how ECG criteria for LVH perform in patients with concentric (cLVH) and eccentric LVH (eLVH).
Methods
In the setting of the Corinthia cross‐sectional study, ECGs were analyzed in 1,570 participants of the study. Seven ECG LVH criteria were calculated (Sokolow–Lyon voltage, index, and product, sex‐specific Cornell voltage and product, Lewis voltage, and the Framingham), whereas LVH was defined, based on echocardiographic data, as left ventricular mass indexed for body surface area (BSA) of at least 125 g/m2 in men and at least 110 g/m2 in women.
Results
Regarding the frequency encountered for each ECG LVH criterion, there was no difference between eLVH and cLVH. However, when ECG criteria were compared as continuous variables between LVH groups, Cornell voltage and product were higher in cLVH individuals, with a value of Cornell voltage >13.95 mV having 61% sensitivity and 62% specificity to differentiate cLVH from eLVH (p = .05). Even after adjustment for age, sex, body mass index, and hypertension, the occurrence of Cornell voltage or product increased the odds of cLVH by 1.6 times (p = .001).
Conclusion
Cornell voltage and product criteria disclosed a superior discriminative ability for the detection of LVH via ECG. When further categorizing LVH as concentric and eccentric, Cornell product depicted the higher discriminative ability for cLVH.
Keywords: concentric left ventricular hypertrophy, eccentric left ventricular hypertrophy, electrocardiogram, left ventricular hypertrophy
1. INTRODUCTION
Echocardiography has long been established as the method of choice for the diagnosis of left ventricular hypertrophy (LVH). However, the electrocardiogram (ECG) is considered the initial screening method not only in every‐day clinical practice but also in research trials (Hancock et al., 2009). Several ECG criteria have been developed over the past century and are characterized by high specificity while their sensitivity is below average, even after adjustment for anthropometric factors (Levy et al., 1990; Okin, Devereux, et al., 2000; Okin, Jern, Devereux, Kjeldsen, & Dahlöf, 2000; Okin et al., 2002). Additionally, the existence of various LVH criteria in the ECG has been associated with adverse cardiovascular events such as myocardial infarction, stroke, and sudden cardiac death (Leigh, O'Neal, & Soliman, 2016; Levy, Salomon, D'Agostino, Belanger, & Kannel, 1994; Okin et al., 2004; Prineas, Rautaharju, Grandits, & Crow, 2001; Salles, Cardoso, Fiszman, & Muxfeldt, 2010; Wachtell et al., 2007).
According to overload condition and the pattern of left ventricle (LV) remodeling and adaptation, LVH is classically classified according to the relative wall thickness (RWT) which is calculated based on the ratio of the LV wall thickness to the diameter of the LV (Ganau et al., 1992; Lang et al., 2015a,2015b). Beyond differences in etiologic and risk factors, distinct pattern of hypertrophy may have prognostic implications and may impose therapeutic challenges (Bang et al., 2014; Ganau et al., 1992).
On primary physician setting, the evaluation of patients with arterial hypertension with or without heart failure relies primarily on the combination of physical examination with the ECG findings. However, there are no data on how ECG criteria for LVH perform in patients with concentric (cLVH) and eccentric LVH (eLVH). Therefore, based on echocardiographic diagnosis of LVH and in the concept of Corinthia study, we sought to examine the diagnostic performance of seven commonly used ECG LVH criteria in determining the presence of cLVH or eLVH.
2. METHODS
2.1. Study's sample
The Corinthia cross‐sectional epidemiological study was carried out in the homonymous region of the Peloponnese area in Greece. From October 2015 to February 2017, two thousand forty‐three permanent inhabitants aged 40 years or older were voluntarily enrolled in the study. All participants underwent a standard 12‐lead ECG followed by a complete transthoracic echocardiographic examination. Subsequently, they were interviewed by trained personnel (i.e., cardiologists, general practitioners, nurses, and social scientists) using a standard questionnaire. Inhabitants with lacking anthropometric data, poor‐quality echocardiographic, or ECG tracings were excluded leading to a final sample size of 1,592 individuals.
All individuals were informed about the aims of the study and provided written informed consent. The study was approved by the Ethics Committee of our institution and was carried out in accordance with the Declaration of Helsinki (1989).
2.2. Clinical and anthropometric measurements
Weight and height were measured following standard protocols, followed by BMI calculation in kg/m2, with obesity being defined by BMI higher than 29.9 kg/m2. Body surface area (BSA) was calculated according to Mosteller's equation ( ) in m2 (Mosteller, 1987). Resting arterial blood pressure was measured three times in the right arm at the end of the physical examination with the individual in sitting position. Those with average blood pressure levels greater or equal to 140/90 mmHg or those under antihypertensive medication were classified as hypertensive subjects. Total serum cholesterol levels exceeding 200 mg/dl or the use of lipid‐lowering drugs defined hypercholesterolemia. Smoking of at least one cigarette per day was defined as current smoking, while diabetes mellitus was determined based on fasting plasma glucose levels in accordance with the American Diabetes Association diagnostic criteria (fasting plasma glucose ≥126 mg/dl or use of specific medication; American Diabetes A, 2019).
2.3. Electrocardiogram measurements
A resting 12‐lead ECG with 10 s of duration was performed during quiet respiration in each individual by the use of SE‐1010 PC ECG with DP‐12 ECG amplifier (EDAN Instruments Inc.). Smart ECG Measurement and Interpretation Program (SEMIP version 1.5), which is part of EDAN SE series electrocardiograph and PC ECG, was used for automated measurement and interpretation of amplitudes and duration of ECG waves in each of 12 leads. The ECG bandwidth that was used for these recordings was between 0.05 and 150 Hz, whereas the low‐pass filter was configured in six steps from the user between 25 and 150 Hz. The automated measurements were based on averaged representative complexes for each lead, and QRS duration was based on “global” complexes, while QT, QTC, and QTD were based on each individual lead. Adjustment of the automatically designated amplitudes and duration of ECG waves was performed by two physicians blinded to the study, with adjudication required in approximately 10% of the recordings. From these measurements, we calculated seven ECG criteria considering their widespread use and recognition in LVH detection, five “pure voltage” and two “time‐voltage” criteria:
Sokolow–Lyon (SL) voltage (sum of the amplitudes of S wave on V1 and R wave on V5 or V6 ≥ 3.5 mV; Sokolow & Lyon, 1949).
SL product (SV1 + RV5 or V6 × QRS duration ≥3,000 mm ms for women and ≥4,000 mm ms for men; Molloy, Okin, Devereux, & Kligfield, 1992).
SL index (amplitude of R wave on lead aVL ≥ 11 mV; Sokolow & Lyon, 1949).
Sex‐specific Cornell voltage (sum of the amplitudes of S wave on V3 and R wave on aVL > 2.0 mV in women and >2.8 mV in men; Casale, Devereux, Alonso, Campo, & Kligfield, 1987).
Cornell product [(RaVL + SV3) +8 mm for women] ×QRS duration ≥2,440 mm ms] (Molloy et al., 1992; Okin, Roman, Devereux, & Kligfield, 1995).
Lewis voltage (sum of the amplitudes of R wave on lead I and S wave on lead III, minus the amplitudes of S wave on lead I and R wave on lead III, ≥1.7 mV; Lewis, 1914).
The Framingham criterion (coexistence of a definite strain pattern and at least one of the following voltage criteria: sum of the amplitudes of the R wave on lead I and the S wave on lead III ≥2.5 mV, sum of the amplitudes of the S wave on lead V1 or V2 and the R wave on lead V5 or V6 ≥ 3.5 mV, the S wave on the right precordial lead ≥2.5 mV, and the R wave on the left precordial lead ≥2.5 mV; Levy et al., 1990).
2.4. Cardiac ultrasonography
Standard transthoracic echocardiographic examination was performed by the same expert in a dimly lit room using a Vivid e cardiovascular ultrasound system (General Electric) equipped with a 2.0–3.6 MHz (harmonics) phased‐array transducer. The two‐dimensional guided M‐mode echocardiographic study of the left ventricle was performed at the parasternal long‐axis view, and left ventricular end‐systolic and end‐diastolic dimensions, as well as posterior wall (PWT) and septal thicknesses, were measured as the mean from three and five consecutive cardiac cycles in those with sinus rhythm and atrial fibrillation, respectively, according to current guidelines (Lang et al., 2015a,2015b).
Left ventricular mass was calculated with the method of Devereux et al (Devereux et al., 1986), namely left ventricular mass = 0.8× (1.04× [(LVID + VST + PWT)3 –LVID3]) +0.6, where LVID is the left ventricular internal diameter; VST, the ventricular septal thickness; and PWT, the posterior wall thickness. The reliability of the echocardiographic measurement of left ventricular mass has been demonstrated in previous studies. Left ventricular mass was subsequently indexed for BSA, and LVH was defined as left ventricular mass indexed for BSA 115 g/m2 or more in men and 95 g/m2 or more in women (Lang et al., 2015a,2015b). In the presence of LVH, further classification was done based on relative wall thickness (RWT = 2 × PWT/LVEDD). In cases of RWT >0.42, cLVH was assumed, while in individuals with LVH and RWT equal or <0.42, the diagnosis of eLVH was set (Lang et al., 2015a,2015b).
In order to estimate a potential relationship of wall stress with different types of LVH, meridional left ventricle wall stress (MLVWS) was calculated based on the following formula: where SBP and WTs were systolic blood pressure and end‐systolic wall thickness averaged between interventricular septum and LV posterior wall, respectively. LVDs represents the end‐systolic LV diameter (Reichek et al., 1982).
2.5. Statistical analysis
Continuous variables that followed a normal distribution are presented as mean ± SD. Categorical variables are presented as percentages. The t test was used for comparisons between means of normally distributed continuous variables. Differences between categorical variables were tested by forming contingency tables and performing chi‐square tests. Diagnostic accuracy of each LVH criterion was estimated via receiver operating characteristic (ROC) curves. Sensitivity, specificity, and negative and positive predictive values of ECG criteria were calculated using standard formulas (Gardner & Greiner, 2006). The areas under the ROC curves of the various ECG criteria were compared using the Z test. All reported p values were based on two‐sided hypotheses. All statistical calculations were performed using SPSS software (version 25.0; SPSS Inc.).
3. RESULTS
Patients with echocardiographically established LVH, that being either eccentric or concentric, compared to those without LVH, were older (70.7 vs. 62.9 years, p < .001), less commonly male (30.4% vs. 41.8%, p = .004), and had significantly higher rates of hypertension (70.3% for LVH vs. 42.5% for no LVH, p < .001) and diabetes mellitus (32.3% in LVH vs. 16.3% in no LVH, p < .001; Table 1). Among nonhypertensive patients with LVH, an underlying condition which possibly accounted for the latter finding was detected in 23% of cases and consisted of heart valve disease in most instances. Specifically, aortic valve disease and mitral regurgitation were equally distributed between subjects with either concentric or eccentric LVH, with conditions causing volume overload associated especially with eLVH. Occasionally, cardiomyopathies and congenital heart disease were found to be responsible for LVH development. A plausible underlying cause of LVH was not unveiled by the initial workup in the remainder 6.7%. Smoking was a more common characteristic in individuals without LVH (28.7% vs. 16.7% in LVH group, p = .001), while no significant difference was noted in the presence of dyslipidemia. As far as echocardiographic parameters are concerned, ejection fraction (EF) was lower (by 2.9%, p < .001) and left atrial (LA) diameter was greater (by 4.7 mm, p < .001) in the LVH group. With regard to LVH detection by ECG criteria, we noted an unprecedentedly low number of fulfilling positivity for SL voltage criterion (0.7% in LVH vs. 1.3% in no LVH, p = .5). The presence of the other ECG criteria of LVH was more often present in subjects with LVH (Table 1).
Table 1.
Clinical, echocardiographic, and electrocardiographic data of the study's sample classified based on the echocardiographic detection of LVH
| LVH (N = 148) | No LVH (N = 1,444) | p | |
|---|---|---|---|
| Age (y) | 70.7 ± 10.5 | 62.9 ± 11.8 | <.001 |
| Sex (male; %) | 30.4 | 41.8 | .004 |
| BMI (kg/m2) | 29.3 ± 5 | 28.6 ± 4.6 | .06 |
| Current smoking (%) | 28.7 | 16.7 | .001 |
| Diabetes mellitus (%) | 32.3 | 16.3 | <.001 |
| Dyslipidemia (%) | 53 | 44.7 | .06 |
| Hypertension (%) | 70.3 | 42.5 | <.001 |
| Systolic BP (mmHg) | 151.6 ± 20.8 | 143.4 ± 20.4 | <.001 |
| Diastolic BP (mmHg) | 84.7 ± 11.1 | 83.7 ± 11.0 | .31 |
| Pulse pressure (mmHg) | 59.7 ± 16.5 | 66.8 ± 17.8 | <.001 |
| EF (%) | 56.3 ± 8 | 59.2 ± 4.2 | <.001 |
| LVEDD (mm) | 49.0 ± 4.6 | 45.0 ± 3.7 | <.001 |
| LVESD (mm) | 31.3 ± 6.1 | 27.4 ± 4.3 | <.001 |
| MLVWS (kPa) | 14.4 ± 4.1 | 13.7 ± 3.5 | .021 |
| LA (mm) | 41.3 ± 5.4 | 36.6 ± 4.8 | <.001 |
| LV mass (BSA; g/m2) | 113.1 ± 18.3 | 78.4 ± 12.8 | <.001 |
| QRS duration (ms) | 99.7 ± 18.7 | 93.4 ± 13.8 | <.001 |
| ECG LVH (SL voltage; %) | 0.7 | 1.3 | .50 |
| ECG LVH (SL product; %) | 4.1 | 1.3 | .01 |
| ECG LVH (SL index; %) | 8.1 | 3.9 | .01 |
| ECG LVH (Cornell voltage; %) | 14.9 | 4.4 | <.001 |
| ECG LVH (Cornell product; %) | 25 | 9.2 | <.001 |
| ECG LVH (Lewis voltage; %) | 16.2 | 8.9 | .004 |
| ECG LVH (Framingham; %) | 1.4 | 0.3 | .04 |
Abbreviations: BMI, body mass index; BP, blood pressure; ECG, electrocardiogram; EF, ejection fraction; LA, left atrium; LVEDD, left ventricular end‐diastolic diameter; LVESD, left ventricular end‐systolic diameter; LVH, left ventricular hypertrophy; MLVWS, meridional left ventricular wall stress; SL, Sokolow–Lyon.
When comparing individuals with eLVH and cLVH, no statistically significant differences were observed in several common risk factors, with hypertension however being more common in subjects with cLVH (by 14.8%, p = .07; Table 2). With echocardiography, larger LA diameter (by 1.9 mm, p = .047), and left ventricular systolic (by 5.1 mm, p < .001) and diastolic diameter (by 5.6 mm, p < .001) were seen in the eLVH group compared with cLVH, with nonsignificant difference in the EF (54.7% vs. 56.9%, p = .13; Table 2; Figure S1). Importantly, MLVWS was found to be significantly higher in subjects with eLVH (by 6.1 kPa, p < .001; Figure S1).
Table 2.
Clinical, echocardiographic, and electrocardiographic data of individuals with echocardiographically proven LVH, classified based on the presence of eccentric or concentric LVH
| Eccentric LVH (N = 45) | Concentric LVH (N = 103) | p | |
|---|---|---|---|
| Age (y) | 70.1 ± 10.1 | 70.9 ± 10.7 | .69 |
| Sex (male; %) | 35.6 | 28.2 | .37 |
| BMI (kg/m2) | 29.1 ± 4.1 | 29.4 ± 5.4 | .76 |
| Current smoking (%) | 20.9 | 14.9 | .37 |
| Diabetes mellitus (%) | 30.8 | 33.0 | .81 |
| Dyslipidemia (%) | 55.3 | 52.1 | .74 |
| Hypertension (%) | 60.0 | 74.8 | .07 |
| Systolic BP (mmHg) | 151.3 ± 20.5 | 152.2 ± 21.6 | .82 |
| Diastolic BP (mmHg) | 85.0 ± 10.9 | 84.1 ± 11.4 | .65 |
| Pulse pressure (mmHg) | 68.1 ± 17.8 | 66.3 ± 17.8 | .59 |
| EF (%) | 54.7 ± 7.7 | 56.9 ± 8.0 | .13 |
| LVEDD (mm) | 52.6 ± 4.3 | 47.5 ± 3.7 | <.001 |
| LVESD (mm) | 35.0 ± 6.2 | 29.4 ± 5.0 | <.001 |
| MLVWS (kPa) | 18.6 ± 3.8 | 12.5 ± 2.6 | <.001 |
| LA (mm) | 42.6 ± 5.1 | 40.7 ± 5.5 | .047 |
| LVmass (BSA; g/m2) | 111.1 ± 14.2 | 114.0 ± 19.9 | .37 |
| QRS duration (ms) | 98.0 ± 18.9 | 100.5 ± 18.7 | .47 |
| ECG LVH (SL voltage; %) | 0.0 | 1.0 | .51 |
| ECG LVH (SL voltage; mV) | 1.85 ± 0.67 | 1.81 ± 0.70 | .69 |
| ECG LVH (SL product; %) | 4.4 | 3.9 | .88 |
| ECG LVH (SL product; mm ms) | 1822 ± 814 | 1788 ± 738 | .80 |
| ECG LVH (SL index; %) | 6.7 | 8.7 | .67 |
| ECG LVH (SL index; mV) | 0.63 ± 0.27 | 0.64 ± 0.33 | .80 |
| ECG LVH (Cornell voltage; %) | 11.1 | 16.5 | .40 |
| ECG LVH (Cornell voltage; mV) | 1.39 ± 0.59 | 1.60 ± 0.73 | .10 |
| ECG LVH (Cornell product; %) | 17.8 | 28.2 | .18 |
| ECG LVH (Cornell product; mm ms) | 1884 ± 860 | 2,225 ± 1,173 | .08 |
| ECG LVH (Lewis voltage; %) | 15.6 | 16.5 | .89 |
| ECG LVH (Lewis voltage; mV) | 1.13 ± 0.59 | 1.00 ± 0.71 | .30 |
| ECG LVH (Framingham; %) | 2.2 | 1.0 | .54 |
Abbreviations: BMI, body mass index; BP, blood pressure; ECG, electrocardiogram; EF, ejection fraction; LA, left atrium; LV, left ventricle; LVEDD, left ventricular end‐diastolic diameter; LVESD, left ventricular end‐systolic diameter; LVH, left ventricular hypertrophy; MLVWS, meridional left ventricular wall stress; SL, Sokolow–Lyon.
Regarding the frequency encountered of each ECG LVH criterion, there was no difference between eLVH and cLVH (Table 2). When ECG criteria were compared as continuous variables between LVH groups, Cornell voltage and product were higher in cLVH individuals (Figure 1) with a value of Cornell voltage >13.95 mV having 61% sensitivity and 62% specificity to differentiate cLVH from eLVH (p = .05).
Figure 1.
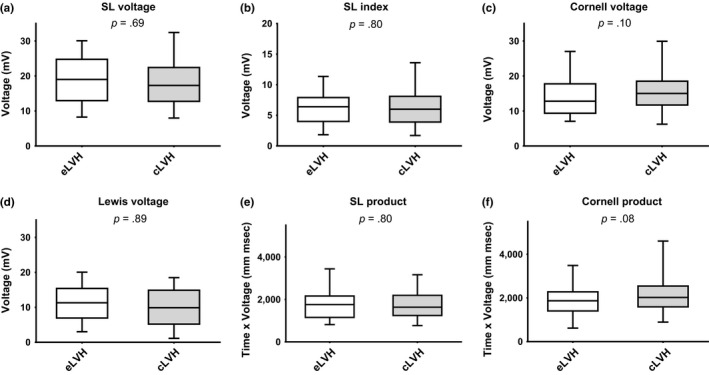
Box plots of ECG LVH criteria as continuous variables in subjects with eLVH and cLVH. Panel (a): Box plots of Sokolow–Lyon (SL) voltage. Panel (b): Box plots of Sokolow–Lyon (SL) index. Panel (c): Box plots of Cornell voltage. Panel (d): Box plots of Lewis voltage. Panel (e): Box plots of Sokolow–Lyon (SL) product. Panel (f): Box plots of Cornell product
Sensitivities, specificities, and negative and positive predictive values of the various ECG LVH criteria according to the type of LVH are presented in Table 3. As far as LVH as a sole entity is concerned, the Cornell product had the greatest sensitivity with a specificity over 90%. In individuals with eLVH, the Cornell product as well as the Lewis voltage criterion had hierarchically higher sensitivity. Regarding those with cLVH, the Cornell product had hierarchically higher sensitivity. Furthermore, all the examined criteria had a negative predictive value over 90% for the detection of both types of LVH.
Table 3.
Sensitivity, specificity, PPV, and NPV of the most commonly used criteria for the electrocardiographic detection of LVH in the diagnosis of eccentric and concentric LVH
| LVH Criterion | Eccentric LVH | Concentric LVH | LVH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| SL voltage | – | – | – | – | 1 | 98.7 | 5 | 93.5 | 0.7 | 98.7 | 5 | 90.7 |
| SL product | 4.4 | 98.5 | 8 | 97.2 | 3.9 | 98.6 | 16 | 93.7 | 4.1 | 98.7 | 24 | 91 |
| SL index | 6.7 | 95.8 | 4.4 | 97.2 | 8.7 | 96 | 13.2 | 93.8 | 8.2 | 96.1 | 17.6 | 91.1 |
| Cornell voltage | 11.1 | 94.8 | 5.8 | 97.3 | 16.5 | 95.4 | 19.8 | 94.3 | 14.9 | 95.6 | 25.6 | 91.6 |
| Cornell product | 17.8 | 89.6 | 4.7 | 97.4 | 28.2 | 90.6 | 17.2 | 94.8 | 25 | 90.9 | 21.9 | 92.2 |
| Lewis voltage | 15.6 | 90.6 | 4.6 | 97.4 | 16.5 | 90.9 | 11.2 | 94 | 16.2 | 91.1 | 15.8 | 91.4 |
| Framingham | 2.2 | 99.7 | 16.7 | 97.2 | 1 | 99.7 | 16.7 | 93.6 | 1.4 | 99.8 | 33.3 | 91.6 |
Abbreviations: LVH, left ventricular hypertrophy; PPV, positive predictive value; NPV, negative predictive value; SL, Sokolow–Lyon.
The presence of SL index, Cornell product and voltage, and Lewis voltage criteria were associated with echocardiographically proven cLVH. However, when adjustment for age, sex, BMI, and the presence of hypertension was performed, Lewis voltage and SL index lost their statistical significance. Indeed, the occurrence of Cornell voltage or product increased the odds of cLVH by 1.6 times (p = .001). However, none of the studied criteria reached statistical significance for the presence of eLVH (Table 4).
Table 4.
Results from logistic regression models examining the association between electrocardiographic criterion and echocardiographic detection of left ventricular hypertrophy (indexed for body surface area) before and after adjustment for age, sex, BMI, and presence of hypertension in individuals with concentric or eccentric left ventricular hypertrophy
| LVH criterion | Concentric LVH | Eccentric LVH | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate OR (95% CI) | p |
Multivariate OR (95% CI) |
p | Univariate OR (95% CI) | p | Multivariate OR (95% CI) | p | |
| SL voltage |
0.762 (0.101–5.753) |
.792 |
1.029 (0.122–8.701) |
.98 | – | – | – | – |
| SL product |
2.84 (0.956–8.434) |
.06 |
2.416 (0.747–7.812) |
.14 |
3.066 (0.7–13.419) |
0.137 |
2.858 (0.624–13.102) |
.176 |
| SL index |
2.319 (1.116–4.821) |
.024 |
2.07 (0.971–4.413) |
.06 |
1.627 (0.491–5.389) |
0.425 | ||
| Cornell voltage |
4.062 (2.289–7.209) |
<.001 |
2.708 (1.474–4.978) |
.001 |
2.259 (0.868–5.878) |
0.095 |
1.746 (0.649–4.695) |
.27 |
| Cornell product |
3.771 (2.372–5.993) |
<.001 |
2.589 (1.593–4.207) |
<.001 |
1.859 (0.851–4.06) |
0.12 |
1.384 (0.62–3.089) |
.427 |
| Lewis voltage |
1.981 (1.144–3.432) |
.015 |
1.589 (0.898–2.81) |
.11 |
1.780 (0.781–4.058) |
0.17 |
1.494 (0.648–3.447) |
.346 |
| Framingham |
2.910 (0.337–25.140) |
.332 |
4.147 (0.454–37.864) |
.21 |
7.009 (0.802–61.255) |
0.078 |
9.647 (1.065–87.417) |
.044 |
Abbreviations: CI, confidence interval; LVH, left ventricular hypertrophy; OR, odds ratio; SL, Sokolow–Lyon.
The performance of each ECG LVH criterion was further evaluated by the analysis of ROC curves (Table 5; Figure 2). All criteria but SL product depicted a good discriminating ability regarding the echocardiographic detection of concentric and eccentric LVH with the Cornell product found to have significantly higher AUC for cLVH compared with eLVH (0.61 vs. 0.52, p = .04).
Table 5.
Areas under the curve regarding the efficacy of ECG LVH criteria in differentiating between eccentric LVH and concentric LVH
| LVH criterion | Eccentric | Concentric | p ** | ||||
|---|---|---|---|---|---|---|---|
| AUC | SE | p * | AUC | SE | p * | ||
| SL voltage | 0.493 | 0.043 | .882 | 0.498 | 0.029 | .959 | |
| SL product | 0.515 | 0.045 | .736 | 0.513 | 0.03 | .672 | .97 |
| SL index | 0.512 | 0.045 | .780 | 0.524 | 0.031 | .413 | .83 |
| Cornell voltage | 0.53 | 0.046 | .499 | 0.56 | 0.032 | .041 | .59 |
| Cornell product | 0.515 | 0.039 | .399 | 0.61 | 0.027 | .001 | .04 |
| Lewis voltage | 0.531 | 0.045 | .483 | 0.538 | 0.031 | .202 | .9 |
| Framingham | 0.509 | 0.044 | .828 | 0.503 | 0.03 | .913 | .91 |
Abbreviations: AUC, area under the curve; SE, standard error.
Within‐group comparison.
Between‐group comparison.
Figure 2.
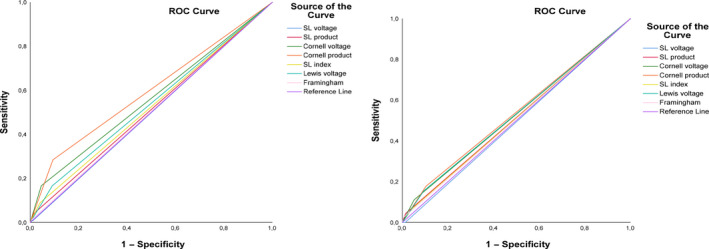
Receiver operating characteristic curve analysis of studied electrocardiographic criteria for the detection of different LVH types. Panel (a): Receiver operating characteristic curve analysis concerning eccentric left ventricular hypertrophy. Panel (b): Receiver operating characteristic curve analysis concerning concentric left ventricular hypertrophy. SL: Sokolow–Lyon
4. DISCUSSION
ECG preserves its paramount importance overtime as a valuable front‐line tool for the initial medical assessment either in the context of a routine examination or in emergent situations. This study conducted in an unselected and apparently healthy population provides further insights into the performance of the available LVH criteria in a primary care setting.
In the present work, the efficiency of seven classic criteria of ECG detection of LVH was assessed especially concerning their performance in differentiating concentric and eccentric LVH. As demonstrated, none of those can safely predict the type of LVH, with Cornell voltage and product having diagnostic accuracy for the detection of cLVH even after adjustment for important confounders such as age, sex, BMI, and the presence of hypertension. Moreover, diagnostic accuracy of Cornell product is significantly higher regarding cLVH compared with eLVH.
ECG has not only proven to be a powerful risk assessment tool in the general population but continues to be extensively used as one of the most reliable, cost‐effective, and reproducible diagnostic tests. Importantly, the prognostic significance of ECG when LVH is evident is irrespective of the presence or not of arterial hypertension. Moreover, when treatment of hypertension results in the improvement of ECG, this is accompanied by favorable outcome (Havranek, Emsermann, et al., 2008; Havranek, Froshaug, et al., 2008; Kannel, Gordon, Castelli, & Margolis, 1970; Okin et al., 2004). However, hypertension may be associated not only to concentric remodeling but also to left ventricular eccentric remodeling or even true left ventricular dilatation with possible clinical and prognostic implications (Bang et al., 2014). Moreover, eLVH is a common finding in situations associated with volume overload, valvular heart disease, or even dilated cardiomyopathy needing special attention, diagnostic approach, and management (Carabello, 2002; Kehat & Molkentin, 2010).
The most commonly used ECG criteria for diagnosis of LVH are based on the voltage of the QRS complex. However, the ECG voltage beyond left ventricular mass may depend on the composition of myocardial tissue and by extracardiac factors such as body and thorax profile, subcutaneous fat, air in the lungs, and gender (Levy et al., 1990). Correction may be achieved by incorporating sex‐specific factors in the determination of simple voltage criteria (Rautaharju et al., 2000). The duration of QRS complex is less dependent on extracardiac factors and is mostly affected by inherent properties of the heart, of the conduction system properties, and remodeling of the left ventricle (Quintanilla et al., 2017). Therefore, adaptation for the QRS duration in combined time‐voltage criteria may overcome limitations of the ECG diagnosis of LVH (Okin et al., 1995; Okin, Roman, Devereux, & Kligfield, 1996).
In the present study beyond the relatively low sensitivity of all examined criteria for the diagnosis of LVH, we documented differences in the performance of ECG criteria for LVH according to the type of left ventricular remodeling. Specifically, we found that sex‐dependent criteria and especially when they incorporate QRS duration characteristic (i.e., Cornell voltage and Cornell product) have the best performance for the diagnosis of cLVH independently of sex, age, BMI, and the presence of hypertension. Furthermore, we documented that Cornell product has greater diagnostic performance for cLVH compared with eLVH highlighting the possible differences in the pathophysiologic background of the two entities resulting in differences in the QRS amplitude and duration generated. However, none of the criteria examined in the present study appeared to be predictive for the occurrence of eLVH with results concerning the Framingham criterion being limited by the small number of subjects fulfilling it. Interestingly, the increased wall stress observed in subjects with eLVH may account for the strain ECG pattern which is already included in this criterion and can justify its performance in relevant cases. However, the multiplicity of pathophysiologic mechanisms related to eccentric remodeling and eLVH may have weaken the performance of the Framingham criterion in our study population.
As far as clinical implications are concerned, the high negative predictive value of all ECG criteria for both patterns of hypertrophy may possibly exclude a meaningful LVH in the absence of any of the pertinent criteria. Moreover, our data provide the rationale to apply different LVH ECG criteria according to possible clinical scenarios and cumulative risk factors in an individual basis. For instance, when pressure overload in the context of hypertension is the most plausible situation, Cornell voltage and product should be employed since they depict the highest sensitivity for the detection of cLVH.
This study has potential limitations. The primary limitation to the generalization of these results consists in the relatively small number of subjects identified with LVH and especially eLVH. Moreover, since ECG is usually applied to subjects with comorbidities or clinical indications related to LVH, selection bias exists. Accordingly, based on our unselected population we cannot conclude on the true diagnostic performance in subjects with high pretest probability. Furthermore, our study was neither designed nor powered to assess the possible influence of the different etiologies associated with LVH evolvement on the performance of the examined ECG LVH criteria. Further adequately powered studies may be needed to confirm our observations.
5. CONCLUSION
ECG, the most readily available and essential tool in the hands of a cardiovascular physician, remains the primordial screening tool for LVH, even though its sensitivity is underwhelming. We found that Cornell voltage and product criteria have superior discriminative ability for the detection of LVH via ECG. When further categorizing LVH as concentric and eccentric, the abovementioned criteria were more prevalent in cLVH. Translating this into predictive value, Cornell voltage and product criteria emerged as independent predictors of the presence of cLVH even after adjustment for common confounders in subjects with hypertension and pressure overload.
Supporting information
ACKNOWLEDGMENTS
We would like to thank Konstantinos Chronakis, Biomedical Engineer, PhD, for his technical support concerning ECG recordings.
Oikonomou E, Theofilis P, Mpahara A, et al. Diagnostic performance of electrocardiographic criteria in echocardiographic diagnosis of different patterns of left ventricular hypertrophy. Ann Noninvasive Electrocardiol. 2020;25:e12728 10.1111/anec.12728
Oikonomou and Theofilis contributed equally to this work.
REFERENCES
- American Diabetes A (2019). 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes‐2019. Diabetes Care. 42(Suppl 1):S13–S28. 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- Bang, C. N. , Gerdts, E. , Aurigemma, G. P. , Boman, K. , de Simone, G. , Dahlof, B. , … Devereux, R. B. (2014). Four‐group classification of left ventricular hypertrophy based on ventricular concentricity and dilatation identifies a low‐risk subset of eccentric hypertrophy in hypertensive patients. Circulation Cardiovascular Imaging, 7(3), 422–429. 10.1161/CIRCIMAGING.113.001275 [DOI] [PubMed] [Google Scholar]
- Carabello, B. A. (2002). Concentric versus eccentric remodeling. Journal of Cardiac Failure, 8(6 Suppl), S258–S263. 10.1054/jcaf.2002.129250 [DOI] [PubMed] [Google Scholar]
- Casale, P. N. , Devereux, R. B. , Alonso, D. R. , Campo, E. , & Kligfield, P. (1987). Improved sex‐specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: Validation with autopsy findings. Circulation, 75(3), 565–572. 10.1161/01.cir.75.3.565 [DOI] [PubMed] [Google Scholar]
- Devereux, R. B. , Alonso, D. R. , Lutas, E. M. , Gottlieb, G. J. , Campo, E. , Sachs, I. , & Reichek, N. (1986). Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. The American Journal of Cardiology, 57(6), 450–458. 10.1016/0002-9149(86)90771-x [DOI] [PubMed] [Google Scholar]
- Ganau, A. , Devereux, R. B. , Roman, M. J. , de Simone, G. , Pickering, T. G. , Saba, P. S. , … Laragh, J. H. (1992). Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. Journal of the American College of Cardiology, 19(7), 1550–1558. 10.1016/0735-1097(92)90617-v [DOI] [PubMed] [Google Scholar]
- Gardner, I. A. , & Greiner, M. (2006). Receiver‐operating characteristic curves and likelihood ratios: Improvements over traditional methods for the evaluation and application of veterinary clinical pathology tests. Veterinary Clinical Pathology, 35(1), 8–17. [DOI] [PubMed] [Google Scholar]
- Hancock, E. W. , Deal, B. J. , Mirvis, D. M. , Okin, P. , Kligfield, P. , Gettes, L. S. ; Heart Rhythm Society (2009). AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part V: Electrocardiogram changes associated with cardiac chamber hypertrophy: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: Endorsed by the International Society for Computerized Electrocardiology. Circulation, 119(10), e251–e261. 10.1161/CIRCULATIONAHA.108.191097 [DOI] [PubMed] [Google Scholar]
- Havranek, E. P. , Emsermann, C. D. , Froshaug, D. N. , Masoudi, F. A. , Krantz, M. J. , Hanratty, R. , … Steiner, J. F. (2008). Thresholds in the relationship between mortality and left ventricular hypertrophy defined by electrocardiography. Journal of Electrocardiology, 41(4), 342–350. 10.1016/j.jelectrocard.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havranek, E. P. , Froshaug, D. B. , Emserman, C. D. , Hanratty, R. , Krantz, M. J. , Masoudi, F. A. , … Steiner, J. F. (2008). Left ventricular hypertrophy and cardiovascular mortality by race and ethnicity. The American Journal of Medicine, 121(10), 870–875. 10.1016/j.amjmed.2008.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel, W. B. , Gordon, T. , Castelli, W. P. , & Margolis, J. R. (1970). Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease. The Framingham study. Annals of Internal Medicine, 72(6), 813–822. 10.7326/0003-4819-72-6-813 [DOI] [PubMed] [Google Scholar]
- Kehat, I. , & Molkentin, J. D. (2010). Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation, 122(25), 2727–2735. 10.1161/CIRCULATIONAHA.110.942268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, R. M. , Badano, L. P. , Mor‐Avi, V. , Afilalo, J. , Armstrong, A. , Ernande, L. , … Voigt, J. U. (2015a). Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal of Cardiovascular Imaging, 16(3), 233–270. 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- Lang, R. M. , Badano, L. P. , Mor‐Avi, V. , Afilalo, J. , Armstrong, A. , Ernande, L. , … Voigt, J. U. (2015b). Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography : Official Publication of the American Society of Echocardiography, 28(1), 1–39.e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Leigh, J. A. , O'Neal, W. T. , & Soliman, E. Z. (2016). Electrocardiographic left ventricular hypertrophy as a predictor of cardiovascular disease independent of left ventricular anatomy in subjects aged >/=65 years. The American Journal of Cardiology, 117(11), 1831–1835. 10.1016/j.amjcard.2016.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, D. , Labib, S. B. , Anderson, K. M. , Christiansen, J. C. , Kannel, W. B. , & Castelli, W. P. (1990). Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation, 81(3), 815–820. 10.1161/01.cir.81.3.815 [DOI] [PubMed] [Google Scholar]
- Levy, D. , Salomon, M. , D'Agostino, R. B. , Belanger, A. J. , & Kannel, W. B. (1994). Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation, 90(4), 1786–1793. 10.1161/01.cir.90.4.1786 [DOI] [PubMed] [Google Scholar]
- Lewis, T. (1914). Observations upon ventricular hypertrophy with especial reference to preponderance of 1 or other chamber. Heart, 5, 367. [Google Scholar]
- Molloy, T. J. , Okin, P. M. , Devereux, R. B. , & Kligfield, P. (1992). Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage‐duration product. Journal of the American College of Cardiology, 20(5), 1180–1186. 10.1016/0735-1097(92)90376-x [DOI] [PubMed] [Google Scholar]
- Mosteller, R. D. (1987). Simplified calculation of body‐surface area. The New England Journal of Medicine, 317(17), 1098 10.1056/NEJM198710223171717 [DOI] [PubMed] [Google Scholar]
- Okin, P. M. , Devereux, R. B. , Jern, S. , Kjeldsen, S. E. , Julius, S. , & Dahlof, B. (2000). Baseline characteristics in relation to electrocardiographic left ventricular hypertrophy in hypertensive patients: The Losartan intervention for endpoint reduction (LIFE) in hypertension study. The life study investigators. Hypertension, 36(5), 766–773. 10.1161/01.hyp.36.5.766 [DOI] [PubMed] [Google Scholar]
- Okin, P. M. , Devereux, R. B. , Jern, S. , Kjeldsen, S. E. , Julius, S. , Nieminen, M. S. ; Investigators LS (2004). Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. Journal of the American Medical Association, 292(19), 2343–2349. 10.1001/jama.292.19.2343 [DOI] [PubMed] [Google Scholar]
- Okin, P. M. , Jern, S. , Devereux, R. B. , Kjeldsen, S. E. , Dahlöf, BjörN. (2000). Effect of obesity on electrocardiographic left ventricular hypertrophy in hypertensive patients : The losartan intervention for endpoint (LIFE) reduction in hypertension study. Hypertension, 35(1 Pt 1), 13–18. 10.1161/01.hyp.35.1.13 [DOI] [PubMed] [Google Scholar]
- Okin, P. M. , Roman, M. J. , Devereux, R. B. , & Kligfield, P. (1995). Electrocardiographic identification of increased left ventricular mass by simple voltage‐duration products. Journal of the American College of Cardiology, 25(2), 417–423. 10.1016/0735-1097(94)00371-v [DOI] [PubMed] [Google Scholar]
- Okin, P. M. , Roman, M. J. , Devereux, R. B. , & Kligfield, P. (1996). Time‐voltage area of the QRS for the identification of left ventricular hypertrophy. Hypertension, 27(2), 251–258. 10.1161/01.hyp.27.2.251 [DOI] [PubMed] [Google Scholar]
- Okin, P. M. , Wright, J. T. , Nieminen, M. S. , Jern, S. , Taylor, A. L. , Phillips, R. , … Devereux, R. B. (2002). Ethnic differences in electrocardiographic criteria for left ventricular hypertrophy: The LIFE study. Losartan intervention for endpoint. American Journal of Hypertension, 15(8), 663–671. 10.1016/s0895-7061(02)02945-x [DOI] [PubMed] [Google Scholar]
- Prineas, R. J. , Rautaharju, P. M. , Grandits, G. , & Crow, R. ; Group MR (2001). Independent risk for cardiovascular disease predicted by modified continuous score electrocardiographic criteria for 6‐year incidence and regression of left ventricular hypertrophy among clinically disease free men: 16‐year follow‐up for the multiple risk factor intervention trial. Journal of Electrocardiology, 34(2), 91–101. 10.1054/jelc.2001.23360 [DOI] [PubMed] [Google Scholar]
- Quintanilla, J. G. , Moreno, J. , Archondo, T. , Alfonso‐Almazan, J. M. , Lillo‐Castellano, J. M. , Usandizaga, E. , … Filgueiras‐Rama, D. (2017). QRS duration reflects underlying changes in conduction velocity during increased intraventricular pressure and heart failure. Progress in Biophysics and Molecular Biology, 130(Pt B), 394–403. 10.1016/j.pbiomolbio.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Rautaharju, P. M. , Park, L. P. , Gottdiener, J. S. , Siscovick, D. , Boineau, R. , Smith, V. , & Powe, N. R. (2000). Race‐ and sex‐specific ECG models for left ventricular mass in older populations. Factors influencing overestimation of left ventricular hypertrophy prevalence by ECG criteria in African‐Americans. Journal of Electrocardiology, 33(3), 205–218. 10.1054/jelc.2000.7667 [DOI] [PubMed] [Google Scholar]
- Reichek, N. , Wilson, J. , St John Sutton, M. , Plappert, T. A. , Goldberg, S. , & Hirshfeld, J. W. (1982). Noninvasive determination of left ventricular end‐systolic stress: Validation of the method and initial application. Circulation, 65(1), 99–108. 10.1161/01.cir.65.1.99 [DOI] [PubMed] [Google Scholar]
- Salles, G. F. , Cardoso, C. R. , Fiszman, R. , & Muxfeldt, E. S. (2010). Prognostic impact of baseline and serial changes in electrocardiographic left ventricular hypertrophy in resistant hypertension. American Heart Journal, 159(5), 833–840. 10.1016/j.ahj.2010.02.012 [DOI] [PubMed] [Google Scholar]
- Sokolow, M. , & Lyon, T. P. (1949). The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. American Heart Journal, 37(2), 161–186. 10.1016/0002-8703(49)90562-1 [DOI] [PubMed] [Google Scholar]
- Wachtell, K. , Okin, P. M. , Olsen, M. H. , Dahlof, B. , Devereux, R. B. , Ibsen, H. , … Thygesen, K. (2007). Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: The LIFE Study. Circulation, 116(7), 700–705. 10.1161/CIRCULATIONAHA.106.666594 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


